Abstract
Rationale: The effect of early life wheezing on respiratory function and continued symptoms through adolescence has not been fully described. Using data from a population-based birth cohort in Tucson, Arizona, we previously described four phenotypes based on the occurrence of wheezing lower respiratory illnesses before age 3 yr and active wheeze at age 6 yr: never wheezers (n = 425), transient early wheezers (n = 164), persistent wheezers (n = 113), and late-onset wheezers (n = 124).
Objective: We sought to determine the prognosis for these phenotypes, with reference to lung function and symptoms, through adolescence.
Methods: Current wheeze was assessed by questionnaire, lung function was measured by conventional spirometry, and atopy was determined by skin prick tests.
Results: The prevalence of atopy and wheeze by age 16 yr was similar for never and transient wheezers and for persistent and late-onset wheezers. Both transient early, and persistent wheezers had significantly lower FEF25–75 (–259 ml/s, p < 0.001, and –260 ml/s, p = 0.001, respectively), FEV1 (–75 ml, p = 0.02, and –87 ml, p = 0.03, respectively), and FEV1:FVC ratio (–1.9%, p = 0.002, and –2.5%, p = 0.001, respectively) through age 16 yr compared with never wheezers. Late-onset wheezers had levels of lung function similar to those of never wheezers through age 16 yr. There was no significant change in lung function among subjects with any of the four phenotypes, relative to their peers, from age 6 to 16 yr.
Conclusion: Patterns of wheezing prevalence and levels of lung function are established by age 6 yr and do not appear to change significantly by age 16 yr in children who start having asthmalike symptoms during the preschool years.
Keywords: adolescent, preschool child, respiratory function tests
Successful strategies for the primary and secondary prevention of asthma remain elusive. Perhaps one of the most significant obstacles for further progress in this area has been the lack of solid data regarding the natural history of the disease. However, cohorts in which data collection was started at birth or during the school years have provided important new insights (1). It now seems established that, in the majority of cases of persistent asthma, symptoms begin during the preschool years and track significantly with age thereafter (2). Moreover, the two longest ongoing longitudinal studies of asthma, conducted in Australia (3) and New Zealand (4), have shown that deficits in lung function are already present by age 9–10 yr among children with asthma, and that these deficits persist up to mid-adulthood. These findings clearly point to the toddler years as the time during which the most important alterations in lung structure and function may develop in most individuals with persistent asthma.
In 1995 we reported the results of a follow-up study of children enrolled at birth in Tucson, Arizona, and in whom wheezing and asthma-associated characteristics had been studied up to the age of 6 yr (5). We have now completed follow-up for these children up to the age of 16 yr, and report herein their outcomes both in terms of lung function and respiratory symptoms during the school years.
Some of the results of these studies have been previously reported in the form of an abstract (6).
METHODS
Participant Characteristics and Assessment of Wheeze
See the online supplement for detailed methods. Briefly, healthy infants were enrolled at birth in the Tucson Children's Respiratory Study in Tucson, Arizona (n = 1,246) (7, 8). Four phenotypes characterizing preschool age wheeze were defined according to the presence or absence of “at least one physician-diagnosed wheezing lower respiratory illness (LRI) in the first 3 years of life” and at least one episode of “parent-reported wheeze during the past year for the child at age 6”: no wheeze from birth to age 6 yr (never wheeze), wheezing LRI before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze) (5). Informed consent was obtained from the parents of participants, and the Institutional Review Board of the University of Arizona (Tucson, AZ) approved the study.
The prevalence of wheeze (with and/or without colds) at ages 8, 11, 13, and 16 yr was obtained from a parent-completed questionnaire that asked whether, and how often during the past year, the child had wheezed. Current wheeze at each age was categorized as follows: no wheeze, infrequent wheeze (one to three episodes), or frequent wheeze (four or more episodes) during the previous year.
Pulmonary Function Tests
Pulmonary function tests were performed at age 11 yr with a custom-built, pneumotachometer-based system running software on a portable computer and at age 16 yr with a portable Spirovit SP-1 (Schiller AG, Baar, Switzerland) (9). Both systems were calibrated with a Jones syringe. Forced vital capacity (FVC, milliliters), (FEV1, milliliters), and the forced expiratory flow between 25 and 75% of the FVC (FEF25–75, milliliters per second) were measured. Concurrent height, weight, and age were recorded by the study nurses.
