Abstract
Rationale: Hypercapnic respiratory failure because of inspiratory muscle weakness is the most important cause of death in chronic obstructive pulmonary disease (COPD). However, the pathophysiology of failure of the diaphragm to generate force in COPD is in part unclear. Objectives: The present study investigated contractile function and myosin heavy chain content of diaphragm muscle single fibers from patients with COPD. Methods: Skinned muscle fibers were isolated from muscle biopsies from the diaphragm of eight patients with mild to moderate COPD and five patients without COPD (mean FEV1 % predicted, 70 and 100%, respectively). Contractile function of single fibers was assessed, and afterwards, myosin heavy chain content was determined in these fibers. In diaphragm muscle homogenates, the level of ubiquitin-protein conjugation was determined. Results: Diaphragm muscle fibers from patients with COPD showed reduced force generation per cross-sectional area, and reduced myosin heavy chain content per half sarcomere. In addition, these fibers had decreased Ca2+ sensitivity of force generation, and slower cross-bridge cycling kinetics. Our observations were present in fibers expressing slow and 2A isoforms of myosin heavy chain. Ubiquitin-protein conjugation was increased in diaphragm muscle homogenates of patients with mild to moderate COPD. Conclusions: Early in the development of COPD, diaphragm fiber contractile function is impaired. Our data suggest that enhanced diaphragm protein degradation through the ubiquitin-proteasome pathway plays a role in loss of contractile protein and, consequently, failure of the diaphragm to generate force.
Keywords: contractility, myosin, single fiber, ubiquitin
Chronic obstructive pulmonary disease (COPD) has been predicted to become the third leading cause of death and the fifth commonest cause of disability in the world by 2020 (1). Hypercapnic respiratory failure because of inspiratory muscle weakness (2) is the most important cause of death in COPD (3), and maximum inspiratory pressure is an independent determinant of survival in these patients (4).
The diaphragm is the most important inspiratory muscle. In severe COPD, the diaphragm undergoes a fiber-type shift toward more fatigue-resistant fibers (5, 6). In addition, changes in sarcomere length, mitochondrial density, and enzyme activity occur within diaphragm muscle fibers of these patients (7, 8). However, no conclusive studies have been published on direct contractile properties of the diaphragm of healthy subjects and patients with COPD. Consequently, the pathophysiology of failure of the diaphragm to generate force in COPD is in part unclear.
Muscle force is generated by cross bridges, which are formed by myosin heavy chain heads that attach to actin filaments (9). Force-generating capacity of single muscle fibers depends on contractile protein content of the fiber (10). Loss of skeletal muscle mass occurs in cachexia-associated conditions, such as cancer, AIDS, and congestive heart failure (11). There is growing evidence that, under cachectic conditions, cytokines initiate muscle wasting and induce muscle atrophy through activation of the ubiquitin–proteasome pathway, by selectively targeting myosin (12).
The present study investigated contractile function of diaphragm muscle single fibers from patients with COPD. We hypothesized that, in patients with mild to moderate COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage I/II), diaphragm single-fiber contractile function is impaired. In addition, it was hypothesized that loss of myosin in these fibers contributes to the reduction in contractile performance. On the basis of previous observations (12), we hypothesized that protein ubiquitination is enhanced in the diaphragm of these patients compared with patients without COPD.
Some of the results of these studies have been previously reported in the form of an abstract (13).
METHODS
Subjects and Pulmonary Function Testing
Diaphragm muscle biopsies were obtained from eight patients with COPD (six men) and five patients without COPD (three men). Biopsies of the right anterior costal diaphragm were obtained during thoracotomy for lung cancer (stage T1–3 N0–1 MX in both groups). Patients with more than 10% weight loss in the last 6 months before surgery were excluded from the study. General characteristics and pulmonary function data are shown in Table 1. Informed consent was obtained from each subject. The study was approved by the local ethics committee.
TABLE 1.
Patient characteristics
| Non-COPD (n = 5) | COPD (n = 8) | |
|---|---|---|
| Male/female | 3/2 | 6/2 |
| Age, yr | 59 ± 4 (46–72) | 60 ± 4 (42–75) |
| BMI, kg/m2 | 28 ± 2 (22–33) | 28 ± 2 (21–36) |
| FEV1, % predicted | 100 ± 3 (95–110) | 70 ± 4 (51–94) |
| VC, % predicted | 103 ± 6 (87–121) | 89 ± 9 (40–128) |
| FEV1/VC, % | 75 ± 3 (70–86) | 58 ± 3 (44–69) |
| TLC, % predicted | 99 ± 5 (84–116) | 105 ± 6 (77–125) |
| DLCO/VA, % predicted | 103 ± 8 (88–135) | 87 ± 7 (54–110) |
| PaO2, kPa | 10.9 ± 0.8 (8.9–13.3) | 11.0 ± 0.5 (8.3–12.6) |
| PaCO2, kPa | 5.2 ± 0.1 (4.9–5.6) | 5.2 ± 0.2 (3.8–5.7) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; DLCO/VA = carbon monoxide transfer coefficient per alveolar volume; TLC = total lung capacity; VC = vital capacity.
