Abstract
Rationale: Little is known about the respiratory-related discharge properties of motor units driving any of the eight muscles that control the movement, shape, and stiffness of the mammalian tongue.
Objectives: To characterize the respiratory-related discharge of genioglossus motor units as synaptic drive to the hypoglossal motoneuron pool is increased with hypercapnia.
Measurements: We recorded airflow, genioglossus muscle EMG activity, and the respiratory-related discharge of 30 genioglossus muscle motor units in spontaneously breathing, urethane-anesthetized rats under control conditions and in hypercapnia (inspired CO2: 3, 6, 9, and 12%, 3–5 min at each level).
Main Results: All motor units were active throughout all or most of inspiration. Nine of 30 units showed “preinspiratory” activity (discharge onset within the last 20% of expiration), with continued discharge into inspiration. Six inspiratory units transitioned to a preinspiratory pattern when inspired CO2 exceeded 6%. For the majority of units (23/30), discharge rate increased with hypercapnia, with the maximum increase averaging about 50%. The average variability of interspike intervals within a spike train increased from 33% under baseline conditions to 50% with maximal hypercapnia.
Conclusions: (1) The discharge pattern of genioglossus muscle motor units can be altered by hypercapnia; (2) most, but not all, genioglossus motor units receive synaptic input from CO2-sensitive chemoreceptors; (3) individual motor units have a wide range of CO2 sensitivities; and (4) hypercapnia significantly increases the variability of motor unit discharge, which may enhance muscle force output.
Keywords: electrophysiology, respiratory muscles, tongue
The muscles of the mammalian tongue adjust its position, shape, and stiffness, and thus play an important role in breathing, swallowing, mastication, and speech. The mammalian tongue is controlled by four extrinsic muscles that originate outside the tongue and insert into the tongue body, and four intrinsic muscles that originate and terminate within the tongue (1–3). Previous findings on the respiratory-related activity of hypoglossal motoneurons (4–6) are based on recordings from single motor axons dissected from the main trunk of the hypoglossal nerve or one of its major branches (6), or by impaling cells in the hypoglossal motor nucleus (7–9). But because hypoglossal motoneurons and motor axons project to any one of the eight tongue muscles (1–3), it is unclear to which of these muscles the motoneuronal activities corresponded. In contrast to previous work, we sought to characterize the respiratory-related discharge pattern of a select group of motor units within the hypoglossal motoneuron pool. Specifically, we targeted the motor units of the genioglossus muscle, an extrinsic muscle that depresses and protrudes the tongue (10), and which is believed to play a critical role in preventing pharyngeal airway collapse during sleep (11–13). Experiments were done in tracheotomized, anesthetized rats at various levels of respiratory drive, which was altered by adding CO2 to the inspired gas mixture (“hypercapnia”). Our main hypothesis was that an increase in motoneuron discharge rate contributes substantially to increases in respiratory-related genioglossus whole muscle EMG activities as respiratory drive is increased with hypercapnia.
In addition, the hypoglossal motoneuron pool provides a model system that can be used to examine the discharge pattern of motor units that receive convergent synaptic inputs from intrinsic oscillatory networks in the brainstem, from chemoreceptors in the brain and vasculature, and from mechanoreceptors in the upper airways and lungs. Recent theoretical studies suggest that noisy synaptic inputs evoke highly variable neuronal discharge patterns, whereas neurons driven by intrinsic oscillators (i.e., the respiratory central pattern generator) exhibit more regular discharge patterns (14). Discharge variability may also be important functionally (see Discussion), as increased variability in motor unit spike trains has been shown to enhance muscle torque by activating the “catchlike” property of muscle (15, 16). Accordingly, we computed the discharge variability of genioglossus muscle motor units as neural drive to the hypoglossal motoneuron pool was increased progressively with hypercapnia. This enabled us to test the hypothesis that the variability in the motor unit spike train will increase when additional excitatory synaptic inputs from CO2-sensitive chemoreceptors are superimposed on the oscillator-driven activity that predominates under baseline conditions.
METHODS
Genioglossus muscle motor unit recordings were obtained from 17 spontaneously breathing male Sprague-Dawley rats weighing 250 to 400 g. Animals were initially anesthetized with isofluorane and a cannula was inserted into a femoral vein. Isofluorane anesthesia was then discontinued, and animals were anesthetized with intravenous urethane at a dose of 1.6 g/kg. Measurement and analysis of respiratory airflow, genioglossus muscle EMG activities, and single motor unit potentials were recorded using methods described in detail previously (17–20), and in the online supplement.
Experimental Protocol
Single motor unit potentials were isolated and identified while the animal breathed 50% oxygen, 3% CO2, balance N2. By elevating the CO2, we ensured that drive to the muscle was sufficient to activate a larger population of motor units. We focused exclusively on units that exhibited unequivocal respiratory-modulated activities, and did not record the activity of tonically discharging motor units, as it is not possible to determine whether tonic discharge underlies respiratory or nonrespiratory activity.