Atopy, Medication Use, and Parental Information
Children were skin prick tested for six local aeroallergens at ages 11 and 16 yr (10). Atopy was defined as one or more tests producing a wheal size of 3 mm or more. Total serum IgE (international units per milliliter) and peripheral blood eosinophils (percent) were measured at ages 11 and 16 yr. Inhaled corticosteroid use was determined from questionnaires. Parental information obtained at enrollment included ethnicity, history of asthma, years of education, age, and current cigarette smoking. Parents were skin prick tested when their child was age 6 yr.
Statistics
Lung function measures, weight, and height were all approximately normally distributed at ages 11 and 16 yr. Cross-sectional differences in lung function between groups were evaluated by analysis of variance with Bonferroni's multiple comparison test. The two forced expiratory flow measures (V̇maxFRC at ages 2.4 mo and 6 yr and FEF25–75 at ages 11 and 16 yr) were converted to z scores to make these measures comparable (z scores have a mean of 0 and a standard deviation of 1 and describe each child's position relative to that of other individuals in the distribution). A random effects model was used to test for differences in longitudinal lung function measures between the preschool wheeze phenotypes (11). Proportions were compared with contingency tables and χ2 statistics. General estimating equations were used to assess the relation between the preschool wheeze phenotypes and longitudinal wheeze from age 8 to 16 yr (12). A two-sided α level of 0.05 defined statistical significance. SPSS for Windows version 12.0 (SPSS, Chicago, IL) and Stata version 8.0 (StataCorp, College Station, TX) were used for statistical analyses.
RESULTS
Study Flow Chart
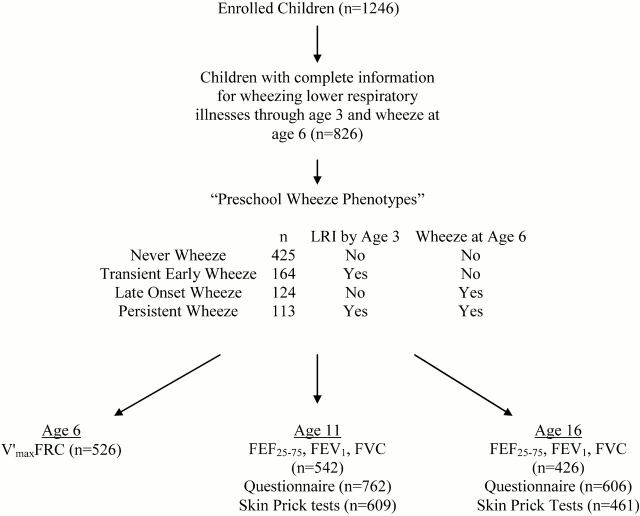
The overall study flow chart is summarized in Figure 1. Of the 1,246 children enrolled, 826 had complete information describing the occurrence of physician-confirmed wheezing lower respiratory illnesses before age 3 yr and, at age 6 yr, the occurrence of wheezing episodes during the previous year (5). These 826 children were more likely to belong to families with older, better educated, nonsmoking, non-Hispanic white parents compared with the children with incomplete wheezing information (n = 420) (5). Of these 826 children, 542 had lung function testing at age 11 yr, 426 had testing at age 16 yr, and 373 were tested at both ages. There were no differences between the 595 children who had pulmonary function testing and the 231 children who did not have such testing in terms of frequencies of wheeze and skin test reactivity at age 6 yr or in terms of parental level of education, history of asthma, smoking status, or age (see data in Table E1 in the online supplement).
Figure 1.
Study flow chart. Definitions of the preschool wheeze phenotypes were previously published (5) and are defined as follows: no wheeze from birth to age 6 yr (never wheeze), wheezing lower respiratory illness (LRI) before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze). FEF25–75 = forced expiratory flow between 25 and 75% of the FVC; V̇maxFRC = maximal expired flow at functional residual capacity.
