Abstract
Rationale: Most models of acute lung injury in mice have yet to be fully characterized. Objectives: To directly compare and contrast endotoxin and oleic acid models of acute lung injury in mice in terms of their physiologic, biochemical, histopathologic, and imaging manifestations. Methods: Survival studies, lung weights, x-ray computed tomographic scanning, light and electron microscopy, bronchoalveolar lavage, lung uptake of (18F)fluorodeoxyglucose, tissue myeloperoxidase, arterial blood gases, mean arterial pressure, and lung tissue prostanoids were measured in separate groups of C57Bl/6 mice (normal animals, endotoxin only [20 μg/g], oleic acid only [0.15 μl/g], or endotoxin + oleic acid). Results: Endotoxin alone caused only mild pulmonary neutrophilic inflammation with little functional or structural damage to the alveolar architecture. In contrast, oleic acid caused severe alveolar damage with the development of alveolar edema of the increased-permeability type with associated abnormalities in gas exchange. When given together, endotoxin and oleic acid acted synergistically to increase pulmonary edema and to worsen gas exchange and hemodynamics, thereby increasing mortality. This synergism was significantly attenuated by the prior administration of the endotoxin antagonist E5564 (eritoran). Conclusions: Under the conditions of these studies, only mice exposed to oleic acid showed both structural and functional characteristics of acute lung injury. Nevertheless, endotoxin had potent synergistic physiologic effects that increased mortality. Overall, these models, which can be translated to genetically altered mice, are amenable to study with state-of-the-art imaging techniques, and with experimental interventions that can probe the underlying mechanisms of injury.
Keywords: fluorodeoxyglucose F18, mice, positron-emission tomography, respiratory distress syndrome (adult)
Animal models of acute lung injury (ALI) are important tools for studying mechanisms relevant to the acute respiratory distress syndrome (ARDS) in humans. Many methods, in a variety of different animal species, can generate ALI, each with its own set of advantages and limitations (1–3). Importantly, however, there are relatively few mouse models of ALI, despite the obvious value of being able to manipulate the genetic background of mice as a means of understanding the pathogenesis of ALI. In many cases, the models that do exist have yet to be fully characterized, often because of the difficulties in obtaining relevant physiologic or similar data from such small animals. Recent developments in microinstrumentation (4), however, make it possible to now address these deficiencies.
Because one of the most common causes of ALI is bacterial sepsis, many investigators have used intraperitoneal or intratracheal administration of endotoxin (Etx) as a method of provoking ALI. Recently, Kabir and colleagues (5) and Rojas and coworkers (6) both reported that intraperitoneal Etx administered to C57Bl/6 mice caused increased expression of various inflammatory mediators, increased lung water, and increased neutrophil accumulation in lung tissue. However, the reported changes were mild and transient, resolving over 48 hours.
In contrast, oleic acid (OA)–induced ALI is another standard and widely used model, with significant histopathologic changes similar to those observed in ARDS (7). Furthermore, in our own studies, we have used low-dose Etx in dogs to modify the physiologic and biochemical expression of OA-induced ALI (8, 9), further enhancing its resemblance to human ARDS (10). OA-induced ALI is neutrophil-independent (11–13), providing a useful contrast to many other models of ALI.
The purpose of the present study, then, was to directly compare and contrast the Etx and OA models of ALI in mice in terms of their physiologic, biochemical, histopathologic, and imaging manifestations, providing an important database for future studies with these models.
METHODS
The methods for these studies are described in detail in an online supplement.
Reagents
Wild-type C57Bl/6 mice, 8 to 11 weeks old, were used. All experiments were approved by the Animal Studies Committee of Washington University. The Etx antagonist E5564 (eritoran) (14) was a generous gift of the Eisai Research Institute. (E5564 is a synthetic Etx antagonist with a structure similar to that of lipid A, derived from the noninfectious gram-negative bacteria Rhodobacter sphaeroides. E5564 blocks Etx triggering of the inflammatory response by binding to toll-like receptor 4, thereby inhibiting its activation [14, 15].) Lipopolysaccharide from Escherichia coli 055:B5 was used in these studies; OA was prepared as a 7.5% solution in 0.1% bovine serum albumin (16). (18F)Fluorodeoxyglucose ([18F]FDG) was manufactured on-site, as previously described (17).
Experimental Groups
In general, four groups of mice were studied at a time. These group clusters included a control group (no interventions), a group administered Etx only (20 μg/g, intraperitoneally), a group administered OA only (0.15 μl/g body weight, intravenously), and a group administered Etx + OA (OA given 30 minutes after Etx). E5564 (eritoran; 4 μg/g bw) was administered 30 minutes before Etx. Mice were monitored for 24 hours for survival studies. For physiologic and biochemical or imaging studies, the measurements were obtained 60 minutes after OA. To measure lung (18F)FDG uptake, the tracer was injected 60 minutes after OA, the mice were killed 60 minutes later, and tissue radioactivity was measured in a well counter in harvested lungs.
Instrumentation
For studies involving mechanical ventilation, mice were orally intubated and connected to a rodent ventilator set at Vt 23.5 μl/g and rate 110/minute. A high-fidelity micromanometer catheter was placed into the right carotid artery. At the end of the study, blood was drawn from the abdominal aorta for blood gas measurements.
Gravimetrics, Histology, and Electron Microscopy
After mice were killed, the left lungs of some mice were harvested, weighed, and then dried to constant weight in an oven at 60°C for 3 to 4 days. In other mice, the lungs were flushed to remove blood, then inflated with 10% buffered formalin (20 cm H2O) before processing for routine light microscopy with hematoxylin–eosin. For electron microscopy, the lungs were inflation-fixed (20 cm H2O) with a 3% solution of glutaraldehyde in cacodylate buffer (0.1 M, pH 7.4) for 1 hour. Blocks (1 mm3) were cut from each lung and immersed in additional fixative until preparation for routine electron microscopy.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) was performed in situ; the recovered BAL fluid was centrifuged, the pellet was resuspended in phosphate-buffered saline, and the total leukocyte count was determined using a hemocytometer. The differential cell count was performed on the cytocentrifuge preparation stained with hematoxylin–eosin. Protein measurements were performed by the bicinchoninic acid method.