Values are means ± SEM with range (in parentheses).
Diaphragm Biopsies
The fresh biopsy specimen was divided in two parts: one part for determination of single-fiber contractile properties and the other part for ubiquitin-conjugation analysis. Both parts were processed and stored as presented in the online supplement.
Tissue Preparation and Single-Fiber Dissection
Approximately 1 hour before determination of single-fiber contractile properties, the muscle bundle was transferred to cold (5°C) relaxing solution containing 1% Triton X-100 to permeabilize the plasma membrane. From the muscle bundle, segments of single fibers of approximately 2 mm were isolated. Subsequently, the fiber ends were attached to aluminum foil clips, and mounted on the single-fiber apparatus. Fibers that appeared injured (e.g., sarcolemmal damage, loss of cross-striation, or other irregularities) during microscopic examination (×400 magnification) were excluded from the study.
Single-Fiber Contractile Measurements
The compositions of activating and relaxing solutions used for contractile measurements were reported previously (14), and are presented in the online supplement. Force generation, the fraction of strongly attached cross bridges and the rate constant of force redevelopment were determined in the single fibers during maximal Ca2+ activation. In addition, Ca2+ sensitivity of single-fiber force generation was recorded. Single-fiber contractile measurements and experimental protocol were performed according to previously described methods (15, 16), with minor modifications, and are presented in the online supplement.
Myosin Heavy Chain Isoform Composition Determination
At the end of the single-fiber contractile measurement protocol, myosin heavy chain isoform composition of the fiber was identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, as described previously (14), with minor modifications (17). Our methodology is presented in the online supplement.
Myosin Heavy Chain Content per Half-Sarcomere Measurements
Myosin heavy chain content per half sarcomere in the same single fibers was determined (18), as presented in the online supplement.
Assay of Ubiquitin-Protein Conjugates
The methodology for determination of content of ubiquitin-protein conjugates in diaphragm muscle is presented in the online supplement.
Statistical Methods
Differences within parameters describing single-fiber characteristics and contractile properties were tested using a random intercept mixed linear model for repeated measurements with group (COPD or non-COPD) as fixed factor. The model was used to predict the individual (fiber) measurements. Thereafter, residuals were calculated (observed minus expected value) to verify the model assumption that these residuals follow a normal distribution. Model estimates of mean values were computed per group. To evaluate statistical significance of difference between patients with and without COPD regarding ubiquitin-conjugation data, a t test was used. Two-sided level of significance was fixed at 5%. Because the present study was explorative and not confirmative, of nature, no correction was necessary for type I errors.
RESULTS
Subject Characteristics
Subject characteristics and pulmonary function data are shown in Table 1. On the basis of GOLD classification (19, 20), two patients had mild COPD (stage I) and the others were classified as moderate COPD (stage II). The patients with COPD were comparable to the patients without COPD with respect to age, body mass index, PaO2, and PaCO2.
Diaphragm Muscle Single-Fiber Data
A total of 227 single fibers from patients with and without COPD were used for contractile measurements. These fibers expressed a single myosin heavy chain isoform (110 myosin heavy chain slow fibers and 117 myosin heavy chain 2A fibers). Fibers that coexpressed myosin heavy chain slow and 2A (n = 20), or myosin heavy chain 2A and 2X (n = 2) were excluded from further analysis. Because of technical circumstances, in some fibers not all parameters describing contractile properties or parameters based on myosin heavy chain quantification could be determined. Therefore, the number of single fibers analyzed can differ per parameter.
Maximum force per cross-sectional area.
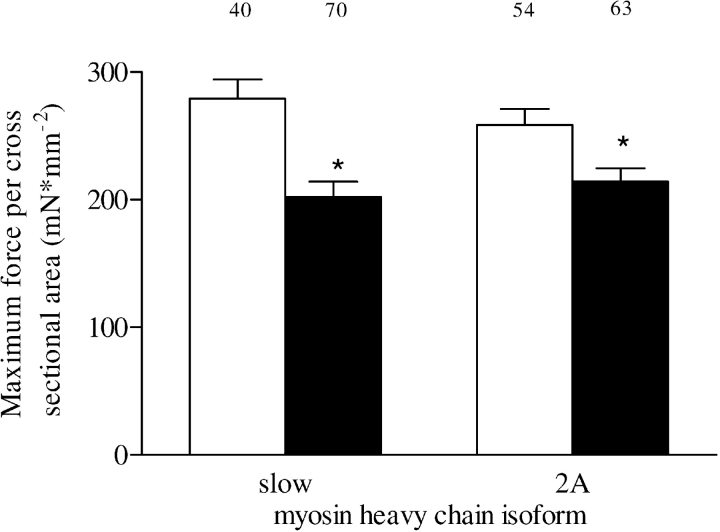
Usually, maximum force generation in single fibers is normalized to fiber cross-sectional area, assuming that increases in cross-sectional area parallel increased myosin content. Single-fiber cross-sectional area was not significantly different between patient groups (3.9 ± 0.4 · 10−3 and 3.1 ± 0.5 · 10−3 mm2 for type slow (p = 0.19) and 3.5 ± 0.5 · 10−3 and 3.5 ± 0.6 · 10−3 mm2 for type 2A fibers (p = 0.98); patients with COPD and without COPD, respectively). Maximum force, normalized to cross-sectional area, of single fibers from patients with COPD was lower in type slow (p < 0.001) and 2A fibers (p = 0.008), respectively, compared with patients without COPD (Figure 1).