Wheeze, Atopy, and Total Serum IgE
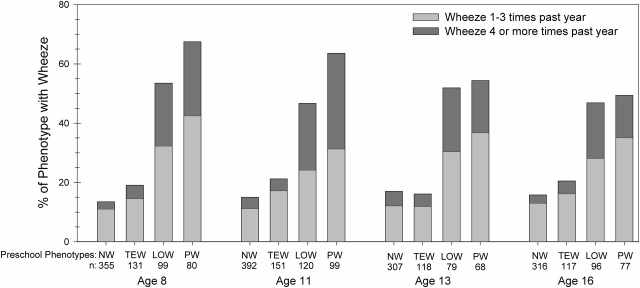
The prevalence of wheeze during the previous year at ages 8, 11, 13, and 16 yr for each of the preschool wheeze phenotypes is shown in Figure 2. More than 75% of never and transient early wheezers reported no wheezing between ages 8 and 16 yr; among the small proportion of children in these groups who reported wheezing, most had infrequent episodes. We used a longitudinal model (general estimating equations) to evaluate the risk for wheezing symptoms from age 8 to 16 yr in the preschool wheeze phenotypes. Both late-onset and persistent wheezers were significantly more likely to continue wheezing compared with never wheezers (late-onset wheeze relative risk, 3.12 [95% confidence interval, 2.5–3.9], p < 0.001; and persistent wheeze relative risk, 3.8 [95% confidence interval, 3.1–4.7], p < 0.001). The risk for subsequent wheeze was not significantly higher for transient early wheezers compared with never wheezers (relative risk, 1.28 [95% confidence interval, 0.97–1.70], p = 0.09). In addition, at ages 8 and 11 yr, a slightly higher proportion of persistent wheezers was symptomatic than late-onset wheezers (67.5 vs. 53.6% at age 8 yr [p = 0.07] and 63.6 vs. 46.7% at age 11 yr [p = 0.01], respectively). However, no significant difference in the prevalence of wheeze was observed at ages 13 and 16 yr between persistent and late-onset wheezers (54.4 vs. 51.9% at age 13 yr [p = 0.8] and 50.0 vs. 46.9% at age 16 yr [p = 0.7], respectively). At age 16 yr, 86% of the children with frequent wheeze and 61% of the children with infrequent wheeze during the previous year had received a diagnosis of asthma at some time during their life.
Figure 2.
Prevalence of infrequent and frequent wheeze at ages 8, 11, 13, and 16 yr for the preschool wheeze phenotypes. The preschool wheeze phenotypes were defined as follows: no wheeze from birth to age 6 yr (never wheeze, NW), wheezing LRI before age 3 yr only (transient early wheeze, TEW), wheeze at age 6 yr only (late-onset wheeze, LOW), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze, PW). Group sample sizes at each age are listed below the acronyms.
The prevalence of skin test reactivity and total serum IgE levels at ages 11 and 16 yr for the preschool wheeze phenotypes are shown in Table 1. As previously reported (5), persistent and late-onset wheezers were more atopic and had higher IgE levels compared with never wheezers at age 6 yr (data not shown). At ages 11 and 16 yr, persistent and late-onset wheezers continued to be more atopic than never and transient early wheezers. Late-onset and persistent wheezers also had higher total serum IgE levels at age 11 yr compared with never wheezers (Table 1).
TABLE 1.
SKIN TEST REACTIVITY AND TOTAL SERUM IgE LEVELS AT AGES 11 AND 16 yr FOR PRESCHOOL WHEEZE PHENOTYPES
| Skin Test Reactivity†
|
Total Serum IgE (IU/ml)¶
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Age 11 yr
|
Age 16 yr
|
Age 11 yr
|
Age 16 yr
|
|||||
| Preschool Wheeze Phenotype* | n | % Pos (95% CI) | n | % Pos (95% CI) | n | GM (95% CI) | n | GM (95% CI) |
| Never wheeze | 299 | 51.2 (45–57) | 228 | 68.4 (62–74) | 252 | 45 (35–57) | 168 | 63 (49–83) |
| Transient early wheeze | 126 | 51.6 (43–61) | 96 | 62.5 (52–72) | 114 | 56 (42–78) | 74 | 58 (40–83) |
| Late-onset wheeze | 97 | 64.9 (55–74)‡§ | 75 | 78.7 (68–87)§ | 83 | 109 (74–158)** | 61 | 96 (66–138) |
| Persistent wheeze | 87 | 63.2 (52–73)‡ | 62 | 85.5 (74–93)‡§ | 73 | 93 (59–148)** | 49 | 83 (51–132) |
| p Value | 0.04‖ | 0.006‖ | 0.001†† | 0.2†† | ||||
Definition of abbreviations: CI = confidence interval; GM = geometric mean; Pos = positive.
Preschool wheeze phenotypes were defined as follows: no wheeze from birth to age 6 yr (never wheeze), wheezing LRI before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze).
Skin test positive (Pos) was defined as at least one positive skin test (wheal, ⩾ 3 mm sum of the orthogonal diameters) to any of the six local aeroallergens tested.
p < 0.05 compared with never wheezers by Pearson 2 × 2 χ2 analysis.
p < 0.05 compared with transient early wheezers by Pearson 2 × 2 χ2 analysis.
p Value based on Pearson χ2 statistic with 3 degrees of freedom.
Total serum IgE was normally distributed after log base 10 transformation; geometric means and 95% confidence intervals are presented as international units per milliliter.
p < 0.05 compared with never wheezers by Bonferroni's multiple comparison test.
p Value based on one-way analysis of variance.
