Abstract
Rationale: Long-term intermittent hypoxia (LTIH) exposure in adult mice, modeling oxygenation patterns of moderate–severe obstructive sleep apnea, results in lasting hypersomnolence and is associated with nitration and oxidation injuries in many brain regions, including wake-active regions. Objectives: We sought to determine if LTIH activates inducible nitric oxide synthase (iNOS) in sleep/wake regions, and if this source of NO contributes to the LTIH-induced proinflammatory gene response, oxidative injury, and wake impairments. Methods: Mice with genetic absence of iNOS activity and wild-type control animals were exposed to 6 weeks of long-term hypoxia/reoxygenation before behavioral state recordings, molecular and biochemical assays, and a pharmacologic intervention. Measurements and Main Results: Two weeks after recovery from hypoxia/reoxygenation exposures, wild-type mice showed increased iNOS activity in representative wake-active regions, increased sleep times, and shortened sleep latencies. Mutant mice, with higher baseline sleep times, showed no effect of long-term hypoxia/reoxygenation on sleep time latencies and were resistant to hypoxia/reoxygenation increases in lipid peroxidation and proinflammatory gene responses (tumor necrosis factor α and cyclooxygenase 2). Inhibition of iNOS after long-term hypoxia/reoxygenation in wild-type mice was effective in reversing the proinflammatory gene response. Conclusions: These data support a critical role for iNOS activity in the development of LTIH wake impairments, lipid peroxidation, and proinflammatory responses in wake-active brain regions, and suggest a potential role for inducible NO inhibition in protection from proinflammatory responses, oxidative injury, and residual hypersomnolence in obstructive sleep apnea.
Keywords: basal forebrain, carbonylation, chronic intermittent hypoxia, locus coeruleus, oxidative
Obstructive sleep apnea (OSA) with significant sleepiness is present in 2 to 4% of the adult population (1), and despite therapy, many patients with obstructive sleep apnea have small improvements in the objective measures of sleepiness (2, 3). The mechanisms of persistent sleepiness in treated sleep apnea are presently not known, but severity of oxyhemoglobin desaturations best predicts sleepiness in persons with obstructive sleep apnea (4–7).
Animal studies have been instrumental in establishing that hypoxia/reoxygenation causes both neural injury and neurobehavioral impairments, including hypersomnolence (8–13). Long-term intermittent hypoxia (LTIH), modeling the oxygenation patterns of moderate–severe obstructive sleep apnea, results in lasting hypersomnolence and a vast array of oxidative changes in sleep/wake brain regions (12). Drugs that reduce the availability of superoxide free radicals reduce the neural injury (9, 13). However, wake-active brain regions of mice exposed to LTIH show evidence of both reactive oxygen and nitrogen species with the formation of nitrosylation, nitration, and lipid peroxidation (12).
Enzymes controlling the production of nitric oxide (NO•) in brain tissue, therefore, should play important roles in LTIH hypersomnolence, sleepiness, and other LTIH-induced impaired neural functions. The generation of reactive nitrogen species underlies nitration of proteins (14) and lipid peroxidation (15) and, under specific conditions, promotes carbonylation (16, 17). Of the NO synthase isoforms in brain tissue, inducible nitric oxide synthase (iNOS) produces large quantities of NO• that may diffuse from the extracellular matrix into neurons (18). Moreover, iNOS has been implicated in several oxidative neurodegenerative disorders (19–24). However, iNOS has also been shown to contribute to hypoxia preconditioning responses in cardiac and brain tissue (25–27), so that alternatively, iNOS could play a protective role in LTIH. Presently, it is unclear whether LTIH activates iNOS in wake-active brain regions and whether changes in iNOS activity would protect from LTIH oxidative injury and/or lessen hypersomnolence, or if iNOS activation contributes to the oxidative injuries and/or hypersomnolence.
In an effort to begin to define the mechanisms and identify potential therapeutic targets for the neurobehavioral morbidities of obstructive sleep apnea, we designed the following studies to determine what roles iNOS plays in the development of LTIH wakefulness impairments, and the proinflammatory and oxidative/nitrative responses. We first determined if iNOS activation occurs in wake-active brain regions. We then compared the effects of LTIH on hypersomnolence, oxidative injury, and the proinflammatory response in mice with absent iNOS with wild-type control animals. To complement studies performed in mutant mice, we characterized the effect of acute iNOS inhibition on the proinflammatory gene responses in wild-type mice after LTIH.
METHODS
Animals
Ten-week-old male C57BL/6J (B6) mice (iNOS−/− mice [B6.129P2-NOS2tm1Lau/J] backcrossed 10 generations to C57BL/6J000664, and iNOS+/+ C57BL/6J 000664 mice; Jackson Laboratory, Bar Harbor, ME) were studied. The mutant mice have a neomycin replacement for the calmodulin binding domain on the iNOS gene, preventing induction of the protein (28). Methods and study protocols were approved in full by the Institutional Animal Care and Use Committee of the University of Pennsylvania, and conformed with the revised National Institutes of Health Office of Laboratory Animal Welfare Policy. Food and water were provided ad libitum.