Computed Tomography Imaging
Mice were anesthetized with isoflurane gas. Micro–x-ray computed tomography images were obtained using a MicroCAT II scanner (ImTek, Inc., Knoxville, TN). Lung density measurements were obtained by recording mean and pixel-by-pixel Hounsfield units within an image region of interest, where a value of 0 = density of water and −1,000 = density of air.
Lung Glucose Uptake
The pulmonary uptake of (18F)FDG was measured as the tissue–blood radioactivity ratio, as previously described (18).
Biochemistry Assays
Lung tissue myeloperoxidase (MPO) activity and measurements of the stable metabolites of thromboxane (TxB2) and prostacyclin (6-ketoPGF1α) were measured by standard techniques, as described previously (18–20).
Statistics
Kaplan-Meier curves were analyzed by the log-rank test. Group data were expressed as the mean ± SD. Standard one-way analysis of variance tests were used to compare results among groups. Post hoc comparisons were done using the Holm-Sidak multiple comparison test. Statistical significance was set at p < 0.05.
RESULTS
In pilot studies (not shown), we determined that an intravenous dose of 0.15 μl/g body weight OA resulted in an approximate 24-hour survival rate of 75%. Using this dose of OA, we then compared the 24-hour survival rates (n = 20 each) of mice administered 20 μg/g body weight Etx alone, OA alone, or the combination of Etx and OA (Figure 1A). Mortality was markedly increased by the combination of both agents (24-hour survival only 30%).
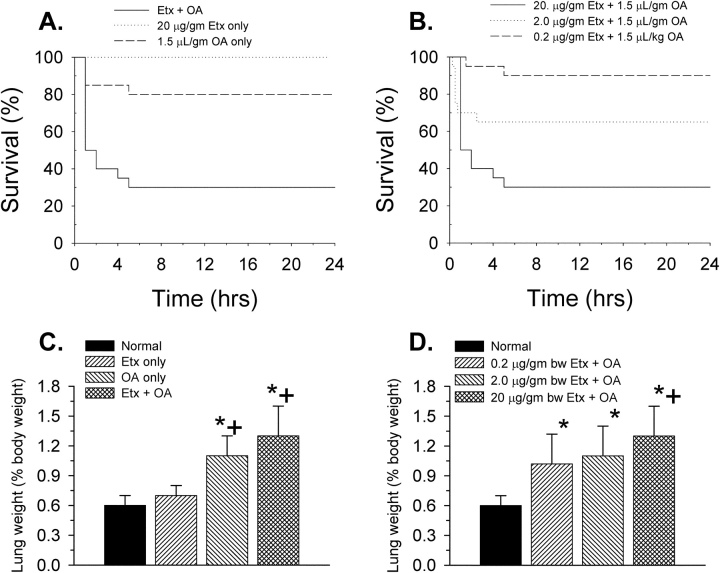
Figure 1.
(A) Kaplan-Meier survival curves for three groups of mice (n = 20 each): oleic acid only (OA), endotoxin only (Etx), and Etx + OA. Both the OA-only and Etx + OA survival curves are statistically different (p < 0.05) from the Etx-only curve. (B) Kaplan-Meier survival curves for three groups of mice (n = 20 each) given both Etx and OA. The group given the lowest dose of Etx had a significantly different survival curve from the group given the highest dose of Etx. (C) Lung weights in the three groups of mice from A compared with a group of normal mice (n = 9). (D) Lung weights in the three groups of mice from B compared with the same group of normal mice as in C. *p < 0.05 compared with the normal group; +p < 0.05 compared with the Etx-only group.
The group given the combination of Etx and OA was also compared with two other groups given 10- and 100-fold lower doses of Etx (Figure 1B). Survival was related to the dose of Etx. All deaths occurred within the first 6 hours after OA.
Postmortem measurements showed that only the animals that received OA had significant increases in lung weight (Figure 1C; a group of mice that received no intervention [n = 9] was included to establish normal values for lung weight). The lung weights of mice that received Etx + OA were higher than those that received OA only, but the difference was not statistically significant. However, an Etx effect on lung weight (when combined with OA) is suggested by a progressive increase in lung weight depending on the dose of Etx given (Figure 1D).
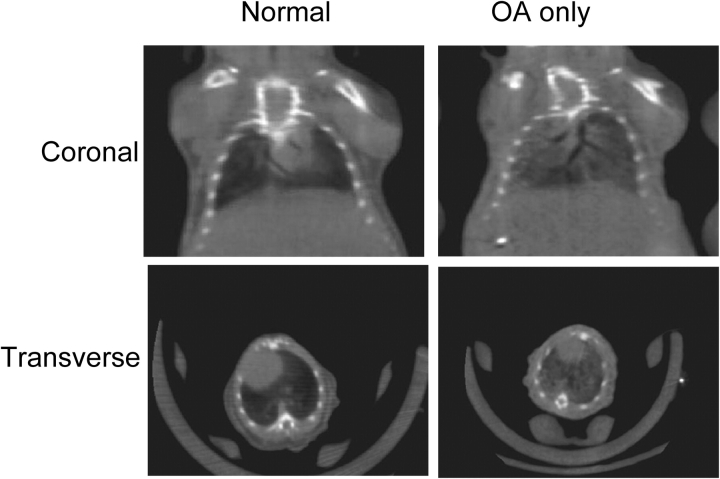
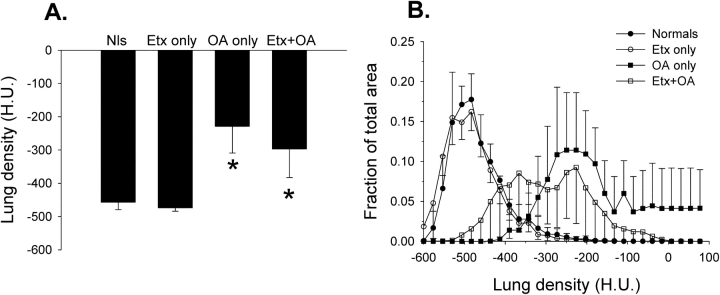
Additional groups of mice were studied to obtain imaging, physiologic, and biochemical data. Examples of micro–x-ray computed tomography scans of mice from the normal and OA-only groups are shown in Figure 2. A mean increase in lung density (Figure 3A) after OA, manifested as lung consolidation with air bronchograms (Figure 2), is clearly visible. A more complete image analysis is given in Figure 3B, showing a rightward shift in the lung density histograms in animals receiving OA.