Taken together, these results suggest that wheezing at age 6 yr, regardless of whether the children wheezed previously, is associated with continued wheezing symptoms through age 16 yr and that the increased prevalence of atopy, present in persistent and late-onset wheezers at age 6 yr, continues through adolescence.
Lung Function at Ages 11 and 16 yr for the Preschool Wheeze Phenotypes
Cross-sectional relationships between preschool wheeze phenotypes and lung function at ages 11 and 16 yr are presented in Table E2. We used a random effects model to examine the association between preschool wheezing and longitudinal lung function because this model adjusts for the serial correlation between paired lung function values at ages 11 and 16 yr and allows for the inclusion of data from participants whose lung function was measured only once (at age 11 or 16 yr). Height, weight, age, and current wheeze were included in the models as time-dependent covariates; sex and preschool wheeze phenotypes were included as fixed covariates. In this longitudinal analysis, both transient early and persistent wheezers had significantly lower FEF25–75, FEV1, and FEV1:FVC ratio at ages 11 and 16 yr compared with never wheezers (Table 2). There were no significant deficits in lung function associated with late-onset wheeze. Current frequent wheeze was also associated with deficits in FEF25–75, FEV1, and FEV1:FVC ratio compared with those children who did not wheeze during the previous year (Table 2).
TABLE 2.
LONGITUDINAL RANDOM EFFECTS MODELS OF LUNG FUNCTION AT AGES 11 AND 16 yr FOR PRESCHOOL WHEEZE PHENOTYPES, CURRENT WHEEZE, AND SEX*
| FEF25–75 (ml/s)
|
FEV1 (ml)
|
FEV1:FVC Ratio (%)
|
||||
|---|---|---|---|---|---|---|
| Coefficient§ (95% CI) | p Value | Coefficient§ (95% CI) | p Value | Coefficient§ (95% CI) | p Value | |
| Preschool wheeze phenotype† | ||||||
| Never wheeze | Ref | — | Ref | — | Ref | — |
| Transient early wheeze | −259 (−392 to −127) | < 0.001 | −75 (−139 to −11) | 0.02 | −1.9 (−3.1 to −0.7) | 0.002 |
| Late-onset wheeze | −99 (−248 to 50) | 0.2 | −23 (−95 to 49) | 0.5 | −0.8 (−2.2 to 0.5) | 0.2 |
| Persistent wheeze | −260 (−419 to −101) | 0.001 | −87 (−164 to −9.4) | 0.03 | −2.5 (−3.9 to −1.0) | 0.001 |
| Current wheeze‡ | ||||||
| No wheeze | Ref | — | Ref | — | Ref | — |
| Infrequent | −69 (−171 to 34) | 0.2 | −66 (−120 to −12) | 0.02 | 0.03 (−0.9 to 0.9) | 0.9 |
| Frequent | −341 (−492 to −191) | < 0.001 | −136 (−215 to −58) | 0.001 | −2.8 (−4.1 to −1.5) | < 0.001 |
| Sex | ||||||
| Male | Ref | Ref | Ref | |||
| Female | 24 (−79 to 128) | 0.6 | −153 (−204 to −103) | < 0.001 | 2.1 (1.2 to 3.0) | < 0.001 |
Definition of abbreviations: CI = confidence interval; FEF25–75 = forced expiratory flow between 25 and 75% of the FVC; Ref = reference.
Adjusted for height, weight, and age. Time-dependent covariates included in the model were height (cm), weight (kg), age (yr), and current wheeze. Preschool wheeze phenotypes and sex were included as fixed covariates. For the models, the number of observations was 955, the number of groups was 591, and the average number of observations per group was 1.6 (minimum, 1; maximum, 2).
Preschool wheeze phenotypes were defined as follows: no wheeze from birth to age 6 yr (never wheeze), wheezing lower respiratory illness (LRI) before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze).
Infrequent wheeze was defined as one to three episodes of wheeze during the previous year and frequent wheeze was defined as four or more episodes of wheeze during the previous year.
Model-based coefficients represent deviations from the reference group; for example, persistent wheezers had a predicted FEF25–75 that was –259 ml/s lower than that of never wheezers and, if they were also frequent wheezers, these children had a predicted FEF25–75 that was –341 ml/s lower than that of children with no wheeze during the previous year, for an estimated total deficit of –600 ml/s.