Once a stable unit recording was obtained, the animal breathed CO2-free gas (40–50% O2, balance N2) for 10 min. At this time we began recording baseline data for 5 min, and then the animal was consecutively exposed to four levels of hyperoxic hypercapnia (inspired CO2 concentration: 3, 6, 9, and 12%). EMG and unit activities were recorded for 3 to 5 min at each level of CO2. Only those units that maintained stable activities throughout each of the five experimental conditions were included in the subsequent analysis (Figure 1, and see the online supplement). After recording at each of these levels, another motor unit was sought on the same side of the genioglossus muscle belly. If a significantly different firing pattern and shape (as seen on the oscilloscope) was found in a single motor unit potential on the same side, it was considered to be a distinct motor unit, and the protocol was repeated as described above. If we could not find a unique single motor unit potential on the same side, the ipsilateral hypoglossal nerve was sectioned, and that half of the muscle became silent. We then searched for motor unit potentials on the contralateral side, and the protocol was repeated. Although this method resulted in a low yield of motor unit recordings from each animal (1 unit from each of seven rats, 2 units from each of eight rats, 3 units from one rat, and 4 units from one rat), it ensured that we were not sampling the activity of the same genioglossus motor unit more than once.
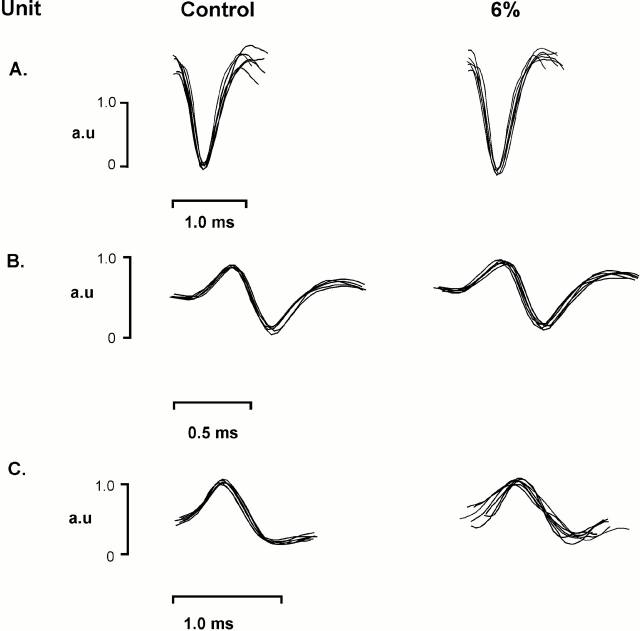
Figure 1.
Three motor units recorded from the same rat, under control conditions and when the animal was breathing 6% inspired CO2. A through C show six superimposed spikes, demonstrating clear discrimination of motor units on the basis of waveform shape and amplitude. Also note that the shape and amplitude of each potential remained constant when respiratory drive increased with CO2 breathing. a.u. = arbitrary units.
Statistical Analysis
All data were averaged across all motor units within each level of hypercapnia and for the normocapnic baseline period. Ventilatory parameters (Vt, breathing frequency, and the rate of pulmonary ventilation), the average integrated EMG (iEMG) activity, the mean motor unit firing frequency, the number of spikes per respiratory cycle, and the coefficient of variation (CV) of the interspike interval (ISI) were analyzed with repeated-measures analysis of variance; details of the data analysis and statistical procedures are provided in the online supplement.
RESULTS
Ventilatory responses to progressive hypercapnia are given in Table 1. Hypercapnia increased pulmonary ventilation monotonically, with the average value rising to 181% of baseline at 12% CO2. The increase in ventilatory output was due exclusively to changes in Vt, as breathing frequency did not increase; in fact, breathing frequency declined slightly at the most severe hypercapnic levels. The iEMG of the genioglossus muscle during hypercapnia paralleled the increase in pulmonary ventilation rate, showing that hypercapnia increased neural drive to the muscle (Table 1). Hypercapnia had no significant effects on mean arterial blood pressure.
TABLE 1.
AVERAGE VALUES FOR EXPIRED VENTILATION RATE, VT, BREATHING FREQUENCY, INTEGRATED GENIGLOSSUS MUSCLE EMG ACTIVITY, AND MEAN ARTERIAL BLOOD PRESSURE AT FIVE LEVELS OF INSPIRED CO2
| Inspired CO2 (%) | 0 | 3 | 6 | 9 | 12 | p Value |
|---|---|---|---|---|---|---|
| Ventilation, % baseline | 100 | 120.8 ± 2.8* | 140.2 ± 4.2* | 163.8 ± 7.0* | 181.0 ± 9.2* | < 0.001 |
| Vt, % baseline | 100 | 118.4 ± 1.6* | 139.4 ± 3.3* | 169.6 ± 6.0* | 191.0 ± 7.3* | < 0.001 |
| Frequency, min−1 | 115 ± 3.2 | 116.9 ± 3.4 | 115.4 ± 3.0 | 109.9 ± 3.0 | 106.9 ± 3.3 | NS |
| Genioglossus iEMG, % baseline | 100 | 108 ± 5.0 | 133.7 ± 8 | 166.5 ± 15.6* | 179.1 ± 22.2* | < 0.001 |
| MABP, mm Hg | 87.8 ± 17.0 | 84.1 ± 17.3 | 86.5 ± 14.5 | 85.4 ± 15.2 | 86.9 ± 15.2 | NS |
Definition of abbreviations: iEMG = integrated EMG; MABP = mean arterial blood pressure; NS = not significant.
p values reflect the results of analysis of variance and appropriate post hoc analyses (see Methods and the online supplement).
p < 0.05 versus % inspired CO2 = 0.