Figure 2.
Examples of micro–x-ray computed tomography scans of mice from the normal and OA-only groups. Both coronal and transverse images are shown. After OA, there is an increase in pulmonary parenchymal infiltrates with air bronchograms, consistent with acute diffuse pulmonary edema.
Figure 3.
Lung density as measured by x-ray computed tomography in all four experimental groups (n = 3–4 mice/group). (A) Mean lung density obtained from analysis of a single tomographic slice, as in Figure 2. *p < 0.05 compared with the normal (Nls) or Etx-only groups of mice. H.U. = Hounsfield units, where 0 = density of water and −1,000 = density of air. (B) Histograms of lung density when sorting the pixel-by-pixel data from the same regions of interest used to obtain mean data in A into 30 separate “bins.” The density pattern after Etx only is virtually identical to that in normal mice, whereas there is a rightward shift toward higher densities in mice administered OA.
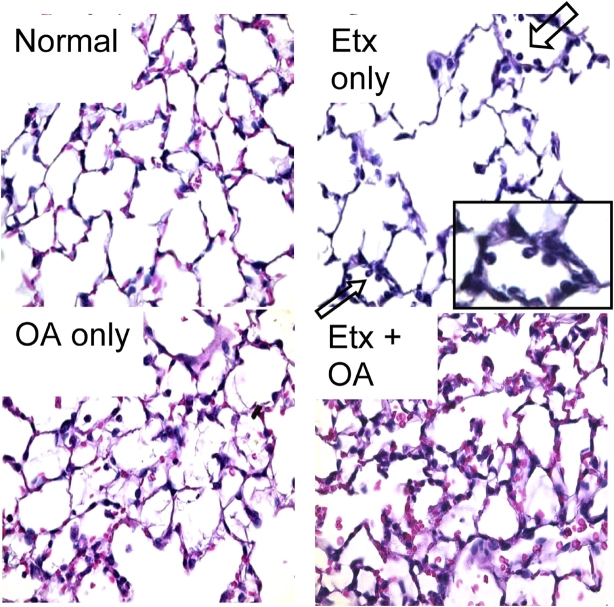
Examples of light micrographs from all four experimental groups are shown in Figure 4. Neutrophil sequestration in small parenchymal vessels was the only abnormal finding in mice that received Etx only. In contrast, inflammatory infiltrate, alveolar hemorrhage, and proteinaceous alveolar edema were all prominent in mice that received OA.
Figure 4.
Examples of light micrographs from all four experimental groups. Arrows in the Etx-only panel point to neutrophils that are adherent to small parenchymal vessels (see enlargement in inset), indicative of sequestration. The micrographs of both OA panels show evidence of inflammatory infiltrate, alveolar hemorrhage, and proteinaceous alveolar edema. Original magnification, ×40.
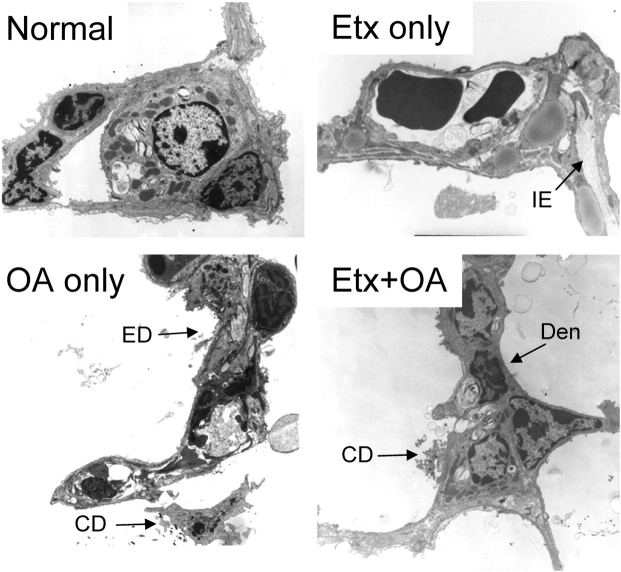
A similar disparity was seen in the electron micrographs obtained from similarly treated mice (Figure 5). Alveolar linings were intact in mice receiving Etx only (although interstitial edema was identified in some sections), whereas alveolar epithelial damage and fibrin and other cellular debris in the alveolar spaces were evident in mice that received OA.
Figure 5.
Electron micrographs from all four experimental groups. The alveolocapillary membranes in the panels from normal and Etx-only mice are normal, although mild interstitial edema (IE) is present in the Etx-only panel. In contrast, there is widespread epithelial membrane damage (ED) and denudation (Den), fibrin and other cellular debris (CD), and proteinaceous edema in the alveolar space in the panels from mice given OA. Original magnification, ×3,000.
