Abstract
Background: Children with mild sleep-disordered breathing (SDB), who may not be recommended for adenotonsillectomy, frequently exhibit neurocognitive and behavioral morbidity, and may benefit from alternative therapeutic interventions, such as leukotriene modifier therapy. Methods: Twenty-four children with SDB completed an open-label intervention study for 16 weeks with daily montelukast therapy. Sleep studies and adenoid size estimates from lateral X-ray films of the neck were obtained before and after treatment. In a parallel study, adenoid and tonsillar tissues from children with obstructive sleep apnea or recurrent throat infections were subjected to quantitative polymerase chain reaction, immunohistochemistry, and Western blotting for gene and protein expression of leukotriene receptors LT1-R and LT2-R, and for concentrations of LTB4 and LTC4/D4/E4. Results: Montelukast treatment induced significant reductions in adenoid size and respiratory-related sleep disturbances, which were absent in 16 children with SDB who did not receive treatment. LT1-R and LT2-R mRNA was similarly abundant in adenoid tissues, but increased LT1-R and LT2-R protein expression and higher levels of LTB4 and LTC4/D4/E4 emerged in children with obstructive sleep apnea. Conclusions: Oral therapy with a leukotriene modifier appears to be associated with improved breathing during sleep. Double-blind, placebo-controlled trials will be needed to corroborate current findings and solidly establish antiinflammatory strategies, such as leukotriene modifiers, as therapeutic alternatives in children with SDB too mild to justify referral for adenotonsillectomy.
Keywords: leukotriene receptors, lymphoid hyperplasia, sleep apnea, adenotonsillectomy, tonsils
Obstructive sleep apnea (SA) is a common and highly prevalent disorder in the pediatric age range, affecting 2 to 3% of all children (1). This disorder is usually due, at least in part, to adenotonsillar hypertrophy (2, 3). If left untreated, SA can result in serious morbidity, primarily affecting neurobehavioral and cardiovascular systems (4–11). Thus, adenotonsillectomy (T&A) is currently the most common treatment for children with SA (12). However, although definitive polysomnographic criteria are not available, T&A is usually reserved for children whose respiratory disturbance (apnea–hypopnea index [AHI]) during sleep exceeds 5 events/hour of sleep. Although most clinicians agree that snoring children with an AHI of less than 1 event/hour of sleep do not require any intervention, there is presently no consensus on the appropriate management of children with an AHI of more than 1 but less than 5 events/hour of sleep, even if such children are at risk for associated morbidity (13).
Nonsurgical antiinflammatory approaches have been cautiously advocated for SA in children (14, 15). Indeed, nasal and oropharyngeal mucosal inflammation are present in adult patients with obstructive sleep apnea syndrome (16–19), and C-reactive protein, a systemic marker for inflammation, was recently reported to be increased in the serum of children with SA, and to correlate with the severity of their respiratory disturbance during sleep (20). Thus, systemic antiinflammatory agents with safe therapeutic profiles for use in children with sleep-disordered breathing (SDB) could serve as an alternative intervention to T&A.
Montelukast is an orally bioavailable cysteinyl leukotriene (LT) receptor antagonist that is effective, safe, well tolerated, and approved by the U.S. Food and Drug Administration for preventive therapy for the inflammatory component in asthma and allergic rhinitis in children 2 years and older (21–24), with no demonstrable development of tolerance in long-term studies (25, 26). We have recently found that the cloned human cysteinyl LT receptors 1 and 2 (LT1-R, LT2-R) (27, 28) have increased expression in the tonsillar tissues of children with SA (29). On the basis of such findings, we compared the relative abundance of LTs and their receptors in the lymphoid tissue of children with or without SDB, and furthermore investigated the effects of a 16-week course of montelukast on sleep and airway patency in children with mild SDB.
METHODS
Patients
The study was approved by the University of Louisville Human Research Committee, and informed consent was obtained from the legal caretaker of each participant. Assent was also obtained from children if they were older than 6 years.
Open-label treatment with montelukast.
Twenty-four consecutive patients evaluated for SDB in the Kosair Children's Hospital Sleep Medicine and Apnea Center who fulfilled inclusion criteria were recruited to the study, and these patients completed a 16-week treatment with daily montelukast. As control subjects, 16 additional children fulfilling the same inclusion criteria, and who were not offered this therapeutic modality while receiving care from other attending physicians at the sleep center, were identified and recruited to the study.
Criteria for inclusion included the following: children older than 2 and younger than 10 years who were habitual snorers (reported to snore by parents > 4 nights/week), who were found to have an obstructive AHI of more than 1 but less than 5 events/hour of sleep in an overnight polysomnographic evaluation, and in whom a lateral neck X-ray film was obtained as part of their clinical evaluation. Exclusion criteria included the following: craniofacial, neuromuscular, syndromic, or defined genetic abnormalities; current or previous use of montelukast; acute upper respiratory tract infection; use of any corticosteroids or antibiotics in the 4 weeks preceding the initial sleep study; and T&A in the past.
Oral montelukast therapy consisted of the daily administration of a 4-mg chewable tablet (Singulair; Merck, Whitehouse Station, NJ) for children younger than 6 years, and a 5-mg tablet for children 6 years and older. Parents were instructed to give the tablet at bedtime. Parents were contacted weekly by the investigators to determine compliance, and to follow-up on potential side effects. On completion of the 16-week course, patients underwent a second overnight sleep study and lateral neck X-ray.
Lateral Neck X-Rays
For assessment of airway patency, lateral neck X-rays were performed using standard techniques in the radiology department of the hospital. The neck was extended and the patient was instructed to breathe through the nose. The adenoidal/nasopharyngeal ratio was measured according to the method of Fujioka and colleagues (30) by two of the present investigators (A.D.G. and D.G.), who were blinded to the polygraphic findings of the subjects.