LTIH Protocol
A detailed description of the LTIH protocol was recently published (12, 13). Briefly, an automated nitrogen/oxygen delivery profile system (Oxycycler Model A84XOV; Biospherix, Redfield, NY) was used to change ambient oxygen levels from 21 to 10% for 5 seconds at 90-second intervals, resulting in arterial oxyhemoglobin saturation fluctuations for LTIH between 96 and 98% and 83 and 86%, and for sham LTIH between 96 and 98% and 94 and 98%. LTIH was produced for 10 hours of the lights-on period for 6 weeks. Humidity, ambient CO2, and environmental temperature were held constant.
iNOS Activity Measurement in Wake-Active Regions
The iNOS activity was measured for two sets of experiments: the first was to determine if iNOS activity increased in LTIH in wake-active regions and the second was to confirm iNOS inhibition for the pharmacologic trials (see later). To determine LTIH effects on iNOS activity in wake-active regions, LTIH (n = 5) and sham LTIH (n = 5) B6 mice, after 2 weeks' recovery in normoxia, were decapitated, and brains were flash-frozen before micropunch sampling of laterobasal forebrain (magnocellular preoptic/substantia inominata and horizontal diagonal band regions) and the posterior and lateral (perifornicular) hypothalamus. A commercially available NOS activity kit was used according to directions (Cayman Chemical, Ann Arbor, MI), using 20-μg protein samples and measuring the conversion of (14C) l-arginine (Amersham Biosciences, Piscataway, NJ) to l-citrulline, expressed as counts/minute, calculated with standards. To examine predominantly iNOS activity in brain tissue, we used a selective neuronal NOS inhibitor, 5-μM S-ethyl-N-(4-[trifluoromethyl]phenyl)isothiourea (29). NOS activities were measured with a multipurpose scintillation counter (LS-6500; Beckman Coulter, Fullerton, CA), and data were analyzed with ImageQuant 5.2 software (Amersham Biosciences).
Sleep/Wake Recording and Analysis of Sleepiness
After 6 weeks of LTIH or sham LTIH exposures, 25 to 30 mice of each strain and intermittent hypoxia (IH) condition were returned to normoxic conditions for 1 week before surgical implantation of electrodes for electrophysiologic recordings, as previously described (12). Baseline sleep was recorded for 5 days. On Recording Day 6, a baseline murine multiple sleep latency test was performed (four nap opportunities between 2:00 and 4:00 p.m.) to measure baseline sleep propensity (30). On Recording Day 7, sleep deprivation (forced wakefulness, watching EEG signals) was performed for 6 hours of the light period (8:00 a.m. to 2:00 p.m.), followed by a second multiple sleep latency text; recovery sleep was then recorded for 12 hours. The behavioral state acquisition and analysis program used for these studies was ACQ 3.4 (31). Primary variables were total sleep time/24 hours, total non-REM sleep time/24 hours and REM sleep time/24 hours, and average sleep latency, before and after short-term sleep loss (32). Secondary variables were correlates of behavioral state consolidation (mean state bout lengths, arousal index).
Measurement of Proinflammatory Gene Responses to LTIH in Genetic and Pharmacologic iNOS Inhibition Models
Real-time Taqman polymerase chain reaction for tumor necrosis factor α (TNF-α), cyclooxygenase-2 (COX-2), and iNOS was performed on macropunches of selected brain regions in mice exposed to sham LTIH or LTIH, using methods as previously published (33). Briefly, 2 weeks after completion of sham LTIH or LTIH, mice were perfused with phosphate-buffered saline; brains were immediately frozen and sectioned (300 μm) for macropunches of the following brain regions: frontal cortex, magnocellular preoptic/substantia inominata/horizontal diagonal band (laterobasal forebrain), hippocampus CA1, and posterior and lateral hypothalamus. RNA was purified and cDNA created for primer/probe sets for Taqman real-time polymerase chain reaction (SDS-7900HT; ABI, Foster City, CA). All primer probe sets showed excellent sensitivity and linearity (detection of ⩾ 100 copies/sample, r2 ⩾ 0.99).
Systemic iNOS Inhibition
An additional series of mice was used to determine if LTIH gene responses could be blocked with iNOS inhibition after LTIH. After LTIH exposure and 2 weeks in normoxia, C57Bl/6J mice were injected with iNOS inhibitor (1400W [Sigma-Aldrich, St. Louis, MO]; 0.01, 0.1, 1, or 2 mg/kg subcutaneously) or saline (vehicle) every 12 hours for three doses (n = 2–6/dose/condition, allowing n = 18 for correlation for each region). Linear regression was performed using the within-mouse percentage of iNOS inhibition (forebrain) versus copy numbers of TNF-α and COX-2 (basal forebrain and posterior/lateral hypothalamus).
Protein Carbonyl ELISA
Concentrations of protein carbonyls in macrodissections of laterobasal forebrain were determined using a commercially available ELISA kit, including antibodies (Zentec PC Test; Zenith Technology, Dunedin, New Zealand) developed from established techniques (34). In brief, a 20-μg protein homogenized sample was incubated with 2,4-diphenylhydrazine solution for 45 minutes, yielding 2,4-dinitrophenylhydrazone groups on protein carbonyls. Carbonylated proteins were bound to a 96-well plate by anti–2,4-dinitrophenylhydrazone. A standard was made using five concentrations of hypochlorous-oxidized protein. Oxidation of O-phenylediamine by horseradish peroxidase was used in the chromatogenic reaction, reading absorbance measured at 490 nm in a spectrophotometer.
Measurement of F2 Isoprostanes
Isoprostane, d4-8,12-iso-iPF2α-VI (F2-iPs), analysis was performed as previously described (35) using macrodissections (1 mm3) from selected regions of the basal forebrain and brainstem for mice under conditions of LTIH and sham IH mice of both iNOS−/− and iNOS+/+ strains. The areas selected for F2-iPs analysis were as follows: (1) magnocellular preoptic, substantia inominata, and horizontal diagonal band regions and (2) lateral and posterior hypothalamus. Thin-layer chromatography was used for purification of the eluate, and negative-ion chemical ionization gas chromatography–mass spectrometry was used to assay F2-iPs (36).
Statistical Analysis
Values reported represent mean ± SEM. Parameter differences were analyzed with one- and two-way analysis of variance, with LTIH conditions, brain region, strain, or drug treatment as the independent variables. When significant overall differences were observed, a priori within-group comparisons of means were performed using Bonferroni posttests for preselected groups. The null hypothesis was rejected for Bonferroni-corrected probabilities less than 0.05.