We recorded the activity of 30 motor units, identified as genioglossus muscle units using anatomic methods, and selected on the basis of strong respiratory-related modulation (see Methods and the online supplement). All units discharged in inspiration, with 9 of the 30 units classified as “preinspiratory” units, as they commenced discharge within the last 20% of expiration, with continued discharge into inspiration. Six of the units discharged only in inspiration under baseline conditions, but transitioned to the preinspiratory pattern when the inspired CO2 level exceeded 6%. Segments of a recording from a representative motor unit are shown in Figure 2. Note that, under baseline conditions (Figure 2A), the unit discharged in inspiration only, but transitioned to a preinspiratory discharge pattern in hypercapnia (Figure 2B). Note that hypercapnia was also associated with parallel increases in flow, genioglossus EMG activity, and motor unit discharge rate. The insets in Figures 2A and 2B, together with the data shown in Figure 1, confirm that despite increased muscle activity with hypercapnia, the shape and amplitude of the motor unit potential remained stable.
Figure 2.
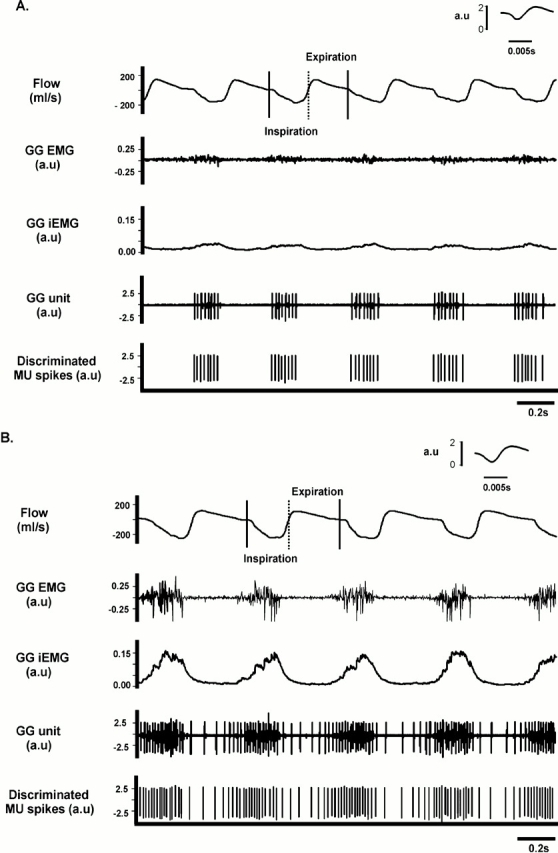
Original recordings of (from top down) airflow (with inspiration represented by a downward deflection), nonprocessed genioglossus EMG activity, the integrated EMG (iEMG) of the genioglossus, the nonprocessed genioglossus motor unit recording, and the discriminated motor unit spikes. (A) A segment of a recording obtained under baseline conditions; (B) recordings obtained during hypercapnia (inspired CO2 = 9%) in the same animal. Note that hypercapnia increased airflow and genioglossus EMG activity, as well as the frequency and train length of the active motor unit. Also note that the pattern of motor unit bursts transitioned from an inspiratory-only pattern under baseline conditions (A), to a preinspiratory–inspiratory pattern with hypercapnia (B). The insets shown in A and B represent a single spike recorded at high speed. See text for further details. MU = motor unit.
Average data for motor unit discharge rate and iEMG activity are shown as a function of the inspired CO2 in Figure 3. Discharge rate rose progressively, with an increase of approximately 50% between baseline conditions and the maximal hypercapnic level (inspired CO2 concentration of 12%; Figure 3A). This rise in discharge rate with hypercapnia paralleled the rise in iEMG activity (Figure 3B). Although the average discharge rate rose smoothly as a function of inspired CO2, the change in discharge rate among units varied widely. Figure 4A shows the discharge rate for all genioglossus motor units, at each level of inspired CO2. Although 23 motor units increased their discharge rate with hypercapnia by 20% or more, in 7 of 30 units discharge rate did not increase with hypercapnia. To determine if the change in motor unit discharge rate with hypercapnia was correlated with the overall level of drive to the muscle (i.e., the whole muscle EMG), we performed a correlation analysis of discharge rate and iEMG activity. As shown in Figure 4B, the correlation was relatively weak, with an r2 of 0.152.
Figure 3.