Figure 1.
Single-fiber maximum force per cross-sectional area in type slow and 2A fibers from patients with (solid bars) and without (white bars) chronic obstructive pulmonary disease (COPD). The numbers above the bars represent the total number of single fibers analyzed. Data are presented as model estimates ± SEM. *p < 0.05 difference from group without COPD.
Myosin heavy chain concentration.
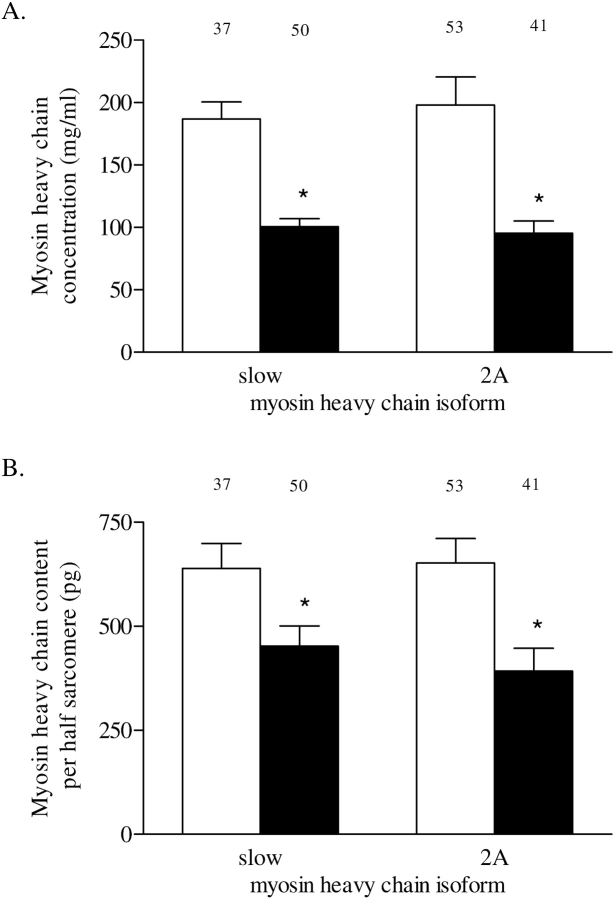
Myosin heavy chain concentration in single fibers from patients with COPD was significantly lower compared with those from patients without COPD in both fiber types (p < 0.001; Figure 2A).
Figure 2.
(A) Myosin heavy chain concentration in type slow and 2A fibers from patients without COPD (white bars) and those with COPD (solid bars). Myosin heavy chain concentration was determined from sodium dodecyl sulfate–polyacrylamide gel electrophoresis densitometry of single fibers and fiber volume. (B) Myosin heavy chain content per half sarcomere in type slow and 2A fibers from patients without COPD (white bars) and those with COPD (solid bars). Myosin heavy chain content per half sarcomere was determined from single-fiber myosin heavy chain concentration and half-sarcomere volume. Myosin heavy chain content per half sarcomere estimates the number of cross bridges in parallel per single fiber. The numbers above the bars represent the total number of single fibers analyzed. Data are presented as model estimates ± SEM. *p < 0.05 difference from group without COPD.
Myosin heavy chain content per half sarcomere.
Myosin heavy chain content in the single fibers normalized to half sarcomere volume reflects the number of cross bridges in parallel in the single fibers, independent of cross-sectional area (21). Figure 2B shows that, in patients with COPD, compared with those without COPD, myosin heavy content per half sarcomere is significantly reduced in type slow (p = 0.018) and 2A fibers (p = 0.002).
Force per half sarcomere myosin heavy chain content.