RESULTS
LTIH Increases iNOS Activity in Two Representative Wake-Active Regions, the Laterobasal Forebrain and Posterior/Lateral Hypothalamus
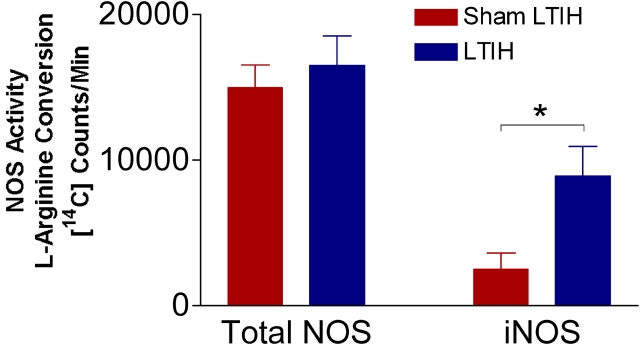
There were no differences in total NOS activity (expressed as counts/minute) in sham LTIH (n = 6) and LTIH (n = 6; 14,957 ± 1,600 vs. 16,509 ± 2,088, not significant in the laterobasal forebrain, and 21,386 ± 2,032 vs. 20,344 ± 1,087, not significant in the lateral and posterior hypothalamus). In contrast, iNOS activity was increased in both regions as follows: laterobasal forebrain: 2,500 ± 1,124 versus 8,901 ± 2,017 (p < 0.001; Figure 1); and lateral and posterior hypothalamic regions: 3,192 ± 419 versus 9,977 ± 1,485 (p < 0.01).
Figure 1.
Effects of long-term intermittent hypoxia (LTIH) on total nitric oxide synthase (NOS) and inducible NOS (iNOS) activity. 14C-arginine to citrulline conversion was measured in homogenates from the laterobasal forebrain of adult wild-type mice exposed to intermittent hypoxia for 6 weeks (n = 5) or sham intermittent hypoxia (n = 5) for total NOS activity (left columns) and iNOS activity (right columns). *p < 0.05.
Mice Lacking iNOS Activity Are Resistant to LTIH-induced Hypersomnolence and Sleepiness
Electrophysiologic recordings of sleep/wake states were successfully obtained in both iNOS−/− and iNOS+/+ mice: iNOS−/− sham LTIH, n = 15; iNOS−/− LTIH, n = 18; iNOS+/+ sham LTIH, n = 24; and iNOS+/+ LTIH, n = 24. There were strain differences across sham LTIH treatments; the iNOS null genotype was associated with increased total sleep time (mean difference, 92 minutes; t = 3.7, p < 0.01) and non-REM sleep times/24 hours (mean difference, 92 minutes; t = 3.6, p < 0.01), as shown in Figure 2. In contrast, REM sleep time/24 hours did not differ by genotype. Wild-type mice exposed to LTIH showed increased total sleep times (mean difference, 152 minutes; t = 7.2, p < 0.001) and non-REM sleep times (mean difference, 120 minutes; t = 5.4, p < 0.001). REM sleep increased in LTIH-exposed wild-type mice relative to control animals (mean difference, 35 minutes; t = 3.0, p < 0.05). There was no significant effect of LTIH in iNOS null mice on total sleep time (mean difference, 24 minutes; t = 0.9), non-REM sleep (mean difference, 16 minutes; t = 0.6), or REM sleep (mean difference, –0.2 minutes; t = 0.01).
Figure 2.
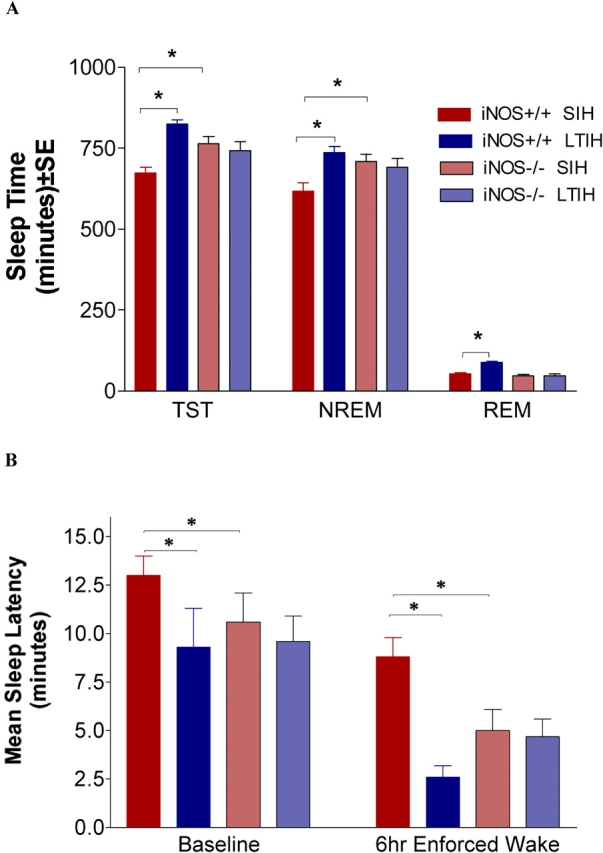
Effect of LTIH on 24-hour sleep times and average sleep latencies in iNOS null and wild-type mice. (A) The 24-hour mean ± SE sleep times in minutes, with sample sizes of 15–24 mice/strain and condition. NREM = non–REM; SIH = sham intermittent hypoxia; TST = total sleep time. (B) Average multiple sleep latency test values (minutes) for the same groups of mice as above (use same color legend as A to identify strain/condition). Left bars represent baseline average sleep latencies from multiple sleep latency testing, and right bars show sleep latency responses after 6 hours forced wakefulness. *p < 0.05 for Bonferroni-corrected comparisons.
