Abstract
Lung transplantation is the only definitive therapy for many forms of end-stage lung diseases. However, the success of lung transplantation is limited by many factors: (1) Too few lungs available for transplantation due to limited donors or injury to the donor lung; (2) current methods of preservation of excised lungs do not allow extended periods of time between procurement and implantation; (3) acute graft failure is more common with lungs than other solid organs, thus contributing to poorer short-term survival after lung transplant compared with that for recipients of other organs; (4) lung transplant recipients are particularly vulnerable to pulmonary infections; and (5) chronic allograft dysfunction, manifest by bronchiolitis obliterans syndrome, is frequent and limits long-term survival. Scientific advances may provide significant improvements in the outcome of lung transplantation. The National Heart, Lung, and Blood Institute convened a working group of investigators on June 14–15, 2004, in Bethesda, Maryland, to identify opportunities for scientific advancement in lung transplantation, including basic and clinical research. This workshop provides a framework to identify critical issues related to clinical lung transplantation, and to delineate important areas for productive scientific investigation.
Keywords: allograft dysfunction, infection, ischemia-reperfusion injury, lung transplantation, obliterative bronchiolitis, rejection
The transplantation of organs offers the promise of life-saving and life-enhancing therapy for a variety of ailments. Lung transplantation (LTx) has become an acceptable therapy to palliate patients with a variety of end-stage lung diseases. However, lung transplantation has not turned out to be a panacea for chronic lung disease because of significant limitations in the entire transplant process (Figure 1). First, there are not nearly enough suitable donor lungs to meet the needs of all patients with end-stage lung disease. As a result, more patients die waiting for transplants than from mortality associated with a lung transplant. Second, early graft survival after LTx is worse than for other types of organs transplanted (1). Finally, late survival after successful LTx is hampered by the development of chronic allograft dysfunction and by the life-limiting complications associated with conventional immunosuppressive medications. Two factors impede progress to address these problems: (1) lung transplant centers lack the infrastructure to facilitate prospective, multicenter clinical studies of sufficient power to address clinical questions and (2) there are too few investigators studying the biology of lung transplantation.
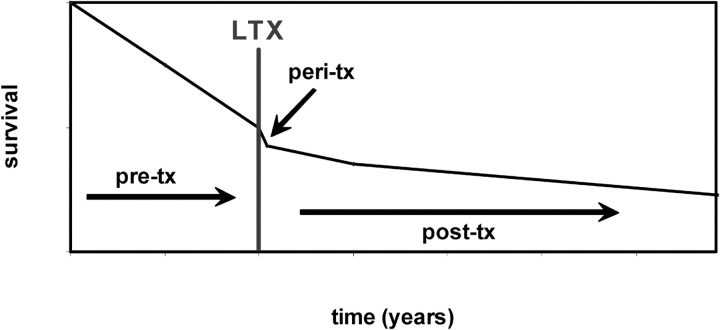
Figure 1.
Conceptual representation of the survival curve for prospective transplant patients before and recipients after lung transplant (LTX). This figure illustrates that survival rates decline for patients with end-stage lung disease pretransplant (pre-tx), and that the rate of decline is only impacted moderately after transplantation (post-tx).
The NHLBI convened a workshop on June 14–15, 2004, to better understand these complex problems and to identify strategies to address these and prioritize them. The topics addressed by participants were wide-ranging. They included the following: lung use rates from conventional brain-dead organ donors; safe limits for donor lung function; consideration of the use of lungs from non–heart-beating donors (NHBDs); ischemia-reperfusion injury (IRI); other causes of primary graft dysfunction; the host immune response to a lung graft; the consequences of current toxic immunosuppressive drugs, including infection and malignancy; the discouraging incidence of bronchiolitis obliterans syndrome (BOS) as a manifestation of chronic allograft dysfunction; and mechanisms associated with development of BOS. These discussions required input from a variety of disciplines, including lung transplant clinicians and experts in inflammation, immunology, infectious disease, biology of structural matrix, pulmonary epithelium and endothelium, and pharmacology. This article summarizes these discussions and attempts to identify key issues that should be the focus of future research in lung transplantation.
INADEQUATE NUMBER OF LUNGS FOR TRANSPLANT
There are many obstacles to organ donation, resulting in a reduced number of actual organ donors among the potential pool of brain-dead individuals. There are large differences in organ donation consent rates based on geographic area, cultural background, ethnicity, and age (2). In general, the level of lung procurement and transplantation has leveled off (Figure 2), providing an inadequate pool of transplantable lungs from deceased donors and providing an impetus to alternative sources of organs. The Division of Transplantation of the Health Resources and Services Administration, part of the Department of Health and Human Services, is beginning to address these issues (3). Unfortunately, there are even fewer suitable lungs than other solid organs among deceased donors because of lung injury from a combination of the high incidence of aspiration (4), neurogenic pulmonary edema (5), nosocomial infection, lung contusion, and acute respiratory distress syndrome/acute inflammation in trauma patients who become donors. Because each of these factors may adversely affect the outcome of lung transplantation, there is reluctance among lung transplant surgeons to use grafts from donors that may function poorly. The net result is a growing disparity between the number of candidates listed for transplantation and the number of transplants performed in the United States (Figure 2).
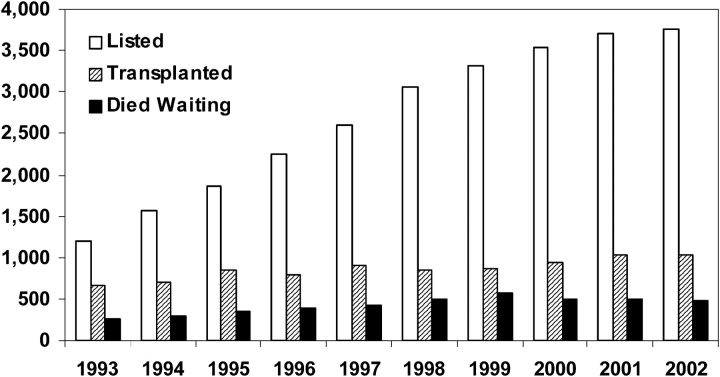
Figure 2.
Lung transplant patients waiting at years' end (open bars), patients undergoing lung transplant (hatched bars), and lung transplant patients dying while waiting (black bars), by year. Adapted by permission from Reference 3.
In the lung transplant community, there is considerable controversy over the specific characteristics of an acceptable donor/organ, and there is an ongoing effort to establish a classification system of lung donors to help determine the limits of acceptability while expanding the pool of available organs. There are substantial differences among organ procurement organizations in lung retrieval rates and among transplant programs in lung use rates. Options to increase the number of lungs available for transplant include encouraging adoption of more uniform, and evidence-based, policies by the Organ Procurement and Transplant Network, such as requirements to always offer potentially suitable lungs to transplant centers, to educate organ procurement organizations with poor lung retrieval rates, and to sanction organ procurement organizations consistently below acceptable performance standards. Such policies are under consideration by the United Network for Organ Sharing Thoracic Organ Committee.
The number of lung transplants being performed in recent years has increased only minimally, despite the increasing utilization of more “marginal” donors (3, 6, 7). Regardless of efforts made to maximize consent and retrieval rates from conventional donors, the number of lungs and other organs remains inadequate to meet the demand. According to Langone and Helderman (8), “Even in a utopian situation in which consent was obtained for all potential donors so that all potential organ donations were retrieved, there would be an inadequate number to satisfy our country's current and future needs.” Xenotransplantation offers the promise of an unlimited supply of donor organs, but the immunologic barriers and potential infectious risks have not yet been overcome (9–12). The working group and NHLBI made a decision to exclude discussion of lung xenotransplantation from this workshop.
Living-donor transplantation poses greater challenges in lung recipients compared with kidney and liver recipients. The morbidity and mortality associated with donor pneumonectomy pose significant ethical considerations. Bilateral, living, lower lobe transplants have been established as an acceptable alternative at some centers (8, 13, 14), but size constraints and the requirement for two donors for each recipient preclude widespread use of this procedure and limit the impact on the present shortage of donor lungs (15).
Interest is growing in the prospect of using lungs from deceased, circulation-arrested individuals, known as NHBDs. Before the concept of brain death was introduced, all lung transplants were performed using lungs retrieved quickly from circulation-arrested donors. Since brain death was introduced and widely accepted in North America and Europe in the early 1980s, virtually all lung transplants have been performed using organs retrieved from circulation-intact, brain-dead organ donors. Considerable experience with transplantation of kidneys retrieved from NHBDs is being accrued, with reports of equivalent or better long-term function of kidneys from NHBDs compared with conventional heart-beating donors (16, 17). A much smaller experience exists with liver transplants from NHBDs (16, 18, 19). Perceived limitations on warm ischemic time led to the classification of NHBDs into four categories, defined at the Maastricht conference in 1995 (20). The largest number of NHBDs are individuals who die outside of the hospital or arrive dead to the emergency service (Maastricht category I), and it is likely that few of these have kidneys or a liver that are salvageable for transplant.
However, there is some evidence that lung tissue remains viable for prolonged periods after circulatory arrest (21), an idea that is supported by the routine culture of airway epithelial cells from morgue specimens (22). There is considerable evidence in animal models that lungs may function adequately after transplantation even when retrieved hours after death (23). Variability in donor conditions and in warm ischemic times inherent in lung retrieval from individuals who arrive dead or who die in emergency rooms mandates that a reliable method be developed to predict function of lungs procured from human NHBDs. In renal grafts from NHBDs, pump perfusion has enhanced post-transplant graft function and also provided a rough measure (flow and resistance) of the expected function of the grafts after implantation. The development of comparable assays for lungs poses logistic and mechanical challenges; the feasibility of assessing gas exchange and circulation ex vivo has been demonstrated in porcine experiments (24, 25) and also performed with human lungs (26) (D. E. Van Raemdonck, personal communication, 2003; T. M. Egan, personal communication). Recently, Varela (27) has performed in vivo gas exchange evaluation in NHBDs to select lungs for transplant. If lungs can be retrieved from NHBDs and substantial numbers can be used for transplant, the shortage of lungs could be reduced. Ex vivo perfusion and ventilation for assessment of lung function also offers an opportunity for manipulation of the graft that may enhance performance post-transplant (28, 29).
