Abstract
GLI proteins, highly conserved in vertebrates and invertebrates, are critical in modulating embryonic development and adult tissue homeostasis. These proteins are zinc-finger-containing transcription factors that were originally identified by genetic screening of embryonic lethal mutants of Drosophila melanogaster. Alterations in GLI activity can lead to tumor development in tissues of different origin including the pancreas. GLI activity is mainly regulated by the Hedgehog pathway, via a ligand-receptor complex that triggers a signaling cascade that activates GLI transcription factors, which in turn regulate gene expression, an essential step of Hegdehog-mediated cellular effects. Interestingly, recent reports show the ability of other signaling cascades to modulate GLI function in cancer cells including RAS and TGF-β, two pathways implicated in pancreatic carcinogenesis. Thus, these findings suggest that GLI proteins are not an exclusive downstream target of Hedgehog but rather a common effector of a network of signaling pathways controlling pancreatic carcinogenesis.
Key Words: GLI proteins, Hedgehog, Pancreatic cancer
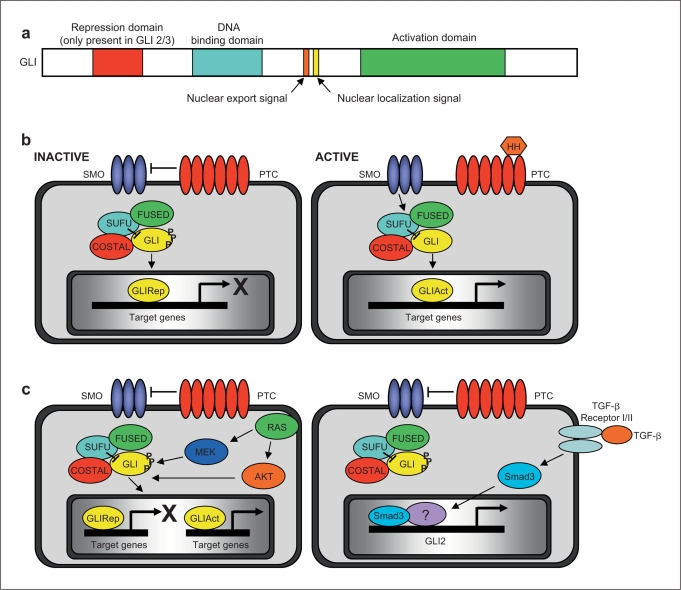
The vertebrate GLI family consists of 3 different members (GLI 1, 2 and 3), which have a highly conserved DNA-binding domain comprising C2-H2 zinc finger motifs that bind to the consensus sequence GACCACCCA, as well as nuclear localization and export signals that regulate GLI subcellular location [1,2,3]. GLI proteins also contain a C-terminal activation domain, but only GLI2 and GLI3 are known to have an N-terminal repression domain [4] (fig. 1a). Analysis of the mechanisms revealed that the C-terminal activation domain modulates transcription through the interaction with specific coactivator molecules such as the acetyltransferase p300 [5]. Regarding the repressor activity, it has been reported to be SKI- or mSIN3A-dependent; these corepressors bind to GLI and recruit the histone deacetylase complex to its target genes [6, 7]. Interestingly, the high homology between the DNA binding of GLI proteins suggested that competition could be an additional mechanism for the modulation of transcription. The molecular basis underlying the competition is the similarities present within their zinc finger motifs. In particular, the amino acids that are predicted to mediate interactions between these proteins and DNA are identical. However, whether this mechanisms is taking place, remains to be established.
Fig. 1.
GLI transcription factors. a Schematic representation of the functional domains of GLI proteins. b Model of Hedgehog-dependent activation of the GLI transcription factors. c Hedgehog-independent regulation of GLI proteins. GLIRep = GLI transcriptional repressor; GLIAct = GLI transcriptional activator; SUFU = Suppressor of FUSED; SMO = SMOOTHENED; PTC = PATCHED.