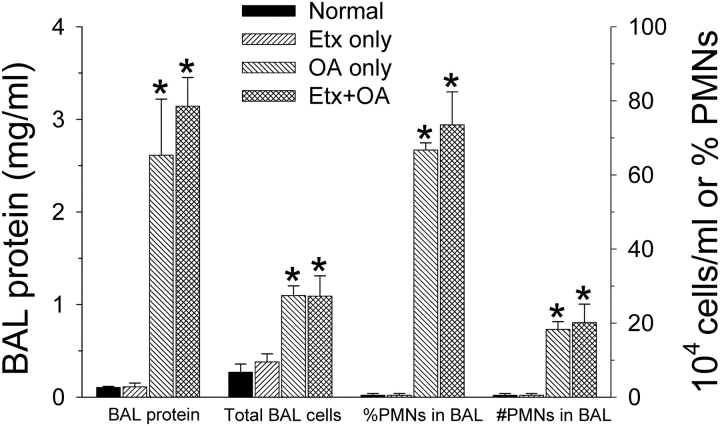
Protein and cell counts from mice (n = 3–4 in each group) that underwent BAL (Figure 6) were consistent with the histologic findings. The protein concentration and neutrophil counts were higher than in normal mice only in the groups given OA.
Figure 6.
Bronchoalveolar lavage (BAL) protein and cell counts from all four experimental groups (n = 3 each except for the Etx + OA group where n = 5). The protein concentration and neutrophil counts were higher only in the groups given OA. *p < 0.05 compared with the normal or Etx-only groups. PMN = polymorphonuclear white cells (i.e., neutrophils).
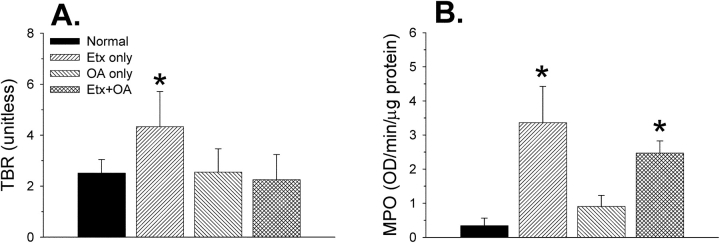
Measurements of neutrophil activation and infiltration performed on whole lungs (n = 10–13 in each group) gave a different picture from the BAL or histologic data (Figure 7). Glucose uptake by the lungs (assayed as the tissue-to-blood radioactivity ratio for [18F]FDG) was increased only in the Etx-only group of mice. In contrast, tissue MPO activity was increased in both groups that received Etx, but not in the group that received OA only.
Figure 7.
Lung (18F)fluorodeoxyglucose ([18F]FDG) uptake and neutrophil burden in animals from all four experimental groups (n = 10–13 mice/group). (A) Lung (18F)FDG uptake was measured as the tissue-to-blood radioactivity ratio (TBR; see METHODS). *p < 0.05 compared with all three other groups. (B) Lung neutrophil burden measured as myeloperoxidase (MPO) activity. *p < 0.05 compared with the normal and OA-only groups. OD = optical density.
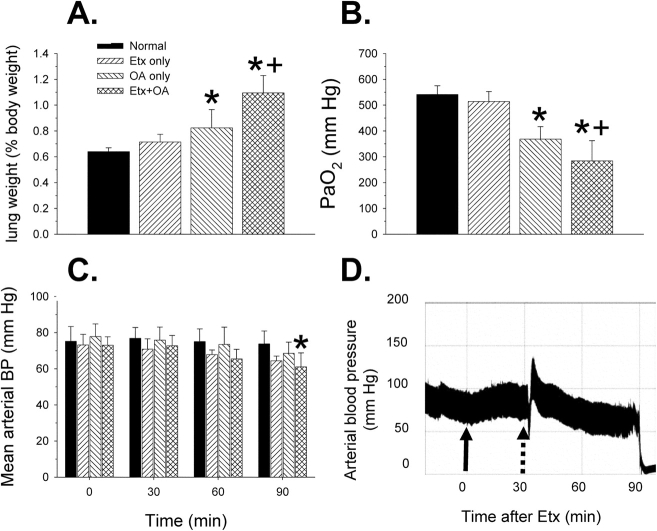
Physiologic effects during, or at the end of 90 minutes of mechanical ventilation for all four experimental groups (n = 5–6 mice each), are shown in Figure 8. As in the survival studies, postmortem lung weight increased significantly only in mice that received OA. In addition, wet-to-dry (W/D) weight ratios were determined in lung tissue samples from these mice: 4.8 ± 0.26, 4.8 ± 0.39, 5.6 ± 0.37, 6.5 ± 0.96 for the normal, Etx only, OA only, and Etx + OA groups, respectively. Only the Etx + OA group W/D ratio was significantly increased statistically.
Figure 8.
Physiologic effects during or at the end of 90 minutes of mechanical ventilation for all four experimental groups (n = 5–6 mice each). (A) Lung weights. Only mice given OA showed a significant increase in lung weight. (B) PaO2 at the end of the ventilation period. Only mice given OA showed a significant decrease in PaO2. (C) Mean arterial blood pressure (BP) at the times indicated. Only mice given Etx + OA showed a significant fall in BP 90 minutes after Etx (or placebo) compared with the baseline pressure at 0 minutes. (D) Example of an arterial BP tracing from one mouse in the Etx + OA group. Solid arrow: time of intraperitoneal Etx injection. Dotted arrow: time of tail vein injection of OA. Note progressive decrease in BP after OA. *p < 0.05 compared with normal mice or at baseline (C); +p < 0.05 compared with Etx group.
PaO2 (Figure 8B) decreased significantly only in mice given OA, and the fall in PaO2 was greatest in mice that received the combination of Etx and OA. Additional blood gas data are shown in Table 1. Mice that received Etx + OA showed a significant respiratory acidosis and concomitant decrease in arterial pH.
