Abstract
The interface between apoptosis (programmed cell death) and the cell cycle is essential to preserve homeostasis and genomic integrity. Here, we show that survivin, an inhibitor of apoptosis over-expressed in cancer, physically associates with the cyclin-dependent kinase p34cdc2 on the mitotic apparatus, and is phosphorylated on Thr34 by p34cdc2-cyclin B1, in vitro and in vivo. Loss of phosphorylation on Thr34 resulted in dissociation of a survivin-caspase-9 complex on the mitotic apparatus, and caspase-9-dependent apoptosis of cells traversing mitosis. These data identify survivin as a mitotic substrate of p34cdc2-cyclin B1 and suggest that survivin phosphorylation on Thr34 may be required to preserve cell viability at cell division. Manipulation of this pathway may facilitate the elimination of cancer cells at mitosis.
Apoptosis or programmed cell death couples to surveillance mechanisms, i.e., checkpoints, to eliminate unneeded, damaged, and potentially harmful cells (1). This process requires the assembly of an evolutionary conserved “apoptosome” complex, which comprises the upstream cell death protease caspase-9, the adapter/cofactor protein Apaf-1, mitochondria-derived cytochrome c and ATP/dATP, and culminates with downstream activation of effector caspases (2). A similar paradigm linking apoptosis control to surveillance mechanisms has been extended to checkpoint presiding over cell cycle transitions (3), which, at mitosis, control the assembly of a bipolar mitotic apparatus, preservation of ploidy, and timing of cytokinesis (4).
A candidate molecule for the interface between apoptosis and cell cycle was recently identified as survivin (5), a member of the inhibitor of apoptosis (IAP) gene family (6), selectively expressed at G2/M and localized to mitotic spindle microtubules (7). Antisense targeting of survivin resulted in increased caspase-3 activity at mitosis (7), with spontaneous apoptosis and dysregulation of mitotic progression (8), whereas interference with survivin homologs in Caenorhabditis elegans and yeast caused a lethal defect of cytokinesis (9, 10). Altogether, these data suggested a role of survivin in maintaining cell viability at mitosis, potentially coupling apoptosis control to regulation of cell division. This pathway may be dramatically exploited in cancer, where survivin was identified as the top fourth “transcriptome” expressed in human tumors, but not in normal tissues (11). Here, we investigated the potential requirements of survivin regulation of apoptosis at cell division.
Materials and Methods
Cells and Antibodies.
HeLa cells were from American Type Culture Collection. YUSAC2 melanoma cells were characterized previously (12). Cell-cycle synchronization was carried out as described (7). The antibodies to survivin were described previously (7, 12). A rabbit antibody was raised against the survivin peptide L28EGCACT*PERMAEAGFI44 containing phosphorylated Thr34 (T*). The serum was precleared over a nonphosphorylated peptide-Sepharose column, and unbound material was affinity-purified over a phosphorylated peptide-Sepharose column. Rabbit antibodies to caspase-9, p34cdc2, and Cdk2 were from PharMingen, Zymed, and Santa Cruz Biotechnology, respectively. A rat antibody to hemagglutinin epitope (HA) was from Roche Molecular Biochemicals.
Plasmids, Recombinant Proteins, and Transfections.
Site-directed mutagenesis of the survivin cDNA (5) with generation of survivin(T34A) in pEGFPc1 and pcDNA3, with or without HA tag, was carried out by using the GeneEditor system (Promega) (8). A caspase 9 dominant negative mutant (Cys287→Ala) was generated by overlapping PCR and directionally cloned in pcDNA3 or pEGFPc1. A kinase-dead p34cdc2(Asp146→Asn) mutant was generated as described (13), and inserted in pcDNA3. All mutant constructs were confirmed by DNA sequencing. Wild-type survivin or survivin(T34A) were expressed as glutathione S-transferase (GST) fusion proteins in BL21 Escherichia coli, isolated by affinity chromatography on GST-Sepharose and released from the GST frame by cleavage with thrombin (1 unit/ml) overnight (7). Transient transfections were carried out by Lipofectamine, as described (7, 8). Comparable expression of the various transfected constructs in HeLa cells was confirmed by flow cytometry by gating on the green fluorescent protein (GFP)-expressing population. A survivin(T34A) cDNA was inserted into pTet-splice, transfected (0.8 μg) in YUSAC2 cells with 0.8 μg of the transactivation/selection plasmid ptTA-Neo by Lipofectamine. Stable lines were selected in 1.5 mg/ml Geneticin (G418; Life Technologies, Grand Island, NY), 2 mM sodium hydroxide, and 0.5 μg/ml Tet (Sigma). Three clones were isolated for differential growth in the presence or absence of Tet, recloned by limiting dilution, and one of them (YUSAC2/T34A-C4) was selected for further investigation.