If the number of lungs for transplant were unlimited, many more lives could be enhanced or extended, and progress would be accelerated in other areas of investigation, including clinical studies of the mechanisms underlying acute lung injury and chronic allograft dysfunction/BOS. In addition, indications for lung transplantation might be extended to individuals who currently are not considered candidates due to the organ shortage.
EARLY GRAFT DYSFUNCTION
The in-hospital survival after LTx is poorer than for other solid organs, as reflected in the 3-month survival of lung recipients of 85% (3). The two main reasons for this poor survival are early graft dysfunction and infection, and frequently the two clinical conditions coexist. Early graft dysfunction may be impacted by the condition of the donor lung. For example, data from Fisher and colleagues (30) indicate that high levels of interleukin 8 (IL-8) in the donor lung was a key risk factor for early graft dysfunction. In those patients, increased levels of IL-8 in the donor lung were associated with increased levels of neutrophils and worse oxygenation post-transplantation. Infection in the recipient may be related to contamination of the donor graft, preexisting infection in the recipient (as in cystic fibrosis), or nosocomial infection while intubated after surgery, and is aggravated by the need for potent immunosuppression after transplant. Early graft dysfunction is attributed to IRI, but there is little relationship between death due to early graft failure and the duration of ischemic time, except for that in older donors (31).
Understanding of the mechanisms contributing to IRI after lung transplantation would allow the interruption of these pathologic effector mechanisms and reduce the incidence of primary graft failure. Early graft injury also contributes to the risk for subsequent episodes of rejection and perhaps even lymphocytic bronchitis and bronchiolitis obliterans (32). A better understanding of lung IRI would facilitate the use of lungs from NHBDs to alleviate the donor shortage. Endothelial cells and mononuclear phagocytes are likely cellular mediators of lung IRI. Pulmonary macrophages have a major role in the elaboration of cytokines that contribute to IRI (33, 34). Although studies applying large-scale genomic technologies to lung IRI are preliminary, experiments using gene arrays suggest that hundreds of genes demonstrate up- or downregulation after reperfusion of transplanted lungs in both humans and rats (35, 36).
The pulmonary vasculature is a dynamically regulated, semipermeable barrier to the lung interstitium and epithelial surface of the alveolar membranes. Pulmonary endothelial cells serve as gatekeepers to trafficking inflammatory and immune cells. Profound vascular leak is a central component of the physiologic derangement that occurs in IRI; this provides a rationale to focus on the role of the pulmonary endothelium in the pathophysiology of IRI. Given the importance of hypoxia in organ procurement and reimplantation, the phenotypic changes of endothelial cells during oxygen deprivation have been investigated. Surprisingly, it is not even clear that hypoxia is a significant component of lung graft ischemia given that lungs are frequently retrieved from donors who have been ventilated with 100% oxygen. Nevertheless, hypoxia is associated with a decline in intracellular cAMP levels (37). As a consequence, endothelial cells retract from one another, promoting edema formation (38). Also, subjacent vascular smooth muscle cells increase their tonus (i.e., become vasoconstricted) (39). Endothelial cells become proadhesive for circulating leukocytes, and leukocytes and platelets tend to aggregate in microvessels. Supplementing deficient cyclic nucleotide signaling systems can prevent some of these changes after lung implantation (40).
After ischemia-reperfusion or transplantation, there are early changes in the endothelial cell cytoskeleton. Abutting edges between endothelial cells contract, resulting in gaps in the endothelial cell monolayer. Selective filtration mediated by the monolayer is abrogated, leading to mass transport of fluid and solutes into the alveolar space. The cells undergo many phenotypic and functional changes that facilitate injury (reviewed in References 41, 42). For example, glycoprotein adhesion receptors are strongly expressed on endothelium, and Weibel-Palade bodies undergo exocytosis (43). P-selectin is translocated to the endothelial surface, transcription of E-selectin increases, and expression of intracellular adhesion molecule 1 is upregulated (44). These events contribute to leukocyte diapedesis across the endothelial monolayer and tissue inflammation. Complement activation occurs early in lung ischemia and reperfusion (45), and contributes directly to injury both by a local deposition of the membrane attack complex as well as by release of circulating anaphylotoxins, C3a and C5a. Nitric oxide (NO), which is produced in abundance in the normal lung vasculature, is rapidly quenched by reactive oxygen species, such as superoxide (46). As a consequence, the homeostatic mechanisms normally maintained by NO are disrupted. Subsequently, there is thrombosis and leukostasis in the inflamed postischemic lung graft. Early expression of transcriptional mediators, such as early growth response gene 1 (Egr-1), as well as AP1 and nuclear factor-κB can also contribute to the inflammatory and procoagulant milieu seen early on after lung transplantation (47).
Depletion of pulmonary macrophages resulted in substantial reduction of lung IRI and improved graft function after transplantation (33, 48). Proinflammatory cytokines, such as IL-1, tumor necrosis factor α (TNF-α), and IL-6 play a pivotal role in IRI and cellular trafficking (49). These effects are modulated by the elaboration of antiinflammatory cytokines IL-4 and IL-10 (50). The balance of proinflammatory and antiinflammatory cytokines in lung allografts may predict primary graft dysfunction (51). IRI also has profound effects on extracellular matrix biology that is poorly understood, inducing injury repair mechanisms and regeneration, through pathways presumably that could be manipulated with therapeutic intent.
There are a number of strategies that have been shown to mitigate lung IRI after experimental transplantation. These can be broadly viewed as those in which normal homeostatic signaling mechanisms are bolstered, transcription is modulated, chemical reactions are inhibited, and inflammation or coagulation is suppressed. Specific strategies include delivery of antioxidants, β-agonists, or cAMP- or cyclic guanosine monophosphate–enhancing agents. For example, transcriptional blockers, such as antisense Egr-1, administered during the preservation period prevent both coagulant and leukoadhesive events after lung transplantation (47). Other strategies to block leukoadhesion or coagulation have also been used successfully in the setting of experimental lung transplantation (44, 52).
Endothelial barrier protective strategies include hepatocyte growth factor (53) inhibitors of endothelial myosin light-chain kinase activity (54, 55), oxidized phospholipids (41, 54–56), and the statins or hydroxymethylglutaryl-coenzyme A coreductase inhibitors (41, 54–58). Sphingosine 1-phosphate, a platelet-derived phospholipid growth factor, is involved in both angiogenesis and vascular hemostasis (59, 60) and recently has been shown to prevent lung edema in models of LPS-induced lung injury (61) and ventilator-induced lung injury (62).
Gene transfer strategies may be uniquely suited to address IRI after lung transplantation, because IRI is a short-lived injury that may not require prolonged gene expression, a problem that plagues the clinical application of other gene transfer strategies. Also, the ability to selectively transfect the lungs via the airway without systemic transfection is unique to the lung. In a proof-of-concept experiment, IRI was reduced in a rodent lung transplant model by gene transfer of the antiinflammatory cytokine IL-10 (63, 64), which modifies the environment of the transplanted lung by reducing inflammation and promoting apoptosis rather than necrosis (65). Endothelial NO synthase augmentation by gene transfer is another strategy that has been successful (65, 66). To circumvent the potentially deleterious inflammatory effects of adenoviral vectors, administration of conjugates of the free radical scavenging enzyme catalase to anti–platelet/endothelial cell adhesion molecule-1 antibodies successfully localized the enzyme to the pulmonary endothelium and reduced IRI after experimental lung transplantation (67). This is another potential strategy to target agents to the pulmonary endothelium.
Although not fully elucidated, IRI may contribute to rejection episodes through generation of novel antigens. For example, IRI may release novel antigens from the interstitium of the lung graft or alter the structure/function of molecules that under normal conditions contribute immune homeostasis in the lung. Accordingly, addressing IRI may have far reaching effects on the overall health of the lung graft.
In summary, it is unlikely that a single strategy will succeed as the one to trump all others in reducing lung IRI. However, understanding each possible unique contributing mechanism may help unravel clues toward the possibility of inhibiting common pathologic pathways. The goal of this approach would be to reduce early morbidity and mortality from the lung transplant procedure, and potentially facilitate the use of lungs retrieved from NHBDs, and to modify later immunology of the rejection response.
IMMUNOLOGY OF THE HOST RESPONSE: ACUTE AND CHRONIC REJECTION
Acute and chronic rejection are causes of significant morbidity and mortality. In-depth discussions and reviews of these processes have been reported recently (68, 69). The current section will provide a brief overview with an emphasis on those areas in need of greater investigation.
During the alloimmune response, T cells recognize foreign major histocompatibility complex (MHC) antigens by two distinct pathways of direct and indirect allorecognition. The direct pathway is important in the vigorous alloimmune response seen early post-transplantation when abundant donor-derived antigen-presenting cells are present within the graft. Indirect allorecognition may also occur when processed host antigen-presenting cells present donor allopeptides; this pathway is responsible for development of T-helper cells that promote alloantibody production and possibly obliterative bronchiolitis (OB). OB is the histologic hallmark of chronic allograft rejection in transplanted lungs and OB remains the single most important problem limiting the long-term survival of the graft and recipient. OB presents clinically with progressive dyspnea and airflow obstruction. The term “bronchiolitis obliterans syndrome” is used to define the clinical problem of chronic rejection without histologic proof of OB (70).
Acute rejection is characterized by two key histologic findings: perivascular infiltration of mononuclear cells and by an influx of CD4+ and CD8+ lymphocytes and macrophages into the subepithelial layer of the airway forming a lesion known as lymphocytic bronchiolitis; this appears to be a harbinger of OB/BOS (71, 72). A fibroproliferative phase develops later in the airways in which macrophages and myofibroblasts predominate. Cytokines (TNF-α, IL-1β, IL-6, IFN-γ, and IL-2) have been implicated in the pathogenesis of OB/BOS (71–76). The regulatory cytokines IL-12 and IL-10 also likely play a key role in this process, but have not been studied in detail. Although much work has been done in allografts other than the lung, there is a lack of information regarding allorecognition networks and cytokine pathways involved in lung allograft rejection.