TABLE 1.
Arterial pH, paco2, and bicarbonate values 90 minutes after oleic acid and/or endotoxin
| pH | PaCO2 | HCO3− | |
|---|---|---|---|
| Normal | 7.32 ± 0.03 | 43 ± 3 | 22 ± 2 |
| Etx only | 7.34 ± 0.03 | 33 ± 6 | 18 ± 2 |
| OA only | 7.29 ± 0.01 | 38 ± 2 | 18 ± 1 |
| Etx + OA | 7.16 ± 0.01*,† | 50 ± 10† | 19 ± 3 |
Definition of abbreviations: Etx = endotoxin; OA = oleic acid.
Compared with the normal group.
Compared with the Etx-only and OA-only groups.
Mean arterial pressure tended to fall in all groups over time (Figure 8C), but by analysis of variance, the decrease was only statistically significant at the end of the 90-minute observation period in animals that received Etx + OA (Figure 8D).
Lung tissue prostanoid measurements from all four experimental groups are shown in Figure 9 (same mice as in Figure 8). None of the groups showed any significant differences in tissue TxB2 concentrations. In contrast, all three experimental groups showed significant increases in the tissue concentration of 6-ketoPGF1α compared with the normal group, but these were not different from one another.
Figure 9.
Lung tissue prostanoid measurements from all four experimental groups (same mice as in Figure 8). None of the groups showed any significant differences in tissue thromboxane (TxB2) concentrations from one another. In contrast, all three experimental groups showed significant increases in the tissue concentration of the stable metabolite of prostacyclin (6-ketoPGF1α) compared with the normal group, but were not different from one another. *p < 0.05 compared with the normal group.
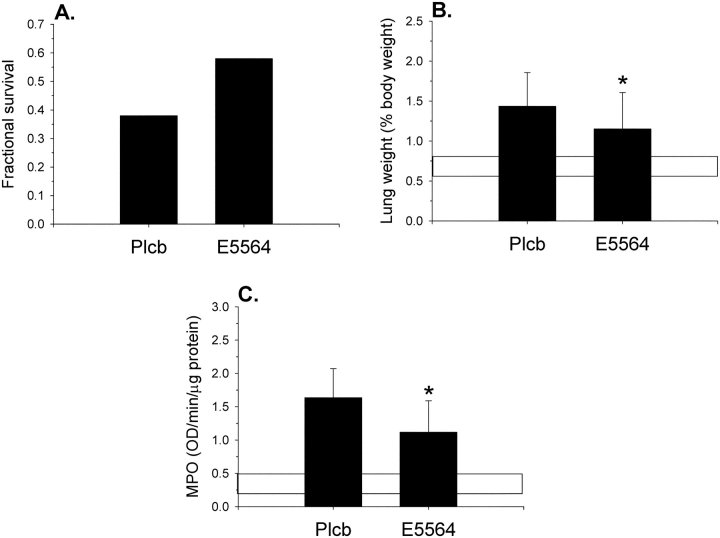
Finally, the effects of the toll-like receptor 4 antagonist E5564 (eritoran) are shown in Figure 10. These mice (n = 26 in each group) all received Etx + OA and, in addition, either a placebo or E5564 30 minutes before Etx. Mice that received E5564 showed a nonsignificant trend toward improved 24-hour survival. However, lung weights and MPO activity after Etx + OA were lower in the E5564 group, indicating less pulmonary edema and pulmonary inflammation, although both were higher than normal control lungs.
Figure 10.
Effects of the toll-like receptor 4 antagonist E5564. All mice received Etx + OA and either a placebo (Plcb) or E5564, 30 minutes before endotoxin. (A) Twenty-four-hour survival rates. The trend in improved survival in the E5564 group was not statistically significant (p = 0.36). (B) Lung weights. Lung weights after Etx + OA were lower in the E5564 group, indicating less pulmonary edema. Horizontal bar indicates normal range in lung weight (from Figure 1). (C) MPO activity. MPO activity was lower in the E5564 group, indicating less neutrophil recruitment to the lungs. Horizontal bar indicates normal range in MPO (from Figure 7B). *p < 0.05 compared with the Plcb group.
DISCUSSION
The main findings of this study, under the conditions used in this study, are as follows: (1) Etx alone causes only mild pulmonary neutrophilic inflammation with little functional or structural damage to the alveolar architecture, (2) OA causes severe alveolar damage with the development of alveolar edema of the increased-permeability type with associated abnormalities in oxygenation, and (3) Etx and OA act synergistically to increase pulmonary edema and worsen gas exchange and hemodynamics, thereby increasing mortality. Furthermore, we demonstrate that the models are amenable to study with state-of-the-art imaging techniques and with experimental interventions that can probe the underlying mechanisms of injury.
Definition of ALI
In patients, ARDS is usually defined as a particularly severe subset of ALI, which in turn is defined as “a syndrome of inflammation and increasing permeability that is associated with a constellation of clinical, radiologic, and physiologic abnormalities that cannot be explained by, but may coexist with, left atrial or pulmonary capillary hypertension” (21). Accordingly, characteristic radiographic and gas exchange abnormalities, together with clinical information about timing and associated “risk factors,” are generally used as criteria to establish the diagnosis of ARDS. Although the performance of these criteria in the clinical setting (e.g., sensitivity and specificity) is a matter of ongoing debate, we are unaware of any similarly agreed-on criteria for ALI in experimental animals. We have previously argued that ARDS should be considered a specific form of lung injury (not simply the most severe form of any lung injury)—one in which structural changes and functional abnormalities are causally linked (22). The structural, histopathologic hallmark of ALI is diffuse alveolar damage (DAD) (23, 24), and the functional abnormalities associated with ALI are a direct consequence of the breakdown in the pulmonary endothelial and epithelial barrier that is characteristic of DAD, leading to proteinaceous alveolar edema and other physiologic effects (e.g., altered respiratory system mechanics and gas exchange).
