Abstract
Rationale: Elevated proinflammatory cytokines are associated with severity of pneumonia, but the role of preinfection cytokine levels in the predisposition to pneumonia in humans is less clear.
Objective: To ascertain role of preinfection inflammatory markers on susceptibility to community-acquired pneumonia (CAP).
Methods: Longitudinal analysis over 6.5 yr of a cohort that consisted of 70- to 79-yr-old well-functioning elderly individuals.
Measurements: Association between preinfection tumor necrosis factor (TNF), interleukin 6 (IL-6), and C-reactive protein (CRP) levels and CAP requiring hospitalization.
Results: Of the 3,075 participants, 161 (5.2%) developed at least one episode of CAP requiring hospitalization over a median duration of 3.3 yr. The highest tertiles of TNF (> 3.7 pg/ml) and IL-6 (> 2.4 pg/ml) were associated with increased risk of CAP, and the adjusted odds ratios were 1.6 (95% confidence interval [CI], 1.02–2.7) and 1.7 (95% CI, 1.1–2.8), respectively. The adjusted risk of CAP with at least one of these markers in the highest tertile was 1.6 (95% CI, 1.1–2.3). TNF and IL-6 levels in the highest tertile had a synergistic effect (p = 0.01 for interaction), and risk of CAP for both markers in the highest tertile was 2.8 (95% CI, 1.8–4.3). An FEV1 of 50% or less of predicted was associated with the highest risk of CAP (adjusted odds ratio, 3.6; 95% CI, 2.3–5.6). Furthermore, TNF and IL-6 levels modified risk of CAP in participants with coexisting medical conditions and history of smoking.
Conclusion: In the well-functioning elderly subjects, preinfection systemic levels of TNF and IL-6 were associated with higher risk of CAP requiring hospitalization in smokers and those with coexisting medical conditions.
Keywords: inflammatory markers, interleukin 6, tumor necrosis factor, pneumonia
Community-acquired pneumonia (CAP) is the most common infectious cause of death in the United States, with the highest incidence at the extremes of age (1, 2). Coexisting medical conditions and nutritional factors increase susceptibility to CAP and have been studied extensively (1, 3–5), but their frequency in the elderly population diminishes their ability to discriminate those at risk. Improving risk stratification to identify elderly individuals at increased risk of CAP will enhance our ability to target appropriate preventive measures. The level of inflammatory markers induced during an episode of acute disease indicates the efficiency of immune clearance and correlates with severity of CAP, but is measured too late to be of predictive value in healthy individuals (6, 7). Recently, however, cytokines have been shown to play an active role in the molecular mechanisms of bacterial invasion that initiates pneumonia (8). Although this raises the possibility that elevated preinfection levels of circulating inflammatory markers may cause invasive disease and therefore be indicative of higher risk for the development of CAP, it remains poorly studied in humans (9, 10).
Systemic administration of tumor necrosis factor (TNF) in rats before inoculation of bacteria into the lungs worsens pneumonia by reducing alveolar neutrophil recruitment and bacterial clearance (11, 12). Furthermore, activation of human cells by cytokines upregulates eukaryotic receptors used by bacteria to traverse cells and enter the systemic circulation (8, 9). On the other hand, animals lacking specific cytokines fail to control bacterial multiplication in host tissues (13–19). Thus, although the absence of cytokines is clearly associated with poor outcome of infection, overproduction of cytokines either at steady state or induced by disease may not be advantageous. In humans, circulating inflammatory markers are elevated in medical conditions that increase risk of CAP, such as chronic obstructive pulmonary disease and congestive heart failure (20, 21). Although the exact mechanisms that increase risk of CAP in patients with these underlying conditions are poorly understood, high baseline levels of inflammatory markers may be a potential link.
The Health Aging and Body Composition (Health ABC) study is a prospective observational cohort of well-functioning elderly participants with longitudinal data for 6.5 yr after enrollment. We examined the association between preinfection circulating levels of inflammatory markers, including TNF, interleukin 6 (IL-6), and C-reactive protein (CRP), and the risk of hospitalization due to CAP over the ensuing 6 yr. Some of the results of this study have been previously reported in the form of an abstract (22).
METHODS
Population
The study population consisted of 70- to 79-yr-old participants residing in Memphis, Tennessee, and Pittsburgh, Pennsylvania. Health ABC was designed to prospectively assess the impact of weight and weight-related health conditions on incident mobility limitation in a community- dwelling population. Eligibility criteria included no reported difficulty with walking a quarter mile, climbing 10 steps, or performing activities of daily living. Those with recent treatment for cancer, severe dementia, or plans of leaving the area within 3 yr were also excluded. The institutional review boards at both sites approved the study.
Study Design
The study design was a longitudinal analysis of the Health ABC cohort.
Outcome Measure
The primary outcome was CAP requiring hospitalization. Hospitalization was ascertained on the basis of participant self-report and active surveillance by study personnel. Adjudication of CAP was performed by an adjudicator at each site, and criteria to adjudicate CAP were established prospectively. We used a combination of discharge summary, diagnoses by International Classification of Diseases, ninth edition (ICD-9), admission history and physical examination, and radiology reports for each hospitalization to ascertain CAP requiring hospitalization (see online supplement).