Despite initial expectations that GLI proteins would have redundant functions, they, in fact, have individually important biological roles. Results from knockout studies have provided valuable insights into some critical and unique functions of these transcription factors in morphogenetic cascades, suggesting that an altered expression or function of these molecules could play a role in carcinogenesis [1, 4, 8, 9]. In fact, aberrant activation of these transcription factors could lead to the development of tumors in different tissues including the pancreas [10,11,12]. The proposed mechanism for the regulation of carcinogenesis is the transcriptional modulation of specific target genes that contribute to the transformed phenotype. For instance, GLI1, a candidate oncogene for a number of neoplasms, increases the expression of antiapoptotic molecules (BCL-2, BFL-1/A1 and 4-1BB) [13,14,15], thus increasing the survival of cancer cells. Further characterization of GLI target genes will be key to better define the role of these molecules in carcinogenesis.
GLI activity is regulated by the Hedgehog ligand via 2, multitransmembrane proteins, named PATCHED (PTC) and SMOOTHENED (SMO). In this receptor complex, PTC is the ligand-binding subunit, while SMO is the signaling component. Upon binding of Hedgehog to its receptor PTC, an inhibitory effect of PTC on SMO is released, allowing SMO to trigger a signaling cascade that activates the GLI transcription factors [2, 4]. Figure 1b demonstrates the inactive and active states of this pathway that was largely assembled from genetic studies in Drosophila melanogaster. In the absence of ligand (fig. 1b, left), the Hedgehog signaling pathway is inactive. In this case, the transcription factor GLI is sequestered by the cytoplasmic complex including COSTAL, FUSED and Suppressor of FUSED (SUFU) [2, 4]. Phosphorylation of GLI by PKA, GSK3 and CK1 targets GLI proteins to proteasome-dependent processing to generate the repressor form of these transcription factors. As a consequence, transcriptional activation of Hedgehog target genes is repressed via SIN3-HDAC or SKI-HDAC-dependent mechanisms. Activation of the pathway (fig. 1b, right) results in derepression of SMO, initiating a cascade disassembling the GLI-COSTAL-FUSED-SUFU complex, thereby allowing the activator forms of GLI to translocate to the nucleus and activate gene transcription. In cancer cells, recent reports suggest that GLI activity can be modulated in a Hedgehog-independent manner through the RAS-MEK/AKT pathway, which regulates the subcellular localization and protein stability of GLI transcription factors [16,17,18] (fig. 1c, left). In addition, Dennler et al. [19] demonstrate that TGF-β can increase GLI expression and prolonged Hedgehog signaling through SMAD3-dependent mechanisms [18] (fig. 1c, right). Thus, together these results expand our understanding of the complex network implicated in the regulation of GLI function in cancer cells and serve as a foundation for the development of new therapeutic approaches targeting the activity of these transcription factors.
References
- 1.Ruiz I Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J. 2007;403:369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- 3.Hallikas O, Palin K, Sinjushina N, Rautiainen R, Partanen J, Ukkonen E, Taipale J. Genome-wide prediction of mammalian enhancers based on analysis of transcription- factor-binding affinity. Cell. 2006;124:47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 6.Dai P, Shinagawa T, Nomura T, Harada J, Kaul SC, Wadhwa R, Khan MM, Akimaru H, Sasaki H, Colmenares C, Ishii S. Ski is involved in transcriptional regulation by the repressor and full-length forms of Gli3. Genes Dev. 2002;16:2843–2848. doi: 10.1101/gad.1017302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng SY, Bishop JM. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci USA. 2002;99:5442–5447. doi: 10.1073/pnas.082096999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz i Altaba A, Sánchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 9.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 10.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Zapico ME. A defined survival pathway underlies the critical effects of Hedgehog on pancreatic carcinogenesis. Pancreas. 2006;33:460. [Google Scholar]
- 14.Bigelow RL, Chari NS, Unden AB, Spurgers KB, Lee S, Roop DR, Toftgard R, McDonnell TJ. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 15.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 16.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 17.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz I, Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauth M, Toftgard R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle. 2007;6:2458–2463. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- 19.Dennler S, André J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-β: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]