Overnight Polysomnography
All children participating in the study were studied twice using polysomnography, once before inclusion in the study and at the end of the 16-week intervention period. Sleep studies were performed in a dedicated quiet, dark room. No sleep deprivation or sedation was used. Children were studied for at least 8 hours in a quiet, darkened room with an ambient temperature of 24°C in the company of one of their parents. The following parameters were measured: chest and abdominal wall movement by respiratory impedance or inductance plethysmography and heart rate by ECG. Airflow was monitored with a side-stream end-tidal capnograph, which also provides breath-by-breath assessment of end-tidal carbon dioxide levels (Pryon, Menomonee Falls, WI), as well as a nasal pressure transducer (Braebon, Carp, ON, Canada) and an oronasal thermistor. SaO2 was assessed by pulse oximetry (Nellcor N100; Nellcor, Inc., Hayward, CA), with simultaneous recording of the pulse waveform. The bilateral electro-oculogram, eight channels of EEG, chin and bilateral anterior tibial and forearm EMG, and analog output from a body position sensor were also monitored. All measures were digitized using a commercially available polysomnographic system (Rembrandt; MedCare Diagnostics, Amsterdam, The Netherlands). Tracheal sounds were monitored with a microphone sensor, and a digital time-synchronized video recording was performed.
Analysis of the polysomnogram was performed using standard techniques by an experienced sleep technologist who was not aware that the sleep studies belonged to study participants. In brief, sleep staging was assessed using standard criteria (31). The obstructive AHI was defined as the number of apneas and hypopneas per hour of total sleep time, and obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths (32, 33). Hypopneas were defined as a decrease in nasal flow of 50% or more with a corresponding decrease in SpO2 of 4% or more and/or arousal (32). The mean SpO2 together with SpO2 nadir were determined. Arousals were defined as recommended by the American Sleep Disorders Association Task Force report (34) and include respiratory-related (occurring immediately after an apnea, hypopnea, or snore), technician-induced, and spontaneous arousals. Arousals were expressed as the total number of arousals per hour of sleep time (ARtotI). In addition, as a surrogate measure for sleepiness, the recently developed sleep pressure score (35) was calculated for each subject's polysomnographic record as follows: sleep pressure score = RAI/ARtotI × (1 − SAI/ARtotI), where RAI represents the respiratory-related arousal index and SAI the spontaneous arousal index. A sleep pressure score of 0.25 or more was used as the threshold for evidence of increased sleepiness (35, 36).
Adenotonsillar Tissue Collection
Because adenotonsillar tissue cannot be obtained from normal children for obvious ethical reasons, a cohort different from the one described above and consisting of consecutive children undergoing T&A for either SA or recurrent infectious tonsillitis (RI) were identified before surgery and recruited to the study. The diagnosis of obstructive sleep apnea syndrome was established by standard overnight polysomnography in the sleep laboratory, and required the presence of an AHI of more than 5 events/hour of sleep (12). Patients referred for RI were selected on the basis of a history of at least five tonsillar infections in less than 6 months; because the absence of any symptoms suggestive of SA essentially negates the presence of this condition (37), the patients were not evaluated by an overnight polysomnogram, since our questionnaire-based evaluation is highly sensitive and specific in ruling out SDB in children (37). For inclusion, children with RI were required to have received their last dose of antibiotic therapy 6 weeks or longer from the day of surgery. Children with known asthma, allergic rhinitis, or history of allergies, and/or those having received corticosteroid or LT modifier therapy within 1 year from surgery were excluded. Both palatine tonsils and adenoids were removed by a pediatric ear, nose, and throat specialist; a portion of each tonsil was snap-frozen in liquid nitrogen and stored at −80°C. Another portion of each tonsil was fixed in 4% formalin, cryoprotected with 30% sucrose, and kept at 4°C. Adenoids were randomly assigned to be kept either in formalin or at −80°C. All samples were numbered and coded by one of the research coordinators and were subsequently assayed by one of the authors (A.D.G.), who was blinded to the specific diagnosis of each of the samples.