Mean sleep latency values were compared for genotype and LTIH conditions for the same mice analyzed, as described previously, for sleep times. Overall, there were both genotype and LTIH effects on sleep latency (p < 0.001; see Figure 2). Comparing sham LTIH groups of iNOS null and wild-type mice, the iNOS null mice had shortened baseline average multiple sleep latency values (mean difference, −2 minutes; t = 3.4, p < 0.05), and an even larger difference in sleep latencies was observed for the null mice after 6 hours of forced wakefulness (mean difference, –4 minutes; t = 5.9, p < 0.001). LTIH in wild-type mice resulted in a shortened sleep latency (−3 minutes, t = 4.1, p < 0.001) and a significant reduction in the sleep latency after 6 hours of forced wakefulness (mean difference, −6 minutes; t = 7.5, p < 0.001). In contrast, LTIH had no significant effect on either the baseline (mean difference, –1 minute; t = 1.7) or sleep deprivation sleep latencies (mean difference, 0 minutes; t = 1.8). On the basis of sample size, means, and deviations observed in sham LTIH iNOS−/− mice, the experiment was adequately powered (> 0.85) to detect a two-way 3-minute change in sleep latency for both baseline and forced wakefulness conditions. LTIH had no effect on average duration of wake or sleep bouts, arousal index, or REM sleep latencies in the iNOS null mice, whereas wake bouts shortened in the wild-type mice exposed to LTIH (mean difference, 3 minutes; t = 5.8, p < 0.01).
Effects of iNOS Absence on the Long-term Hypoxia/Reoxygenation Proinflammatory Gene Expression
Proinflammatory gene expression (TNF-α, COX-2, and iNOS) was measured in four brain regions with LTIH-increased p67phox gene expression (cortex, laterobasal forebrain, hippocampus CA1, and lateral hypothalamus) in iNOS−/− and iNOS+/+ mice exposed to LTIH or sham LTIH (n = 15, each group), using 20 μg of the same purified RNA sample obtained from each mouse for each region. Values are presented in Figure 3.
Figure 3.

Proinflammatory gene responses to LTIH vary with presence of iNOS activity. Tumor necrosis factor α (TNF-α; top panel), cyclooxygenase-2 (COX-2; middle panel), and mRNA copy numbers (lower panel) were measured in 20 μg RNA from micropunches from the following brain regions: frontal cortex (Cortex); magnocellular preoptic/substantia inominata/horizontal diagonal band or laterobasal forebrain (MCPO/SI/HDB); hippocampus CA1; and posterior and lateral hypothalamus (Lat Hypothalamus). sh = sham. *Bonferroni-corrected p < 0.05.
Overall, large effects of LTIH and genotype were observed for TNF-α mRNA (F = 1500, p < 0.0001). In wild-type mice, LTIH was associated with increased TNF-α mRNA in all four brain regions (p < 0.05; see Figure 3, top panel), whereas in iNOS−/− mice, LTIH increases in TNF-α were observed only in the laterobasal forebrain and CA1 hippocampus (p < 0.05). Cortical TNF-α gene levels in LTIH-exposed mice were significantly higher in wild-type mice than in iNOS−/− mice (t = 14, p < 0.001).
In response to LTIH, COX-2 gene expression was increased in brains of both iNOS−/− and wild-type mice (F = 30, p < 0.01), as summarized in Figure 3, middle panel. LTIH increases in wild-type mice were found in the cortex (Bonferroni t = 3.5, p < 0.05) and in laterobasal forebrain (Bonferroni t = 3.4, p < 0.01). Increases in iNOS mutant mice were also observed in the cortex and laterobasal forebrain (t = 3.1, 3.3; p < 0.05), but the LTIH effect was of less magnitude than in wild-type mice cortex and laterobasal forebrain compared with the iNOS+/+ mice exposed to LTIH, as shown in Figure 3. COX-2 gene expression after sham LTIH did not vary significantly with strain in any region.
Large increases were observed in iNOS gene expression response to LTIH in iNOS null mice (F = 334, p < 0.001). In the iNOS−/− mice, large increases were observed in each brain region assayed in response to LTIH (Figure 3, lower panel; t = 5–13, p < 0.01). In contrast, there was no LTIH on iNOS copy numbers in wild-type mice, except in the posterior and lateral hypothalamus.
Lack of iNOS Activity Confers Resistance to LTIH-induced Oxidative Protein Damage in Laterobasal Forebrain
Isoprostane levels measured in dissected laterobasal forebrain tissue blocks from iNOS−/− and iNOS+/+ mice exposed to LTIH or sham LTIH (n = 6/strain and condition). iPF2α sham LTIH levels did not vary with strain (Figure 4, top panel). iPF2α levels in wild-type mice were increased compared with sham LTIH (F = 65, p < 0.01), and did not increase significantly in iNOS mutants across LTIH conditions. A separate group of mice were used for carbonyl protein measurement. Macrodissections of the laterobasal forebrain were obtained from iNOS−/− and iNOS+/+ mice exposed to LTIH or sham LTIH (n = 10–11/strain and condition). Carbonyl content in the two sham LTIH groups was lower in iNOS−/− mice compared with iNOS+/+ mice (p < 0.05; Figure 4, lower panel). Both wild-type (t = 3.9, p < 0.05) and mutant mice (t = 3.2, p < 0.05) showed increases in carbonyl content with LTIH.
Figure 4.