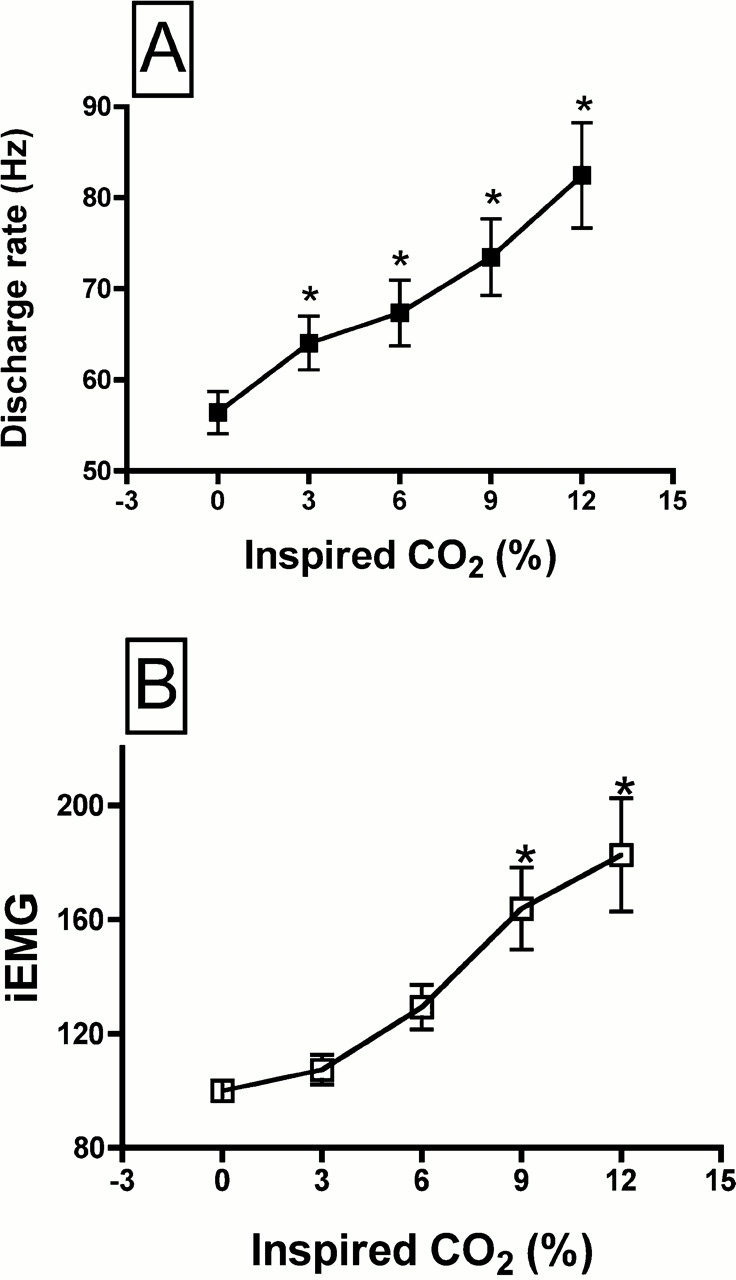
Average discharge rate of genioglossus motor units (A) and the average iEMG of the whole genioglossus muscle as a function of the % inspired CO2 (B). * Significantly different than corresponding value at 0% inspired CO2. iEMG expressed as a % of baseline activity.
Figure 4.
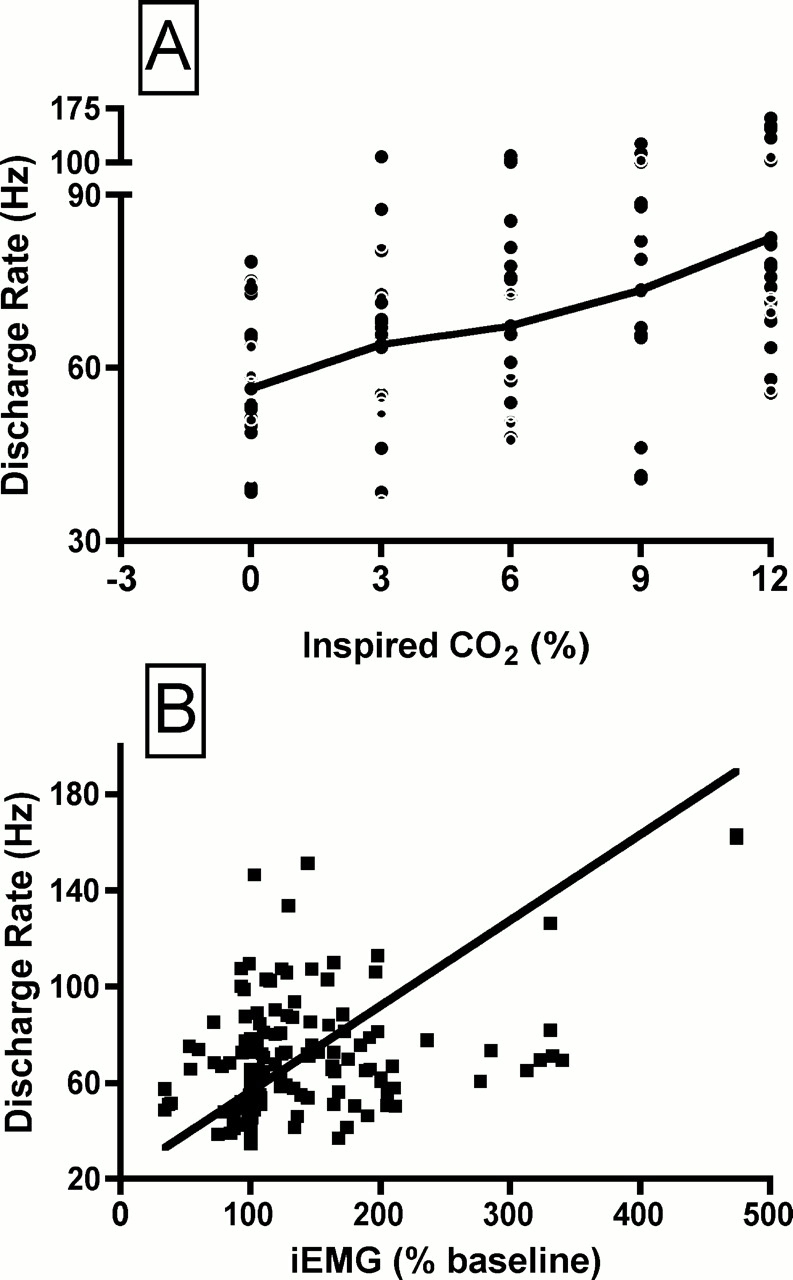
(A) Discharge rate of all 30 genioglossus motor units, at each level of inspired CO2. The solid black line connects the mean values at each level of inspired CO2. (B) Discharge rate as a function of the iEMG activity, expressed as a % of the baseline activity. Each point represents the average rate for a given motor unit, at a given level of inspired CO2. There are 150 data points (30 motor units, each studied at five levels of inspired CO2). Solid black line is the result of linear regression analysis (r2 = 0.152, p < 0.001).