Chemokines in the Rejection Response
The predominant function of chemokines is to induce the migration and activation of leukocytes, including neutrophils, monocytes, lymphocytes, eosinophils, and dendritic cells (77). Leukocyte chemotactic and activating effects of chemokines are vital to the development of effective host response to an “antigen” (i.e., xeno-, allo-, autoantigens) and the subsequent shift from innate to polarized adaptive immunity with the generation of a predominance of T-helper (Th) type 1 versus Th2 cytokine expression within the local microenvironment. Moreover, chemokines have broad effects on other facets of lung allograft rejection related to homeostasis, inflammation, and fibrosis.
Studies indicate that chemokines play a major role in directing alloantigen-primed T cells and other effector leukocytes into grafts to mediate rejection (78, 79). During the rejection of allografts in rodent models, the production of chemokines in grafts occurs in two temporal cascades. Chemokines in the first cascade appear shortly after reperfusion and at equivalent levels in iso- and allografts and include chemokines directing the recruitment of cellular components of the innate immune system (e.g., neutrophils, macrophages, and natural killer cells). These chemokines are induced in response to the surgical trauma to tissue and IRI imposed on the graft. Studies show attenuation of early inflammation and tissue damage during reperfusion of ischemic organs, including allografts, by interfering with the activity of neutrophil chemoattractants (52). This strategy might be useful in promoting long-term survival of allografts.
The second chemokine cascade begins as the early cascade subsides, and occurs only in allografts. These chemokines direct recruitment of alloantigen-primed T cells and other effector leukocytes into the allograft (80–82). These chemokines include the CXCR3 ligands and the CCR5 ligands. Antagonism of CXCR3, CCR5, and their respective CCR5 ligands can attenuate recruitment of primed T cells and other effector leukocytes trafficking into allografts and prolong allograft survival (81). In addition to their chemoattractant properties, chemokines may also influence the level and phenotype of T-cell priming. The presence of specific CCR5-binding chemokines in the lymphoid tissue draining the graft supports a model of chemokine-mediated regulation of alloreactive T-cell priming in response to the allograft. Collectively, these studies indicate diverse and complex functions of chemokines regulating recipient immune responses to allografts. Indeed, IL-8 (neutrophil chemotaxis), the C-C chemokines (RANTES [regulated on activation, normal T-cell expressed and secreted], macrophage inflammatory protein 1α, macrophage inflammatory protein 1β), and the IFN-γ–induced chemokines (interferon-γ–inducible 10kd protein, interferon-induced monokine, and interferon-inducible T-cell alpha chemoattractant) all have been implicated in the pathogenesis of OB/BOS in both human and animal studies (80, 82, 83). Because chemokines have key roles in trafficking, remodeling, and priming immune responses, more study is required to understand the function of these critical molecules in lung transplantation in general, and OB in particular.
Autoimmunity, Regulatory T cells, and Tolerance
A clear role for alloimmunity in the pathogenesis of the rejection response has been documented in many studies. An ideal approach to preventing rejection would be to introduce a state of tolerance to the graft rather than generalized suppression of acquired immunity. The cells that mediate or regulate tolerance to allografts are the same cells that mediate tolerance to self-antigens. Accordingly, loss of tolerance to alloantigens could also predispose an individual to autoimmunity. In turn, autoimmunity could participate in the rejection response (84–87). Release of novel self-antigens could result from repeated acute rejection episodes in which sequestered self-antigens are released after a cycle of damage and repair. For example, collagen V, a highly conserved self-antigen sequestered in the perivascular and peribronchial spaces of the lung, is released into the transplanted lung after IRI or after rejection episodes (87). This may account for the presence of collagen V–specific T cells isolated from rat lung allografts during rejection (87, 88). The role of collagen V reactivity in the rejection response is highlighted by reports showing that collagen V–specific T cells induce rejection-like disease in rat isograft lungs (87), and that human lung transplant recipients who develop immunity to collagen V are at greater risk for graft loss and development of OB (W. Burlingham and D. S. Wilkes, unpublished manuscript).
Regulatory T cells (Tregs) are CD4+CD25+ cells that function to suppress auto- and alloimmune responses. Developing an immune response to self- and alloantigens implicates that Treg dysfunction occurs during lung allograft rejection. Data are limited regarding the role of Tregs in lung transplant rejection. One report in humans indicates that the rejection response and OB were associated with fewer numbers of CD4+CD25+ T cells (89). These cells are believed to mediate immune suppression in a contact-dependent manner by membrane-associated transforming growth factor β (TGF-β). Therefore, for Tregs to mediate suppression, the responding cells should be permissive to TGF-β–mediated signaling. Evidence for this is a report showing that pathogenic autoreactive cells isolated from rat lung allograft recipients did not express SMAD7 transcripts, a key negative regulator of TGF-β signaling (88).
Common pharmacologic mediators used to prevent rejection may actually diminish protective tolerance. Modulation of intracellular calcium levels by calcineurin is a major mechanism of immunosuppressive drugs, such as cyclosporine and FK506. Calcineurin inhibitors suppress IL-2 transcription. Although suppression of IL-2 has been documented to downregulate alloimmune responses, it may cause a breach of tolerance. Treg function is dependent on IL-2. As initially suggested by other investigators, perhaps immunosuppressive drugs that interfere with IL-2 have detrimental effects on Tregs and the development of tolerance to transplanted organs (90). Therefore, because Tregs function to suppress immune responses to self-antigens, calcineurin inhibitor–induced suppression of Treg function could precipitate the autoimmune responses observed during lung allograft rejection. These data highlight the need for a better understanding of the properties of current treatment regimens in organ transplantation, including lymphocyte depletion strategies, to prevent adverse immune responses and to promote tolerance induction. In addition, there is a great need for in-depth analysis of interaction of allo- and autoimmunity in the rejection response, and the roles of Tregs in the modulation of OB.
Humoral Immunity
Although much attention has been focused on cellular immunity that could mediate OB, recent evidence suggests that humoral events may also be involved. For example, the presence of anti-HLA antibodies was associated with steroid-resistant allograft rejection and increased soluble C4d as measured in bronchoalveolar lavage (BAL) (91, 92). Detection of C4d, a component of the complement cascade, is a strong indicator of active humoral immunity. HLA antibodies develop in a significant proportion of lung transplant recipients (25%), predominately in the first year (91). Anti-HLA antibodies are associated with an increased frequency of refractory acute rejection in the first 6 months, and with a higher prevalence of BOS in the late post-transplant period (91). During acute cellular rejection, there is an increased ratio of IgG2/IgG1 within the lung allograft, suggesting local upregulation of Th1 activity (93). Although non–complement-fixing antibodies can activate bronchial epithelial cells by cross-linking antigens (94), recent studies in transplants other than the lung implicate complement activation in the pathogenesis of rejection. For example, systemic activation of complement by cobra venom in rats causes pulmonary vascular leakage that can be prevented by blocking C5a (95). Parallel findings have been reported for complement activation by ischemia-reperfusion of limbs, cardiopulmonary bypass, and sepsis. OKT-3 activates complement (96) and can also cause an accumulation of neutrophils in pulmonary capillaries that might be confused with “capillaritis” (97). Indeed, reports from several groups using microarrays in a rodent model indicate that antibody and complement genes are upregulated in the transplanted lung (45, 98, 99). These data are similar to prior reports from other investigators (45, 98). Antibody-producing plasma cells can be identified by immunohistology in transplanted lungs. Because macrophages and epithelial cells are sources of complement, both immune cells (e.g., macrophages) and traditionally nonimmune cells (e.g., epithelium) could contribute to the pathogenesis of BO. However, the incidence of antibody/complement-mediated injury to lung transplants is unknown, and the primary cause of complement activation in the lung has not been established. Finally, the target antigens of the locally produced antibodies have not been fully identified.
Innate immune mechanisms.
In recent years, the complexity and central importance of innate immunity in host defense have been recognized. In contrast to adaptive immunity, which relies on highly specific B- or T-cell receptor interactions with foreign antigen presented in the context of host MHC, the secreted cellular and intracellular components of host innate defense recognize and respond to pathogen-associated molecular patterns (PAMPs). These PAMPs represent molecular structures highly conserved among large classes of microbial pathogens, not present in the host. Endotoxin, or LPS, for example, is a prototypic trigger of innate immunity commonly found on gram-negative bacteria. Cellular components of innate immunity, such as macrophages and dendritic cells, rely on the Toll-like receptors (TLRs) to recognize PAMPs. Currently, 11 TLRs have been identified (100), most associated with recognition of distinct PAMPs.
Activation of specific innate immune pathways through TLRs is now recognized as critical to the development of adaptive immune responses. Important effects on adaptive immunity include differentiation of dendritic cells, upregulation of MHC, upregulation of costimulatory molecules (e.g., CD80 and CD86), and production of various chemokines and cytokines (101). The presence of appropriate costimulation is essential for naive T cells to develop an allo-specific response (i.e., clonal expansion) to a foreign peptide presented in the context of self-MHC. Recent evidence also suggests that complement activation might regulate B-cell antibody production and T-cell responses.
Goldstein and colleagues (102) were first to report a critical role for a TLR signaling pathway in allograft rejection. Compared with wild-type mice, skin allograft rejection was downregulated in mice deficient in MyD88, a key TLR signaling molecule. Using the same approach, these investigators found decreased skin graft rejection in TLR2−/− mice but not TLR4−/− mice. These data show MyD88-dependent and -independent signaling is involved in TLR-mediated allograft rejection, and demonstrate a key role for TLRs in linking the innate and adaptive immune response during rejection.
The role of polymorphisms in TLR4 in human lung allograft rejection has been reported recently. Using polymerase chain reaction allelic discrimination assays, specific TLR4 polymorphisms at Asp299 and Thr399 alleles in the recipient were associated with a lower incidence of rejection (29% rate of rejection in TLR4 mutants vs. 56% rate of rejection in wild-type recipient) (103). In contrast, no effect was seen with the 299/399 polymorphisms on rejection by donor genotype. There was also a trend toward a significant delay in the time to first acute rejection in TLR4 polymorphic recipients as compared with wild-type recipients. In a larger cohort monitored over a 3-year period, post-transplant individuals with the 299 or 399 polymorphisms had a significant reduction in rejection episodes (104).