Thus, we consider DAD to be the sine qua non of ALI of the ARDS type. The distinguishing features of DAD are alveolar epithelial cell necrosis, inflammatory cell infiltration, proteinaceous alveolar and interstitial edema, alveolar hyaline membranes, and, eventually, type II pneumocyte proliferation and varying degrees of alveolar/interstitial fibrosis (23, 24). In the current study, animals in the OA groups clearly showed the early features of DAD (Figures 4 and 5). Using doses of OA that were up to approximately 1.5 times the dose used here, Ulrich and coworkers (25) recently reported similar histologic and electron micrographic findings. In contrast, in the current study, animals given Etx alone showed virtually no structural evidence of DAD.
Etx and OA models of ALI
Mice are known to be highly resistant to Etx (2), and the relatively mild nature of the lung injury associated with Etx administration to mice has been repeatedly observed and reported by others (5, 6). Kabir and colleagues (5) reported a 17% increase in lung weight within 6 hours of Etx administration, but no increase in the W/D ratio. Rojas and colleagues (6) reported a 10% increase in the W/D ratio. In the present study, lung weight increased 17% in nonventilated mice (Figure 1) and 12% in mice that were ventilated for 1 hour after OA administration (Figure 8). In the latter group, however, there was no significant difference from normal mice in the W/D ratio. The imaging data (Figure 3) are consistent with these gravimetric results in that lung density was found to be increased only in groups given OA. As noted by Parker and Townsley (26), the W/D ratio accounts for any simultaneous increases in lung dry weight and therefore is the more specific measure of increases in lung water per se. Given the increase in MPO activity associated with Etx administration (Figure 7) coupled with the lack of change in the W/D ratio in both this and the Kabir study (5), we believe the relatively modest increases in total lung weight (in the current as well as other studies) may simply reflect the additional water content of the inflammatory cell infiltrate. We cannot exclude, however, a minor component due to increased interstitial edema (Figure 5), which would imply an increase in endothelial permeability caused by Etx, as previously shown to occur in sheep (27).
An increased protein concentration in pulmonary edema fluid suggests increased capillary permeability as the cause of the pulmonary edema and is a major criterion of ALI. In the current study, BAL protein concentrations were only markedly elevated in animals that received OA (Figure 6), confirming recent measurements by others (16, 25).
Alveolar neutrophilia is another universal finding in ALI/ARDS, and like the BAL protein concentrations, alveolar neutrophilia was only documented in animals that received OA (Figure 6). Guimaraes and others (16) also reported the presence of alveolar neutrophilia after OA in mice. These observations are to be contrasted with the whole-lung MPO data (Figure 7). As have others (5, 28), we observed a significant increase in lung tissue MPO after Etx. Because neutrophils did not appear in the BAL of mice given Etx alone (Figure 6), and given that neutrophils were frequently found to be adherent to the endothelium of small parenchymal vessels (Figure 4), we believe these findings suggest that Etx-induced neutrophil influx into the lungs was limited to vascular sequestration. Using a dose of Etx that was 1/20th that used in our study, Ghosh and coworkers (29) recently arrived at a similar conclusion. In contrast, neutrophil penetration into the alveolar space after OA (with or without Etx) appears to be the result of either a breach in the alveolocapillary barrier or the establishment of a chemokine gradient caused by epithelial tissue damage. A similar disparity between the Etx and OA models with respect to the development of alveolar neutrophilia was recently reported by us in dogs (30) and by Ghosh and colleagues (29) in mice.
Interestingly, the overall MPO content of the lungs was only minimally elevated in mice that received OA alone (Figure 7), suggesting that the overall influx of neutrophils was less in this group of mice (despite the degree of alveolar neutrophilia). A similar observation has also recently been reported in dogs (30).
An additional disparity is revealed by the measurements of glucose uptake (tissue–blood radioactivity ratio; Figure 7A). The pulmonary uptake of radiolabeled FDG has repeatedly been linked to neutrophil activation and infiltration (30–33). In the current study, mice in the Etx-only group showed an increase in tissue–blood radioactivity ratio, whereas mice in the OA-only group showed no significant increase, consistent with previous reports. However, mice in the Etx + OA group also showed no increase in tissue–blood radioactivity ratio, which is different from a previous report in dogs. This observation suggests that injury in mice resulted in deactivation of neutrophils (and/or lung parenchymal cells otherwise activated by Etx). Similar disparities between neutrophil infiltration into the lungs and neutrophil activation have been reported by Jones and colleagues (32) in the clinical setting of chronic bronchitis versus acute pneumonia, and by others in mouse models of bleomycin-induced lung injury (34, 35). Thus, in future studies, it should be possible to exploit these different patterns of neutrophil burden (MPO), alveolar neutrophilia (BAL), and neutrophil activation (tissue–blood radioactivity ratio) among the different experimental groups to study the role of neutrophils in the development and/or exacerbation of ALI.
Acute refractory hypoxemia requiring ventilatory support is yet another universal marker of clinical ARDS. For this reason, we obtained arterial blood gas measurements in mice after 90 minutes of mechanical ventilation (Figure 8). Only mice that received OA showed a significant decrease in PaO2. Ghosh and colleagues (29) recently compared intravenous Etx (1/20th the dose used here) with intravenous OA (one-half the dose used here) in mice; again, only mice treated with OA showed hypoxemia.