We also computed the number of spikes discharged in each respiratory cycle, at each level of hypercapnia. The number of spikes per cycle increased as a function of inspired CO2, reaching significance at the three highest levels (Figure 5A). The variability within each spike train was estimated by computing the CV of the ISI. An example of the instantaneous discharge rate of a motor unit under baseline conditions and at 9% CO2 is shown in Figures 2A and 2B, respectively. It is evident that the variability of successive ISIs increases with hypercapnia. This is borne out by the average data shown in Figure 5B, as the CV ISI increased significantly at the three highest levels of inspired CO2.
Figure 5.
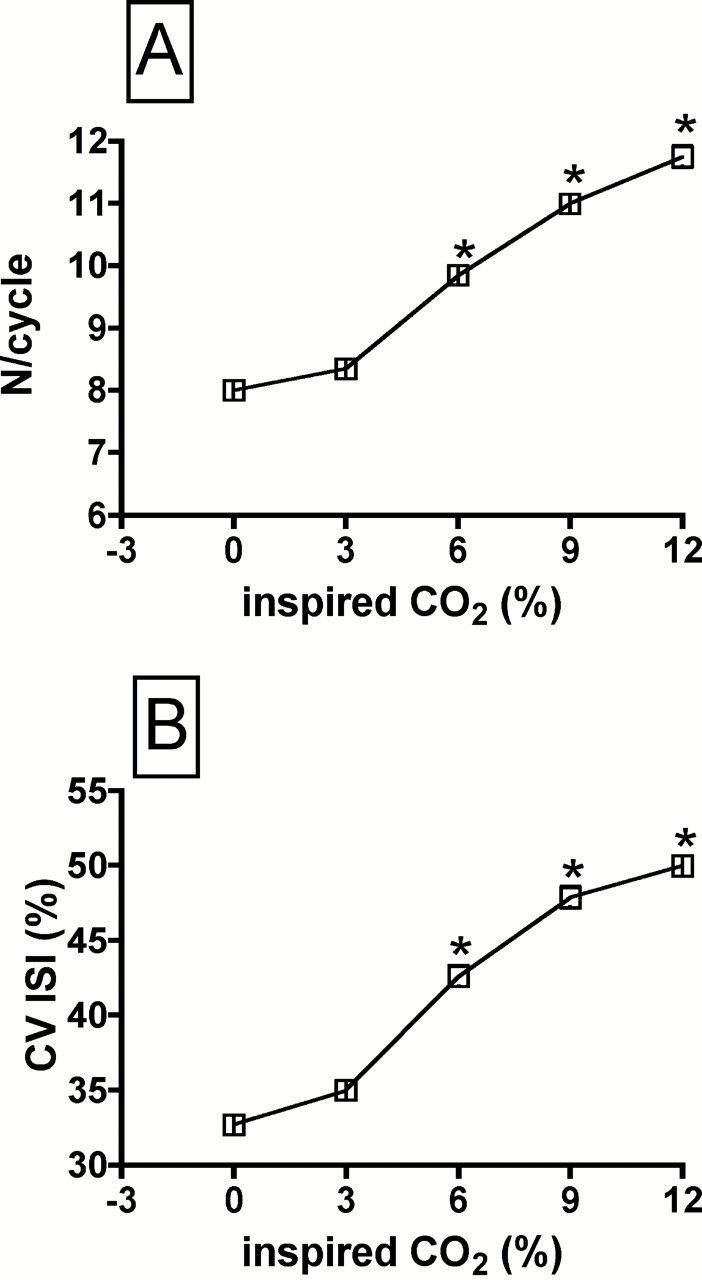
Changes in the number of spikes per respiratory cycle (N/cycle; A) and the coefficient of variation of the interspike interval (CV ISI; B) as a function of the % inspired CO2. * Different than corresponding values at 0% inspired CO2.