Immunoprecipitation and Western Blotting.
Two-hundred micrograms of precleared, detergent-solubilized HeLa cell extracts were immunoprecipitated with antibodies to p34cdc2 (5 μg/ml), Cdk2 (5 μg/ml), survivin (12) (20 μg/ml), or HA (2.5 μg/ml) for 16 h at 4°C, with precipitation of the immune complexes by addition of 50 μl of a 50:50 protein A slurry. After separation by SDS gel electrophoresis, samples were transferred to nylon membranes and incubated with antibodies to p34cdc2 (1 μg/ml), survivin (12) (2 μg/ml), survivinT34* (5 μg/ml), caspase-9 (1:2000 dilution), or HA (0.1 μg/ml) followed by HRP-conjugated anti-mouse (Amersham Pharmacia), anti-rabbit (Amersham Pharmacia), or anti-rat (Roche Molecular Biochemicals) antibodies and chemiluminescence (Amersham Pharmacia). In some experiments, HeLa cells transfected with HA-survivin were labeled with 200 μCi/ml 32PI (New England Nuclear) in 10% phosphate-free serum, detergent-solubilized, and immunoprecipitated with an antibody to HA (2.5 μg/ml) or control IgG, followed by autoradiography.
Kinase Assays.
Baculovirus-expressed human p34cdc2-cyclin B1 or Cdk2-cyclin E were incubated with histone H1 (1 μg), wild-type survivin, or survivin(T34A) (6 μg) in kinase buffer containing 10 μCi of [γ-32P]ATP (Amersham Pharmacia). Samples were separated by SDS gel electrophoresis, and phosphorylated bands were visualized by autoradiography. Equal protein loading was confirmed by Coomassie blue staining of the gel.
Flow Cytometry, Immunofluorescence, and Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL).
DNA content analysis by propidium iodide staining, scoring of apoptotic nuclei by 4′,6-diamidino-2-phenylindole (DAPI) staining, and double immunofluorescence labeling and confocal microscopy were carried out as described (7, 8). In some experiments, HeLa cells were treated with 5 μM taxol for 18 h at 37°C before labeling for survivin, Thr34 phosphorylated survivin, and caspase-9 and confocal microscopy analysis. Internucleosomal DNA fragmentation was carried out by end-labeling with terminal deoxynucleotidyl transferase and peroxidase-conjugated anti-digoxigenin antibody, as described (12).