One intriguing hypothesis that emerges from these studies is that the failure of currently available, primarily T-cell–based therapies to completely prevent rejection, particularly in the lung, might not reflect failure at the T-cell level, but failure to prevent innate immune responses from activating T cells or other acquired immune responses. Specifically, local activation of innate immunity (through macrophage TLRs, complement, and other innate effectors) might drive the alloimmune response toward rejection despite the use of effective T-cell–based immunosuppression. The innate response to pathogens, especially viruses, may also be important because community-acquired viral infections have been reported to be associated with BOS (105). However, the molecular and cellular mechanisms that regulate the response to microbes in the transplanted lung are in need of more in-depth investigation.
Immunosuppressants used to prevent rejection suppress both humoral and T-cell–mediated immunity. Thus, these patients are more dependent on innate immunity to prevent and clear potential pathogens. Therefore, other key effectors of lung innate immunity, including alveolar macrophages (AMs), and the collectins, such as surfactant proteins SP-A and SP-D (106, 107), assume a critical role in host defense against invading pathogens. AMs bind, phagocytose, and kill pathogens by producing reactive oxygen/nitrogen intermediates (108), such as NO (109), and trigger specific immunity by presenting antigen to T lymphocytes (110). SP-A induces opsonization of pathogens by binding mannose and N-acetylglucosamine residues on microbial cell walls (111), which enhances their attachment and phagocytosis by AMs via specific and nonspecific receptors (112–118). SP-A also induces AM chemotaxis and killing that stimulate release of reactive oxygen intermediates, such as NO (107, 118). Therefore, in addition to its well defined effects in downregulating AM function, in response to infection, SP-A can function as an opsonin-enhancing, AM-mediated killer of pathogens.
Homeostasis of the lung microenvironment is altered dramatically in the transplant recipient. For example, although SP-A decreases NO production in AMs obtained from the BAL of normal volunteers, SP-A significantly increased NO production in 50% of AMs from transplant patients (109). Thus, SP-A appears to turn on the innate immune response in the presence of inflammation and infection while suppressing inappropriate activation of AMs in the normal lung. This has significant implications in the transplant setting where inappropriate release of NO by AMs could account for lung pathology commonly observed in these patients. Further complicating this issue are data showing that nitration of SP-A limits its effectiveness, and nitrated proteins are increased markedly in BAL fluid from lung allograft recipients (119). These reports show a complex relationship between AMs and SP-A in the context of normal homeostatic mechanisms and regulation of local innate immunity in the allograft lung. However, the extent of SP-A nitration in the alveolar spaces in transplanted lungs is not known, and the potential effects of commonly used immunosuppressants on SP-A and AM biology are largely unknown. Further research is needed to determine if targeting specific innate immune receptors/pathways or mediators of innate immunity effectively reduces allograft rejection and improves transplant outcomes. Finally, more studies are required to discern how innate immune responses affect acquired immunity in the transplant recipient.
Chronic Allograft Dysfunction: OB/BOS Markers, Mechanisms, Potential Therapies
Long-term survival rates for lung transplant recipients are considerably lower than those observed in kidney, heart, and liver recipients. This is due both to the poorer early survival rate and to the development of chronic rejection manifest clinically as BOS. This rejection is defined by progressive airflow obstruction and deterioration of graft function (70). BOS is often associated with a specific histologic lesion, OB, which is characterized by inflammation and fibrosis of small airways (120). On a conceptual basis, BOS and OB represent progressive loss of the cross-section of the airways in the transplant patient (Figure 3).
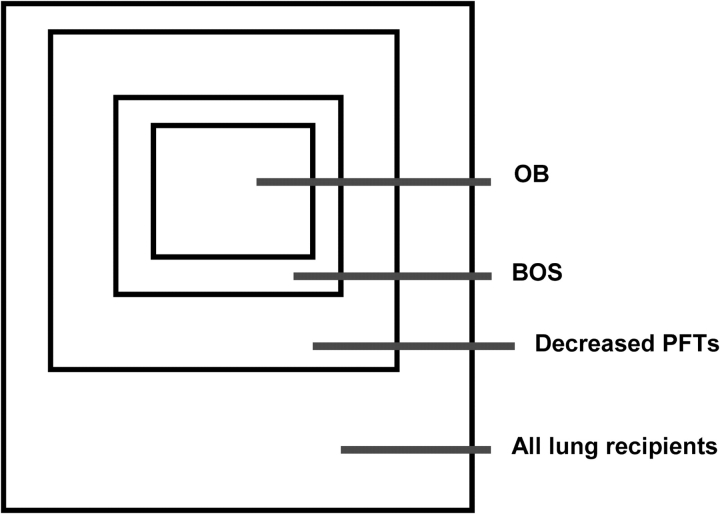
Figure 3.
Venn diagram of airway dysfunction after lung transplantation. Schematic representing the relative caliber of the airways, which often presages the fate of lung transplant recipients. Many patients post-transplant will develop airflow obstruction as measured by pulmonary function testing (PFT). This is sometimes related to infection, but often due to development of bronchiolitis obliterans syndrome (BOS). Often, but not always, BOS is associated with histologic demonstration of obliterative bronchiolitis (OB). (Courtesy of Marshall Hertz, M.D., University of Minnesota.)
Current therapies for OB/BOS are sometimes able to slow or arrest disease progression; however, recovery of lost lung function is unusual (121). Therefore, identification of reliable, reproducible, and noninvasive risk biomarkers for prevention and preclinical detection of OB/BOS are important goals. Acute rejection histology, characterized by perivascular and peribronchiolar lymphocytic inflammation, has repeatedly been identified as a major risk factor for OB/BOS (122). However, putative markers of acute rejection, such as profiles of cellular activation (123) or cytokine levels in BAL fluid observed during acute rejection (124), are not reliable indices of OB/BOS. Markers of granulocyte activation in BAL fluid have also been identified before or concurrently with the development of OB/BOS (125, 126). In addition, profibrotic cytokines have been identified in BAL fluid from these patients (127, 128).
Gimino and colleagues (129) performed analyses of gene expression in BAL and peripheral blood mononuclear cells from lung transplant recipients with acute rejection and with no rejection. Two-dimensional hierarchic clustering based on the 135 most overexpressed genes in the acute rejection samples grouped all acute rejection samples into one cluster, and the majority of the nonrejection samples into a second cluster. Genes that were upregulated in the acute rejection group included the following: genes previously known to be involved in acute rejection, immune-response genes with an unknown role in rejection, genes not known to have a role in rejection, and genes of unknown function. Gene expression patterns of peripheral blood mononuclear cells are markedly different than those of BAL cells. Tracking the evolution of gene expression patterns in subjects who develop OB/BOS may provide valuable clues to pathophysiology and elaboration of therapeutic strategies for intervention.
Protein biomarkers of chronic rejection have been identified using archived BAL fluid from lung transplant recipients whose clinical outcomes are now known (129). Human neutrophil peptides 1–3, members of the α-defensin family of peptides, have been associated with development of BOS, independent of episodes of acute rejection or cytomegalovirus (CMV) and fungal infections. Identification of other protein biomarkers in BAL fluid of lung transplant recipients that will predict subsequent development of BOS would be of value.
The important role of alloantigen-independent factors in the development of BOS is underscored by the observation of an association between gastroesophageal reflux disease and the development of BOS (130, 131), and the recent observation of an association between the presence of bile acids in BAL fluid and the development of BOS (132).
BOS is a diagnosis of exclusion, because acute rejection and infection can also cause airflow obstruction. Thus, invasive maneuvers like transbronchial biopsy and BAL are usually indicated for establishing the diagnosis. Currently, therapy for new-onset BOS usually starts with augmenting or altering the patient's immunosuppressive regimen (121, 133, 134). Because methotrexate has been used in patients with recurrent acute rejection (135), it has been used to treat BOS. Photopheresis therapy has been advocated by some centers to treat patients with BOS, but there are limited reports documenting its efficacy (136).
Gerhardt and colleagues (137) reported that five of six patients with BOS had significant improvement in pulmonary function with oral azithromycin therapy, suggesting a potential role for azithromycin in lung transplant recipients. Patients with cystic fibrosis experienced clinical improvement and fewer exacerbations on maintenance azithromycin therapy (138–140). Macrolide antibiotics exert both antiinflammatory and nonbactericidal antimicrobial effects (141–143). In a retrospective analysis of more than 200 lung transplant recipients, Johnson and colleagues (144) demonstrated a strong association between the use of statins to control hypercholesterolemia, and freedom from the development of BOS. Other effects of the HMG coreductase inhibitors other than their cholesterol-reducing properties have been alluded to above, and statins have been shown to reduce lung IRI (145). These and other treatment strategies would be potential candidates for multicenter prospective clinical trials to establish efficacy and delineate mechanisms of action.
In summary, the current paradigm of T-cell–based allorecognition driving the development of acute lung rejection and BOS is built, in part, on an understanding of alloimmunity acquired through studies of other solid organ transplants. However, this model does not adequately explain why acute and chronic rejection rates are dramatically higher in lung transplant recipients as compared with other solid organ transplant recipients. Furthermore, the effect of the unique and constant environmental exposures in the lung and the role of recurring infections (both endogenous, such as CMV, and exogenous, including bacterial and fungal) on immune activation is likely important. Similarly, the central role of AMs and innate immunity in pulmonary host defense must also be considered.
INFECTION AND REJECTION
The role of infection in lung transplantation is well established. CMV causes both latent infection in seropositive individuals and invasive infection in the transplant recipient. CMV and other viruses (respiratory syncytial virus, influenza, Epstein-Barr virus) contribute not only to direct graft injury (i.e., pneumonia) but also to an array of indirect effects, including predisposition to other opportunistic infections (e.g., pneumocystis and Aspergillus infections) and to the risk of rejection and post-transplant malignancy. CMV seropositivity in the donor or recipient is associated with an increased risk of death (146–148). Viral infections, including CMV, respiratory syncytial, parainfluenza, and adenoviral infections, are risk factors for BOS (discussed below) (105, 149–152). Allorejection may be enhanced by coinfection of grafts by viral or other pathogens through a process termed “heterologous immunity.” This is believed to involve upregulation of MHC antigens and presentation of novel or cross-reacting epitopes on the surfaces of infected cells (153–155). In general, the mechanisms by which infection impacts the outcomes of lung transplantation remain poorly defined and are important areas for future investigation. The role of viral infection in the development of malignancy (Epstein-Barr virus and post-transplant lymphoma) is beginning to be explored. Better models of infection and malignancy in lung transplantation should be developed.