Inflammatory Markers
Two weeks after the baseline interview to assess coexisting health conditions and spirometry (Year 1 visit), blood samples were obtained in the morning after an overnight fast in the absence of infection (mean time, 9:25 a.m.; 50% of specimens were collected between 8:55 and 9:58 a.m.). Plasma TNF and IL-6 levels were measured in duplicate by ELISA kits (R&D Systems, Minneapolis, MN) using HS600 Quantikine and HSTA50 kits, respectively. Serum levels of CRP were also measured in duplicate by ELISA based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, Darmstadt, Germany). The minimal detectable limits for TNF, IL-6, and CRP were 0.18 pg/ml, 0.1 pg/ml, and 0.007 mg/L, respectively. Reliability was ascertained using a blind duplicate system, and interassay coefficients of variation for TNF, IL-6, and CRP assays were 16, 10, and 8%, respectively (see online supplement).
Demographics, Comorbid Conditions, andNutritional Markers
Age, sex, and race were assessed along with a detailed health history and a medication inventory at the baseline (Year 1) visit. Prevalent health conditions, such as congestive heart failure, diabetes, coronary artery disease, hypothyroidism, and smoking, were determined by a combination of self-report and use of specific medications. Spirometry was performed approximately 2 wk at baseline during clinic visit with a horizontal dry rolling seal spirometer to ascertain lung function. FVC and FEV1 had to meet American Thoracic Society (ATS) criteria for acceptability and reproducibility (23, 24). Whole body lean mass was assessed by dual-energy X-ray absorptiometry (Hologic 4500A; Hologic, Inc., Waltham, MA). Serum albumin was measured using a Vitros chemistry kit (Ortho-Clinical Diagnostics, Johnson and Johnson) from plasma obtained at the Year 1 visit. Reliability was assessed using blind duplicate system on 5% of samples, and the coefficient of variation was 2%.
Statistical Analysis
Univariate analysis was performed using χ2 and t tests to compare baseline characteristics of participants with and without CAP. A Wilcoxon test was used to compare levels of inflammatory markers. Two-tailed p values were used for all analyses. Kaplan-Meier survival curves and failure plots were plotted to ascertain relationship between tertiles of inflammatory markers and CAP events (Figure 1). A logistic regression model was used for multivariable analysis. Model fit was ascertained by the Hosmer-Lemeshow test. Interaction effects were also ascertained and reported, if p value was less than 0.05 (see online supplement).
Figure 1.
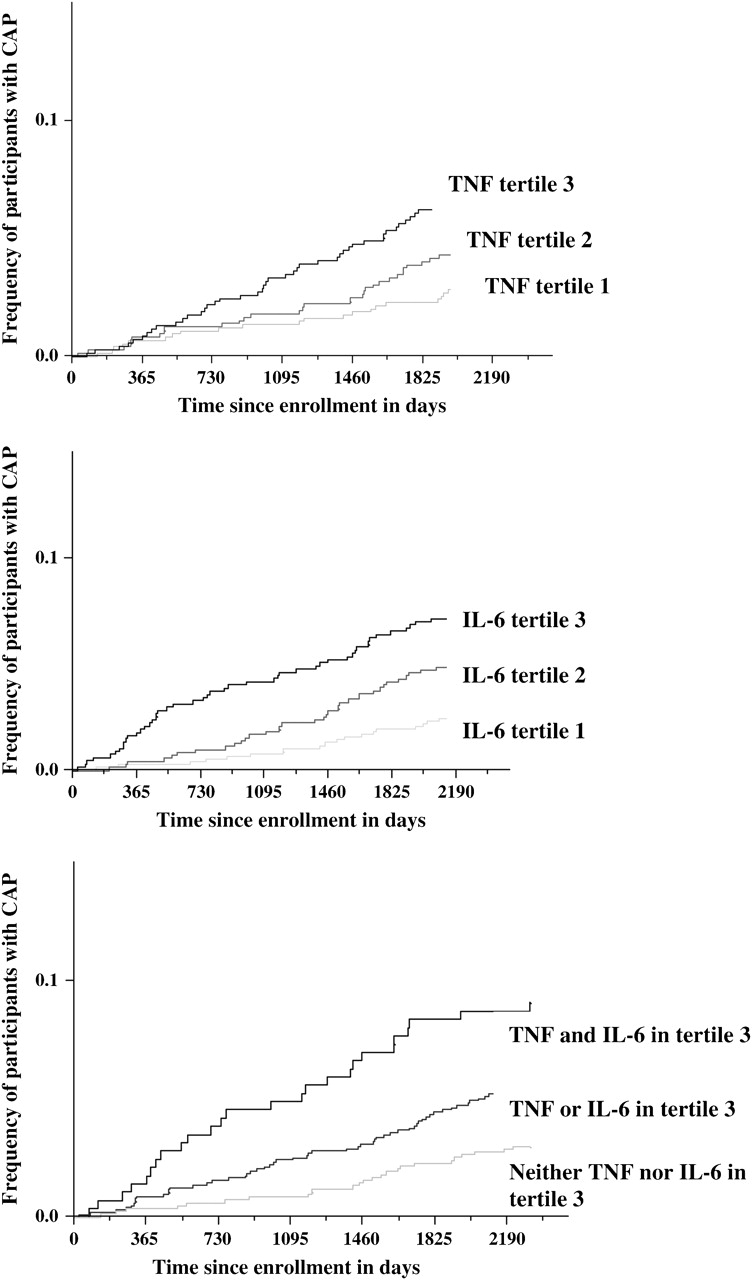
Frequency of participants with at least one episode of community-acquired pneumonia (CAP) requiring hospitalization stratified by tertiles of tumor necrosis factor (TNF) and interleukin (IL)-6 levels (Failure plot).