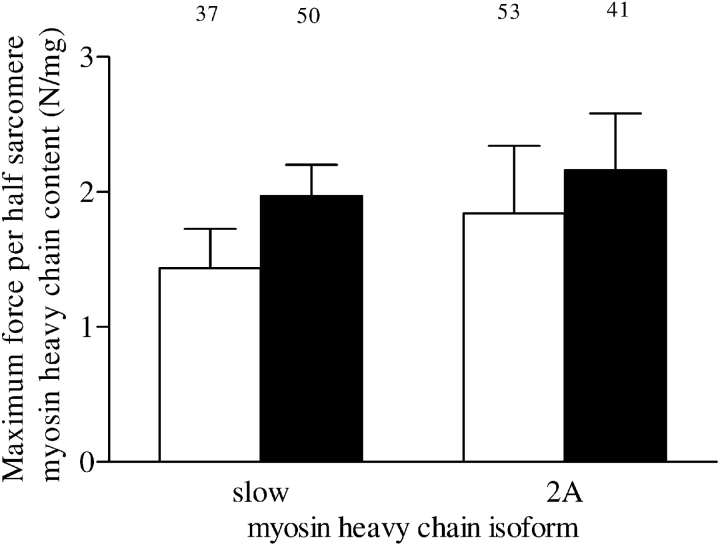
Maximum force values of single fibers were normalized to myosin heavy chain content per half sarcomere to evaluate the effect of cross-bridge number on maximum force per cross-sectional area. Figure 3 shows that maximum force in both fiber types was similar in patients with and without COPD when normalized for myosin heavy chain content (type slow fibers, p = 0.15; type 2A fibers, p = 0.63).
Figure 3.
Maximum force per half sarcomere myosin heavy chain content in type slow and 2A fibers from patients without COPD (white bars) and those with COPD (solid bars). Maximum force normalized for half-sarcomere myosin heavy chain content estimates the average force generated per cross bridge. The numbers above the bars represent the total number of single fibers analyzed. Data are presented as model estimates ± SEM.
Fraction of cross bridges in strongly attached state.
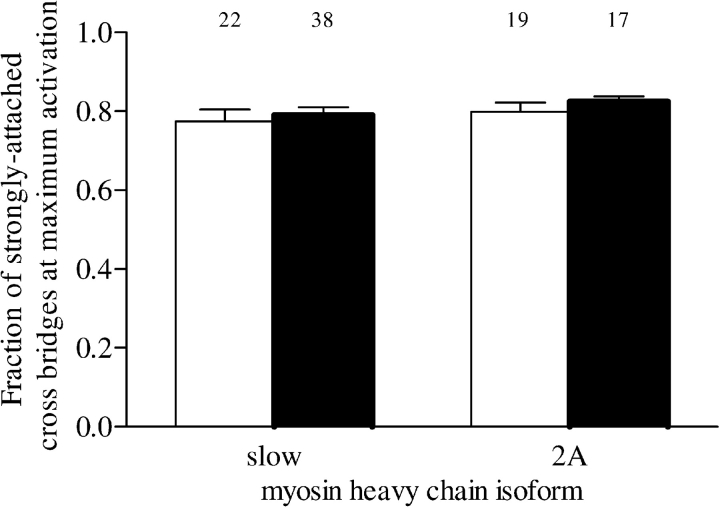
Maximum force depends on the fraction of cross bridges in the strongly attached state. This can be derived from measuring single-fiber stiffness (10). Figure 4 shows that, in both type slow (p = 0.62) and 2A (p = 0.37) fibers, the fraction of strongly attached cross bridges at maximum activation was not significantly different between patient groups.
Figure 4.
Fraction of strongly attached cross bridges at maximal activation in type slow and 2A fibers from patients without COPD (white bars) and those with COPD (solid bars). The fraction of strongly attached cross bridges was estimated by determining the ratio of single-fiber stiffness at maximal activation in the presence of adenosine triphosphate to stiffness at maximal activation in the absence of adenosine triphosphate. The numbers above the bars represent the total number of single fibers analyzed. Data are presented as model estimates ± SEM.
Cross-bridge cycling kinetics.
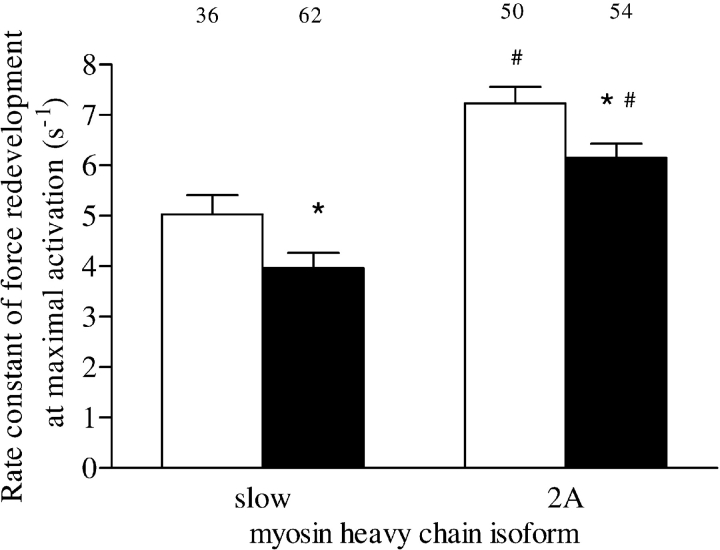
The rate constant of force redevelopment was used as a measure for cross-bridge cycling kinetics (10). The maximally activated fiber was rapidly released to a shorter length, and then rapidly restretched. During this procedure, all cross bridges detach and force decreases abruptly to zero. Subsequently, force redevelops as cross bridges reattach to actin. Figure 5 shows that, in diaphragm muscle fibers from patients with COPD, the rate constant of force redevelopment at maximum activation was slower in both type slow (p = 0.03) and type 2A fibers (p = 0.01) than in fibers from patients without COPD. As expected, rate constant of force redevelopment was faster in type 2A fibers than in type slow fibers within both patients without COPD (p < 0.001) and those with COPD (p < 0.001).