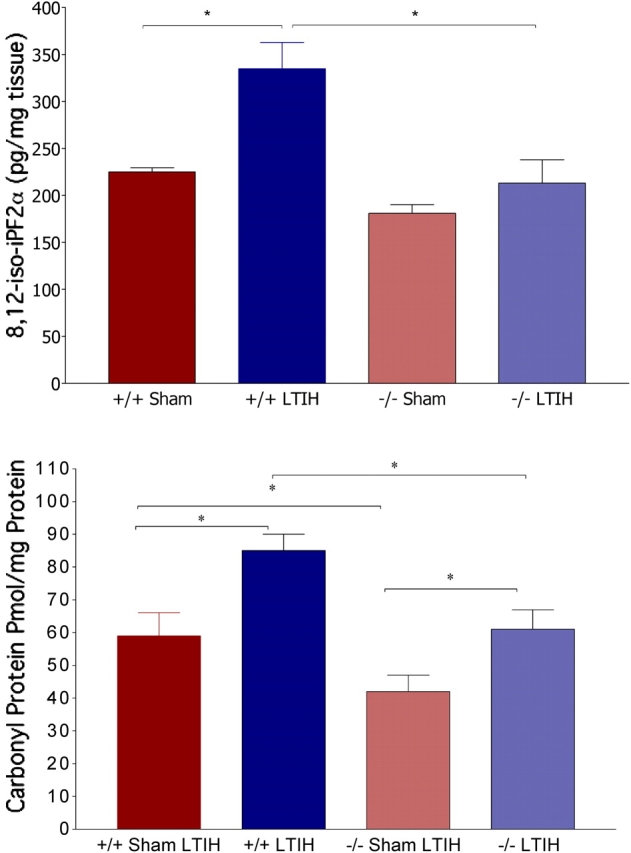
Effects of LTIH on lipid peroxidation (top) and carbonylation (bottom) in the laterobasal forebrains of iNOS null and wild-type mice. Macrodissections, as performed for gene response assays, of the laterobasal forebrain were used to measure isoprostane (8,12-iso-iPF2α) levels. Average isoprostane values ± SE for sham LTIH wild-type (+/+sh LTIH, n = 10), sham LTIH iNOS null (−/−sh LTIH, n = 11), LTIH wild-type (+/+LTIH, n = 10), LTIH iNOS null (−/−sh LTIH, n = 11). *p < 0.05 Bonferroni-corrected IH and strain comparisons.
Effects of iNOS Inhibition on Proinflammatory Gene Responses and iNOS Activity after LTIH
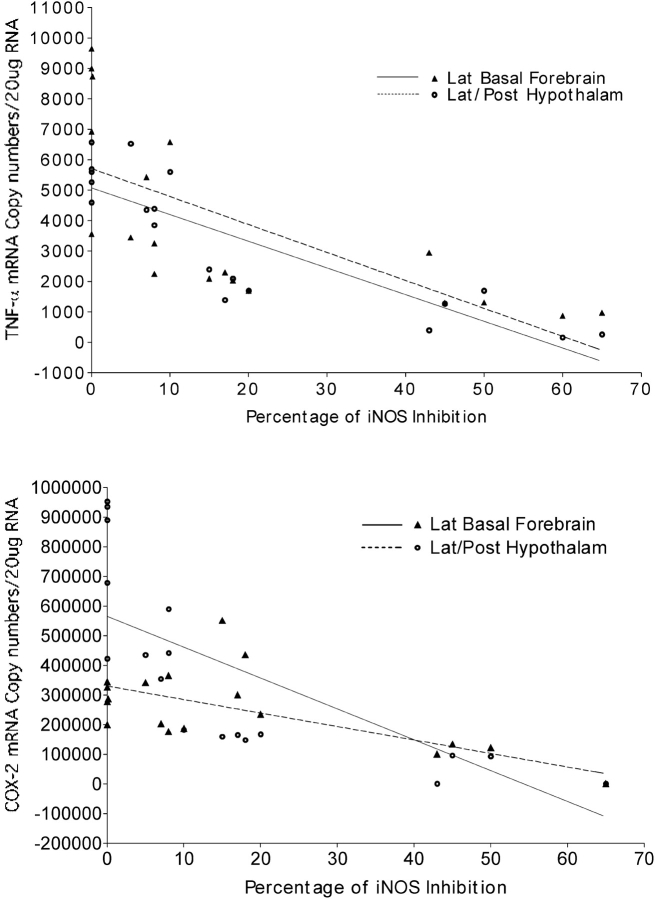
Systemic injection of iNOS inhibitor 1400W significantly reduced iNOS activity for doses of 1 and 2 mg/kg (F = 84, p < 0.01). Overall, there was a dose-dependent response in iNOS activity across the five doses (r2 = 0.75). There were significant effects of iNOS inhibition on the proinflammatory gene responses of TNF-α and COX-2, as shown in Figure 5. In the laterobasal forebrain, the goodness of fit for TNF-α versus percentage of iNOS inhibition was r2 = 0.44, F = 13, p < 0.003, and in the lateral hypothalamus/posterior hypothalamus, the goodness of fit for TNF-α versus percentage of iNOS inhibition was r2 = 0.7, F = 38, p < 0.0001. In the laterobasal forebrain, the goodness of fit for COX-2 versus percentage of iNOS inhibition was r2 = 0.55, F = 22, p < 0.001, and in the lateral hypothalamus/posterior hypothalamus, the goodness of fit for COX-2 versus percentage of iNOS inhibition was r2 = 0.60, F = 27, p < 0.0001.
Figure 5.
Effects of iNOS inhibition on LTIH proinflammatory gene responses. After LTIH, a series of mice received varying doses of a selective iNOS inhibitor (1400W 0.01–2 mg/kg) or saline vehicle systemically. iNOS activity was measured and analyzed in two brain regions: laterobasal forebrain (Lat Basal Forebrain; solid triangles) and the lateral and posterior regions of the hypothalamus (Lat/Post Hypothalam; open circles) as percentage reduction from vehicle iNOS activity to perform linear regression with percentage of iNOS inhibition after LTIH and copy numbers of TNF-α (top) and COX-2 (bottom).