RESULTS
A cohort of 3,075 well-functioning volunteers, aged 70 to 79 yr, was monitored prospectively for 6.5 yr. One hundred and sixty-one (5.2%) participants had at least one hospitalization for CAP over the ascertainment period. Only 19 participants had more than one episode of CAP. The median time to onset of the first CAP event requiring hospitalization was 1,208 d (3.3 yr), and the last CAP event was recorded 6.3 yr after the baseline visit.
Table 1 compares baseline characteristics of participants hospitalized at least once to those never hospitalized for CAP. Participants with CAP were more likely to be males, were current and past smokers, have higher frequency of coexisting medical conditions, such as congestive heart failure, coronary heart disease, diabetes, renal failure, a reduced FEV1, and were more likely to use oral steroids. Nutritional markers, including weight, body mass index (BMI), and serum albumin as well as lean body mass were equally distributed between the two groups. Less than a third of the participants reported receiving pneumococcal vaccination, and the frequency of pneumococcal vaccination was similar in participants with and without CAP requiring hospitalization.
TABLE 1.
BASELINE CHARACTERISTICS OF COHORT
| Variable | All Participants (n = 3,075) | Hospitalized for CAP (n = 161) | Not Hospitalized for CAP (n = 2,914) | p Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age | 73.6 ± 2.9 | 73.7 ± 2.8 | 73.6 ± 2.6 | 0.5 |
| Sex, % males | 48.5 | 55.9 | 48.1 | 0.05 |
| Race, % Blacks | 41.7 | 35.4 | 42 | 0.1 |
| Site, % Memphis | 50.3 | 46 | 51 | 0.3 |
| Comorbid conditions | ||||
| Congestive heart failure, % | 3.1 | 6.3 | 2.9 | 0.007 |
| Coronary heart disease, % | 20.8 | 30.1 | 20.3 | 0.0003 |
| Diabetes, % | 15.3 | 22.6 | 14.9 | 0.008 |
| Serum creatinine, mg/dl | 1.06 ± 0.17 | 1.17 ± 0.5 | 1.06 ± 0.15 | 0.048 |
| FEV1, % predicted | ||||
| FEV1 ⩾ 80% | 70.7 | 50.9 | 71.8 | < 0.0001 |
| 80% > FEV1 > 50% | 19.2 | 24.3 | 18.9 | < 0.0001 |
| FEV1 ⩽ 50% | 10.1 | 24.8 | 9.3 | < 0.0001 |
| Hypothyroidism, % | 7.4 | 10.1 | 7.2 | 0.4 |
| Smoking | ||||
| Current | 10.4 | 17.5 | 10 | < 0.0001 |
| Past | 45.7 | 54 | 45.2 | |
| Pack-yr of smoking | 19.1 ± 28 | 27.5 ± 32 | 18.6 ± 27 | < 0.0001 |
| Inflammatory markers* | ||||
| TNF, pg/ml | 3.2 (2.4–4.1) | 3.6 (2.7–4.9) | 3.2 (2.4–4.1) | < 0.0001 |
| IL-6, pg/ml | 1.8 (1.3–2.8) | 2.6 (1.7–3.5) | 1.8 (1.2–2.7) | < 0.0001 |
| CRP, mg/L | 1.7 (1–3) | 2.1 (1.2–3.8) | 1.6 (1–3) | 0.002 |
| Pneumococcal vaccination, % | 29.1 | 30.1 | 29 | 0.8 |
| Nutritional markers | ||||
| Weight, kg | 75.8 ± 15 | 75.5 ± 15.1 | 75.8 ± 15 | 0.8 |
| BMI, kg/m2 | 27.4 ± 4.8 | 26.9 ± 5.8 | 27.4 ± 4.9 | 0.2 |
| Serum albumin, g/dl | 4 ± 0.3 | 4 ± 0.4 | 4 ± 0.3 | 0.5 |
| Lean body mass, kg | 47.5 ± 10.1 | 48.4 ± 10.1 | 47.5 ± 10.1 | 0.2 |
| Medications, % | ||||
| Oral steroids | 2.3 | 5.4 | 2.1 | 0.02 |
| Aspirin | 37.4 | 38.3 | 37.4 | 0.8 |
Definition of abbreviations: BMI = body mass index; CAP = community-acquired pneumonia; CRP = C-reactive protein; TNF = tumor necrosis factor.
Values are expressed as mean ± SD.
Expressed as median with interquartile range.
TNF, IL-6, and CRP levels measured in blood at the time of entry into the study (i.e., in the absence of infection) were higher among participants who experienced subsequent CAP (Table 1). Median TNF, IL-6, and CRP levels among the 19 participants with more than one hospitalization for CAP were 3.7 pg/ml, 2.8 pg/ml, and 2.3 mg/L, respectively. A weak correlation was noted between TNF with IL-6 and CRP, with Spearman correlation coefficients of 0.27 and 0.11, respectively. IL-6 modestly correlated with CRP and the Spearman correlation coefficient was 0.44. We estimated risk of each inflammatory marker for susceptibility to CAP requiring hospitalization after adjusting for age, race, sex, site, smoking status, coexisting medical conditions, and oral steroid use (Table 2). TNF and IL-6 levels at the baseline visit were associated with increased risk of CAP susceptibility, but CRP levels did not confer higher risk. Although the risk of CAP increased for every tertile increase in baseline TNF and IL-6 levels, the results were statistically significant only for the highest tertiles of TNF and IL-6 levels (Figure 1). In 411 participants, inflammatory markers were measured approximately a year apart, and the correlation coefficients were TNF = 0.41, IL-6 = 0.63, and CRP = 0.66.