The Combination of Etx with OA
In a series of previous studies (8, 9, 11, 30, 36, 37), we have used low-dose Etx in dogs to modify the physiologic and biochemical expression of OA-induced ALI. Low-dose Etx during OA-induced ALI produces severe systemic hypotension, the ablation of hypoxic vasoconstriction, and a failure for pulmonary perfusion to be distributed away from edematous lung regions, which causes profound hypoxemia. These hemodynamic effects do not occur, and the effects on gas exchange are much less severe, when either Etx or OA are administered alone (8). The synergistic hemodynamic effects of low-dose Etx with OA-induced ALI are associated with dramatic increases in prostacyclin production within the lungs, mediated primarily by cyclooxygenase 2 (9). They are not due to “priming” by Etx per se (36) nor can they be ablated by experimentally induced neutropenia or inhibition of two likely biochemical mediators (platelet-activating factor and secretory phospholipase A2 [11]).
The combination of Etx + OA in the current study produced similar effects, although with some potentially notable exceptions. For instance, only animals that received Etx + OA showed a statistically significant decrease in arterial blood pressure (Figure 8C), and the decrease in PaO2 was greatest in animals that received Etx + OA. As in dogs (8), lung tissue prostacyclin production was markedly increased compared with normal control mice (Figure 9). However, unlike our data in dogs, the increase in prostacyclin production was not greater than in animals given either agent alone. And, although animals that received Etx + OA were hypotensive relative to the other groups, the differences were small and unlike the shock state that develops in dogs.
These observations are probably important when trying to explain why mortality was so greatly affected by the addition of Etx to OA (Figure 1) or why the synergistic effects of Etx + OA could be partially attenuated by the use of an Etx antagonist (Figure 10). As in previous dog studies, we found, as discussed above, that the dose of Etx used here, when administered alone, was relatively benign. However, mortality was much greater when this same dose of Etx was used in combination with OA than when either agent was used alone. Because oxygenation was also worse in the Etx + OA group (and undoubtedly would have been even greater in the absence of mechanical ventilatory support with supplemental oxygen), we initially expected to find that prostacyclin levels would also be much higher in the Etx + OA group as well (leading then, as in dogs, to systemic hypotension, impaired hypoxic vasoconstriction, and lethal hypoxemia). Instead, we found no synergistic effect on prostacyclin production and little synergistic effect on systemic blood pressure (Figures 8 and 9).
A key, and probably crucial, difference among the groups was that severe respiratory acidosis developed only in the group of mice given Etx + OA (Table 1). In addition, pulmonary edema was more severe in this group (Figures 1 and 7), and all deaths occurred within the first 6 to 8 hours after OA (Figure 1). These observations lead us to speculate that the most likely explanation for the excess mortality associated with the combination of Etx and OA was an increase in pulmonary edema that crossed a critical threshold, leading to excessive work of breathing in the nonventilated animal. The combination of ventilatory failure with oxygenation failure produced by alveolar flooding proved highly lethal.
Figure 1 shows that the observed effects of Etx on OA-associated mortality were dose related. The bacterial source of Etx, serotype, route of administration, and strain of mouse may also each affect the manifestations of ALI due to Etx (2, 38). Likewise, varying the dose of OA (25, 29) or observation period will likely affect the expression of ALI in these models. Varying these factors may provide useful new information and insights about the pathogenesis of ALI produced by either of the two agents used in the current study.
Conclusions
In summary, mice given Etx alone showed an increase in lung tissue MPO, an increase in the rate of glucose uptake, and slight increases in lung weight (all consistent with the vascular sequestration of activated neutrophils), but no other significant physiologic, biochemical, or histopathologic abnormalities. In contrast, animals that received OA showed less overall pulmonary inflammation but had severe alveolar edema, epithelial cell damage, and arterial hypoxemia. Despite the lack of significant structural or functional abnormalities associated with Etx administration alone, the combination of Etx with OA-induced lung injury was highly lethal. The synergistic effects of Etx with OA were significantly attenuated by the prior administration of an Etx antagonist. These lung models, combined with the availability of noninvasive imaging methods, can be used to provide new insights into the pathogenesis of ALI and ARDS.
Supplementary Material
Acknowledgments
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Supported in part by National Institutes of Health grants HL32815, HL52535, and EB04732.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
References
- 1.Flick M. Mechanisms of acute lung injury: what have we learned from experimental animal models. Crit Care Clin 1986;2:455–470. [PubMed] [Google Scholar]
- 2.Opal S. The value of animal models in endotoxin research. In: Braude H, Opal S, Vogel S, Morrison D, editors. Endotoxin in health and disease. New York: Marcel Dekker; 1999. pp. 809–816.