Several covariates were added to the basic random effects models presented in Table 2 to adjust for factors potentially related to lung function and/or preschool wheeze. Neither blood eosinophils nor skin test reactivity, total serum IgE, or ethnicity modified the relations under study. Likewise, parental factors (education, age, smoking at enrollment, skin test reactivity, and asthma) had no significant effect on the relationship between preschool wheeze and lung function. Only 6.7% of the wheezers at age 11 yr and 3.3% of the wheezers at age 16 yr had used inhaled corticosteroids frequently or daily during the previous year and this use did not significantly modify the associations under study (see Table E3 for details concerning steroid use). At age 16 yr, 10.7% of participants described themselves as active smokers and the prevalence of smoking was similar between the preschool wheeze phenotypes. Taken together, these results suggest that transient wheezers and persistent wheezers have decreased lung function at ages 11 and 16 yr even after adjusting for relevant covariates and current wheeze at these ages.
Forced Expiratory Flows from Age 6 to 16 Yr for the Preschool Wheeze Phenotypes
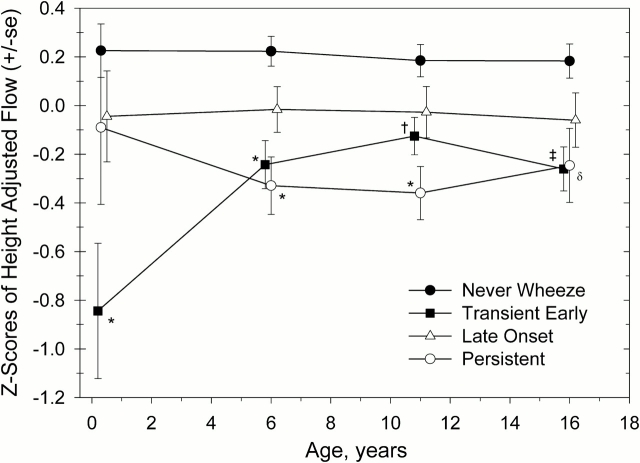
Tracking of forced expiratory flows among children with the various preschool wheezing phenotypes is shown cross-sectionally from age 2.4 mo to age 16 yr in Figure 3. As reported previously for a subset of this population (n = 125), persistent wheezers had flows that were slightly lower but not significantly different from those of never wheezers at age 2.4 mo, whereas transient early wheezers had significantly diminished flows at that age (5). By age 6 yr, both these groups had significantly lower flows than never wheezers, whereas late-onset wheezers had flows that were not significantly different from those of never wheezers (5). Diminished flows were still present at ages 11 and 16 yr for persistent wheezers; however, there did not appear to be any further decline with age relative to their peers. Transient early wheezers did not change their position, relative to their peers, from age 6 to 16 yr. Late-onset wheezers showed slightly, but not significantly, diminished flows as compared with never wheezers, and their position, relative to their peers, did not change with age.
Figure 3.
Cross-sectional z scores of height-adjusted maximal expiratory flows at ages 2.4 mo and 6, 11, and 16 yr for the preschool wheeze phenotypes. The preschool wheeze phenotypes were defined as follows: no wheeze from birth to age 6 yr (never wheeze), wheezing LRI before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze). One-way analysis of variance with Bonferroni's multiple comparison test was used to assess differences between groups. Sample sizes for each of the preschool wheeze phenotypes (never wheeze, transient early wheeze, late-onset wheeze, and persistent wheeze) were as follows: at 2.4 mo, n = 67, 21, 21, and 16; at age 6 yr, n = 260, 104, 81, and 81; at age 11 yr, n = 266, 113, 84, and 78; and at age 16 yr, n = 206, 91, 69, and 56, respectively. *p < 0.001 compared with never wheeze; †p = 0.03 compared with never wheeze; ‡p = 0.002 compared with never wheeze; δp = 0.02 compared with never wheeze.
These apparent trends were tested statistically using a longitudinal random effects model for flow at ages 6, 11, and 16 yr with height, weight, and age as time-dependent covariates, and sex and the preschool wheeze phenotypes as fixed covariates. Because current wheeze at age 6 yr was part of the definition of the preschool wheeze phenotypes, current wheeze at ages 11 and 16 yr was included in the model as a fixed covariate as described in Table 3. In addition, because infrequent wheeze was not significant in the longitudinal models for flow at ages 11 and 16 yr (Table 2), infrequent wheeze was combined with the no-wheeze group. Transient early and persistent wheezers had significantly lower flows at ages 6, 11, and 16 yr compared with never wheezers (Table 3). Flows observed for persistent wheezers were also significantly lower than those for late-onset wheezers (z score difference of –0.27 [95% confidence interval, 0.04–0.51], p = 0.02). As with the cross-sectional analyses, late-onset wheezers had flows that were not significantly different from those of never wheezers. Finally, an interaction term for the preschool wheeze phenotypes and age was tested in the model and was not significant for any of the phenotypes (p = 0.5 for transient early, p = 0.9 for late-onset, and p = 0.6 for persistent wheezers), indicating no significant change in lung function from age 6 to 16 yr for the preschool wheeze phenotypes relative to their peers.