Quantitative (Real-Time) Polymerase Chain Reaction
Total RNA was prepared from adenoid or tonsillar tissues using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Isolated total RNA was quantified using a spectrophotometer (Beckman DU-530, Fullerton, CA). Aliquots of total RNA (1 μg) were reverse-transcribed using random primers and Superscript II-Reverse Transcriptase (Invitrogen) according to the manufacturer's protocol. cDNA equivalent to 20 ng of total RNA was subjected to real-time polymerase chain reaction analysis (MX4000; Stratagene, La Jolla, CA) following the manufacturer's protocol. Polymerase chain reaction primers (Invitrogen) and Taqman probes (Biosearch Technologies, Novato, CA) for LTR-1, LTR-2 and β-actin were designed by Beacon Designer 2.0 software (Premier Biosoft International, Palo Alto, CA). The primer and probe for LTR-1 were as follows: forward primer, 5′-TTATGTTCACAAAGGCATTTGG-3′; reverse primer, 5′- GCTCATGGCTGTCTAAAGAA-3′; Taqman probe, 5′-FAM-GGTGACTTCTTGTGCCGCCTC-BHQ-1-3′. The primer and probe for LTR-2 were as follows: forward primer, 5′-ACTATATTGCCTTGGTGGTGGG-3′; reverse primer, 5′-ATGATGGTGGTCAGTGCCTTC-3′; Taqman probe, 5′-(FAM)-TGTGAGAAACCCGCAGCCCCGA-(BHQ-1)-3′; and for β-actin: forward primer, 5′-GACTACCTCATGAAGATCCTCACC-3′; reverse primer, 5′-TCTCCTTAATGTCAC GCACGATT-3′; Taqman probe, 5′-FAM-CGGCTACAGCTTCACCACCACGG-BHQ-1-3′. Each reaction (25 μl) contained 2.5 μl reaction buffer (10×), 6 mM MgCl2, 0.2 μM dNTP, 0.6 μM each primer, 0.25 μl SureStar Taq DNA polymerase (Cerestar, Cedar Rapids, IA), and 2 μl cDNA dilutions. The cycling conditions consisted of one cycle at 95°C for 10 minutes and 40 two-segment cycles (95°C for 30 seconds and 55°C for 60 seconds). Standard curves for target gene (LTR-1 or LTR-2) and the housekeeping gene (β-actin) were performed for each assay. Briefly, 10-fold serial dilutions of control cDNA were amplified by the MX-4000 polymerase chain reaction machine (Stratagene). The CT value (initial amplification cycle) of each standard dilution was plotted against standard cDNA copy numbers. On the basis of the standard curves for each gene, the sample cDNA copy number was calculated according to the sample CT value. Finally, each of the calculated copy numbers for either LTR-1 or LTR-2 was normalized against the corresponding β-actin copy numbers, and are therefore expressed as ratios of the gene of interest and corresponding β-actin value. Standard curves and polymerase chain reaction results were analyzed using MX-4000 software (Stratagene).
Immunohistochemistry
Coronal sections (30 μm) of both tonsil and adenoid tissues were initially incubated in 0.3% H2O2 for 30 minutes, washed several times in phosphate-buffered saline (PBS), and blocked with a PBS/0.4% Triton X-100/0.5% Tyramide Signal Amplification (Perkin Elmer Life Sciences, Boston, MA) blocking reagent/10% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 hour. Sections were then serially incubated with LTR-1 antibody (1:1500; Cayman Chemical, Ann Arbor, MI) at 4°C for 24 hours, and then washed in PBS six times for 5 minutes each wash. Sections were then incubated at room temperature for 1 hour in biotinylated antirabbit antibody (1:600; Vectastain Elite ABC kit; Vector Laboratories) in a PBS/0.5% Tyramide Signal Amplification blocking reagent /10% goat serum solution. After three 5-minute washes, sections were incubated at room temperature with streptavidin–horseradish peroxidase diluted 1:100 in PBS/0.5% Tyramide Signal Amplification blocking reagent. Subsequently, the sections were incubated with tetramethyl rhodamine tyramide (red) diluted 1:50 in amplification diluent (Perkin Elmer Life Sciences) for 2 minutes. Sections were processed the same way again and subsequently incubated with serum raised against myeloperoxidase (1:1000; Labvision, Fremont, CA) at 4°C for 24 hours, followed by a biotinylated antirabbit antibody (1:600; Vectastain Elite ABC kit; Vector Laboratories), and by fluorescein tyramide reagent (green) diluted 1:50 for 3 minutes. Sections were then washed in PBS, and mounted onto glass slides. Negative controls were prepared by either omitting the primary or the secondary antibodies for either of the primary antibodies. Sections were prepared from seven sets of tonsils and of adenoids from either SA or RI groups, and were visualized using a fluorescent microscope by an investigator who was blinded to the sample source.
Western Blotting
Tonsils and adenoids were homogenized in a lysis buffer (50 mM Tris, pH 7.5, 0.4% NP-40, 10% glycerol, 150 mM NaCl, 10 mg/ml aprotinin, 20 mg/ml leupeptin, 10 mM ethylenediaminetetraacetic acid, 1 mM sodium orthovanadate, 100 mM sodium fluoride), and the protein concentration was determined using the Bradford method (Bio-Rad DC, Hercules, CA). Samples (40 μg protein) were resolved on 12% sodium dodecyl sulfate-polyacrylamide gels using electrophoresis (Novex/Invitrogen, Carlsbad, CA) for 120 minutes at 120 V, and electroblotted onto 0.2-μm nitrocellulose membranes for 90 minutes at 30 V. Membranes were blocked with 5% nonfat dry milk in total buffered saline-T (total buffered saline + 0.05% Tween 20), and were then incubated overnight at 4°C with primary antibodies recognizing the human LT1-R (1:500; Cayman Chemical), or the LT2-R (1:500; Cayman Chemical), and later with anti–β-actin (1:20,000; Sigma, St. Louis, MO), both diluted in 5% milk. Lanes were also incubated with a mixture of the primary antibody and the receptor-blocking peptide (1:2 ratio) to establish a competition assay. Membranes were washed with TBS-T, and incubated with either horseradish peroxidase–linked antirabbit or antimouse antibodies (for LT receptors and β-actin, respectively). Proteins were visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ). The intensities of the bands corresponding to the protein of interest were quantified using scanning densitometry, expressed as the ratio of the density corresponding to the protein of interest and corresponding β-actin, and compared using t tests or analysis of variance as appropriate.