TABLE 2.
ADJUSTED RISK OF SUSCEPTIBILITY TO COMMUNITY-ACQUIRED PNEUMONIA FOR LEVELS OF TUMOR NECROSIS FACTOR, INTERLEUKIN 6, AND C-REACTIVE PROTEIN (LOGISTIC REGRESSION)
| Variable | OR (95% CI) | p Value |
|---|---|---|
| TNF, pg/ml | ||
| Tertile 3 (median = 4.6, range = 3.7–29.6) | 1.9 (1.2–3) | 0.02 |
| Tertile 2 (median = 3.2, range = 2.7–3.72) | 1.5 (0.95–2.5) | 0.5 |
| Tertile 1 (median = 2.2, range = 0.6–2.69) | Referent | — |
| Per log (TNF) increase | 1.9 (1.2–2.9) | 0.003 |
| Per TNF SD increase | 1.3 (1.1–1.5) | 0.003 |
| IL-6, pg/ml | ||
| Tertile 3 (median = 3.5, range = 2.41–16) | 1.6 (1.04–2.6) | 0.02 |
| Tertile 2 (median = 1.8, range = 1.43–2.4) | 1.2 (0.7–1.9) | 0.7 |
| Tertile 1 (median = 1.1, range = 0.2–1.42) | Referent | — |
| Per log (IL-6) increase | 1.4 (1.1–1.9) | 0.009 |
| Per IL-6 SD increase | 1.3 (1.1–1.5) | 0.009 |
| CRP, mg/L | ||
| Tertile 3 (median = 3.9, range = 2.5–85.2) | 1.2 (0.7–2) | 0.6 |
| Tertile 2 (median = 1.7, range = 1.15–2.4) | 1.1 (0.7–1.8) | 0.9 |
| Tertile 1 (median = 0.9, range = 0.2–1.14) | Referent | — |
| Per log (CRP) increase | 1.2 (0.9–1.4) | 0.2 |
| Per CRP SD increase | 1.1 (0.9–1.4) | 0.2 |
Definition of abbreviations: CI = confidence interval; CRP = C-reactive protein; IL = interleukin; OR = odds ratio; TNF = tumor necrosis factor.
Values adjusted for age, race, sex, site, congestive heart failure, coronary artery disease, smoking status, diabetes, renal failure, oral steroid use, and FEV1. Each inflammatory marker was entered in the logistic regression model separately.
TNF and IL-6 were then entered in the model concurrently, and the risk of CAP was estimated after adjusting for demographic characteristics and coexisting medical conditions (Table 3). The highest tertiles of TNF and IL-6 were independent predictors of CAP susceptibility, and the adjusted odds ratios (ORs) were 1.6 (1.02–2.7) and 1.7 (1.1–2.8), respectively. Linear dose–effect relationships between tertiles of TNF and IL-6 and increased risk of CAP were also seen. Although the second tertiles of both these markers were also associated with higher risk, the results were not statistically significant. A second logistic regression model was constructed to ascertain effect of nutritional markers and the association between the highest tertile of TNF and IL-6, and susceptibility to pneumonia remained significant and risk estimates remained similar (see online supplement and Table 1).
TABLE 3.
UNADJUSTED AND ADJUSTED RISK OF SUSCEPTIBILITY TO COMMUNITY-ACQUIRED PNEUMONIA FOR INFLAMMATORY MARKERS
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| TNF tertile 3 vs. tertile 1 | 2.1 (1.4–3.3) | 1.6 (1.02–2.7) |
| TNF tertile 2 vs. tertile 1 | 1.5 (0.9–2.3) | 1.4 (0.9–2.2) |
| IL-6 tertile 3 vs. tertile 1 | 2.6 (1.6–4) | 1.7 (1.1–2.8) |
| IL-6 tertile 2 vs. tertile 1 | 1.7 (1.04–2.7) | 1.3 (0.8–2.1) |
| Severity of lung disease | ||
| Severe (FEV1 ⩽ 50%) | 3.8 (2.6–5.7) | 3.6 (2.3–5.6) |
| Moderate (65 > FEV1>50) | 2.2 (1.2–4.1) | 1.7 (0.8–3.4) |
| Mild (80 > FEV1 ⩾ 65) | 1.7 (1.1–2.6) | 1.3 (0.8–2.2) |
| History of coronary artery disease | 1.7 (1.2–2.4) | 1.2 (0.8–1.7) |
| History of congestive heart failure | 2.2 (1.1–4.4) | 1.3 (0.6–2.8) |
| Serum creatinine > 1.5 mg/dl | 2.4 (1.3–4.3) | 1.3 (0.7–2.7) |
| History of diabetes | 1.7 (1.1–2.5) | 1.3 (0.8–2) |
| Smoking status, ever smoked vs. never smoked | 1.7 (1.2–2.4) | 1.4 (0.97–2.1) |
| Oral steroid use | 2.5 (1.2–5.3) | 1.3 (0.5–3.2) |
| Age, per yr increase | 1.01 (0.9–1.1) | 1.01 (0.9–1.1) |
| Sex, males vs. females | 1.4 (0.9–1.9) | 1.2 (0.8–1.7) |
| Race, Whites vs. Blacks | 1.3 (0.9–1.8) | 1.4 (0.9–2.1) |
| Site, Memphis vs. Pittsburgh | 0.8 (0.6–1.1) | 0.7 (0.5–1.01) |
Definition of abbreviations: CI = confidence interval; IL = interleukin; OR = odds ratio; TNF = tumor necrosis factor.