DISCUSSION
Results from this collection of studies advance our understanding of the mechanisms through which LTIH results in lasting hypersomnolence and sleepiness. The transgenic absence of iNOS confers resistance to the recently described LTIH-induced wake impairments (8). In addition, transgenic absence of iNOS is associated with a blunting of the LTIH-induced proinflammatory gene responses in the cortex and laterobasal forebrain. Mice without functional iNOS were resistant to the LTIH increase in lipid peroxidation. In contrast, there was no strain effect observed for the LTIH effect on carbonyl content. In wild-type mice, iNOS inhibition was effective in reversing the LTIH proinflammatory gene response. Together, these data show that iNOS activity is not only necessary for LTIH hypersomnolence; it also contributes, in part, to the proinflammatory gene response and lipid peroxidation in mice. In addition, it is possible to reverse the LTIH proinflammatory response with iNOS inhibition.
Methodologic Considerations
The presented studies implemented an established murine model of the hypoxia/reoxygenation patterns observed in persons with severe obstructive sleep apnea (10, 12, 37). This model isolates the effects of hypoxia/reoxygenation from the other physiologic disturbances in sleep apnea. The model excludes upper airway and hypercapneic responses, and fragments sleep only during the first 2 weeks of exposure (12). Although work with the model highlights the importance of hypoxia/reoxygenation events in obstructive sleep apnea, end-organ injury, sleep fragmentation, hypercapnia, and upper airway responses and injury may also contribute to lasting impairments in brain function. The model should be developed further. We must next determine if hypersomnolence and cognitive impairments are dependent on the severity of LTIH, and equally, if the severity of LTIH affects the reversibility of neurobehavioral sequelae.
An unexpected difference observed in iNOS mutants was a significant increase in sham LTIH 24-hour non-REM sleep time, relative to wild-type control animals. A recent study comparing sleep times in iNOS null and wild-type mice found similar 24-hour non-REM sleep times across strains (38). Non-REM sleep times, in iNOS null mice without any IH condition, were also increased (data not shown). Differences in sleep state scoring (quiet waking vs. non-REM sleep time) most likely explain variance in non-REM sleep state times, because age, light/dark, temperature, and background strains were similar across studies. This finding highlights the need to standardize murine behavioral scoring across laboratories.
LTIH Results in Lasting Increases in iNOS Activity in Wake-Active Regions
LTIH resulted in increased iNOS activity in brain regions tested. iNOS protein is increased in palatine tissue in persons with obstructive sleep apnea (39); however, iNOS activity in sleep apnea has not been reported. Acute hypoxia increases iNOS through hypoxia inducible factor (HIF)-1α binding to the iNOS promoter and/or nuclear factor κ β (NFκ-β) activation of p38 mitogen-activated protein kinase (MAPK) (40–45). Two weeks of IH results in elevated iNOS activity in the cortex (46). We extend the characterization to show that iNOS activity is elevated 2 weeks after LTIH in the laterobasal forebrain and hypothalamic wake-active regions. Our iNOS inhibition trials show that this elevated iNOS activity is, at least in part, responsible for the LTIH proinflammatory response. Whether this persistent increase in iNOS activity contributes to the persistence of wake impairments and whether iNOS inhibition after LTIH would facilitate recovery of neural function should now be studied.
The increase in iNOS activity without a change in total NOS activity suggests that one or more of the other NOS isoforms are reduced in LTIH. One of the other major isoforms in neural tissue is neuronal NOS (47). The present article raises the possibility that neuronal NOS activity is reduced in LTIH. Neuronal NOS in cholinergic neurons may contribute to wakefulness and REM sleep through enhanced cholinergic neural transmission (48, 49). Thus, LTIH-induced reduction in neuronal NOS activity should be explored as a potential mechanism through which LTIH induces hypersomnolence.
Transgenic Absence of iNOS Activity Confers a Resistance to LTIH Hypersomnolence, Sleepiness, Oxidative Changes, and the Proinflammatory Response
Mice with a transgenic absence of the ability to induce iNOS synthesis are resistant to LTIH hypersomnolence. The lack of effect on sleep times may not be attributed to a ceiling effect from higher baseline total sleep times in the mutants, because sleep in the mutants did increase after sleep loss (data not shown). Unlike in wild-type mice, LTIH did not result in shortened wakefulness bouts in iNOS−/− mice or in shorter sleep latencies. Thus, all known LTIH-induced wake impairments are iNOS-dependent processes. Whether iNOS impairs wakefulness through neural injury or nitration/nitrosylation signaling mechanisms is presently unknown.
LTIH resulted in a proinflammatory gene response in many brain regions. Magnitudes of gene responses varied with the gene assayed, the regions sampled, and iNOS strain. Of particular interest, an LTIH TNF-α gene response was evident in wild-type mice in all brain regions tested, whereas COX-2 mRNA was increased only in the laterobasal forebrain and cortex. The cDNAs for TNF-α and COX-2 were created from the same purified RNA samples; thus, the difference cannot be explained by sampling quality differences. The magnitude of increase in COX-2 mRNA in LTIH-exposed cortex tissue (2.5-fold) is consistent with a previous study immediately after shorter IH in rats (10). COX-2 upregulation in the brain results in prostaglandin E2 synthesis and oxidative injury (50) and is implicated in the pathogenesis of several neurodegenerative disorders, including Parkinson's and Alzheimer's disease (51, 52). The reported regional differences in COX-2 gene responses must be confirmed with enzymatic activity.