LT Concentrations
All adenoid and tonsillar tissue specimens (n = 20 for SA group, n = 9 for RI group) were processed as described by Bachert and colleagues (38). In brief, tissues were weighed, and 1 ml of 0.9% NaCl solution was added per 0.1 g of tissue. Tissues were then homogenized with a mechanical homogenizer at 5,000 rpm for 5 minutes (Tissue-Tearor; BioSpec Products, Inc., Bartlesville, OK). After homogenization, suspensions were centrifuged at 3,000 rpm for 10 minutes at 4°C, and supernatants were separated and stored at −80°C. All supernatants were assayed for LTs LTC4/D4/E4 (cysteinyl LTs) and LTB4 by means of a specific immunoassay (enzyme-linked immunoassay) kit with commercially available kits (LTC4/D4/E4: Cayman Chemical; LTB4: Amersham Biosciences). All samples were loaded in duplicates and assayed at two dilutions, and plate reader absorbance results were analyzed with a four-parameter logistic curve fit. The intraassay and interassay variability for LTB4 and LTC4/D4/E4 assays was less than 10%. The specificity of LTB4 and LTC4/D4/E4 assays was 100% (except for LTB4, which was 67%). The detection limit of the assays was 6.2 pg/ml for LTB4 and 7.8 pg/ml for LTC4/D4/E4.
Statistical Analysis
Results are presented as means ± SD, unless stated otherwise. All numeric data were subjected to statistical analyses using either t tests or analysis of variance procedures followed by Newman-Keuls post hoc tests, as appropriate. A two-tailed p value of less than 0.05 was considered statistically significant.
RESULTS
Montelukast Treatment
A total of 13 boys and 11 girls completed the montelukast treatment arm of the study. Their mean age was 5.4 ± 2.0 years (range, 2.5–10 years); their body mass index was 19.5 ± 0.9 kg/m2; and 62.5% were white and 37.5% African American. The adenoidal/nasopharyngeal ratio (Figure 1) significantly decreased from 0.76 ± 0.03 to 0.56 ± 0.03 (p < 0.001) after 16 weeks of montelukast treatment. For sleep-related variables, the obstructive AHI and apnea index significantly decreased with treatment (Table 1). Although ARtotI did not decrease significantly (16.87 ± 1.52 to 14.4 ± 1.26, p = not significant), RAI was significantly improved (7.15 ± 0.79 to 3.01 ± 0.33, p < 0.001; Table 1), as was the sleep pressure score (0.25 ± 0.02 to 0.12 ± 0.02, p = 0.0016; Table 1). No significant changes occurred in total sleep time, mean sleep latency, sleep efficiency, number of awakenings, or in the mean and nadir saturation values recorded during sleep. However, improvements in peak end-tidal carbon dioxide levels were observed after montelukast (Table 1). No significant changes emerged in the distribution of sleep stages except for a decrease in the percentage of time spent in stage 1 non-REM sleep. There were no adverse drug reactions reported throughout the study, with excellent compliance and no attrition. In contrast, with such findings, children who received no therapy displayed no significant changes in any of the anatomic and polysomnographic measures during the 16-week period (Table 1).
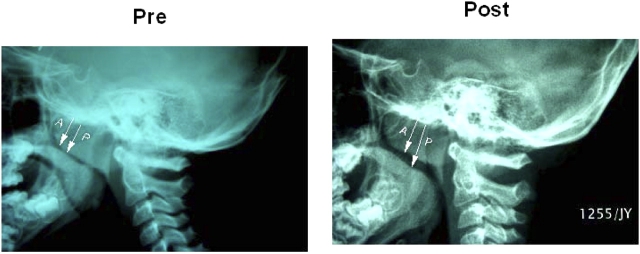
Figure 1.
Lateral neck soft X-ray in a 6-year-old patient with mild sleep-disordered breathing before (Pre) and after (Post) 16-week course of montelukast. Increased upper airway diameter and recession of adenoid tissue are apparent in the post-treatment radiograph. Arrows: A, adenoid; P, pharynx.
TABLE 1.
Demographic and polysomnographic characteristics in 40 children with mild sleep-disordered breathing who were either treated with montelukast or received no therapy at diagnosis and at follow-up
| Montelukast (n = 24)
|
No Treatment (n = 16)
|
|||||
|---|---|---|---|---|---|---|
| Pre | Post | p Value | Pre | Post | p Value | |
| Age, yr | 5.39 ± 2.0 | NS | 5.7 ± 1.8 | NS | ||
| Sex, M/F | 11/ 13 | 7/9 | ||||
| Race, W/AA | 15 W/ 9 AA | 11 W/5 AA | ||||
| BMI, kg/m2 | 19.6 ± 0.9 | 19.7 ± 0.9 | NS | 19.8 ± 0.8 | 19.8 ± 0.9 | NS |
| A/N ratio* | 0.76 ± 0.03 | 0.56 ± 0.03 | < 0.001 | 0.78 ± 0.04 | 0.79 ± 0.04 | NS |