Values adjusted for age, race, sex, site, congestive heart failure, coronary artery disease, smoking status, diabetes, renal failure, oral steroid use, and FEV1. Only 2,674 participants and 139 cases of community-acquired pneumonia included in the adjusted model due to missing data for exposure variables and covariates. Both inflammatory markers were entered in the logistic regression model concurrently.
One hundred and eighty-eight (6.6%) participants reported having a respiratory tract infection 2 wk before the blood draw. The median TNF, IL-6, and CRP levels for these participants were 3.1 pg/ml, 2.3 pg/ml, and 1.9 mg/L, respectively. These 188 participants accounted for only 2.5, 1.8, and 1.8% of subjects in the highest tertile of TNF, IL-6, and CRP, respectively. The frequency of CAP among participants reporting respiratory tract infection and those not reporting a respiratory tract infection before the blood draw were similar (6.9 vs. 4.9%, respectively; p = 0.2). The risk of CAP for the highest tertiles of TNF and IL-6 remained unchanged after excluding these 188 participants (see online supplement and Table 2).
Because the highest tertile of TNF and IL-6 levels were independently associated with risk of CAP, we ascertained the combined effect of these inflammatory markers (Table 4 and Figures 1 and 2). The risk increased substantially for participants with two versus one baseline inflammatory marker level in the highest tertile (OR, 2.8 vs. 1.6). Furthermore, the interaction between TNF and IL-6 levels in the highest tertile and risk of CAP was also significant (p = 0.01), suggesting a synergistic effect for the combination.
TABLE 4.
ADJUSTED RISK ACCORDING TO NUMBER OF INFLAMMATORY MARKERS IN THE HIGHEST TERTILE FOR ALL PARTICIPANTS AND STRATIFIED BY FEV1, SMOKING STATUS, AND COMORBID CONDITIONS
| Variable | Neither Inflammatory Marker in Tertile 3 | At Least One Inflammatory Marker in Tertile 3 | Both Inflammatory Markers in Tertile 3 |
|---|---|---|---|
| All participants | Referent | 1.6 (1.1–2.3) | 2.8 (1.8–4.3)† |
| Stratified by FEV1 | |||
| FEV1 ⩽ 50% (n = 269)* | 2.8 (1.3–6.4) | 6.3 (3.3–11.9) | 6.8 (2.9–16) |
| FEV1> 50% (n = 1,949) | Referent | 1.4 (0.8–2.3) | 2.5 (1.4–4.7) |
| Stratified by smoking status | |||
| Ever smoked (n = 1,140)* | 1.1 (0.6–2.2) | 2.7 (1.5–5) | 3.5 (1.7–7.1) |
| Never smoked (n = 1,015) | Referent | 0.9 (0.4–2.1) | 2.2 (0.9–5.4) |
| Stratified by diabetes | |||
| Diabetes (n = 313)* | 1.4 (0.5–3.6) | 2.2 (1–4.7) | 5.2 (2.4–11.7) |
| No diabetes (n = 1,846) | Referent | 1.8 (1.1–3) | 2.4 (1.3–4.5) |
| Stratified by comorbid conditions | |||
| Two or more comorbid conditions (n = 234)* | 2.5 (0.8–7.6) | 4 (1.8–8.9) | 8.1 (3.6–18.1) |
| One comorbid conditions (n = 627) | 1.9 (0.9–3.9) | 3.6 (1.9–6.9) | 4 (1.8–9.1) |
| No comorbid conditions (n = 1,298) | Referent | 1.3 (0.7–2.7) | 1.6 (0.6–4.5) |
Values adjusted for age, race, sex, and site. Comorbid conditions include history of congestive heart failure, coronary artery disease, FEV1 ⩽ 50% predicted, serum creatinine > 1.5mg/dl, and diabetes.
Numbers do not add to 3,075 due to missing data for inflammatory marker or covariates.
p Value for the interaction between risk of community-acquired pneumonia for tumor necrosis factor (TNF) tertile 3, interleukin (IL)-6 tertile 3, and the combination of TNF and IL-6 in tertile 3 was 0.01.
Figure 2.
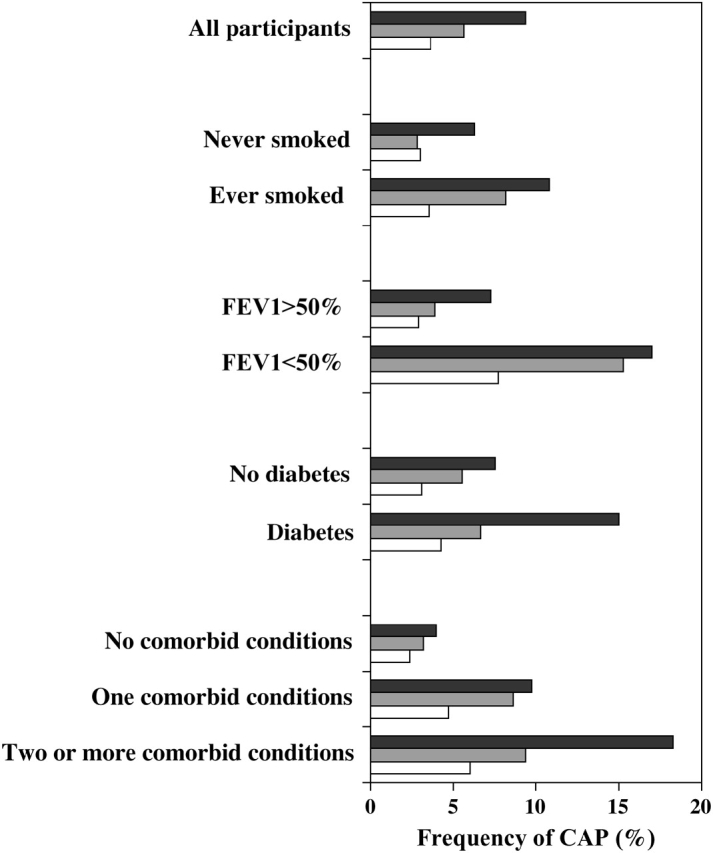
Frequency of CAP for all participants and stratified by FEV1, smoking status, history of diabetes, and comorbid conditions. Comorbid conditions include history of congestive heart failure, coronary heart disease, diabetes, serum creatinine levels greater than 1.5 mg/dl, and FEV1 of 50% predicted or less. Dark gray bars, both inflammatory markers in tertile 3; light gray bars, at least one inflammatory marker in tertile 3; white bars, none of the inflammatory markers in tertile 3.