Results and Discussion
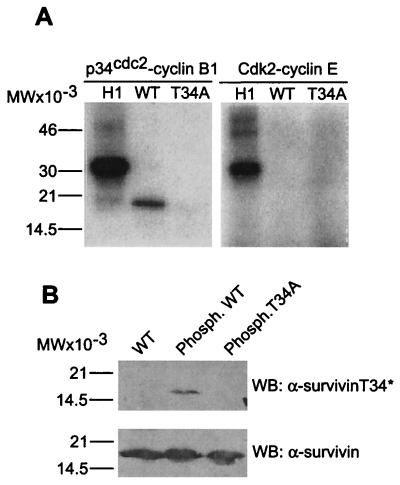
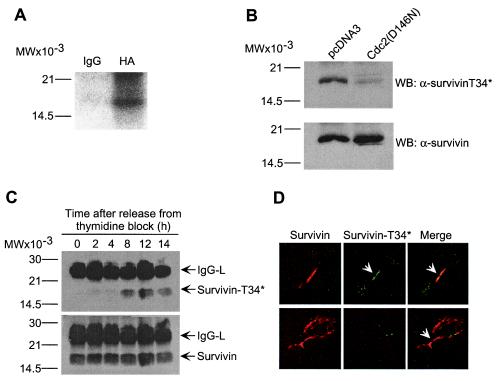
A sequence T34PER matching the consensus phosphorylation site (14) for p34cdc2 is found in human and mouse survivin, but not in other IAP proteins (6). In in vitro kinase assays, baculovirus-expressed p34cdc2-cyclin B1 readily phosphorylated histone H1 and wild-type survivin, whereas substitution of Thr34→Ala, i.e., survivin(T34A), abolished phosphorylation by p34cdc2-cyclin B1 (Fig. 1A). In contrast, baculovirus-expressed Cdk2-cyclin E did not phosphorylate wild-type survivin or survivin(T34A) (Fig. 1A). A rabbit antibody was raised against the survivin peptide L28EGCACT*PERMAEAGFI44 containing phosphorylated Thr34 (T*), sequentially affinity purified on nonphosphorylated/phosphorylated peptide-Sepharose and used in immunoblotting. The antibody to phosphorylated Thr34 (α-survivinT34*) recognized wild-type survivin after in vitro phosphorylation by p34cdc2-cyclin B1, but not unphosphorylated survivin or survivin(T34A) after incubation with p34cdc2-cyclin B1 (Fig. 1B). In contrast, an antibody to survivin (α-survivin) (12) indistinguishably recognized wild-type survivin or survivin(T34A), irrespective of Thr34 phosphorylation (Fig. 1B). In in vivo experiments, a 16.5-kDa phosphorylated survivin band was immunoprecipitated from orthophosphate-labeled, G2/M-arrested HeLa cells, whereas no radioactive bands were immunoprecipitated with a control antibody (Fig. 2A). Transfection of HeLa cells with a kinase-dead p34cdc2 mutant (Asp146→Asn) (13) nearly completely abolished phosphorylation of endogenous survivin on Thr34 by Western blotting with α-survivinT34* (Fig. 2B). In control experiments, comparable amounts of endogenous survivin were immunoprecipitated from HeLa cells transfected with control pcDNA3 or p34cdc2(Asp146→Asn) (Fig. 2B). We next investigated the kinetics of survivin phosphorylation in vivo. The antibody to phosphorylated Thr34 did not recognize survivin immunoprecipitated from synchronized HeLa cells at G1 or S phase 0, 2, and 4 h after thymidine release, respectively (Fig. 2C). In contrast, phosphorylation of survivin on Thr34 was detected beginning at 8 h after thymidine release, which coincides with entry into mitosis of these cells by DNA content analysis (7), and remained sustained throughout cell division (Fig. 2C). In contrast, α-survivin recognized survivin immunoprecipitated from synchronized interphase and mitotic HeLa cells (Fig. 2C). By immunofluorescence and confocal microscopy, both endogenous survivin and phosphorylation-defective survivin(T34A) localized to midbodies at telophase (Fig. 2D) in agreement with previous observations (7). Although endogenous survivin was phosphorylated on Thr34 on microtubules, no reactivity of α-survivinT34* with survivin(T34A) was demonstrated at midbodies by dual immunofluorescence labeling (Fig. 2D).
Figure 1.
Survivin phosphorylation on Thr34 by p34cdc2-cyclin B1. (A) Kinase assay. Wild-type (WT) survivin, survivin(T34A) (T34A), or histone H1 (H1) were incubated with the indicated baculovirus-expressed kinase complexes, and phosphorylated bands were visualized by autoradiography. (B) Recognition of Thr34 phosphorylated survivin by Western blotting. WT survivin or survivin(T34A) were immunoblotted with affinity-purified antibodies to phosphorylated Thr34 (α-survivinT34*) or survivin (α-survivin) (12), before or after kinase assay (phosph.) with p34cdc2-cyclin B1. Relative molecular weight markers are shown on the left of each panel. WB, Western blotting.
Figure 2.