Figure 5.
Rate constant of force redevelopment at maximal activation in type slow and 2A fibers from patients without COPD (white bars) and those with COPD (solid bars). The rate constant of force redevelopment was used as a measure for cross-bridge cycling kinetics. The numbers above the bars represent the total number of single fibers analyzed. Data are presented as model estimates ± SEM. *p < 0.05 difference from group without COPD within fiber type; #p < 0.05 difference from type slow fibers within patient group.
Myofibrillar Ca2+ sensitivity.
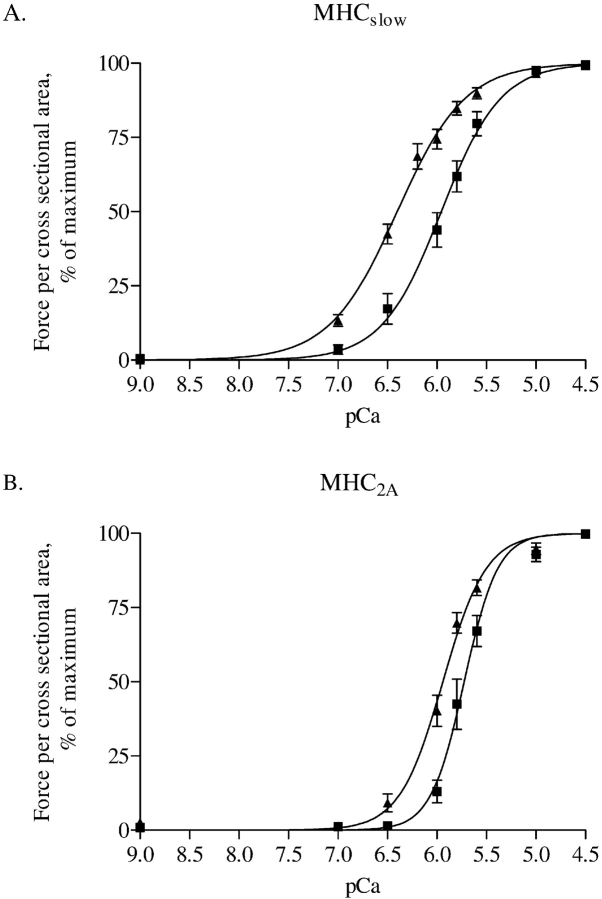
Ca2+ sensitivity of force generation in diaphragm muscle fibers is reduced in patients with COPD, as can be derived from the rightward shift of the force–pCa curves for both type slow and 2A fibers (Figures 6A and 6B, respectively). In slow fibers, pCa50 was 5.95 ± 0.10 versus 6.32 ± 0.11 in patients with and without COPD, respectively (p = 0.02). In type 2A fibers, pCa50 was 5.68 ± 0.06 versus 5.94 ± 0.06 in patients with and without COPD, respectively (p = 0.007).
Figure 6.
Force–Ca2+ characteristics of single fibers from three patients without COPD and four with COPD. Isometric force generated in response to incubation of single fibers with incremental [Ca2+] was determined. Note the rightward shift of the force–Ca2+ relationship in both type slow (panel A) and 2A (panel B) single fibers from patients with COPD. Calcium concentration needed for 50% of maximal force generation was significantly higher (i.e., lower pCa50, see RESULTS) in both fiber types from patients with COPD compared with patients without COPD, indicating decreased Ca2+ sensitivity of force generation. Data are presented as model estimates ± SEM. n = 19 for patients with and without COPD, respectively, for type slow fibers; n = 10 for patients with and without COPD, respectively, for type 2A fibers. Triangles indicate patients without COPD; squares indicate patients with COPD. MHC = myosin heavy chain.