| Arousal index, total/hr TST* | 16.9 ± 1.5 | 14.4 ± 1.3 | NS | 15.7 ± 2.0 | 18.6 ± 2.0 | < 0.02 |
| Arousal index, resp. hr/TST* | 7.2 ± 0.8 | 3.0 ± 0.3 | < 0.001 | 9.4 ± 0.7 | 12.8 ± 1.3 | < 0.03 |
| Sleep pressure score* | 0.25 ± 0.02 | 0.12 ± 0.02 | 0.0016 | 0.26 ± 0.03 | 0.32 ± 0.04 | < 0.01 |
| Apnea index, hr TST* | 1.2 ± 0.2 | 0.8 ± 0.1 | 0.036 | 1.2 ± 0.2 | 2.0 ± 0.2 | < 0.04 |
| Obstructive AHI, hr TST* | 3.0 ± 0.22 | 2.0 ± 0.3 | 0.017 | 3.2 ± 0.2 | 4.1 ± 0.4 | < 0.03 |
| TST, hr | 8.33 ± 0.28 | 8.46 ± 0.16 | NS | 8.28 ± 0.24 | 8.37 ± 0.23 | NS |
| Mean sleep latency, min | 13.7 ± 1.6 | 18.8 ± 2.5 | NS | 14.8 ± 1.8 | 15.1 ± 1.9 | NS |
| Sleep efficiency, % | 87.3 ± 6.5 | 89.0 ± 4.9 | NS | 88.0 ± 5.9 | 89.6 ± 5.6 | NS |
| Minimal SaO2 | 90.3 ± 3.1 | 90.5 ± 3.0 | NS | 90.4 ± 2.9 | 89.0 ± 4.9 | NS |
| Mean saturation | 96.4 ± 1.7 | 96.1 ± 1.9 | NS | 96.2 ± 2.1 | 95.7 ± 2.7 | NS |
| Mean PETCO2 | 45.2 ± 0.7 | 44.1 ± 0.6 | NS | 45.8 ± 0.8 | 46.1 ± 1.0 | NS |
| Peak PETCO2 | 58.1 ± 1.4 | 53.4 ± 1.1 | < 0.002 | 57.4 ± 1.3 | 59.0 ± 1.5 | NS |
| Awakenings | 7.8 ± 5.4 | 7.3 ± 4.2 | NS | 7.7 ± 4.4 | 8.8 ± 4.9 | NS |
| Wake, %TST | 4.1 ± 1.0 | 3.5 ± 1.0 | NS | 4.2 ± 0.9 | 4.1 ± 1.2 | NS |
| Stage 1, %TST | 12.1 ± 1.1 | 8.0 ± 1.0 | 0.008 | 12.5 ± 1.4 | 13.7 ± 1.4 | NS |
| Stage 2, %TST | 41.1 ± 2.0 | 41.3 ± 1.8 | NS | 43.3 ± 2.8 | 45.3 ± 2.5 | NS |
| Stage 3 + 4, %TST | 24.0 ± 1.7 | 24.8 ± 1.2 | NS | 22.1 ± 2.1 | 19.1 ± 2.7 | NS |
| Stage REM, %TST* | 19.0 ± 1.0 | 20.5 ± 1.0 | NS | 20.0 ± 1.3 | 17.5 ± 14 | < 0.04 |
Definition of abbreviations: A/N = adenoidal/nasopharyngeal; AHI = apnea–hypopnea index; BMI = body mass index; M/F = male/female; NS = not significant for montelukast versus no-treatment groups; PETCO2 = end-tidal CO2 pressure; resp = respiratory; TST = total sleep time; W/AA = white/African American.
Montelukast versus no treatment, p < 0.01.
Adenotonsillar Tissue Assessments
Adenoid and tonsillar tissues were obtained from 38 children (20 SA and 18 RI). The mean age for this cohort was 5.2 ± 2.8 years (range, 2–10 years; 21 males; 67% white/33% African American). LT1-R and LT2-R mRNA was present in all 16 adenoids studied. However, no significant differences in gene expression emerged between the SA and the RI groups for either LT1-R (n = 8/group; 0.41 ± 0.13 in SA vs. 0.31 ± 0.07 in RI, p = not significant) or LT2-R (n = 8/group; 0.008 ± 0.003 in SA vs. 0.017 ± 0.013 in RI, p = not significant).
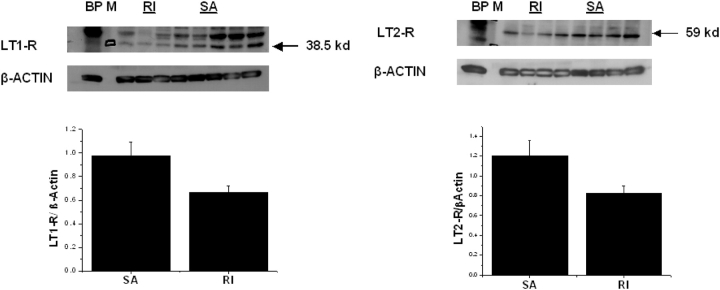
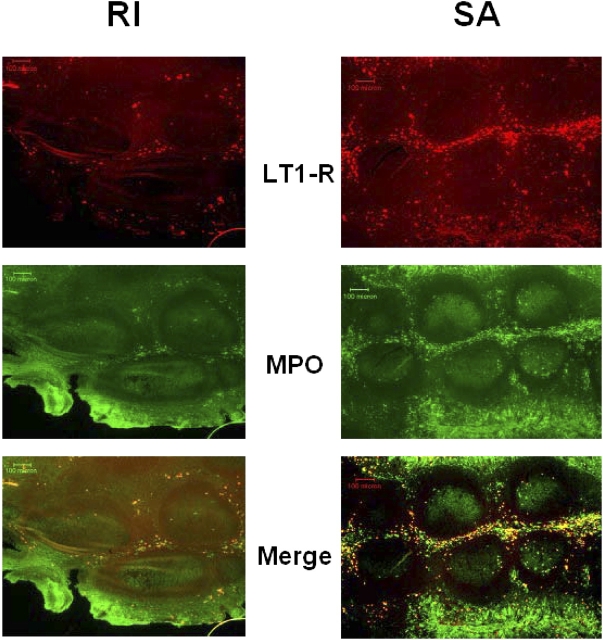
LT1-R immunoreactivity was abundantly expressed in the adenoidal and tonsillar epithelial layers and within the extra follicular area in the tonsillar parenchyma. Clusters of LT1-R positively labeled cells were also present within blood vessels. No staining for LT1-R was detected in the tonsillar germinal centers (Figure 2). In contrast, only a restricted number of cells primarily constrained to the epithelial layer expressed the LT1-R in the tonsils obtained from patients with RI (Figure 2). Colabeling with a myeloperoxidase antibody (for identification of neutrophils and eosinophils) was primarily observed within LT1-R–expressing cells in the patients with SA (Figure 2). Immunoblots of adenoidal lysates for LT1-R detected a protein with the appropriate molecular weight of approximately 38 kD (Figure 3), which was further confirmed by a competition assay with the immunogenic peptide. LT1-R expression in both adenoids and tonsils was significantly higher in the SA group compared with the RI group (n = 8; 0.97 ± 0.11 in SA vs. 0.66 ± 0.05 in RI, p < 0.05; Figure 3). The LT2-R antibody detected a protein with a predicted molecular weight of approximately 59 kD (also confirmed with blocking peptide), and as with LT1-R, LT2-R expression was significantly higher in both adenoids and tonsils of patients with SA compared with RI group (n = 8; 1.20 ± 0.15 in SA vs. 0.82 ± 0.06 in RI, p < 0.05; Figure 3). Furthermore, the relative abundance of LT1-R and LT2-R was higher in adenoid tissues compared with tonsillar tissues in patients with SA (n = 8/group, p = 0.02).