IMMUNOSUPPRESSION
The discovery of the calcineurin inhibitor cyclosporine A ushered in the modern era of successful extrarenal solid organ transplantation (156). However, it is also true that current drugs used as immunosuppressants are (1) relatively nonspecific regarding donor/recipient interactions, (2) toxic, and (3) cause problems that contribute to morbidity and mortality after solid organ transplant, both because of specific organ toxicity (especially renal) and the increased risk of opportunistic infections from the “broad” immunosuppressive strategies used to prevent rejection (157).
The optimal immunosuppression regimen for lung transplant recipients is unknown. Although there is some variability among programs, most use “triple immunosuppression” consisting of a calcineurin antagonist (cyclosporine or tacrolimus), an antimetabolite (azathioprine or mycophenylate), and corticosteroids (158). The danger of the early use of rapamycin in lung recipients has been documented because of its antifibrotic effects on bronchial wound healing (159). However, the same antifibrotic effects may make this a useful agent to arrest progression of BOS. There is considerable variability in the use of induction therapy with antilymphocyte agents (158), but there are some encouraging early data supporting the use of alemtuzumab in a small number of lung transplant recipients with short follow-up periods (160). The lung is ideally suited to the strategy of “local” immunosuppressive therapy because of the ability to use the airway as a conduit for medications. Aerosolized cyclosporin A has been used to treat refractory acute rejection, and may permit reduction of systemic drug levels (161).
Recent advances in genomics may allow for in-depth analyses to determine optimal immune suppression. Genetics may influence the nature of the immune response among individuals. Similarly, the genetic makeup of patients may alter the efficacy or side-effect profiles of pharmacologic agents. Genetics influence drug efficacy and pharmacokinetics in individuals through variability uptake and metabolism of medications. This concept, known as pharmacogenomics, has been shown to have a major impact in determining which medications may be effective in individual patients treated with cancer chemotherapeutics (162). However, the application of pharmacogenomics in solid organ transplantation has not yet been reported. The possibility exists that immunosuppressive regimens may be tailored for an individual patient to minimize toxicities and to address the individual's needs for specific antirejection therapies for the organ transplanted. As such, pharmacogenomics is a field requiring more in-depth study in organ transplant recipients.
STRENGTH THROUGH NUMBERS
Currently, the small number of lung transplant procedures performed at a relatively large number of centers is a serious obstacle to collection of useful information concerning the biology of the transplanted lung. Accordingly, establishing a network of centers to facilitate scientific investigations should lead to improvement in outcomes for the transplant recipient.
Such a network would also facilitate uniformity in classification of donor quality, immunosuppression strategies, standardization of terminology of graft failure and development of BOS, and standardization of tissue and specimen collection and preparation. The development of this network would provide a forum for consistent data collection and an opportunity for lung transplant recipients to participate in multicenter clinical trials to properly evaluate therapeutic strategies to treat OB/BOS that have been promulgated with inadequate evidence-based support.
CONCLUSIONS
Lung transplantation is a relatively new field aimed at the treatment of end-stage lung failure. The challenges facing the lung transplant patient and medical teams are only beginning to be addressed, but are important areas for basic and clinical investigation. Strategies are needed to increase the number of organs available for transplantation and to reduce graft destruction mediated by infection, inflammation, and immunity in the post-transplant period. The condition of the donor lung in the pretransplant period is inextricably linked to function post-transplantation. Accordingly, developing techniques that lead to tolerance of the allograft, the Holy Grail of transplantation for all organs, is the ultimate goal of these approaches. It is the hope of the working group that the recommendations listed in Table 1 will greatly improve our understanding of lung transplant biology, and ultimately will lead to decreased morbidity and mortality for our patients.
TABLE 1.
General recommendations of the working group
| 1. Establish a multicenter collaborative network to enhance the standards of clinical protocols; understand the disease of BOS, including a lung tissue repository to assist in the characterization of the disease; and conduct clinical trials. | |
|---|---|
| 2. Increase use of the present donor pool of lung organs (use in United States is about 15% vs. 30 to 40% in European countries). Public policy issues should increase awareness of organ donation, and explore the feasibility of expanding the donor pool by use of lungs from non–heart-beating donors. | |
| 3. Develop methods to better assess quality of donor lungs before transplantation and to reliably predict graft failure. | |
| 4. Assess new strategies for creating tolerance in the recipient and optimizing immunosuppression using pharmcogenomic methods. | |
| 5. Identify better biomarkers, based on proteomic and genomic approaches, that will predict BOS before patient symptoms and/or irreversible loss of pulmonary function develop. | |
| 6. Investigate methods to ameliorate ischemia/reperfusion injury in the allograft lung. | |
| 7. Develop models to investigate the roles of infection, both viral and nonviral, and the molecular mechanism that regulates innate immunity in the pathogenesis of lung injury after transplantation. | |
| 8. Support and promote research into the whole spectrum of graft dysfunction: primary failure, acute rejection, and BOS. This would focus on innate and adaptive immunity and the emerging awareness that autoimmunity is involved in the rejection response. | |
| 9. Develop novel approaches for immune suppression and tolerance induction to lung allografts. | |
| 10. Continue to assess and develop relevant animal models that reflect human illness. |
Definition of abbreviation: BOS = bronchiolitis obliterans syndrome.
Acknowledgments
The authors thank Margaret Cloud and Gloria Carie for their clerical assistance in the preparation of this manuscript.
Sponsored by the National Heart, Lung, and Blood Institute, Division of Lung Diseases, Department of Health and Human Services.
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Participants: Co-chairs—David S. Wilkes, M.D. (Indiana University School of Medicine) and Thomas M. Egan, M.D. (University of North Carolina at Chapel Hill); William M. Baldwin, M.D. (Johns Hopkins Medical Institutes); William Burlingham, Ph.D. (University of Wisconsin at Madison); Steven R. Duncan, M.D. (University of Pittsburgh School of Medicine); Robert L. Fairchild, Ph.D. (Cleveland Clinic Foundation); Jay A. Fishman, M.D. (Massachusetts General Hospital, Harvard Medical School); David A. Flockhart, M.D., Ph.D. (Indiana University School of Medicine); Joe G. N. Garcia, M.D. (Johns Hopkins University School of Medicine); Marshall I. Hertz, M.D. (University of Minnesota School of Medicine); Shaf Keshavjee, M.D. (University of Toronto School of Medicine); Sadis Matalon, Ph.D. (University of Alabama School of Medicine at Birmingham); Kenneth R. McCurry, M.D. (University of Pittsburgh School of Medicine); Jonathan B. Orens, M.D. (Johns Hopkins Medical Institutes); Scott M. Palmer, M.D. (Duke University Medical Center); G. Alexander Patterson, M.D. (Washington University School of Medicine); David J. Pinsky, M.D. (University of Michigan School of Medicine); Stig Steen, M.D. (University Hospital of Lund, Sweden); Robert M. Strieter, M.D. (David Geffen School of Medicine, University of California in Los Angeles); Dirk E. M. Van Raemdonck, M.D., Ph.D. (University Hospitals Leuven, Belgium); Adriana Zeevi, Ph.D. (University of Pittsburgh School of Medicine); Herbert Y. Reynolds, M.D. (National Heart, Lung, and Blood Institute, National Institutes of Health); Dorothy B. Gail, Ph.D. (National Heart, Lung, and Blood Institute, National Institutes of Health); Shiv Prasad, Ph.D. (National Institute of Allergy and Infectious Diseases).
References
- 1.Boucek MM, Edwards LB, Keck BM, Trulock EP, Taylor DO, Hertz MI. Registry for the International Society for Heart and Lung Transplantation: seventh official pediatric report—2004. J Heart Lung Transplant 2004;23:933–947. [DOI] [PubMed] [Google Scholar]
- 2.Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, Schkade L, Hunsicker L. Estimating the number of potential organ donors in the United States. N Engl J Med 2003;349:667–674. [DOI] [PubMed] [Google Scholar]
- 3.2003 annual report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Rockville, MD, and Richmond, VA: U.S. Department of Health and Human Services/Health Resources and Services Administration and United Network for Organ Sharing; 2004.
- 4.Hsieh AH-H, Bishop MJ, Kublis PS, Newell DW, Pierson DJ. Pneumonia following closed head injury. Am Rev Respir Dis 1992;146:290–294. [DOI] [PubMed] [Google Scholar]
- 5.Rogers FB, Shackford SR, Trevisani GT, Davis JW, Mackersie RC, Hoyt DB. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma 1995;39:860–867. [DOI] [PubMed] [Google Scholar]
- 6.Pierre A, Sekine Y, Hutcheon M, Waddell T, Keshavjee S. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg 2002;123:421–428. [DOI] [PubMed] [Google Scholar]
- 7.Sundaresan S, Semenkovich J, Ochoa L, Richardson G, Trulock EP, Cooper JD, Patterson GA. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg 1995;109:1075–1080. [DOI] [PubMed] [Google Scholar]
- 8.Langone AJ, Helderman JH. Disparity between solid-organ supply and demand. N Engl J Med 2003;349:704–706. [DOI] [PubMed] [Google Scholar]
- 9.Sachs DH. Mixed chimerism as an approach to transplantation tolerance. Clin Immunol 2000;95:S63–S68. [DOI] [PubMed] [Google Scholar]
- 10.Fishman J, Patience C. Xenotransplantation: infectious risk revisited. Am J Transplant 2004;4:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JC, Quinn G, Suling KM, Oldmixon BA, Van Tine BA, Cina R, Arn S, Huang CA, Scobie L, Onions DE, et al. Identification of exogenous forms of human-tropic porcine endogenous retrovirus in miniature swine. J Virol 2004;78:2494–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman J. Infection in xenotransplantation: assessing the risks of novel technology. In: Bowden RA, Ljungman P, Paya C, editors. Transplant infections, 2nd ed. Hagerstown, MD: Lippincott, Williams, and Wilkins; 2003. pp. 687–698.