TABLE 3.
LONGITUDINAL RANDOM EFFECTS MODEL FOR z SCORES OF FORCED EXPIRATORY FLOWS AT AGES 6, 11, AND 16 yr FOR PRESCHOOL WHEEZE PHENOTYPES, FREQUENT WHEEZE AT AGES 11 AND 16 yr, AND SEX*
|
z Score of Flow
|
||
|---|---|---|
| Coefficient§ (95% CI) | p Value | |
| Preschool wheeze phenotype† | ||
| Never wheeze | Ref | — |
| Transient early wheeze | −0.35 (–0.52 to –0.18) | < 0.001 |
| Late-onset wheeze | −0.12 (–0.31 to 0.07) | 0.2 |
| Persistent wheeze | −0.40 (–0.60 to –0.20) | < 0.001 |
| Current frequent wheeze‡ | ||
| 11–16– | Ref | — |
| 11+16– | −0.15 (–0.44 to 0.15) | 0.3 |
| 11–16+ | −0.52 (–0.89 to –0.15) | 0.006 |
| 11+16+ | −0.61 (–0.99 to –0.23) | 0.002 |
| Missing wheeze information | −0.10 (–0.27 to 0.06) | 0.2 |
| Sex | ||
| Male | Ref | |
| Female | 0.05 (–0.08 to 0.18) | 0.5 |
Definition of abbreviations: CI = confidence interval; Ref = reference.
Adjusted for height, weight, and age. Time-dependent covariates included in the model were height (cm), weight (kg), and age (yr). Fixed covariates included in the model were the preschool wheeze phenotypes, sex, and frequent wheeze. For the model, the number of observations was 1,489, the number of groups was 679, and the average number of observations per group was 2.2 (minimum, 1; maximum, 3).
Preschool wheeze phenotypes were defined as follows: no wheeze from birth to age 6 yr (never wheeze), wheezing lower respiratory infection (LRI) before age 3 yr only (transient early wheeze), wheeze at age 6 yr only (late-onset wheeze), and wheezing LRI before age 3 yr and wheeze at age 6 yr (persistent wheeze).
Current frequent wheeze (four or more episodes of wheeze during the previous year) at ages 11 and 16 yr was defined as: no frequent wheeze at either age (11–16–), frequent wheeze at age 11 yr (11+16−), frequent wheeze at age 16 yr (11−16+), and frequent wheeze at both ages (11+16+); children with infrequent wheeze (one to three episodes of wheeze during the previous year) were combined with the no-wheeze group and children with missing wheeze information were included in the model as a separate wheeze category.
Coefficients represent deviations from the reference group in z scores (standard deviation units).
DISCUSSION
In this study, we report for the first time the lung function and wheezing outcomes during the school years of a large group of unselected children in whom wheeze was prospectively ascertained from birth.
This study shows that transient early wheezers, the rather large group of children who wheezed during early life but who were not wheezing at age 6 yr, were as unlikely to wheeze at any age thereafter as children who never wheezed in the first 6 years of life. As reported elsewhere (5), transient early wheezers start life with levels of lung function that are significantly lower than those of children who had no wheezing episodes during the first 6 years of life. We now confirm that these children continue to have lower levels of lung function at the ages of 11 and 16 yr and that, relative to their peers, their levels of lung function remain stable during the school years. It is now established that individuals who enter adult life with lung function deficits are more likely to develop chronic obstructive pulmonary disease during the late adult years, especially if they had lower respiratory illnesses in early life (13). Whether transient early wheezers are also predisposed to chronic obstructive pulmonary disease is at present unknown.
Forced expiratory flows were significantly lower among persistent wheezers as compared with late-onset wheezers. Moreover, the levels of flows were remarkably stable, relative to those of their peers, both for persistent and late-onset wheezers. Our results, therefore, suggest that the deficits in lung function observed in children with asthma are not the consequence of their ongoing disease process during the school years, but are already present by the age of 6 yr. These results support the findings of the Childhood Asthma Management Program study (14), which showed that school children with asthma had relatively stable levels of lung function during a 4- to 6-yr follow-up, even when they were not treated with inhaled antiinflammatory medication. We did not observe an effect of inhaled steroid use on lung function; however, only a small percentage of children who wheezed at age 11 and/or 16 yr reported use of this medication and we did not characterize dose or duration, thus limiting the applicability of this negative finding. Our results are also concordant with those of two extensive prospective studies in which children were enrolled during the school years and in which follow-up for lung function was continued up to the late 20s and early 40s, respectively (3, 4). In those studies, no further deterioration in lung function was observed after the early school years, regardless of the severity of asthma symptoms and of type of asthma therapy during the follow-up.