Figure 2.
Left upper panel: Representative immunoblots of leukotriene 1 receptor (LT1-R; detected at 38.5 kD) and β-actin in adenoids and tonsils from patients with obstructive sleep apnea (SA) and recurrent tonsillitis (RI). BP indicates blocking peptide for competition assay and M indicates molecular marker. Left lower panel: Mean LT1-R/β-actin demonstrate significantly higher LT1-R expression in adenoid tissues of patients with SA (n = 8) compared with RI (n = 8; p = 0.04). Right upper panel: Representative immunoblots of LT2-R (detected at 59 kD) and β-actin in adenoids and tonsils from patients with SA and RI. Right lower panel: Mean LT2-R/β-actin demonstrate significantly higher LT2-R expression in adenoid tissues of patients with SA (n = 8) compared with RI (n = 8; p = 0.02).
Figure 3.
Double-label immunohistochemistry for LT1-R and myeloperoxidase (MPO) in tonsils obtained from patients with SA and RI reveals the enhanced immunoreactivity of LT1-R in SA. In addition, a higher abundance of LT1-R/MPO coexpression is apparent in the patient with SA. Similar findings were detected in five sets of tonsils from patients with SA and RI.
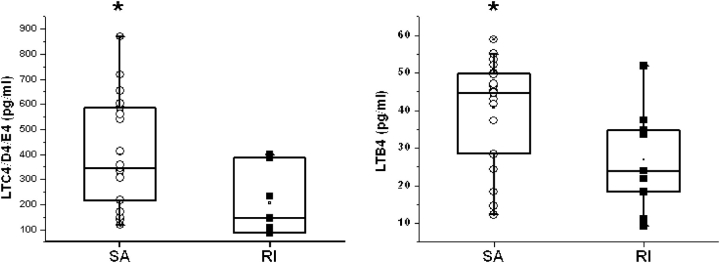
LT concentration assays revealed higher levels in the SA group for both LTB4 and LTC4/D4/E4, in comparison to children with RI. Indeed, patients with SA (n = 19) had higher LTC4/D4/E4 concentrations (398.3 ± 50.5 pg/ml in SA vs. 206.1 ± 51.7 pg/ml in RI, p = 0.03; Figure 4, left panel), and also higher LTB4 concentrations (815.7 ± 61.8 pg/ml in SA vs. 538.2 ± 91.5 pg/ml in RI, p < 0.02; Figure 4, right panel).
Figure 4.
Left panel: Individual and mean LTC4/D4/E4 concentrations (pg/ml) in adenotonsillar tissues obtained from children with SA (n = 19) were significantly higher compared with upper airway lymphoid tissues from patients with RI (n = 7; *p < 0.03). Right panel: Individual and mean LTB4 concentrations (pg/ml) in adenotonsillar tissues obtained from children with SA (n = 20) were significantly higher compared with those found in RI (n = 9; p < 0.02).
DISCUSSION
This study shows that a 16-week course of an orally administered LT receptor antagonist is associated with significant improvements in upper airway patency and in the severity of SDB, and that these improvements fail to occur when no treatment is administered. Furthermore, in a different set of children, we show that enhanced expression of LT receptors and elevated concentrations of LTs are present in the upper airway lymphoid tissues of pediatric patients with SDB compared with those with recurrent tonsillitis, and propose that such differences could underlie the favorable response to LT modifier therapy.
The conceptual premises under which therapy for SDB in children is based are not yet well defined. Indeed, although it has become increasingly apparent that SDB is associated with substantial morbidities, particularly affecting cognitive, behavioral, and cardiovascular functions (5, 7, 8, 11, 13), the thresholds of disease severity that delineate the cost–benefit ratios for T&A, the primary line of treatment for pediatric SDB, are currently unknown. Thus, the recently published empirically based consensus guidelines that were developed for the treatment of SDB in children (12) do not mention any specific polysomnographic measures as firmly established criteria for T&A referral. As such, different pediatric sleep centers have adopted quite disparate obstructive AHI cut-off values for surgical treatment. Notwithstanding such uncertainties, when an AHI of greater than 5 is present in snoring symptomatic children, this AHI value is consistently considered as a condition requiring T&A. Similarly, when an AHI of less than 1 is found during overnight sleep studies, patients are uniformly considered as having primary snoring and will not be viewed as candidates for any specific treatment. However, substantial debate exists when an AHI of more than 1 but less than 5 is present in symptomatic children referred for evaluation of snoring (39). In this clinical setting, the mortality and morbidity of T&A need to be weighed against SDB-associated consequences, and the lack of evidence-based analyses makes this task particularly arduous. Therefore, although some professionals will opt for the surgical approach, others will withhold any intervention. Such considerations have led to the search for nonsurgical therapeutic alternatives. Systemic steroids were initially explored, but failed to yield any substantial benefits (40). In contrast, topical intranasal application of high-potency corticosteroids revealed significant improvements in AHI and oxygenation in a cohort of children with SDB (AHI > 5) (14). Unfortunately, this latter study did not examine the role of topical steroids in children with mild SDB (1 < AHI < 5), even if the expression pattern of glucocorticoid receptors suggests a favorable therapeutic profile for adenotonsillar tissue in children with SDB (41).