Ubiquitin-Protein Conjugation
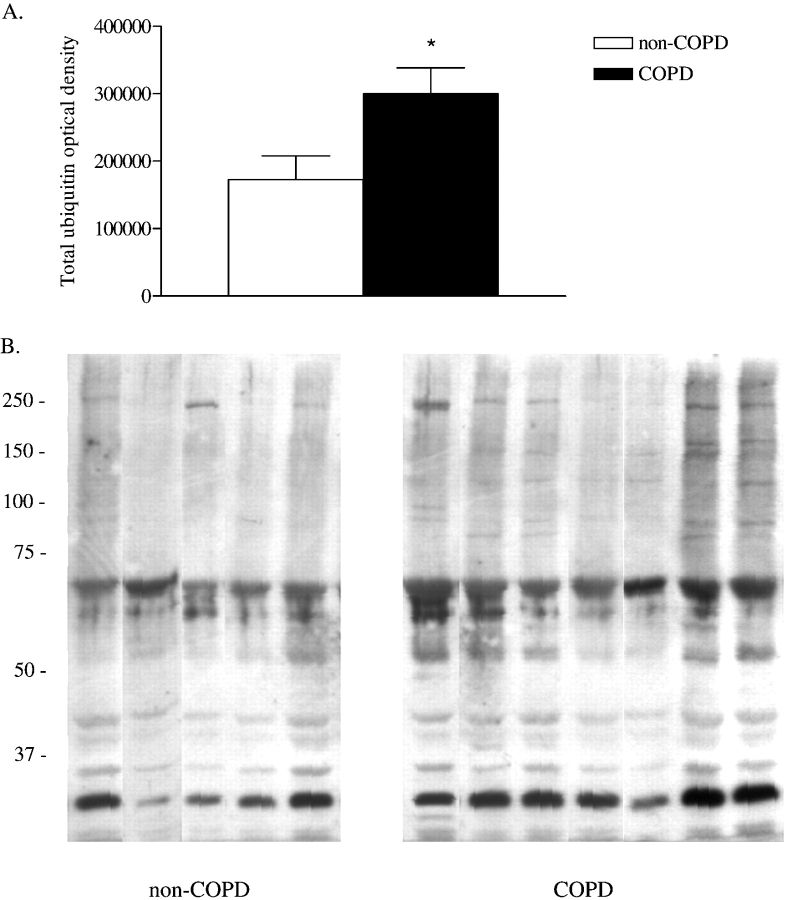
Ubiquitin-protein conjugation marks the substrate protein for proteasomal degradation. Figure 7 shows increased ubiquitin-protein conjugation in diaphragm muscle homogenates of patients with COPD compared with those without COPD. Total optical density of ubiquitin-protein conjugates in diaphragm muscle homogenates was higher in patients with COPD compared with patients without COPD (Figure 7A; p = 0.02). The antiubiquitin immunoblot of diaphragm muscle homogenates from patients with and without COPD showed the appearance of additional ubiquitin-protein conjugates with molecular masses between approximately 75 and 250 kD in diaphragm muscle homogenates from patients with COPD (Figure 7B). Also, optical density of ubiquitin–protein conjugates with molecular masses of approximately 30 and approximately 70 kD, present in diaphragm muscle homogenates from patients without COPD, was increased in diaphragm muscle homogenates from patients with COPD (Figure 7B).
Figure 7.
Ubiquitin–protein conjugation in diaphragm muscle homogenates of patients with COPD (n = 7) compared with the patients without COPD (n = 5). (A) Total optical density of ubiquitin–protein conjugates in diaphragm muscle homogenates. Data are presented as mean ± SEM. *p < 0.05 difference from group without COPD. (B) Antiubiquitin immunoblot of diaphragm muscle homogenates from patients with and without COPD. Each lane shows result for a diaphragm muscle homogenate from one patient. Molecular weight markers are shown in kilodaltons. Note the appearance of additional ubiquitin-protein conjugates with molecular masses between approximately 75 and 250 kD in diaphragm muscle homogenates from patients with COPD. Also, optical density of ubiquitin–protein conjugates with molecular masses of approximately 30 and approximately 70 kD, present in diaphragm muscle homogenates from patients without COPD, was increased in diaphragm muscle homogenates from patients with COPD.
DISCUSSION
Our results clearly demonstrate that diaphragm muscle single fibers from patients with COPD have reduced force-generating capacity, concomitant with loss of myosin heavy chain in these specific fibers. In the diaphragm of these patients with COPD, elevated levels of ubiquitin–conjugated proteins were found, suggesting accelerated muscle protein degradation. In addition to loss of contractile protein, the remaining contractile proteins in these fibers are dysfunctional because cross-bridge cycling was slower. Also, the calcium sensitivity of force generation is reduced in single fibers from patients with COPD, which could contribute to muscle weakness at submaximal activation. Importantly, these changes appear to occur in patients with only mild to moderate COPD (GOLD stage I/II).