DISCUSSION
Remarkably few studies have examined the discharge behavior of respiratory-related genioglossus muscle motor units, despite the fact that understanding how these units are modulated is a critical first step toward understanding mechanisms of pharyngeal airway defense. In the current study, we recorded the discharge of respiratory-related motor unit spike trains in the rat genioglossus muscle, under baseline conditions and when neural drive to the muscle was increased with hypercapnia. Our major findings are as follows: (1) all 30 respiratory-related units had inspiratory-modulated discharge patterns, with some units showing a preinspiratory discharge pattern under baseline conditions and/or in hypercapnia; (2) motor unit discharge rate increased with hypercapnia in 23 of 30 units, and the temporal pattern of the spike trains became significantly more variable; and (3) there is only a weak correlation between changes in discharge rate and whole muscle EMG activity with progressive hypercapnia.
Methodologic Considerations
Denervation of the lateral hypoglossal nerve branch could alter the discharge pattern of genioglossus muscle motor units in unpredictable ways, because this procedure removes the major antagonist muscles, which are generally coactive with the genioglossus in rats and human subjects (18, 21, 22). However, because the fibers from all major tongue muscles are so extensively intermeshed, we used lateral branch denervation to ensure that we were sampling genioglossus muscle motor units (see Methods in the online supplement), which was our priority for this initial study.
We chose not to study tonically discharging units because such tonic activity may or may not be respiratory related. The influence of hypercapnia on tonically discharging motor units is certainly of interest, and future studies are needed. If CO2 modulates such activities, one could assume that they have a respiratory-related function, unless all central neurons are CO2 sensitive, as some believe (23); if this were the case, interpretation of such data would be difficult. It should be noted that the number of tonic units that we did find was very low, as stated in the online supplement.
Finally, we searched for units against a background of 3% inspired CO2 to increase the odds of finding CO2-sensitive single motor units. Although this technique is very common when searching for respiratory-related motor unit or motoneuron activities in anesthetized animal models (4, 5), it can bias the results toward only those units that are CO2 sensitive. However, all units in our sample were active under baseline normocapnic conditions, indicating that bias was not a problem in this study. We noted that 6 of the 30 motor units in our sample did not increase their discharge rate with hypercapnia, indicating that the genioglossus muscle does have motor units with respiration-related discharge, but an absence of CO2 sensitivity. The significance of these units to tongue motor control is unknown.
Discharge Pattern of Genioglossus Muscle Motoneurons
The genioglossus motor unit discharge pattern was defined on the basis of the temporal relationship between the spike train and the subdivisions of the respiratory cycle. All 30 genioglossus motor units that we recorded showed inspiratory modulation. Twenty-one of the 30 units discharged exclusively in inspiration, whereas the remaining nine were defined as preinspiratory units (those units that commenced discharge within the last 20% of expiration, with continued discharge into inspiration). Six of the 21 inspiratory units transitioned to the preinspiratory discharge pattern when the inspired CO2 exceeded 6%. On the basis of these observations, it appears that inspiratory and preinspiratory units are derived from a single population of hypoglossal motoneurons with a range of thresholds to excitatory inputs from central chemoreceptors. Thus, units with preinspiratory discharge patterns may have comparatively low thresholds to stimulation by central chemoreceptor afferent inputs relative to inspiratory units.
Two or more respiratory-related discharge patterns (e.g., inspiratory, preinspiratory, expiratory, phase-spanning, tonic) have been reported previously in recordings obtained from hypoglossal motoneurons (4, 5, 7), although the proportion and characteristics of motoneuron discharge patterns reported in each of the aforementioned studies varied substantially. However, it is important to note that, in these studies, activities were recorded from motor axons dissected from the hypoglossal nerve (4), one of its main branches (5), or from the somata of neurons within the hypoglossal motor nucleus (7). Thus, it is likely that a substantial proportion of the units recorded represent the discharge of motoneurons driving tongue muscles other than the genioglossus. This is not the case for the present findings, which are based on recordings from anatomically confirmed genioglossus muscle motor units.
Our finding that all motor units discharged during inspiration, with some of the units showing preinspiratory bursting, is consistent with recent data in human subjects (22). From a functional standpoint, the preinspiratory discharge of some units, found by us and others, underlies the common observation that tongue muscle EMG activity precedes the onset of inspiratory airflow (2, 4, 18, 24, 25), particularly when respiratory drive is elevated. This preinspiratory discharge is presumed to stiffen and dilate the pharyngeal airway in advance of the negative intrapharyngeal pressure that accompanies contraction of the diaphragm and other inspiratory pumping muscles. Importantly, these new findings indicate that the preinspiratory discharge is not the result of a separate population of genioglossus motoneurons; rather, when synaptic input is sufficient, a substantial population of genioglossus motoneurons can transition to the preinspiratory pattern and participate in airway defense.