The current study opens a new therapeutic modality for symptomatic pediatric patients with mild SDB. Indeed, we have now confirmed and further expanded on the enhanced expression levels of LT receptors in the upper lymphoid tissues of patients with SA (29). Furthermore, we now show that LT concentrations in such tissues are also increased, indicating that an active inflammatory process is present in the upper airway of these children, and that the coordinated increase in LT production and receptor expression may underlie signaling pathways leading to proliferation and hyperplasia of the lymphoid tissue in these children. Thus, if these biological processes are indeed pathophysiologically relevant to the increased size of adenotonsillar tissue, treatment with LT receptor blockers should abrogate the proliferative signals, and thereby lead to progressive reductions in overall lymphoid tissue volume within the upper airway, thus ameliorating the respiratory disturbances during sleep (42).
Montelukast, a selective LT1-R blocker, is now a widely used and safe pharmacologic option in the treatment of asthma and allergic rhinitis in children. The recent cloning of the human genes for LT1-R (27) and LT2-R (28), and the subsequent generation of antibodies to their cognate proteins, enabled us to explore the differential expression patterns of these receptors in upper airway lymphoid tissues (29). We found that the expression of LT receptors is higher in patients with SDB. Furthermore, we describe how LT1-R is primarily expressed in myeloperoxidase-positive cells within upper airway lymphoid tissues in children with SDB. The rather selective and peripheral location of LT1-R in relation to germinal centers within the tonsils suggests that either LT1-R expression occurred during late stages of maturation of lymphoid tissue or, as proposed by others, LT1-R–positive cells may have migrated from the vasculature to occupy their sites within the tonsils (43). Notwithstanding such considerations, the overarching concept emanating from this study supports the existence of a chronic inflammatory process in children with SDB. Indeed, C-reactive protein serum levels, an important systemic marker for inflammation, may be elevated in both children and adults with SA (20, 44, 45), even though this finding may not be consistently observed (46). Such elevations of C-reactive protein suggest that systemic processes may either initiate or maintain the localized inflammatory process and associated proliferative signaling within upper airway lymphoid tissues. In addition, inflammatory changes in the upper airway mucosa elicited by the recurrent vibratory mechanical stress of snoring could also contribute to the upregulation of LT receptor expression, and to the accelerated growth of the adenotonsillar tissues. Some corroboration to the slow, albeit progressive nature of these processes is exemplified by the relatively small, albeit significant increases in respiratory disturbance during sleep in the cohort of historical control children who received no treatment. In contrast, we observed statistically significant improvements in airway patency, as documented by the substantial changes in the adenoidal/nasopharyngeal ratio and by the parallel small but significant reductions in respiratory-related sleep disturbances, after a 16-week course of oral montelukast therapy. It is likely that, based on the LT2-R expression patterns found herein and the modifying role that LT2-R has on LT1-R function (47), implementation of combined therapy using both LT1-R and LT2-R blockers may be even more effective in reducing the size of lymphoid tissue in children with SDB. Although it is evident that randomized double-blind, placebo-controlled trials are needed to confirm our current findings, and further define the optimal duration of therapy and improved delineation of the patient population most likely to benefit from LT1-R antagonist therapy, the present study clearly establishes the beneficial role of montelukast therapy as a nonsurgical alternative for symptomatic children with mild SDB.
In summary, we have delineated the expression of LT receptor and LT levels in the upper airway lymphoid tissue of children with SDB. Although double-blind, placebo-controlled trials are clearly needed to corroborate our findings, the use of LT receptor antagonists emerges as a potential therapeutic consideration in children with mild SDB.
Acknowledgments
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The authors thank Kenneth R. Brittian for technical assistance in the immunohistochemical stainings.
Supported by grants from the National Institutes of Health HL62570, HL63912, HL69932, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund (D.G.).