Reduced Maximum Force Generation in Diaphragm Muscle Single Fibers in COPD
The present study shows reduced maximum force generation in diaphragm single fibers from patients with COPD (see Figure 1). Maximum force generation in permeabilized single fibers is determined by the following factors: (1) myosin heavy chain content per half sarcomere, (2) force generated per half sarcomere myosin heavy chain content, and (3) the fraction of strongly attached cross bridges (10). Therefore, the reduced force generation in single fibers from patients with COPD should be reflected by a change in one or more of its determinants. Our results show a decrease in myosin heavy chain content per half sarcomere in patients with COPD in both type slow and 2A fibers (see Figure 2B). The magnitude of the decrease of myosin heavy chain content can completely explain the reduced force generation in these fibers. In line with previous findings, the other two determinants of force generation, force generated per half sarcomere myosin heavy chain content and the fraction of strongly attached cross bridges, were not different between single fibers from patients with and without COPD (see Figures 3 and 4, respectively). Although diaphragm function in vivo is also determined by other factors (e.g., neural drive, muscle fiber recruitment, calcium homeostasis), our results strongly indicate that loss of myosin is a key player in the etiology of diaphragm muscle weakness in COPD. Only one previous study has reported changes in diaphragm single-fiber contractile properties in patients with COPD (22). That study showed that, in two patients with severe COPD, maximum force per cross-sectional area was reduced in single fibers. However, myosin content was not determined, thus the underlying pathophysiology remained unclear.
In vivo, the diaphragm does not perform maximum isometric contractions but shortens against a submaximal load. Thus, submaximal and kinetic parameters of muscle function provide more physiologic information. Ca2+ sensitivity of force generation is determined by binding of Ca2+ to troponin C, which in turn affects the probability of myosin binding to actin, or cross-bridge formation. The rightward shift of the force–pCa curve (see Figure 6) indicates that, at a certain Ca2+ concentration, generated force relative to maximum force is reduced in fibers from patients with COPD. In other words, Ca2+ sensitivity of force generation is reduced. This appears to be an important finding, because it could compromise diaphragm function at submaximum activation, which reflects in vivo activation of the diaphragm. The rate constant of force redevelopment is determined by the rate of cross-bridge formation and detachment (23). The decreased rate constant of force redevelopment in diaphragm fibers from patients with COPD (see Figure 5) shows that cross-bridge cycling kinetics are impaired in the diaphragm in COPD. These data show that, besides loss of contractile protein in the diaphragm in COPD, the remaining contractile proteins are dysfunctional. The nature of these dysfunctional proteins is unclear. However, myosin and troponin might be involved, because function of these proteins is an important determinant of cross-bridge cycling kinetics and Ca2+ sensitivity of force generation.
What Might Cause the Loss of Myosin Heavy Chain in the Diaphragm of Patients with COPD?
The present study is the first to investigate levels of ubiquitin-conjugated proteins in human diaphragm muscle. We found elevated levels of ubiquitin-conjugated proteins in diaphragm muscle of patients with COPD compared with those without COPD (see Figure 7). Ubiquitin conjugation to proteins marks the proteins for proteasomal degradation as ubiquitin accumulates (24). It is well recognized that the ubiquitin–proteasome pathway, and not lysosomal or Ca2+-activated proteinases, degrade myofibrillar proteins, such as actin and myosin (24–27). Our findings suggest involvement of the ubiquitin–proteasome proteolytic pathway in the loss of myosin in the diaphragm of patients with COPD.
Recently, Chamberlain (12) addressed the role of selective degradation of myosin by the ubiquitin–proteasome pathway in cachexia. Cytokines may be at play (12). Resistive breathing has been shown to increase expression of cytokines, such as tumor necrosis factor α and interleukin 6, in the diaphragm (28). Also, circulating levels of these cytokines are increased in a subgroup of patients with COPD (29, 30), supporting a role for proinflammatory cytokines in diaphragm weakness. In our study, the loss of myosin heavy chain in single fibers from patients with COPD is not paralleled by decreased fiber cross-sectional area. Consequently, myosin heavy chain concentration is decreased in these muscle fibers (see Figure 2A). Because preferential loss of myosin heavy chain would increase the average distance between myosin and actin filaments, this might induce muscle atrophy later in the course of the disease (i.e., decreased fiber cross-sectional area) to restore filament lattice spacing needed for optimal contractile function (12). Although speculative, our findings could support the hypothesis that, in mild to moderate COPD, loss of myosin heavy chain is an initial, but functional important step in diaphragm muscle fiber atrophy, as observed in severe COPD (6, 7). Therefore, ubiquitin-induced loss of myosin heavy chain in the diaphragm may play a role in ventilatory failure in severe COPD.
In summary, the present study clearly demonstrates reduced force-generation capacity in single diaphragm muscle fibers from patients with COPD. The reduced force generation in these fibers can be explained by loss of myosin heavy chain. Diaphragm muscle from patients with COPD had increased levels of ubiquitin-conjugated proteins, suggesting accelerated muscle protein degradation. The remaining contractile proteins show signs of impaired function, and thereby may contribute to respiratory muscle dysfunction in patients with COPD. Our findings occur early in the disease process, because our patients had only mild to moderate airway obstruction (GOLD I/II).