The increase in iNOS mRNA levels in iNOS−/− mice in response to LTIH is of particular interest. The mutant mouse line used in our studies has a replacement of the calmodulin binding domain with a neomycin gene; the resultant effect of this transgene is complete loss of inducibility for iNOS (i.e., no iNOS protein) (28), but this will not affect transcription of the gene, and the gene is otherwise intact. Transcriptional regulation of the iNOS gene is complex, and regulated by cytokines, a hypoxia-responsive enhancer element, and stress-activated protein kinase (SAPK)/Jun kinase (JNK) and p38 MAPK (53, 54). In addition, changes in iNOS mRNA stability have been reported, and it is possible that the increase we observe is, in part, a consequence of increased stability. For example, β-adrenergic stimulation enhances the interleukin-1β induction of iNOS, in part through mRNA stabilization (55). Thus, there are many potential reasons for why LTIH increased iNOS mRNA in the mutant mice, and we will need to systematically explore these to determine the mechanisms through which LTIH increases iNOS. High levels of iNOS mRNA in iNOS−/− mice in response to LTIH suggest that the iNOS protein itself must provide a negative feedback to transcription or mRNA stability that we have unmasked in this model.
Transgenic absence of iNOS prevented significant lipid peroxidation within the brain and brainstem of mice exposed to LTIH. iNOS has been shown to contribute to the formation of peroxynitrite (ONOO−) in brain lipid peroxidation (56). Lower isoprostane levels (lipid peroxidation) in LTIH-exposed iNOS null mice, relative to controls exposed to LTIH, suggest that iNOS activity is necessary for LTIH-induced isoprostane formation. Whether long-term iNOS inhibition after LTIH can reverse lipid peroxidation should be addressed. The reduction in sham LTIH protein carbonylation in iNOS null mice surprised us, but has been shown in a lung injury model using iNOS null mice (57).
The present study has characterized the overall role for iNOS in LTIH as injurious, by showing iNOS dependence in all known LTIH wake impairments, oxidative injury, and the proinflammatory gene response. Dose-dependent effects of iNOS inhibition on sleep/wake activity and on LTIH injury and hypersomnolence must now be tested. Whether longer term iNOS inhibition of the LTIH proinflammatory gene response would be followed by reductions in oxidative injury and improved reversal of hypersomnolence will be important to ascertain, as we look to develop therapeutics to lessen residual hypersomnolence in persons treated for sleep apnea.
Acknowledgments
G.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; P.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; D.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; S.C.V. has served as an advisor twice for Sepracor, receiving $2,000 in June 2004 and $2,500 in December 2004. The company has no connections with the topic of this article, and this work was never discussed with Sepracor.
Supported by grants NIH HL65225 and AG 17628.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Douglas NJ, Engleman HM. Effects of CPAP on vigilance and related functions in patients with the sleep apnea/hypopnea syndrome. Sleep 2000;23:S147–S149. [PubMed] [Google Scholar]
- 3.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565–571. [DOI] [PubMed] [Google Scholar]
- 4.Bedard MA, Montplaisir J, Richer F, Malo J. Nocturnal hypoxemia as a determinant of vigilance impairment in sleep apnea syndrome. Chest 1991;100:367–370. [DOI] [PubMed] [Google Scholar]
- 5.Tiihonen M, Partinen M. Polysomnography and maintenance of wakefulness test as predictors of CPAP effectiveness in obstructive sleep apnea. Electroencephalogr Clin Neurophysiol 1998;107:383–386. [DOI] [PubMed] [Google Scholar]
- 6.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep 2000;23:S102–S108. [PubMed] [Google Scholar]
- 7.Naismith S, Winter V, Gotsopoulos H, Hickie I, Cistulli P. Neurobehavioral functioning in obstructive sleep apnea: diferential effects of sleep quality, hypoxemia, and subjective sleepiness. J Clin Exp Neuropsychol 2004;26:43–54. [DOI] [PubMed] [Google Scholar]
- 8.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 2001;21:2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Row BW, Liu R, Wei X, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 2003;167:1540–1547. [DOI] [PubMed] [Google Scholar]
- 10.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 2003;168:469–475. [DOI] [PubMed] [Google Scholar]
- 11.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 2003;167:1540–1547. [DOI] [PubMed] [Google Scholar]
- 12.Veasey SC, Davis C, Zhan G, Hsu YJ, Fenik P, Pratico D, Gow AJ. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004;27:194–201. [DOI] [PubMed] [Google Scholar]
- 13.Veasey SC, Zahn GX, Fenik P, Pratico D. Long-term intermittent hypoxia: reduced excitatory hypoglossal nerve output. Am J Respir Crit Care Med 2004;170:665–672. [DOI] [PubMed] [Google Scholar]
- 14.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol 2004;287:L262–L268. [DOI] [PubMed] [Google Scholar]
- 15.Pratico D, Lee VM, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. FASEB J 1998;12:1777–1783. [DOI] [PubMed] [Google Scholar]
- 16.Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyperpermeability of barrier of intestinal epithelia. J Pharmacol Exp Ther 2003;305:482–494. [DOI] [PubMed] [Google Scholar]
- 17.Cahuana GM, Tejedo JR, Jimenez J, Ramirez R, Sobrino F, Bedoya FJ. Nitric oxide-induced carbonylation of Bcl-2, GAPDH and ANT precedes apoptotic events in insulin-secreting RINm5F cells. Exp Cell Res 2004;293:22–30. [DOI] [PubMed] [Google Scholar]
- 18.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci 2004;75:639–653. [DOI] [PubMed] [Google Scholar]
- 19.Haas J, Storch-Hagenlocher B, Biessmann A, Wildemann B. Inducible nitric oxide synthase and argininosuccinate synthetase: co-induction in brain tissue of patients with Alzheimer's dementia and following stimulation with beta-amyloid 1–42 in vitro. Neurosci Lett 2002;322:121–125. [DOI] [PubMed] [Google Scholar]