References
- 1.Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, Agorogiannis E, Christodoulou S, Pantazidou A, Gourgoulianis K, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004;37:499–509. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2003;167:65–70. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CL. Racial differences in the diagnosis of childhood obstructive sleep apnea (OSA). Am J Respir Crit Care Med 1998;157:A535. [Google Scholar]
- 4.Frank Y, Kravath RE, Pollak CP, Weitzman ED. Obstructive sleep apnea and its therapy: clinical and polysomnographic manifestations. Pediatrics 1983;71:737–742. [PubMed] [Google Scholar]
- 5.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol 1988;4:139–143. [DOI] [PubMed] [Google Scholar]
- 6.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr 1982;100:31–40. [DOI] [PubMed] [Google Scholar]
- 7.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med 1998;157:1098–1103. [DOI] [PubMed] [Google Scholar]
- 8.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616–620. [DOI] [PubMed] [Google Scholar]
- 9.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring-evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr 1982;139:165–171. [DOI] [PubMed] [Google Scholar]
- 10.Owens J, Opipari L, Nobile C, Spirito A. Sleep and daytime behavioral sleep disorders. Pediatrics 1998;102:1178–1184. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien LM, Gozal D. Behavioral and neurocognitive implications of snoring and obstructive sleep apnea in children: facts and theory. Paediatr Respir Rev 2002;3:3–9. [DOI] [PubMed] [Google Scholar]
- 12.Schechter MS. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:e69. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, Raffield TJ, Gozal D. Neurobehavioral implications of habitual snoring in children. Pediatrics 2004;114:44–49. [DOI] [PubMed] [Google Scholar]
- 14.Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, Morielli A. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001;138:838–844. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL. Nasal steroids as treatment for obstructive sleep apnea: don't throw away the scalpel yet. J Pediatr 2001;138:795–797. [DOI] [PubMed] [Google Scholar]
- 16.Rubinstein I. Nasal inflammation is present in patients with obstructive sleep apnea. Laryngoscope 1995;105:175–177. [DOI] [PubMed] [Google Scholar]
- 17.Sekosan M, Zakkar M, Wenig B, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa with obstructive sleep apnea. Laryngoscope 1996;106:1018–1020. [DOI] [PubMed] [Google Scholar]
- 18.Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA, Rubinstein I. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 1997;111:1500–1504. [DOI] [PubMed] [Google Scholar]
- 19.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:541–546. [DOI] [PubMed] [Google Scholar]
- 20.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics 2004;113:564–569. [DOI] [PubMed] [Google Scholar]
- 21.Singulair. Manufacturer's prescribing information. Whitehouse Station, NJ: Merck & Co, Inc.; 1999.
- 22.Becker A. Clinical evidence with montelukast in the management of chronic childhood asthma. Drugs 2000;59(Suppl 1):29–34. [DOI] [PubMed] [Google Scholar]
- 23.Storms W, Michele TM, Knorr B, Noonan G, Shapiro G, Zhang J, Shingo S, Reiss TF. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clin Exp Allergy 2001;31:77–87. [DOI] [PubMed] [Google Scholar]
- 24.Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, Michele TM, Reiss TF, Nguyen HH, Bratton DL. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2–5 years. Pediatrics 2001;108:e48. [DOI] [PubMed] [Google Scholar]
- 25.Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, Becker A. Montelukast for chronic asthma in 6–14 year old children: a randomized double blind trial. JAMA 1998;279:1181–1186. [DOI] [PubMed] [Google Scholar]
- 26.Villaran C, O'Neill SJ, Helbling A, van Noord JA, Lee TH, Chuchalin AG, Langley SJ, Gunawardena KA, Suskovic S, Laurenzi M, et al. Montelukast versus salmeterol in patients with asthma and exercise induces bronchoconstriction. J Allergy Clin Immunol 1999;104:547–553. [DOI] [PubMed] [Google Scholar]
- 27.Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 1999;399:789–793. [DOI] [PubMed] [Google Scholar]
- 28.Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 2000;275:30531–30536. [DOI] [PubMed] [Google Scholar]
- 29.Goldbart AD, Goldman GL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea and recurrent infection. Chest 2004;126:13–18. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 1979;133:401–404. [DOI] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. Washington DC: National Institutes of Health; 1968. Publication No. 204.
- 32.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med 1996;153:866–878. [DOI] [PubMed] [Google Scholar]
- 33.Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis 1992;156:1235–1239. [DOI] [PubMed] [Google Scholar]
- 34.Sleep Disorders Atlas Task Force. EEG arousals: scoring and rules and examples. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 35.Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep 2004;27:274–278. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep 2004;27:279–282. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87–94. [DOI] [PubMed] [Google Scholar]
- 38.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 2001;107:607–614. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin JL, Kaemingk KL, Fregosi RF, Rosen GM, Morgan WJ, Sherrill DL, Quan SF. Clinical outcomes associated with sleep-disordered breathing in Caucasian and Hispanic children–the Tucson Children's Assessment of Sleep Apnea study (TuCASA). Sleep 2003;26:587–591. [DOI] [PubMed] [Google Scholar]
- 40.Al-Ghamdi SA, Manoukian JJ, Morielli A, Oudjhane K, Ducharme FM, Brouillette RT. Do systemic corticosteroids effectively treat obstructive sleep apnea secondary to adenotonsillar hypertrophy? Laryngoscope 1997;107:1382–1387. [DOI] [PubMed] [Google Scholar]
- 41.Goldbart AD, Veling MC, Goldman JL, Li RC, Brittian KR, Gozal D. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res 2004;57:232–236. [DOI] [PubMed] [Google Scholar]
- 42.Brooks LJ, Stephens BM, Bacevice AM. Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr 1998;132:682–686. [DOI] [PubMed] [Google Scholar]
- 43.Ebenfelt A, Ivarsson M. Neutrophil migration in tonsils. J Anat 2001;198:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002;105:2462–2464. [DOI] [PubMed] [Google Scholar]
- 45.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, Zambito AM, Tracy RP, Jenny NS, Redline S. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation 2005;111:1978–1984. [DOI] [PubMed] [Google Scholar]
- 46.Kaditis AG, Alexopoulos EI, Kalampouka E, Kostadima E, Germenis A, Zintzaras E, Gourgoulianis K. Morning levels of C-reactive protein in children with obstructive sleep-disordered breathing. Am J Respir Crit Care Med 2005;171:282–286. [DOI] [PubMed] [Google Scholar]
- 47.Figueroa DJ, Borish L, Baramki D, Philip G, Austin CP, Evans JF. Expression of cysteinyl leukotriene synthetic and signaling proteins in inflammatory cells in active seasonal allergic rhinitis. Clin Exp Allergy 2003;33:1380–1388. [DOI] [PubMed] [Google Scholar]