An exponential relationship was noted between FEV1 and susceptibility to CAP. Mild or moderate reductions in FEV1 were associated with modest increase in risk of CAP (OR, 1.3 and 1.7, respectively). However, an FEV1 of 50% predicted or less was associated with the highest risk (OR, 3.6), and remained an independent predictor of CAP susceptibility. Smoking status and other coexisting medical conditions, such as coronary heart disease, congestive heart failure, serum creatinine level of more than 1.5 g/dl, and diabetes were associated with higher risk of CAP in the univariate analysis (Tables 1 and 3). However, these covariates were no longer significant in the multivariable analysis after adjusting for effects of inflammatory markers.
Finally, we ascertained the role of inflammatory markers in modifying risk of CAP on the basis of comorbid conditions, such as congestive heart failure, coronary heart disease, diabetes, serum creatinine level of more than 1.5 mg/dl, and an FEV1 of 50% predicted or less (Table 4 and Figure 2). Participants without any of these comorbid conditions were associated with a low incidence of CAP (2.9%), and inflammatory markers did not confer higher risk to these participants. The frequency of CAP among participants with one and two or more comorbid conditions were 7 and 10.7%, respectively. In contrast to participants with no comorbid conditions, inflammatory markers increased risk of CAP in participants with one or more comorbid conditions. Again, participants with both inflammatory markers in the highest tertile experienced the highest risk, whereas those with at least one inflammatory marker in the highest tertile were associated with intermediate risk of CAP. The incidence of CAP for those with two or more comorbid conditions for both inflammatory markers in the highest tertile, either in the highest tertile, and neither in the highest tertile were 18.3, 9.4, and 6%, respectively; whereas for those with only one comorbid condition were for the same strata 9.7, 8.6, and 4.7%, respectively (Figure 2). Inflammatory markers also increased risk of CAP, regardless of smoking status, diabetes, and FEV1.
DISCUSSION
We have shown an association between baseline TNF and IL-6 levels in the systemic circulation of well-functioning elderly individuals and an increased risk of subsequently developing CAP requiring hospitalization. These associations were independent of coexisting medical conditions, smoking status, and steroid medication use. Furthermore, we have demonstrated a synergistic effect of TNF and IL-6 and risk of CAP. Participants with the highest tertile of TNF and IL-6 levels had approximately 2.5-fold higher risk of CAP. Although inflammatory markers are elevated among smokers and in the presence of coexisting medical conditions, such as diabetes, congestive heart failure, reduced FEV1, and elevated serum creatinine, preinfection levels of TNF and IL-6 modified risk of CAP in the presence of these coexisting medical conditions and smoking status. Participants with higher CRP levels were associated with higher risk of CAP in the univariate analysis, but this was not an independent predictor in the multivariable analysis.
Comorbid conditions, such as diabetes, congestive heart failure, and reduced FEV1, are associated with increased risk of CAP as well as elevated systemic inflammatory markers in previous studies. In our study, these medical conditions were associated with increased risk of CAP in the univariate analysis, although only TNF and IL-6 levels as well as percent-predicted FEV1 were associated with CAP susceptibility in the multivariable analysis. A potential reason for this association in the multivariable analysis (Table 3) could be that the inflammatory markers are a surrogate marker for a participant's smoking status and comorbid conditions, and one could argue that a detailed history of smoking and comorbid conditions should be sufficient to risk-stratify individuals. Therefore, we analyzed association between inflammatory markers and risk of CAP stratified by comorbid conditions (Table 4 and Figure 2). Our stratified analysis suggests that increased TNF and IL-6 levels are an effect modifier, both in the presence and absence of individual comorbid conditions, such as diabetes and a reduced FEV1. To further clarify whether inflammatory markers modify the risk of CAP independently, we ascertained the associations after combining these comorbid conditions. Compared with participants without any comorbid conditions and TNF as well as IL-6 levels in the lowest tertiles, participants with both markers in the highest tertiles were associated with a four- and eightfold higher risk of CAP in participants with one and two or more comorbid conditions, respectively. Among participants without any comorbid conditions, the frequencies of CAP among participants without any marker in the highest tertile, at least one marker in the highest tertile, and both markers in the highest tertile were 2.3, 3.7, and 7.8%, respectively. However, due to the low incidence of CAP in participants without any comorbid conditions, we did not have adequate power to detect an association in this subgroup. We observed similar results after stratifying our results based on smoking status. Although inflammatory markers modified risk of CAP among smokers, the associations were not significant among participants who never smoked due to low frequency of events in these groups. These analyses suggest that inflammatory markers may modify risk of CAP only among smokers or participants with comorbid conditions.