- 20.Carreras MC, Franco MC, Peralta JG, Poderoso JJ. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med 2004;25:125–139. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Encinas JM, Serrano J, Bentura ML, Munoz P, Martinez-Murillo R, Rodrigo J. Expression of nitric oxide system in clinically evaluated cases of Alzheimer's disease. Neurobiol Dis 2004;15:287–305. [DOI] [PubMed] [Google Scholar]
- 22.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB Life 2003;55:329–335. [DOI] [PubMed] [Google Scholar]
- 23.Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNF alpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci 2001;21:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol 2003;53:S49–S58. [DOI] [PubMed] [Google Scholar]
- 25.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke 2002;33:1889–1898. [DOI] [PubMed] [Google Scholar]
- 26.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biochem (Tokyo) 2003;278:27256–27266. [DOI] [PubMed] [Google Scholar]
- 27.Patel HH, Hsu AK, Gross GJ. COX-2 and iNOS in opioid-induced delayed cardioprotection in the intact rat. Life Sci 2004;75:129–140. [DOI] [PubMed] [Google Scholar]
- 28.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA 1995;92:10688–10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper GR, Mialkowski K, Wolff DJ. Cellular and enzymatic studies of N(omega)-propyl-l-arginine and S-ethyl-N-[4-(trifluoromethyl)phenyl]isothiourea as reversible, slowly dissociating inhibitors selective for the neuronal nitric oxide synthase isoform. Arch Biochem Biophys 2000;375:183–194. [DOI] [PubMed] [Google Scholar]
- 30.Veasey SC, Yeou-Jey H, Thayer P, Fenik P. Murine multiple sleep latency test: phenotyping sleep propensity in mice. Sleep 2004;27:388–393. [DOI] [PubMed] [Google Scholar]
- 31.Benington JH, Heller HC. Monoaminergic and cholinergic modulation of REM-sleep timing in rats. Brain Res 1995;681:141–146. [DOI] [PubMed] [Google Scholar]
- 32.Veasey SC, Valladares O, Fenik P, Kapfhamer D, Sanford L, Benington J, Bucan M. An automated system for recording and analysis of sleep in mice. Sleep 2000;23:1025–1040. [PubMed] [Google Scholar]
- 33.Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience 2002;113:145–154. [DOI] [PubMed] [Google Scholar]
- 34.Buss IH, Winterbourn CC. Protein carbonyl measurement by ELISA. Methods Mol Biol 2002;186:123–128. [DOI] [PubMed] [Google Scholar]
- 35.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 2001;21:4183–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates A-beta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci 2002;22:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004;126:313–323. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res 2003;973:214–222. [DOI] [PubMed] [Google Scholar]
- 39.Wang HW, Su WF, Lin YS, Kang BH. Immunolocalization of inducible nitric oxide synthase and 3-nitrotyrosine in recurrently inflamed, human palatine tonsils. Eur Arch Otorhinolaryngol 2002;259:413–418. [DOI] [PubMed] [Google Scholar]
- 40.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res 2000;86:319–325. [DOI] [PubMed] [Google Scholar]
- 41.Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biochem (Tokyo) 2001;276:39805–39811. [DOI] [PubMed] [Google Scholar]
- 42.Vartiainen N, Goldsteins G, Keksa-Goldsteine V, Chan PH, Koistinaho J. Aspirin inhibits p44/42 mitogen-activated protein kinase and is protective against hypoxia/reoxygenation neuronal damage. Stroke 2003;34:752–757. [DOI] [PubMed] [Google Scholar]
- 43.Warke VG, Nambiar MP, Krishnan S, Tenbrock K, Geller DA, Koritschoner NP, Atkins JL, Farber DL, Tsokos GC. Transcriptional activation of the human inducible nitric-oxide synthase promoter by Kruppel-like factor 6. J Biochem (Tokyo) 2003;278:14812–14819. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Li D, Saldeen T, Mehta JL. TGF-beta(1) modulates NOS expression and phosphorylation of Akt/PKB in rat myocytes exposed to hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 2001;281:H1035–H1039. [DOI] [PubMed] [Google Scholar]
- 45.Matrone C, Pignataro G, Molinaro P, Irace C, Scorziello A, Di Renzo GF, Annunziato L. HIF-1alpha reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem 2004;90:368–378. [DOI] [PubMed] [Google Scholar]
- 46.Row BW, Kheirandish L, Li RC, Guo SZ, Brittian KR, Hardy M, Bazan NG, Gozal D. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem 2004;89:189–196. [DOI] [PubMed] [Google Scholar]
- 47.Lacza Z, Horn TF, Snipes JA, Zhang J, Roychowdhury S, Horvath EM, Figueroa JP, Kollai M, Szabo C, Busija DW. Lack of mitochondrial nitric oxide production in the mouse brain. J Neurochem 2004;90:942–951. [DOI] [PubMed] [Google Scholar]
- 48.Marino J, Cudeiro J. Nitric oxide-mediated cortical activation: a diffuse wake-up system. J Neurosci 2003;23:4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cudeiro J, Rivadulla C, Grieve KL. A possible role for nitric oxide at the sleep/wake interface. Sleep 2000;23:829–835. [PubMed] [Google Scholar]
- 50.Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, Levi G, Minghetti L. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem 2002;81:1028–1034. [DOI] [PubMed] [Google Scholar]
- 51.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci USA 2003;100:5473–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, et al. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J 2002;16:805–813. [DOI] [PubMed] [Google Scholar]
- 53.Rudders S, Gaspar J. madore R. ESE-1 is a novel transcriptional mediator of inflammation that interacts with NF-κβ to regulate inducible nitric oxide synthase gene. J Biochem (Tokyo) 2001;276:3302–3309. [DOI] [PubMed] [Google Scholar]
- 54.Melillo G, Musso T, Sica A. A hypoxia responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 1995;182:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gustafsson AB, Brunton LL. B-adrenergic stimulation of rat cardiofibroblasts enhances induction of nitric oxide synthase by interleukin-1b via message stabilization. Mol Pharmacol 2000;58:1470–1478. [DOI] [PubMed] [Google Scholar]
- 56.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res 1999;31:577–596. [DOI] [PubMed] [Google Scholar]
- 57.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 2003;168:568–574. [DOI] [PubMed] [Google Scholar]