Our results suggest that there are at least two mechanisms that determine lower levels of lung function in early childhood. One mechanism, observed in transient early wheezers, appears to be present in the first 3 months of life and is unrelated to the likelihood of wheezing during the school years. A different mechanism may be present in persistent wheezers, given their different prognoses. We (5, 15, 16) and others (17–19) have reported that children with persistent wheeze are more likely than transient early wheezers to have high serum IgE levels, eosinophilia, and skin test reactivity to local aeroallergens. Lowe and coworkers (20) showed that, regardless of the development of early respiratory symptoms, children with a strong family history of asthma who had become sensitized to local allergens had significantly lower specific airway conductance at age 3 yr compared with nonsensitized children. It is thus tempting to speculate that the early development of allergic immune responses in the airways may facilitate a process of physiologic remodeling occurring at a time of fast lung and airway growth. Therefore, unlike the initial reductions in lung function present in transient early wheezers, the changes observed in persistent wheezers could be acquired during the first 3 years of life. These results appear to be contradictory to those reported by Turner and coworkers (21). They found that children who wheezed at age 6 yr, and who were still wheezing at age 11 yr, had lower levels of lung function shortly after birth than did children who did not wheeze up to age 11 yr. However, our interest was to determine the long-term outcomes of the preschool wheezing phenotypes we described 10 years ago, and not to assess retrospectively the association between different wheezing phenotypes during the school years and lung function in early life. In addition, in the Australian study, early wheeze was parent reported and not ascertained by a physician, as in our study.
Late-onset wheezers started life with levels of lung function that were similar to those of persistent wheezers but did not show the same deficits in lung function observed in the latter group thereafter. This difference in lung function for two groups of children who are equally likely to wheeze during the school years may have important implications for their prognosis into adult life. In a Dutch study of children with asthma, lung function during childhood was strongly associated with the likelihood of having bronchial hyperresponsiveness, lower lung function, and persistent asthma symptoms by the age of 42 yr (22–24). Therefore, the long-term prognosis of wheezing during childhood may be different for children whose symptoms start before age 3 yr than for those whose symptoms start after that age.
Because of the long duration of this study and the requirement for paired data from the first 3 years of life and at age 6 yr, along with data at 11 or 16 yr, approximately one-third of the initially enrolled children were not included in the analysis. However, as noted in Table E1, there were no differences in preschool wheeze phenotype or wheezing at age 11 or 16 yr between the children included and those excluded because of incomplete data. Those included in the analysis were more likely to belong to families with older, better educated, nonsmoking, non-Hispanic white parents; however, it is unlikely that these differences led to systematic bias in the outcomes analyzed.
In summary, our study strongly suggests that both lung function characteristics in early infancy and events occurring during the first 6 years of life determine the expression of asthma and the level of lung function that will be achieved during childhood and into early adult life. Identifying the environmental and genetic factors that influence these processes will be decisive for the prevention of asthma and chronic airway obstruction.
Supplementary Material
Acknowledgments
The authors thank study nurses Marilyn Lindell and Lydia de la Ossa for data collection, Shelley Radford and Darcie Revay for measurement of lung function at age 6 yr, Bruce Saul and Cathy Ward for database maintenance, Maria Cristina Minervini and Marilyn Halonen for helpful comments, and the participants in the Children's Respiratory Study.
Supported by National Institutes of Health grants HL-14136, HL-56177, and HL-03154.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200504-525OC on August 18, 2005
Conflict of Interest Statement: W.J.M. is chair of the Epidemiology Study of Cystic Fibrosis sponsored by Genentech, Inc., for which he received $7,000 in 2004. D.A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.W.G. received $6,000 in 2004, $3,000 in 2003, and $3,500 in 2002 for serving on advisory boards, consulting on designing clinical trials, and speaking on conferences sponsored by GlaxoSmithKline (GSK). She has received $2,500 in 2005, $1,500 in 2004, and $2,100 in 2002 for speaking at conferences sponsored by AstraZeneca (AZ). She has received $12,000 in 2003 from Genentech as a research grant for participating in a multicenter epidemiology trial. She has received $9,000 from an exchange program for consulting in the design of CME courses for asthma. T.W.G. has participated as a speaker in CME-accredited courses sponsored by the following companies: SOMA Medical Education, Innovia Education Institute, Medical World Conferences, and Health Matters. L.M.T. serves on the Pediatric Expert Panel for GSK and received $1,500 in 2004 for this activity. A.L.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.D.M. received $13,100 in 2002, $3,000 in 2003, and $6,500 in 2004 from Merck while serving as a member on the Merck Scientific Advisory Board for participation and lecture fees. He received monies from GSK for presenting at a sponsored event in 2003 ($1,500). As a member of the AZ Speaker's bureau, he received $10,000 in 2002 and $2,500 in 2003 for lectures.