Motor Unit Spike Train Variability
Hypercapnia significantly increased the variability in the temporal pattern of genioglossus motor unit spike trains, as assessed by computing the CV of the ISI. The variability of neural discharge patterns has traditionally been attributed to synaptic noise, which broadly refers to fluctuations in membrane potential resulting from diffuse synaptic inputs (26). More recent studies suggest that fluctuations in spike train discharge rate are correlated with changes in the duration of the after hyperpolarization (27, 28), although this idea is based on data from tonically firing motoneurons, and may not apply to phasically modulated respiratory motoneurons. In the present experiments, the CV of the ISI ranged from 30% under baseline conditions to over 50% at the highest level of hypercapnia. This is consistent with recent data in feline geniohyoid muscle motor units, which showed increased motor unit discharge variability on removal of inhibitory afferent inputs from pulmonary stretch receptors (29). Similarly, pharmacologic elimination of excitatory synaptic inputs to abducens motoneurons reduced the CV of the ISI (30). Together, these observations support the view (14) that excitatory synaptic input increases the variability of neural spike train discharge.
Although our experiments cannot establish the source of the increased discharge variability of genioglossus motor units with hypercapnia, variability itself may be of functional significance. Studies in human and animal subjects have shown that variable frequency trains enhance muscle torque by activating the catchlike property of muscle, particularly during fatiguing contractions (15, 16). According to this view, shorter, randomly occurring interpulse intervals enhance intracellular calcium levels and excitation–contraction coupling (31). Others have suggested that variable spike train patterns increase muscle stiffness by actions on the series elastic component, leading to a greater mechanical advantage (32). It is interesting that the compliance of the series elastic component increases with fatigue (33); under these conditions, a more variable spike train may compensate for the increase in compliance. These observations are of potential clinical significance given recent work that shows hypoxia induces fatigue in the genioglossus muscle in rat during rhythmic contractions (34).
Discharge Rate in Hypercapnia
In a previous study of genioglossus motoneuronal activities, Hwang and colleagues (4) showed that the magnitude of the change in discharge rate with hypercapnia varies widely. Similarly, Mitra and Cherniack (5) reported two large populations of hypoglossal motoneurons that increased their discharge rate with hypercapnia. Although they failed to provide the range of the increase in discharge in these populations (5), the average rate rose from 10 Hz under baseline conditions to 50 Hz with maximal hypercapnic stimulation, which is similar to the average changes reported herein. The magnitude of the increase in discharge rate in other respiratory muscle motor unit pools with hypercapnia also tends to be small and variable. For example, hypercapnia is associated with an increase of approximately 8 Hz in the discharge rate of diaphragm motor units, and a 1- to 2-Hz increase in the discharge rate of intercostal and scalene muscle motor units (35). In comparison, the dynamic range of discharge rate modulation in genioglossus muscle motor units is relatively large, although variable across the population.
Contribution of Discharge Rate to Genioglossus Muscle EMG Activity
The correlation between motor unit discharge frequency and the net change in whole muscle EMG activity has been used previously as a crude estimate of the contribution of discharge rate to changes in muscle force. For example, during natural locomotion in the conscious rat, modulation of discharge rate explained 35% of the change in the slow-twitch soleus muscle EMG activity, but only 1.5% of the change in the EMG of the fast-twitch lateral gastrocnemius (36). The authors suggest that the difference is due either to the diverse physiologic properties of the two muscles or to different synaptic inputs from spinal pattern generators for locomotion. In the present study, discharge rate could explain only about 15% of the variability in genioglossus muscle iEMG activity during hypercapnia. The rat genioglossus muscle has fast contractile properties, consisting of approximately 10% type I, 37% type IIa, and 54% type IIb muscle fibers (37). These data are thus consistent with the idea (36) that muscles with fast contractile properties rely more on recruitment than on an increase in discharge rate to enhance drive to the muscle. In contrast, a previous study from our laboratory showed that discharge rate could explain 57% of the hypoxia-induced change in the respiratory-related activity of the external oblique abdominal muscle in the anesthetized cat (20), which has a preponderance of fast motor units (38). These observations do not support the view that the contribution of discharge rate is low in motor units with fast contractile properties. Perhaps the contribution of discharge rate to muscle force is unique to the mechanical actions of the muscle and its behavioral context.
Supplementary Material
Acknowledgments
The authors thank Liliana Erard for technical assistance.