- 13.Starnes VA, Barr ML, Cohen RG, Hagen JA, Wells WJ, Horn MV, Schenkel FA. Living-donor lobar lung transplantation experience: intermediate results. J Thorac Cardiovasc Surg 1996;112:1284–1291. [DOI] [PubMed] [Google Scholar]
- 14.Starnes V, Woo M, MacLaughlin E, Horn M, Wong P, Rowland J, Durst C, Wells W, Barr M. Comparison of outcomes between living donor and cadaveric lung transplantation in children. Ann Thorac Surg 1999;68:2279–2283. [DOI] [PubMed] [Google Scholar]
- 15.Egan T, Rea J, McSweeney J, Gott K, Detterbeck F, Mill M, Roberts C, Paradowski L, Aris R, Burker E. Bilateral lobe transplant: how often is this a realistic option in CF patients? Pediatr Pulmonol 1998;17:352. [Google Scholar]
- 16.Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Bronsther O, Randhawa P, Broznick B, Fung JJ, Starzl T. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation 1995;59:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kootstra G. The asystolic, or non-heartbeating, donor. Transplantation 1997;63:917–921. [DOI] [PubMed] [Google Scholar]
- 18.D'Alessandro AM, Hoffmann RM, Knechtle SJ, Eckhoff DE, Love RB, Kalayoglu M, Sollinger HW, Belzer FO. Successful extrarenal transplantation from non-heart-beating donors. Transplantation 1995;59:977–982. [DOI] [PubMed] [Google Scholar]
- 19.Jassem W, Koo DD, Muiesan P, Cerundolo L, Rela M, Fuggle SV, Heaton ND. Non-heart-beating versus cadaveric and living-donor livers: differences in inflammatory markers before transplantation. Transplantation 2003;75:1386–1390. [DOI] [PubMed] [Google Scholar]
- 20.Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893–2894. [PubMed] [Google Scholar]
- 21.D'Armini AM, Roberts CS, Griffith PK, Lemasters JJ, Egan TM. When does the lung die? I. Histochemical evidence of pulmonary viability after “death.” J Heart Lung Transplant 1994;13:741–747. [PubMed] [Google Scholar]
- 22.Lechner JF, Stoner GD, Yoakum GH, Willey JC, Grafstrom RC, Masui T, LaVeck MA, Harris CC. In vitro carcinogenesis studies with human tracheobronchial tissues and cells. In: Schiff LJ, editor. In vitro models of respiratory epithelium. Boca Raton, FL: CRC Press; 1986. pp. 143–159.
- 23.Egan T. Non-heart beating donors in thoracic transplantation. J Heart Lung Transplant 2004;23:3–10. [DOI] [PubMed] [Google Scholar]
- 24.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg 2003;76:244–252. [DOI] [PubMed] [Google Scholar]
- 25.Aitchison JD, Orr HE, Flecknell PA, Kirby JA, Dark JH. Functional assessment of non-heart-beating donor lungs: prediction of post-transplant function. Eur J Cardiothorac Surg 2001;20:187–194. [DOI] [PubMed] [Google Scholar]
- 26.Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart beating donor. Lancet 2001;357:825–829. [DOI] [PubMed] [Google Scholar]
- 27.Varela A. Are out hospital non heart beating donors (NHBD) better than brain death lung donors [abstract]? J Heart Lung Transplant 2004;23:S87. [Google Scholar]
- 28.Takashima S, Schlidt S, Koukoulis G, Egan T. Isoproterenol amelioriates ischemia-reperfusion lung injury (IRI) in rat lungs from non-heart-beating donors even with β-blockade [abstract]. Am J Respir Crit Care Med 2003;167:A363. [Google Scholar]
- 29.Aitchison JD, Orr HE, Flecknell P, Kirby JA, Dark JH. Functional assessment of the non-heart-beating donor lung offers improved preservation [abstract]. J Heart Lung Transplant 2002;21:57. [Google Scholar]
- 30.Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med 2001;163:259–265. [DOI] [PubMed] [Google Scholar]
- 31.Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. J Heart Lung Transplant 1999;18:425–431. [DOI] [PubMed] [Google Scholar]
- 32.King RC, Binns OAR, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg 2000;69:1681–1685. [DOI] [PubMed] [Google Scholar]
- 33.Fiser S, Tribble C, Long S, Kaza A, Kern J, Kron I. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg 2001;71:1134–1139. [DOI] [PubMed] [Google Scholar]
- 34.Wilkes DS, Neimeier M, Mathur PN, Soliman DM, Twigg HL, Bowen LK, Heidler KM. Effect of human lung allograft alveolar macrophages on IgG production: immunoregulatory role of interleukin-10, transforming growth factor-beta, and interleukin-6. Am J Respir Cell Mol Biol 1995;13:621–628. [DOI] [PubMed] [Google Scholar]
- 35.Olson E, Jin J, Funkhouser W, Yuan Y, Randell S, Egan T. Gene expression profile of ischemia-reperfusion injury in human lung transplantation [abstract]. Chest 2003;124:99S. [Google Scholar]
- 36.Koukoulis G, Yuan Y, Jin J, Egan T. Gene expression in transplanted rat lungs: conventional vs. non-heart beating donors [abstract]. Am J Respir Crit Care Med 2004;169:A218. [Google Scholar]
- 37.Ogawa S, Koga S, Kuwabara K, Brett J, Morrow B, Morris SA, Bilezikian JP, Silverstein SC, Stern D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol 1992;262:C546–C554. [DOI] [PubMed] [Google Scholar]
- 38.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol 1998;274:H1855–H1894. [DOI] [PubMed] [Google Scholar]
- 39.Pinsky DJ, Yan S-F, Lawson C, Naka Y, Chen J-X, Connolly ES Jr, Stern DM. Hypoxia and modification of the endothelium: implications for regulation of vascular homeostatic properties. Semin Cell Biol 1995;6:283–294. [DOI] [PubMed] [Google Scholar]
- 40.Naka Y, Roy DK, Liao H, Chowdhury NC, Michler RE, Oz MC, Pinsky DJ. cAMP-mediated vascular protection in an orthotopic rat lung transplant model. Circ Res 1996;79:773–783. [DOI] [PubMed] [Google Scholar]
- 41.Birukov KG, Leitinger N, Bochkov VN, Garcia JG. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc Res 2004;67:18–28. [DOI] [PubMed] [Google Scholar]
- 42.Ardehali A, Laks H, Russell H, Levine M, Shpiner R, Lackey S, Ross D. Modified reperfusion and ischemia-reperfusion injury in human lung transplantation. J Thorac Cardiovasc Surg 2003;126:1929–1934. [DOI] [PubMed] [Google Scholar]
- 43.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, et al. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies: a mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 1996;97:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toda K, Kayano K, Karimova A, Naka Y, Fujita T, Minamoto K, Wang CY, Pinsky DJ. Antisense intercellular adhesion molecule-1 (ICAM-1) oligodeoxyribonucleotide delivered during organ preservation inhibits posttransplant ICAM-1 expression and reduces primary lung isograft failure. Circ Res 2000;86:166–174. [DOI] [PubMed] [Google Scholar]
- 45.Naka Y, Marsh HC, Scesney SM, Oz MC, Pinsky DJ. Complement activation as a cause for primary graft failure in an isogeneic rat model of hypothermic lung preservation and transplantation. Transplantation 1997;64:1248–1255. [DOI] [PubMed] [Google Scholar]
- 46.Pinsky DJ, Naka Y, Chowdhury NC, Liao H, Oz MC, Michler RE, Kubaszewski E, Malinski T, Stern DM. The nitric oxide/cyclic GMP pathway in organ transplantation: critical role in successful lung preservation. Proc Natl Acad Sci USA 1994;91:12086–12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada M, Fujita T, Sakaguchi T, Olson KE, Collins T, Stern DM, Yan SF, Pinsky DJ. Extinguishing Egr-1-dependent inflammatory and thrombotic cascades after lung transplantation. FASEB J 2001;15:2757–2759. [DOI] [PubMed] [Google Scholar]
- 48.Fiser S, Tribble C, Long S, Kaza A, Cope J, Laubach V, Kern J, Kron I. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg 2001;121:1069–1075. [DOI] [PubMed] [Google Scholar]
- 49.Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2003;125:261–272. [DOI] [PubMed] [Google Scholar]
- 50.Farivar AS, Krishnadasan B, Naidu BV, Woolley SM, Verrier ED, Mulligan MS. Endogenous interleukin-4 and interleukin-10 regulate experimental lung ischemia reperfusion injury. Ann Thorac Surg 2003;76:253–259. [DOI] [PubMed] [Google Scholar]
- 51.Kaneda H, Guttierez C, de Perrot M, Yamane M, Quadri S, Arenovich T, Waddell T, Liu M, Keshavjee S. Pre-implantation multiple cytokine MRNA expression analysis in donor lung grafts predicts survival after lung transplantation in humans [abstract]. J Heart Lung Transplant 2004;23:S49. [DOI] [PubMed] [Google Scholar]
- 52.DeMeester SR, Molinari MA, Shiraishi T, Okabayashi K, Manchester JK, Wick MR, Cooper JD, Patterson GA. Attenuation of rat lung isograft reperfusion injury with a combination of anti-ICAM-1 and anti-beta 2 integrin monoclonal antibodies. Transplantation 1996;62:1477–1485. [DOI] [PubMed] [Google Scholar]
- 53.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 2002;16:950–962. [DOI] [PubMed] [Google Scholar]
- 54.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 1995;163:510–522. [DOI] [PubMed] [Google Scholar]
- 55.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol 2000;89:2241–2248. [DOI] [PubMed] [Google Scholar]
- 56.Birukov KG, Bochkov VN, Birukova A, Leitinger N, Garcia JGN. Enhancement of human pulmonary endothelial cell barrier function by OxPAPC, a bioactive component of oxidized phospholipids. Microvasc Res 2004;67:18–28. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson JR, Barnard J, Garcia JGN. Attenuation of vascular leak and inflammation in murine acute lung injury by simvastatin. Am J Physiol Lung Cell Mol Physiol 2005;288:L1026–L1032. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 2004;30:662–670. [DOI] [PubMed] [Google Scholar]
- 59.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J 2000;14:2255–2265. [DOI] [PubMed] [Google Scholar]
- 61.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
- 62.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 2004;170:987–993. [DOI] [PubMed] [Google Scholar]