References
- 1.Martinez FD. Toward asthma prevention: does all that really matters happen before we learn to read? N Engl J Med 2003;349:1473–1475. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD, Godfrey S. Wheezing disorders in the preschool child. London: Martin Dunitz, Taylor & Francis Group; 2003.
- 3.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol 2002;109:189–194. [DOI] [PubMed] [Google Scholar]
- 4.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med 1995;332:133–138. [DOI] [PubMed] [Google Scholar]
- 6.Stern DA, Morgan WJ, Sherrill D, Guerra S, Guilbert T, Halonen M, Wright AL, Taussig LM, Martinez FD. Long term outcome of wheezing during the first 6 years of life [abstract 208]. Presented at the AHSC Frontiers in Biomedical Research Poster Forum, Arizona State University, Tempe, AZ, September 15, 2004.
- 7.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG, Group Health Medical Associates. The Tucson Children's Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol 1989;129:1219–1231. [DOI] [PubMed] [Google Scholar]
- 8.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children's Respiratory Study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol 1989;129:1232–1246. [DOI] [PubMed] [Google Scholar]
- 9.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 10.Stern DA, Lohman IC, Wright AL, Taussig LM, Martinez FD, Halonen M. Dynamic changes in sensitization to specific aeroallergens in children raised in a desert environment. Clin Exp Allergy 2004;34:1563–1569. [DOI] [PubMed] [Google Scholar]
- 11.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997;16:2349–2380. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 2004;7:127–150. [Google Scholar]
- 13.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med 1996;154:S208–S211. [DOI] [PubMed] [Google Scholar]
- 14.Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–1063. [DOI] [PubMed] [Google Scholar]
- 15.Karakoc F, Remes ST, Martinez FD, Wright AL. The association between persistent eosinophilia and asthma in childhood is independent of atopic status. Clin Exp Allergy 2002;32:51–56. [DOI] [PubMed] [Google Scholar]
- 16.Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol 1999;104:28–36. [DOI] [PubMed] [Google Scholar]
- 17.Reijonen TM, Korppi M, Kuikka L, Savolainen K, Kleemola M, Mononen I, Remes K. Serum eosinophil cationic protein as a predictor of wheezing after bronchiolitis. Pediatr Pulmonol 1997;23:397–403. [DOI] [PubMed] [Google Scholar]
- 18.Pifferi M, Ragazzo V, Caramella D, Baldini G. Eosinophil cationic protein in infants with respiratory syncytial virus bronchiolitis: predictive value for subsequent development of persistent wheezing. Pediatr Pulmonol 2001;31:419–424. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson EC, Turner G, Heaney LG, Schock BC, Taylor R, Gallagher T, Ennis M, Shields MD. Bronchoalveolar lavage findings suggest two different forms of childhood asthma. Clin Exp Allergy 1997;27:1027–1035. [DOI] [PubMed] [Google Scholar]
- 20.Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet 2002;359:1904–1908. [DOI] [PubMed] [Google Scholar]
- 21.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Cox M, Young S, Goldblatt J, Landau LI, Le Souef PN. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am J Respir Crit Care Med 2004;169:921–927. [DOI] [PubMed] [Google Scholar]
- 22.Grol MH, Postma DS, Vonk JM, Schouten JP, Rijcken B, Koeter GH, Gerritsen J. Risk factors from childhood to adulthood for bronchial responsiveness at age 32–42 yr. Am J Respir Crit Care Med 1999;160: 150–156. [DOI] [PubMed] [Google Scholar]
- 23.Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koeter GH, Rijcken B, Postma DS. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: a 30-year follow-up study. Am J Respir Crit Care Med 1999;160:1830–1837. [DOI] [PubMed] [Google Scholar]
- 24.Vonk JM, Postma DS, Boezen HM, Grol MH, Schouten JP, Koeter GH, Gerritsen J. Childhood factors associated with asthma remission after 30 year follow up. Thorax 2004;59:925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.