Supported by National Institutes of Health grants HL 56876 and DC 05728.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200505-790OC on September 1, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 1995;74:547–555. [DOI] [PubMed] [Google Scholar]
- 2.Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 1997;110:295–306. [DOI] [PubMed] [Google Scholar]
- 3.Sokoloff AJ. Activity of tongue muscles during respiration: it takes a village? J Appl Physiol 2004;96:438–439. [DOI] [PubMed] [Google Scholar]
- 4.Hwang JC, Bartlett D Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 1983;55:793–798. [DOI] [PubMed] [Google Scholar]
- 5.Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fiber activity. Respir Physiol 1983;54:55–66. [DOI] [PubMed] [Google Scholar]
- 6.Mitra J, Prabhakar NR, Haxhiu M, Cherniack NS. Comparison of the effects of hypercapnia on phrenic and hypoglossal activity in anesthetized decerebrate and decorticate animals. Brain Res Bull 1986;17:181–187. [DOI] [PubMed] [Google Scholar]
- 7.Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience 1988;25:1041–1051. [DOI] [PubMed] [Google Scholar]
- 8.Mifflin SW. Arterial chemoreceptor input to respiratory hypoglossal motoneurons. J Appl Physiol 1990;69:700–709. [DOI] [PubMed] [Google Scholar]
- 9.Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep 1996;19:187–195. [PubMed] [Google Scholar]
- 10.Nishikawa KC, Kier WM, Smith KK. Morphology and mechanics of tongue movement in the African pig-nosed frog Hemisus marmoratum: a muscular hydrostatic model. J Exp Biol 1999;202:771–780. [DOI] [PubMed] [Google Scholar]
- 11.Knaack L, Podszus T. Electric stimulation of the upper airway muscle. Curr Opin Pulm Med 1998;4:370–375. [DOI] [PubMed] [Google Scholar]
- 12.Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am 2003;36:501–510. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de Heyning P, Ejnell H, Hochban W, Knaack L, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001;127:1216–1223. [DOI] [PubMed] [Google Scholar]
- 14.Gutkin BS, Ermentrout GB. Dynamics of membrane excitability determine interspike interval variability: a link between spike generation mechanisms and cortical spike train statistics. Neural Comput 1998;10:1047–1065. [DOI] [PubMed] [Google Scholar]
- 15.Callister RJ, Reinking RM, Stuart DG. Effects of fatigue on the catchlike property in a turtle hindlimb muscle. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2003;189(12):857–866. [DOI] [PubMed] [Google Scholar]
- 16.Slade JM, Bickel CS, Warren GL, Dudley GA. Variable frequency trains enhance torque independent of stimulation amplitude. Acta Physiol Scand 2003;177:87–92. [DOI] [PubMed] [Google Scholar]
- 17.Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 2004;96:440–449. [DOI] [PubMed] [Google Scholar]
- 18.Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 2001;532:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol 1997;82:419–425. [DOI] [PubMed] [Google Scholar]
- 20.Mateika JH, Essif E, Fregosi RF. Effect of hypoxia on abdominal motor unit activities in spontaneously breathing cats. J Appl Physiol 1996;81:2428–2435. [DOI] [PubMed]
- 21.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 1998;507:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 1999;160:1976–1982. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol 2004;197:179–191. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett D Jr, St John WM. Influence of lung volume on phrenic, hypoglossal and mylohyoid nerve activities. Respir Physiol 1988;73:97–109. [DOI] [PubMed] [Google Scholar]
- 25.Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol 1979;46:772–779. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard JI, Stenhouse D, Eccles RM. Origin of synaptic noise. Science 1967;157:330–331. [DOI] [PubMed] [Google Scholar]
- 27.Powers RK, Turker KS, Binder MD. What can be learned about motoneurone properties from studying firing patterns? Adv Exp Med Biol 2002;508:199–205. [DOI] [PubMed] [Google Scholar]
- 28.Powers RK, Binder MD. Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones. J Physiol 2000;528:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Respir Physiol 2001;124:23–33. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Forero D, Alvarez FJ, de la Cruz RR, Delgado-Garcia JM, Pastor AM. Influence of afferent synaptic innervation on the discharge variability of cat abducens motoneurones. J Physiol 2002;541:283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchateau J, Hainaut K. Nonlinear summation of contractions in striated muscle. II. Potentiation of intracellular Ca2+ movements in single barnacle muscle fibres. J Muscle Res Cell Motil 1986;7:18–24. [DOI] [PubMed] [Google Scholar]
- 32.Parmiggiani F, Stein RB. Nonlinear summation of contractions in cat muscles. II. Later facilitation and stiffness changes. J Gen Physiol 1981;78:295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigreux B, Cnockaert JC, Pertuzon E. Effects of fatigue on the series elastic component of human muscle. Eur J Appl Physiol Occup Physiol 1980;45:11–17. [DOI] [PubMed] [Google Scholar]
- 34.Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 2000;88:2123–2130. [DOI] [PubMed] [Google Scholar]
- 35.Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med 1999;160:1598–1603. [DOI] [PubMed] [Google Scholar]
- 36.Gorassini M, Eken T, Bennett DJ, Kiehn O, Hultborn H. Activity of hindlimb motor units during locomotion in the conscious rat. J Neurophysiol 2000;83:2002–2011. [DOI] [PubMed] [Google Scholar]
- 37.Oliven A, Carmi N, Coleman R, Odeh M, Silbermann M. Age-related changes in upper airway muscles morphological and oxidative properties. Exp Gerontol 2001;36:1673–1686. [DOI] [PubMed] [Google Scholar]
- 38.Watchko JF, Daood MJ, Vazquez RL, Brozanski BS, LaFramboise WA, Guthrie RD, Sieck GC. Postnatal expression of myosin isoforms in an expiratory muscle–external abdominal oblique. J Appl Physiol 1992;73:1860–1866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.