The results of our study emphasize the role of inflammatory markers in improving risk stratification rather than a causal role in the susceptibility to CAP. Numerous mechanisms have been hypothesized to explain this effect of inflammatory markers (25). Proinflammatory markers, like TNF, upregulate adherence of bacteria to human endothelial and alveolar epithelial cells by increasing expression of receptors that bind to these organisms (8, 26). This critical step facilitates bacterial entry into the alveolar spaces and the systemic circulation. In addition, systemic inflammatory markers may also reduce the effectiveness of the host immune response by impairing alveolar macrophage TNF production, reducing neutrophil recruitment and phagocytosis (12). Once bacteria gain access to the systemic circulation, elevated proinflammatory cytokines may increase bacterial growth (13, 27). Whether these responses are mediated by TNF or IL-6 itself, downstream markers, or IL-10 is an area of ongoing investigation (28–30). In comparison to TNF and IL-6, CRP was not associated with CAP susceptibility after adjusting for coexisting medical conditions. The objective of our study was to ascertain the role of systemic inflammatory markers in the risk stratification, rather than causal mechanisms. The median duration of CAP after measurement of inflammatory markers was 3.3 yr. The confirmation of our results in future studies using measurements of inflammatory markers in the months preceding CAP or measuring levels over time would strengthen the causal role of preinfection systemic inflammatory markers in increasing risk of CAP.
Our study also demonstrates an important relationship between severity of lung disease and increased susceptibility to CAP. Chronic lung disease or chronic obstructive pulmonary disease has been identified as a risk factor for CAP in numerous studies (3, 5, 31). However, pulmonary function tests were seldom performed in these studies. Self-report often underestimates underlying lung dysfunction in elderly individuals (32). Spirometry results that met ATS criteria for acceptability and reproducibility were available in 93% of subjects in our study. The relation between FEV1 and risk of CAP is not linear, but rather exponential. Mild (80 > FEV1 > 65% predicted) or moderate lung disease (50 > FEV1 > 65% predicted) increased risk of CAP modestly and these results were not statistically significant. In comparison, participants with an FEV1 of 50% predicted or less were associated with a 3.5-fold higher risk.
Various nutritional markers have been studied in CAP susceptibility, including BMI, serum albumin, arm muscle area, and triceps skin-fold thickness (5, 31). Compared with previous studies, BMI and serum albumin did not predict CAP susceptibility in our study (3–5). We attempted to identify various cutoffs of BMI or serum albumin, but neither subgroup was associated with higher risk. Nutritional factors are probably important in the susceptibility to CAP, but these measurements were obtained approximately 3 yr before the hospitalization for CAP in our study. Measurements in the months preceding hospitalization or changes in these measures over time may more accurately reflect risk of nutritional markers.
Our study has limitations. First, we adjusted for common comorbid conditions that are associated with susceptibility to CAP. However, residual confounding due to unmeasured comorbidities cannot be excluded. Second, the role of a single cytokine measurement in risk stratification is unclear. The correlation coefficients for inflammatory markers measured a year apart in our study were modest and consistent with previous studies (33–35). The lack of correlation would tend to make the associations weaker than if multiple measures were available, referred to as regression-dilution bias. Cytokines may also fluctuate with episodes of infection, after the initial measurement, and thereby misclassify individuals. However, this would tend to move the estimate toward the null. Another source of variability in circulating cytokine levels could be genetic variation, and polymorphisms within the TNF and IL-6 genes are associated with differences in systemic levels of these cytokines. Whether these cytokine gene polymorphisms are associated with susceptibility to CAP is currently under investigation. Third, we did not adjust for multiple comparisons. We ran three models to adjust for potential confounders and avoid overfitting any individual model. The lower limits of the confidence intervals for TNF was 1.02 in the adjusted analyses and may represent type I error. However, the adjusted risk of CAP for the highest tertiles of TNF and IL-6 remained consistent across each of these models.
In conclusion, comorbid conditions are important predictors of susceptibility to CAP. In particular, an FEV1 reduced to 50% of predicted or less is a strong risk factor for CAP requiring hospitalization in the elderly. Inflammatory markers do not modify risk of CAP in the absence of comorbid conditions. However, in the presence of these comorbid conditions, elevated baseline levels of TNF and IL-6 in the systemic circulation, in the absence of ongoing infection, may modify risk of CAP, particularly in combination and at the highest tertile (TNF > 3.7 and IL-6 > 2.4 pg/ml). Once measured, the predictive value of these markers for high risk of developing CAP extended for more than 5 yr and thus may improve risk stratification in the elderly.
Supplementary Material
Supported by National Institute on Aging contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, and NHLBI-RO1HL74104, NIAID 27913, NIAID 39482, and American Lebanese– and Syrian-associated charities.