- 63.Fischer S, Liu M, MacLean AA, de Perrot M, Ho M, Cardella JA, Zhang XM, Bai XH, Suga M, Imai Y, et al. In vivo transtracheal adenovirus-mediated transfer of human interleukin-10 gene to donor lungs ameliorates ischemia-reperfusion injury and improves early posttransplant graft function in the rat. Hum Gene Ther 2001;12:1513–1526. [DOI] [PubMed] [Google Scholar]
- 64.Itano H, Zhang W, Ritter J, McCarthy T, Mohanakumar T, Patterson G. Adenovirus-mediated gene transfer of human interleukin 10 ameliorates reperfusion injury of rat lung isografts. J Thorac Cardiovasc Surg 2000;120:947–956. [DOI] [PubMed] [Google Scholar]
- 65.Fischer S, de Perrot M, Liu M, MacLean AA, Cardella JA, Imai Y, Suga M, Keshavjee S. IL-10 gene transfection of donor lungs ameliorates post-transplant cell death by a switch from cellular necrosis to apoptosis. J Thorac Cardiovasc Surg (In press) [DOI] [PubMed]
- 66.Suda T, Mora BN, D'Ovidio F, Cooper JA, Hiratsuka M, Zhang W, Mohanakumar T, Patterson GA. In vivo adenovirus-mediated endothelial nitric oxide synthase gene transfer ameliorates lung allograft ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2000;119:297–304. [DOI] [PubMed] [Google Scholar]
- 67.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 2003;21:392–398. [DOI] [PubMed] [Google Scholar]
- 68.Chakinala MM, Trulock EP. Acute allograft rejection after lung transplantation: diagnosis and therapy. Chest Surg Clin N Am 2003;13:525–542. [DOI] [PubMed] [Google Scholar]
- 69.Corris PA. Lung transplantation: bronchiolitis obliterans syndrome. Chest Surg Clin N Am 2003;13:543–557. [DOI] [PubMed] [Google Scholar]
- 70.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310. [DOI] [PubMed] [Google Scholar]
- 71.Yousem SA, Burke CM, Billingham ME. Pathologic pulmonary alterations in long-term human heart-lung transplantation. Hum Pathol 1985;16:911–923. [DOI] [PubMed] [Google Scholar]
- 72.Kelly K, Hertz MI. Obliterative bronchiolitis. Clin Chest Med 1997;18:319–338. [DOI] [PubMed] [Google Scholar]
- 73.Iacono A, Dauber J, Keenan R, Spichty K, Cai J, Grgurich W, Burckart G, Smaldone G, Pham S, Ohori NP, et al. Interleukin 6 and interferon-gamma gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation 1997;64:263. [DOI] [PubMed] [Google Scholar]
- 74.Sundaresan S, Alevy YG, Steward N, Tucker J, Trulock EP, Cooper JD, Patterson GA, Mohanakumar T. Cytokine gene transcripts for tumor necrosis factor-α, interleukin-2, and interferon-γ in human pulmonary allografts. J Heart Lung Transplant 1995;14:512–518. [PubMed] [Google Scholar]
- 75.Smith C, Jaramillo A, Lu KC, Kaleem Z, Patterson G, Mohanakumar T. Neutralization of tumor necrosis factor-alpha or interleukin-1 prevents obliterative airway disease in HLA-A2 transgenic murine tracheal allografts. J Heart Lung Transplant 2001;20:166–167. [DOI] [PubMed] [Google Scholar]
- 76.Saito R, Prehn J, Zuo X-J, Marchevesky A, Castracane J, Waters P, Matloff J, Jordan SC. The participation of tumor necrosis factor in the pathogenesis of lung allograft rejection in the rat. Transplantation 1993;55:967–972. [DOI] [PubMed] [Google Scholar]
- 77.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000;12:121–127. [DOI] [PubMed] [Google Scholar]
- 78.el-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol 2002;14:562–568. [DOI] [PubMed] [Google Scholar]
- 79.Colvin BL, Thomson AW. Chemokines, their receptors, and transplant outcome. Transplantation 2002;74:149–155. [DOI] [PubMed] [Google Scholar]
- 80.Belperio JA, Burdick MD, Keane MP, Xue YY, Lynch JP III, Daugherty BL, Kunkel SL, Strieter RM. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol 2000;165:461–472. [DOI] [PubMed] [Google Scholar]
- 81.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol 2002;169:1037–1049. [DOI] [PubMed] [Google Scholar]
- 82.Belperio JA, Keane MP, Burdick MD, Lynch JP III, Zisman DA, Xue YY, Li K, Ardehali A, Ross DJ, Strieter RM. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol 2003;171:4844–4852. [DOI] [PubMed] [Google Scholar]
- 83.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, Furst H, Briegel J, Vogelmeier C. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation 2000;70:362–367. [DOI] [PubMed] [Google Scholar]
- 84.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol 2004;286:L1129–L1139. [DOI] [PubMed] [Google Scholar]
- 85.Yasufuku K, Heidler KM, O'Donnell PW, Smith GN Jr, Cummings OW, Foresman BH, Fujisawa T, Wilkes DS. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am J Respir Cell Mol Biol 2001;25:26–34. [DOI] [PubMed] [Google Scholar]
- 86.Yasufuku K, Heidler KM, Woods KA, Smith GN Jr, Cummings OW, Fujisawa T, Wilkes DS. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation 2002;73:500–505. [DOI] [PubMed] [Google Scholar]
- 87.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol 2002;169:1542–1549. [DOI] [PubMed] [Google Scholar]
- 88.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN Jr, Cummings OW, Fujisawa T, Blum JS, et al. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol 2003;171:1140–1147. [DOI] [PubMed] [Google Scholar]
- 89.Meloni F, Vitulo P, Bianco AM, Paschetto E, Morosini M, Cascina A, Mazzucchelli I, Ciardelli L, Oggionni T, Fietta AM, et al. Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation 2004;77:762–766. [DOI] [PubMed] [Google Scholar]
- 90.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol 2003;3:199–210. [DOI] [PubMed] [Google Scholar]
- 91.Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, Spichty KJ, Yousem SA, Burckart G, Dauber JH, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant 2004;23:1135–1141. [DOI] [PubMed] [Google Scholar]
- 92.Miller GG, Destarac L, Zeevi A, Girnita A, McCurry K, Iacono A, Murray JJ, Crowe D, Johnson JE. Ninan M, et al. Acute humoral rejection of human lung allografts and elevation of C4d in bronchoalveolar lavage fluid. Am J Transplant 2004;4:1323–1330. [DOI] [PubMed] [Google Scholar]
- 93.Wilkes DS, Heidler KM, Niemeier M, Schwenk GR, Mathur PN, Breite WM, Cummings OW, Weissler JC. Increased bronchoalveolar IgG2/IgG1 ratio is a marker for human lung allograft rejection. J Investig Med 1994;42:652–659. [PubMed] [Google Scholar]
- 94.Reznik SI, Jaramillo A, Zhang L, Patterson GA, Cooper JD, Mohanakumar T. Anti-HLA antibody binding to HLA class I molecules induces proliferation of airway epithelial cells: a potential mechanism for bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg 2000;119:39–45. [DOI] [PubMed] [Google Scholar]
- 95.Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, et al. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest 1996;98:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vallhonrat H, Williams WW, Cosimi AB, Tolkoff-Rubin N, Ginns LC, Wain JC, Preffer F, Olszak I, Wee S, Delmonico FL, et al. In vivo generation of C4d, Bb, iC3b, and SC5b-9 after OKT3 administration in kidney and lung transplant recipients. Transplantation 1999;67:253–258. [DOI] [PubMed] [Google Scholar]
- 97.Bemelman FJ, Parlevliet KJ, Schellekens PT, Surachno S, van Royen EA, ten Berge RJ. Sequestration of labelled granulocytes in the lungs following administration of OKT3 is dose-dependent. Transpl Immunol 1994;2:47–51. [DOI] [PubMed] [Google Scholar]
- 98.Schmid RA, Zollinger A, Singer T, Hillinger S, Leon-Wyss JR, Schöb OM, Hogasen K, Zünd G, Patterson GA, Weder W. Effect of soluble complement receptor type 1 on reperfusion edema and neutrophil migration after lung allotransplantation in swine. J Thorac Cardiovasc Surg 1998;116:90–97. [DOI] [PubMed] [Google Scholar]
- 99.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM III. Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol 2002;169:4620–4627. [DOI] [PubMed] [Google Scholar]
- 100.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987–995. [DOI] [PubMed] [Google Scholar]
- 101.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145. [DOI] [PubMed] [Google Scholar]
- 102.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 2003;111:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palmer SM, Burch LH, Davis RD, Herczyk WF, Howell DN, Reinsmoen NL, Schwartz DA. The role of innate immunity in acute allograft rejection after lung transplantation. Am J Respir Crit Care Med 2003;168:628–632. [DOI] [PubMed] [Google Scholar]
- 104.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, Schwartz DA. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med 2005;171:780–785. [DOI] [PubMed] [Google Scholar]
- 105.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, Mohanakumar T, Trulock EP, Walter MJ. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004;170:181–187. [DOI] [PubMed] [Google Scholar]
- 106.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 2001;63:521–554. [DOI] [PubMed] [Google Scholar]
- 107.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev 1997;77:931–962. [DOI] [PubMed] [Google Scholar]
- 108.Desrois M, Sciaky M, Lan C, Cozzone PJ, Bernard M. L-arginine during long-term ischemia: effects on cardiac function, energetic metabolism and endothelial damage. J Heart Lung Transplant 2000;19:367–376. [DOI] [PubMed] [Google Scholar]
- 109.Hickman-Davis JM. Role of innate immunity in respiratory mycoplasma infection. Front Biosci 2002;7:d1347–d1355. [DOI] [PubMed] [Google Scholar]
- 110.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest 1997;100:2417–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today 1994;15:67–74. [DOI] [PubMed] [Google Scholar]
- 112.Williams MD, Wright JR, March KL, Martin WJ II. Human surfactant protein A enhances attachment of Pneumocystis carinii to rat alveolar macrophages. Am J Respir Cell Mol Biol 1996;14:232–238. [DOI] [PubMed] [Google Scholar]