Supplementary Material
Acknowledgments
The authors thank P. Verhaert and S. van der Locht (NV Organon, The Netherlands), for technical assistance regarding myosin heavy chain isoform determinations, and Drs. A. Verhagen (Radboud University Nijmegen Medical Centre, The Netherlands), F. van den Elshout, S. van Sterkenburg, W. de Vries, T. Bloemen (Rijnstate Hospital Arnhem, The Netherlands), and F. Smeenk and B. van Straten, (Catharina Hospital Eindhoven, The Netherlands) for collecting the diaphragm muscle biopsies.
Supported by an unrestricted educational grant from GlaxoSmithKline, The Netherlands, and National Institutes of Health grant HL-37680.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med 1998;4:1241–1243. [DOI] [PubMed] [Google Scholar]
- 2.Begin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:905–912. [DOI] [PubMed] [Google Scholar]
- 3.Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Howard P, Gorzelak K, Lahdensuo A, Strom K, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997;52:43–47. [PubMed] [Google Scholar]
- 4.Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:961–966. [DOI] [PubMed] [Google Scholar]
- 5.Mercadier JJ, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, Marrash R, Pariente R, Aubier M. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol 1998;274:L527–L534. [DOI] [PubMed] [Google Scholar]
- 6.Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 1997;337:1799–1806. [DOI] [PubMed] [Google Scholar]
- 7.Orozco-Levi M, Gea J, Lloreta JL, Felez M, Minguella J, Serrano S, Broquetas JM. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J 1999;13:371–378. [DOI] [PubMed] [Google Scholar]
- 8.Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, Dudley G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol 2002;92:1205–1213. [DOI] [PubMed] [Google Scholar]
- 9.Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature 1971;233:533–538. [DOI] [PubMed] [Google Scholar]
- 10.Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 1988;85:3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–871. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain JS. Cachexia in cancer: zeroing in on myosin. N Engl J Med 2004;351:2124–2125. [DOI] [PubMed] [Google Scholar]
- 13.Ottenheijm CAC, Heunks LMA, Degens H, Sieck GC, Dekhuijzen PNR. Impaired contractility of diaphragm single muscle fibers in COPD patients [abstract]. Am J Respir Crit Care Med 2004;169:A241. [Google Scholar]
- 14.Degens H, Yu F, Li X, Larsson L. Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol Scand 1998;163:33–40. [DOI] [PubMed] [Google Scholar]
- 15.Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol 1999;87:1894–1900. [DOI] [PubMed] [Google Scholar]
- 16.Heunks LMA, Cody MJ, Geiger PC, Dekhuijzen PNR, Sieck GC. Nitric oxide impairs Ca2+ activation and slows cross-bridge cycling kinetics in skeletal muscle. J Appl Physiol 2001;91:2233–2239. [DOI] [PubMed] [Google Scholar]
- 17.Han YS, Proctor DN, Geiger PC, Sieck GC. Reserve capacity for ATP consumption during isometric contraction in human skeletal muscle fibers. J Appl Physiol 2001;90:657–664. [DOI] [PubMed] [Google Scholar]
- 18.Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 2002;92:1506–1514. [DOI] [PubMed] [Google Scholar]
- 19.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 20.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 21.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol 2000;89:695–703. [DOI] [PubMed] [Google Scholar]
- 22.Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 2003;168:706–713. [DOI] [PubMed] [Google Scholar]
- 23.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 2000;80:853–924. [DOI] [PubMed] [Google Scholar]
- 24.Mitch WE, Goldberg AL. Mechanisms of muscle wasting: the role of the ubiquitin-proteasome pathway. N Engl J Med 1996;335:1897–1905. [DOI] [PubMed] [Google Scholar]
- 25.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 1999;129:227S–237S. [DOI] [PubMed] [Google Scholar]
- 26.Taillandier D, Combaret L, Pouch MN, Samuels SE, Bechet D, Attaix D. The role of ubiquitin-proteasome-dependent proteolysis in the remodelling of skeletal muscle. Proc Nutr Soc 2004;63:357–361. [DOI] [PubMed] [Google Scholar]
- 27.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell 2004;119:907–910. [DOI] [PubMed] [Google Scholar]
- 28.Vassilakopoulos T, Divangahi M, Rallis G, Kishta O, Petrof B, Comtois A, Hussain SN. Differential cytokine gene expression in the diaphragm in response to strenuous resistive breathing. Am J Respir Crit Care Med 2004;170:154–161. [DOI] [PubMed] [Google Scholar]
- 29.Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:1453–1455. [DOI] [PubMed] [Google Scholar]
- 30.Godoy I, Campana AO, Geraldo RR, Padovani CR, Paiva SA. Cytokines and dietary energy restriction in stable chronic obstructive pulmonary disease patients. Eur Respir J 2003;22:920–925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.