Regulation of survivin phosphorylation by p34cdc2 in vivo. (A) In vivo phosphorylation of survivin. HeLa cells were transfected with HA-survivin, labeled with 200 μCi/ml 32PI and immunoprecipitated with control IgG or anti-HA followed by autoradiography. (B) Requirement of p34cdc2 kinase activity for survivin phosphorylation on Thr34. Endogenous survivin was immunoprecipitated from HeLa cells transfected with control pcDNA3 or kinase-dead p34cdc2 mutant (Cdc2(D146N)), and analyzed with α-survivinT34* or α-survivin, by Western blotting. No significant loss in cell viability was observed in HeLa cells expressing Cdc(D146N). (C) Kinetics of survivin phosphorylation on Thr34. Endogenous survivin was immunoprecipitated from cell cycle-synchronized HeLa cells at the indicated time intervals after thymidine release, and immunoblotted with α-survivinT34* or α-survivin. IgG-L, Ig light chain. (D) Localization of Thr34 phosphorylated survivin to midbodies. Endogenous survivin (Top) or HA-survivin(T34A) transfected in HeLa cells (Bottom) were labeled with mAb 8E2 to survivin (8) or an antibody to HA, respectively, plus α-survivinT34*. Binding of the primary antibodies was detected by addition of Texas Red (TR)-conjugated goat anti-mouse (survivin, red) and FITC-conjugated goat anti-rabbit (Thr34 phosphorylated survivin, green) antibodies. Coverslips were analyzed by confocal laser scanning microscopy. Image merging analysis is shown on the right. (A–C) Relative molecular weight markers are indicated on the left. WB, Western blotting.
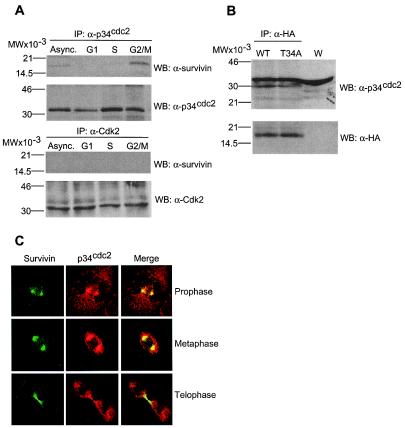
We next asked whether survivin and p34cdc2 physically interacted in vivo. Immunoprecipitates of p34cdc2 from G2/M-arrested HeLa cells contained coassociated 16.5-kDa survivin by Western blotting (Fig. 3A). In contrast, no survivin bands were immunoblotted in p34cdc2 immunoprecipitates from G1- or S-synchronized cultures (Fig. 3A). Similarly, Cdk2 immunoprecipitates from synchronized HeLa cells did not contain 16.5-kDa coassociated survivin at any cell cycle phase (Fig. 3A). In reciprocal experiments, HA-survivin or HA-survivin(T34A) immunoprecipitated from HeLa cells contained coassociated p34cdc2 by Western blotting (Fig. 3B), thus demonstrating that a survivin-p34cdc2 interaction was independent of Thr34. Furthermore, endogenous p34cdc2 and survivin colocalized on mitotic spindle microtubules at prophase and metaphase, and concentrated at midbodies at telophase, by immunofluorescence and confocal microscopy (Fig. 3C), in agreement with their individual localization (7, 15). This suggests that a phosphorylation-defective survivin(T34A) mutant may act as a dominant negative mutant for its ability to associate with p34cdc2 on the mitotic apparatus and prevent phosphorylation of endogenous survivin.
Figure 3.
Physical association between survivin and p34cdc2. (A) Cdk immunoprecipitation. HeLa cells asynchronously growing (Async.) or synchronized to G1, S, or G2/M were detergent-solubilized and immunoprecipitated (IP) with antibodies to p34cdc2 (Upper), or Cdk2 (Lower) followed by Western blotting with antibodies to survivin, p34cdc2, or Cdk2. (B) Survivin immunoprecipitation. HeLa cells transfected with HA-survivin or HA-survivin(T34A) were immunoprecipitated with an antibody to HA followed by Western blotting with antibodies to p34cdc2 or HA. W, whole extract. (C) Colocalization of survivin and p34cdc2 to the mitotic apparatus. Untransfected HeLa cells at the indicated phases of mitosis were labeled with mAb 8E2 to survivin (FITC, green) and a rabbit antibody to p34cdc2 (TR, red), and analyzed by confocal microscopy. Image merging analysis is shown on the right. (A and B) Relative molecular weight markers are on the left. WB, Western blotting.