The results of this study have been presented in an oral presentation and published in abstract form at the American Thoracic Society International Conference in San Diego, California, 2005.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200506-888OC on September 15, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, Kurki S, Ronnberg PR, Seppa A, Soimakallio S, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol 1993;137:977–988. [DOI] [PubMed] [Google Scholar]
- 2.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730–1754. [DOI] [PubMed] [Google Scholar]
- 3.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of US older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep 1989;104:350–360. [PMC free article] [PubMed] [Google Scholar]
- 4.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 2000;160:3082–3088. [DOI] [PubMed] [Google Scholar]
- 5.Koivula I, Sten M, Makela PH. Risk factors for pneumonia in the elderly. Am J Med 1994;96:313–320. [DOI] [PubMed] [Google Scholar]
- 6.Glynn P, Coakley R, Kilgallen I, Murphy N, O'Neill S. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax 1999;54:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes G, Evans SA, Lordan JL, Frew AJ. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur Respir J 2002;20:990–995. [DOI] [PubMed] [Google Scholar]
- 8.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 1995;377:435–438. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AL, Koomey JM, Lee S, Jaffe EA, Fischetti VA. Recombinant human tumor necrosis factor alpha promotes adherence of Staphylococcus aureus to cultured human endothelial cells. Infect Immun 1991;59:3827–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000;55:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White JC, Nelson S, Winkelstein JA, Booth FV, Jakab GJ. Impairment of antibacterial defense mechanisms of the lung by extrapulmonary infection. J Infect Dis 1986;153:202–208. [DOI] [PubMed] [Google Scholar]
- 12.Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis 1997;176:1293–1302. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Del Sorbo L, Khine AA, de Azavedo J, Low DE, Bell D, Uhlig S, Slutsky AS, Zhang H. Modulation of bacterial growth by tumor necrosis factor-alpha in vitro and in vivo. Am J Respir Crit Care Med 2003;168:1462–1470. [DOI] [PubMed] [Google Scholar]
- 14.van der Poll T, Keogh CV, Buurman WA, Lowry SF. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med 1997;155:603–608. [DOI] [PubMed] [Google Scholar]
- 15.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol 2001;166:4042–4048. [DOI] [PubMed] [Google Scholar]
- 16.Mizgerd JP, Peschon JJ, Doerschuk CM. Roles of tumor necrosis factor receptor signaling during murine Escherichia coli pneumonia. Am J Respir Cell Mol Biol 2000;22:85–91. [DOI] [PubMed] [Google Scholar]
- 17.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 1997;176:439–444. [DOI] [PubMed] [Google Scholar]
- 18.Laichalk LL, Bucknell KA, Huffnagle GB, Wilkowski JM, Moore TA, Romanelli RJ, Standiford TJ. Intrapulmonary delivery of tumor necrosis factor agonist peptide augments host defense in murine gram-negative bacterial pneumonia. Infect Immun 1998;66:2822–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun 1997;65:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petretta M, Condorelli GL, Spinelli L, Scopacasa F, De Caterina M, Leosco D, Vicario ML, Bonaduce D. Circulating levels of cytokines and their site of production in patients with mild to severe chronic heart failure. Am Heart J 2000;140:E28. [DOI] [PubMed] [Google Scholar]
- 22.Yende S, Kritchevsky SB, Wunderink RG, de Rekeneire N, Kanaya A, Newman AB, Harris T, Tuomanen E. Inflammatory markers predict susceptibility to pneumonia [abstract]. Proc Am Thorac Soc 2005;2: A246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 25.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997;3:678–681. [DOI] [PubMed] [Google Scholar]
- 26.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med 1995;332:1280–1284. [DOI] [PubMed] [Google Scholar]
- 27.Meduri GU, Kanangat S, Stefan J, Tolley E, Schaberg D. Cytokines IL-1β, IL-6, and TNF-α enhance in vitro growth of bacteria. Am J Respir Crit Care Med 1999;160:961–967. [DOI] [PubMed] [Google Scholar]
- 28.van Westerloo DJ, Schultz MJ, Bruno MJ, de Vos AF, Florquin S, van der Poll T. Acute pancreatitis in mice impairs bacterial clearance from the lungs, whereas concurrent pneumonia prolongs the course of pancreatitis. Crit Care Med 2004;32:1997–2001. [DOI] [PubMed] [Google Scholar]
- 29.Zuckerman SH, Evans GF. Endotoxin tolerance: in vivo regulation of tumor necrosis factor and interleukin-1 synthesis is at the transcriptional level. Cell Immunol 1992;140:513–519. [DOI] [PubMed] [Google Scholar]
- 30.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol 1999;162:392–399. [PubMed] [Google Scholar]
- 31.Riquelme R, Torres A, El Ebiary M, de la Bellacasa JP, Estruch R, Mensa J, Fernandez-Sola J, Hernandez C, Rodriguez-Roisin R. Community-acquired pneumonia in the elderly: a multivariate analysis of risk and prognostic factors. Am J Respir Crit Care Med 1996;154:1450–1455. [DOI] [PubMed] [Google Scholar]
- 32.Waterer GW, Wan JY, Kritchevsky SB, Wunderink RG, Satterfield S, Bauer DC, Newman AB, Taaffe DR, Jensen RL, Crapo RO. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc 2001;49:1032–1038. [DOI] [PubMed] [Google Scholar]
- 33.Tornberg SA, Jakobsson KF, Eklund GA. Stability and validity of a single serum cholesterol measurement in a prospective cohort study. Int J Epidemiol 1988;17:797–803. [DOI] [PubMed] [Google Scholar]
- 34.Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol 1994;102:802–805. [DOI] [PubMed] [Google Scholar]
- 35.Ho GY, Xue XN, Burk RD, Kaplan RC, Cornell E, Cushman M. Variability of serum levels of tumor necrosis factor-alpha, interleukin 6, and soluble interleukin 6 receptor over 2 years in young women. Cytokine 2005;30:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