- 113.Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem 1996;271:16375–16383. [DOI] [PubMed] [Google Scholar]
- 114.Oosting RS, Wright JR. Characterization of the surfactant protein A receptor: cell and ligand specificity. Am J Physiol 1994;267:L165–L172. [DOI] [PubMed] [Google Scholar]
- 115.Strayer DS, Yang S, Jerng HH. Surfactant protein A-binding proteins: characterization and structures. J Biol Chem 1993;268:18679–18684. [PubMed] [Google Scholar]
- 116.Weikert LF, Edwards K, Chroneos ZC, Hager C, Hoffman L, Shepherd VL. SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am J Physiol 1997;272:L989–L995. [DOI] [PubMed] [Google Scholar]
- 117.Pison U, Wright JR, Hawgood S. Specific binding of surfactant apoprotein SP-A to rat alveolar macrophages. Am J Physiol 1992;262:L412–L417. [DOI] [PubMed] [Google Scholar]
- 118.Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem 1989;264:13923–13928. [PubMed] [Google Scholar]
- 119.De Andrade JA, Crow JP, Viera L, Bruce Alexander C, Randall Young K, McGiffin DC, Zorn GL, Zhu S, Matalon S, Jackson RM. Protein nitration, metabolites of reactive nitrogen species, and inflammation in lung allografts. Am J Respir Crit Care Med 2000;161:2035–2042. [DOI] [PubMed] [Google Scholar]
- 120.Burke CM, Theodore J, Dawkins KD, Yousem SA, Blank N, Billingham ME, Van Kessel A, Jamieson SW, Oyer PE, Baldwin JC, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest 1984;86:824–829. [DOI] [PubMed] [Google Scholar]
- 121.Estenne M, Hertz M. Bronchiolitis obliterans after human transplantation. Am J Respir Crit Care Med 2002;166:440–444. [DOI] [PubMed] [Google Scholar]
- 122.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, Garrity ER. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 1999;159:829–833. [DOI] [PubMed] [Google Scholar]
- 123.Brouland J-P, Egan T, Roussi J, Bonneau M, Pignaud G, Bal C, Vaiman M, André P, Hervé P, Mazmanian GM, et al. In vivo regulation of von Willebrand factor synthesis: von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler Thromb Vasc Biol 1999;19:3055–3062. [DOI] [PubMed] [Google Scholar]
- 124.Magnan A, Mege JL, Escallier JC, Brisse J, Capo C, Reynaud M, Thomas P, Meric B, Garbe L, Badier M, et al. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med 1996;153:1431–1436. [DOI] [PubMed] [Google Scholar]
- 125.Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, Schersten H. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J 1999;14:1123–1130. [DOI] [PubMed] [Google Scholar]
- 126.DiGiovine B, Lynch JP III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–4202. [PubMed] [Google Scholar]
- 127.Charpin JM, Valcke J, Kettaneh L, Epardeau B, Stern M, Israel-Biet D. Peaks of transforming growth factor-beta mRNA in alveolar cells of lung transplant recipients as an early marker of chronic rejection. Transplantation 1998;65:752–755. [DOI] [PubMed] [Google Scholar]
- 128.Hertz MI, Henke CA, Nakhleh RE, Harmon KR, Marinelli WA, Fox JM, Kubo SH, Shumway SJ, Bolman RM III, Bitterman PB. Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet-derived growth factor. Proc Natl Acad Sci USA 1992;89:10385–10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gimino VJ, Lande JD, Berryman TR, King RA, Hertz MI. Gene expression profiling of bronchoalveolar lavage cells in acute lung rejection. Am J Respir Crit Care Med 2003;168:1237–1242. [DOI] [PubMed] [Google Scholar]
- 130.Davis RD Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, Palmer SM. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg 2003;125:533–542. [DOI] [PubMed] [Google Scholar]
- 131.Cantu E III, Appel JZ III, Hartwig MG, Woreta H, Green C, Messier R, Palmer SM, Davis RD Jr. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg 2004;78:1142–1151. [Discussion: 1142–1151.] [DOI] [PubMed] [Google Scholar]
- 132.D'Ovidio F, Mura M, Waddell TK, Pierre A, Hutcheon M, Hadjiliadis D, Singer L, Miller L, Darling G, de Perrot M, et al. Bile acids in bronchoalveolar lavage after lung transplantation as a marker of pulmonary aspiration asociated with alveolar neutrophillia [abstract]. J Heart Lung Transplant 2004;23:S42.14986652 [Google Scholar]
- 133.Glanville AR, Imoto E, Baldwin JC, Billingham ME, Theodore J, Robin ED. The role of right ventricular endomyocardial biopsy in the long-term management of heart-lung transplant recipients. J Heart Transplant 1987;6:357–361. [PubMed] [Google Scholar]
- 134.Snell GI, Esmore DS, Williams TJ. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest 1996;109:874–878. [DOI] [PubMed] [Google Scholar]
- 135.Cahill BC, O'Rourke MK, Strasburg KA, Savik K, Jessurun J, Bolman RM III, Hertz MI. Methotrexate for lung transplant recipients with steroid-resistant acute rejection. J Heart Lung Transplant 1996;15:1130–1137. [PubMed] [Google Scholar]
- 136.Salerno CT, Park SJ, Kreykes NS, Kulick DM, Savik K, Hertz MI, Bolman RM III. Adjuvant treatment of refractory lung transplant rejection with extracorporeal photopheresis. J Thorac Cardiovasc Surg 1999;117:1063–1069. [DOI] [PubMed] [Google Scholar]
- 137.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168:121–125. [DOI] [PubMed] [Google Scholar]
- 138.Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 1998;351:420. [DOI] [PubMed] [Google Scholar]
- 139.Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW III. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290:1749–1756. [DOI] [PubMed] [Google Scholar]
- 141.Agen C, Danesi R, Blandizzi C, Costa M, Stacchini B, Favini P, Del Tacca M. Macrolide antibiotics as antiinflammatory agents: roxithromycin in an unexpected role. Agents Actions 1993;38:85–90. [DOI] [PubMed] [Google Scholar]
- 142.Khan AA, Slifer TR, Araujo FG, Remington JS. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents 1999;11:121–132. [DOI] [PubMed] [Google Scholar]
- 143.Suzuki H, Shimomura A, Ikeda K, Furukawa M, Oshima T, Takasaka T. Inhibitory effect of macrolides on interleukin-8 secretion from cultured human nasal epithelial cells. Laryngoscope 1997;107:1661–1666. [DOI] [PubMed] [Google Scholar]
- 144.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med 2003;167:1271–1278. [DOI] [PubMed] [Google Scholar]
- 145.Naidu BV, Woolley SM, Farivar AS, Thomas R. Fraga C, Mulligan MS. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg 2003;126:482–489. [DOI] [PubMed] [Google Scholar]
- 146.Sharples LD, Tamm M, McNeil K, Higenbottam TW, Stewart S, Wallwork J. Development of bronchiolitis obliterans syndrome in recipients of heart-lung transplantation–early risk factors. Transplantation 1996;61:560–566. [DOI] [PubMed] [Google Scholar]
- 147.Sharples LD, Scott JP, Dennis C, Higenbottam TW, Stewart S, Wreghitt T, Large SR, Wells FC, Wallwork J. Risk factors for survival following combined heart-lung transplantation: the first 100 patients. Transplantation 1994;57:218–223. [DOI] [PubMed] [Google Scholar]
- 148.Luckraz H, Sharples L, McNeil K, Wreghitt T, Wallwork J. Cytomegalovirus antibody status of donor/recipient does not influence the incidence of bronchiolitis obliterans syndrome in lung transplantation. J Heart Lung Transplant 2003;22:287–291. [DOI] [PubMed] [Google Scholar]
- 149.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Human cytomegalovirus load in plasma and bronchoalveolar lavage fluid: a longitudinal study of lung transplant recipients. J Infect Dis 2004;190:1076–1083. [DOI] [PubMed] [Google Scholar]
- 150.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation 2003;75:2064–2068. [DOI] [PubMed] [Google Scholar]
- 151.Zamora MR. Controversies in lung transplantation: management of cytomegalovirus infections. J Heart Lung Transplant 2002;21:841–849. [DOI] [PubMed] [Google Scholar]
- 152.Zamora MR. Use of cytomegalovirus immune globulin and ganciclovir for the prevention of cytomegalovirus disease in lung transplantation. Transpl Infect Dis 2001;3:49–56. [DOI] [PubMed] [Google Scholar]
- 153.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 2003;111:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol 2002;169:5387–5391. [DOI] [PubMed] [Google Scholar]
- 155.Williams MA, Tan JT, Adams AB, Durham MM, Shirasugi N, Whitmire JK, Harrington LE, Ahmed R. Pearson TC, Larsen CP. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol 2001;167:4987–4995. [DOI] [PubMed] [Google Scholar]
- 156.Stiller CR, Keown PA. Cyclosporine therapy in perspective. Prog Transplant 1984;1:11–45. [Google Scholar]
- 157.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998;338:1741–1751. [DOI] [PubMed] [Google Scholar]
- 158.Levine SM. A survey of clinical practice of lung transplantation in North America. Chest 2004;125:1224–1238. [DOI] [PubMed] [Google Scholar]
- 159.King-Biggs MB, Dunitz JM, Park SJ, Kay Savik S, Hertz MI. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 2003;75:1437–1443. [DOI] [PubMed] [Google Scholar]
- 160.McCurry KR, Zeevi A, Zaldonis DB, Bertani A, Spichty K, McDade K, Iacono A, Yousem SA, Starzl T. Results of a tolerance-enhancing protocol in human lung transplantation [abstract]. J Heart Lung Transplant 2004;23:S111. [Google Scholar]
- 161.Keenan RJ, Iacono A, Dauber JH, Zeevi A, Yousem SA, Ohori NP, Burckart GJ, Kawai A, Smaldone GC, Griffith BP. Treatment of refractory acute allograft rejection with aerosolized cyclosporine in lung transplant recipients. J Thorac Cardiovasc Surg 1997;113:335–341. [DOI] [PubMed] [Google Scholar]
- 162.Di Paolo A, Danesi R, Del Tacca M. Pharmacogenetics of neoplastic diseases: new trends. Pharmacol Res 2004;49:331–342. [DOI] [PubMed] [Google Scholar]