- 3.Whitsett J, Bachurski C, Barnes K, Bunn P Jr, Case L, Cook D, Crooks D, Duncan MW, Dwyer-Nield L, Elston RC, et al. Functional genomics of lung disease. Am J Respir Cell Mol Biol 2004;31:S23–S34. [Google Scholar]
- 4.Schuster DP, Kovacs A, Garbow J, Piwnica-Worms D. Recent advances in imaging the lungs of intact small animals. Am J Respir Cell Mol Biol 2004;30:129–138. [DOI] [PubMed] [Google Scholar]
- 5.Kabir K, Gelinas J, Chen M, Chen D, Zhang D, Luo X, Yang J, Carter D, Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock 2002;17:300–303. [DOI] [PubMed] [Google Scholar]
- 6.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol 2005;288:L333–L341. [DOI] [PubMed] [Google Scholar]
- 7.Schuster DP. ARDS: clinical lessons from the oleic acid model of acute lung injury. Am J Respir Crit Care Med 1994;149:245–260. [DOI] [PubMed] [Google Scholar]
- 8.Gust R, Kozlowski J, Stephenson AH, Schuster DP. Synergistic hemodynamic effects of low-dose endotoxin and acute lung injury. Am J Respir Crit Care Med 1998;157:1919–1926. [DOI] [PubMed] [Google Scholar]
- 9.Gust R, Kozlowski JK, Stephenson AH, Schuster DP. Role of cyclooxygenase-2 in oleic acid-induced acute lung injury. Am J Respir Crit Care Med 1999;160:1165–1170. [DOI] [PubMed] [Google Scholar]
- 10.Schuster DP, Anderson C, Kozlowski J, Lange N. Regional pulmonary perfusion in patients with acute pulmonary edema. J Nucl Med 2002;43:863–870. [PubMed] [Google Scholar]
- 11.Hill LL, Chen D, Kozlowski J, Schuster D. Role of neutrophils and neutrophil products in mediating the effects of endotoxin on oleic acid induced lung injury. Anesth Analg 2003;98:452–457. [DOI] [PubMed] [Google Scholar]
- 12.Julien M, Hoeffel J, Flick M. Oleic acid lung injury in sheep. J Appl Physiol 1986;60:433–440. [DOI] [PubMed] [Google Scholar]
- 13.Hofman W, Ehrhart I. Permeability edema in dog lung depleted of blood components. J Appl Physiol 1984;57:147–153. [DOI] [PubMed] [Google Scholar]
- 14.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther 2003;304:1093–1102. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol DP, Wasan K, Choo E, Yau E, Wong N, Rose JR, Moran J, Lynn M. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distributino of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother 2004;48:3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guimaraes C, Trentin P, Rae G. Endothelin ETB receptor-mediated mechanisms involved in oleic acid-induced acute lung injury in mice. Clin Sci 2002;103:340s–344s. [DOI] [PubMed] [Google Scholar]
- 17.Fuchtner F, Steinbach J, Mading P, Johannsen B. Basic hydrolysis of 1-[18F]fluoro-1,3,4,5-tetra-O-acetyl-D-glucose in the preparation of 2-[18F]fluoro-2-deoxy-D-glucose. Appl Radiat Isot 1996;47:61–66. [Google Scholar]
- 18.Zhou Z, Kozlowski J, Goodrich A, Markman N, Chen D, Schuster D. Molecular imaging of lung glucose uptake after endotoxin in mice. Am J Physiol Lung Cell Mol Physiol (In press). [DOI] [PubMed]
- 19.Stephenson AH, Lonigro AJ, Holmberg SW, Schuster DP. Eicosanoid balance and perfusion redistribution of oleic acid-induced acute lung injury. J Appl Physiol 1992;73:2126–2134. [DOI] [PubMed] [Google Scholar]
- 20.Schuster DP, Sandiford P, Stephenson AH. Thromboxane receptor stimulation/inhibition and perfusion redistribution after acute lung injury. J Appl Physiol 1993;75:2069–2078. [DOI] [PubMed] [Google Scholar]
- 21.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed]
- 22.Schuster DP. What is acute lung injury? What is ARDS? Chest 1995;107:1721–1726. [DOI] [PubMed] [Google Scholar]
- 23.Crouch EC. Pathobiology of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 1990;3:L159–L184. [DOI] [PubMed] [Google Scholar]
- 24.Katzenstein A, Askin F. Surgical pathology of non-neoplastic lung disease. Philadelphia: W.B. Saunders; 1990. [PubMed]
- 25.Ulrich K, Stern M, Goddard ME, Williams J, Zhu J, Dewar A, Painter HA, Jeffery PK, Gill DR, Hyde SC, et al. Keratinocyte growth factor therapy in murine oleic acid-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005;288:L1179–L1192. [DOI] [PubMed] [Google Scholar]
- 26.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L231–L246. [DOI] [PubMed] [Google Scholar]
- 27.Wiener-Kronish J, Albertine K, Matthay M. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 1991;12:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baboolal H, Ichinoise F, Ullrich R, Kawai N, Bloch K, Zapol W. Reactive oxygen species scavengers attenuate endotoxin-induced impairment of hypoxic pulmonary vasoconstriction in mice. Anesthesiology 2002;97:1227–1233. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Wilson MR, Choudhury S, Yamamoto H, Goddard ME, Falusi B, Marczin N, Takata M. Effects of inhaled carbon monoxide on acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2005;288:L1003–L1009. [DOI] [PubMed] [Google Scholar]
- 30.Chen DL, Schuster DP. Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L834–L840. [DOI] [PubMed] [Google Scholar]
- 31.Jones H, Clark R, Rhodes C, Schofield J, Krausz T, Haslett C. In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med 1994;149:1635–1639. [DOI] [PubMed] [Google Scholar]
- 32.Jones H, Sriskandan S, Peters A, Pride N, Krausz T. Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J 1997;10:795–803. [PubMed] [Google Scholar]
- 33.Jones H, Schofield J, Krausz T, Boobis A, Haslett C. Pulmonary fibrosis correlates with duration of tissue neutrophil activation. Am J Respir Crit Care Med 1998;158:620–628. [DOI] [PubMed] [Google Scholar]
- 34.Wiekowski M, Chen S, Zalamea P, Wilburn B, Kinsley D, Sharif W, Jensen K, Hedrick J, Manfra D, Lira S. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol 2001;167:7102–7110. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Park P, Wilson C, Parks W. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646. [DOI] [PubMed] [Google Scholar]
- 36.Schuster DP, Kozlowski JK, McCarthy T, Morrow J, Stephenson A. Effect of endotoxin on oleic acid lung injury does not depend on priming. J Appl Physiol 2001;91:2047–2054. [DOI] [PubMed] [Google Scholar]
- 37.Brimioulle S, Julien V, Gust R, Kozlowski JK, Naeije R, Schuster DP. Importance of hypoxic vasoconstriction in maintaining oxygenation during acute lung injury. Crit Care Med 2002;30:874–880. [DOI] [PubMed] [Google Scholar]
- 38.Stewart D, Fulton W, Wilson C, Monitto C, Paidas C, Reeves R, De Maio A. Genetic contribution to the septic response in a mouse model. Shock 2002;18:342–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.