We next investigated a potential role of survivin phosphorylation by p34cdc2 in apoptosis control and/or cell cycle progression (8). In the absence of exogenous apoptotic stimuli, transfection of HeLa cells with survivin(T34A) fused to a GFP caused apoptotic morphology of chromatin condensation and DNA fragmentation in GFP-expressing cells (Fig. 4A). This was associated with generation of hypodiploid cells by DNA content analysis and flow cytometry, in a reaction reversed by the caspase inhibitor Z-VAD-fmk (Fig. 4B). Similar results were obtained after transfection of GFP-caspase-9 (Met1-Asp330), whereas expression of GFP vector or wild-type survivin did not affect nuclear morphology in HeLa cells (Fig. 4 A and B). Next, stable lines of survivin-positive YUSAC2 melanoma cells (12) were generated to express survivin(T34A) under the control of a tetracycline (Tet)-regulated promoter (Tet-off system) (16), and one clone (YUSAC2/T34A-C4) was selected for further investigation. On Tet withdrawal, YUSAC2/T34A-C4 cells exhibited loss of the mitotic (G2/M) fraction, appearance of hypodiploid cells, and labeling for internucleosomal DNA fragmentation by TUNEL (Fig. 4C), thus distinguishing this pathway from mitotic catastrophe at G2/M (17). In contrast, Tet+ YUSAC2/T34A-C4 cells had normal viability and DNA content, and did not label by TUNEL (Fig. 4C). To determine the kinetics of apoptosis induced by survivin(T34A) with respect to cell cycle progression, YUSAC2/T34A-C4 cells were thymidine-synchronized in the presence or absence of Tet, released and analyzed for DNA content at increasing 3-h intervals. Tet+ or Tet− YUSAC2/T34A-C4 cells exhibited comparable kinetics of cell cycle progression, approaching the first mitosis 9 h after thymidine release, completing cell division by 15–18 h, and reentering G1 after 21 h (Fig. 4D). However, coinciding with entry into mitosis, Tet− YUSAC2/T34A-C4 cells began to accumulate within the hypodiploid apoptotic fraction, which increased steadily throughout mitosis and in the postmitotic phase (Fig. 4D). In contrast, no changes in the hypodiploid fraction were observed in Tet+ YUSAC2/T34A-C4 cells at the various cell cycle phases (Fig. 4D). Coinciding with entry into mitosis, Tet− YUSAC2/T34A-C4 cells also exhibited progressive proteolytic cleavage of ≈46 kDa proform caspase-9 to active subunits of ≈35 kDa and ≈37 kDa, which peaked in the postmitotic phase with nearly complete disappearance of the ≈46-kDa proform band (Fig. 4E). In contrast, no proteolytic processing of caspase-9 was observed in Tet+ YUSAC2/T34A-C4 cells at the various cell cycle phases (Fig. 4E).
Figure 4.
Regulation of cell viability at mitosis by survivin phosphorylation on Thr34. (A) Nuclear morphology. HeLa cells transfected with GFP-vector (vector), GFP-survivin(T34A) (T34A), or GFP-caspase-9 (Casp.9) were scored morphologically by DAPI staining after a 48-h culture. The percentages of apoptotic cells were: GFP-vector, 3 ± 2.5; WT-survivin (not shown in the figure), 4.8 ± 5; survivin(T34A), 76.5 ± 1.4; caspase-9, 79.5 ± 9.1 (mean ± SD, n = 2–5). (B) Caspase-dependence. HeLa cells transfected with GFP-survivin or GFP-survivin(T34A) with or without 20 μM of the caspase inhibitor Z-VAD-fmk were analyzed for DNA content by propidium iodide staining and flow cytometry. The percentages of apoptotic cells with hypodiploid DNA content are indicated. Data are representative of one experiment of three independent determinations. (C) Tet-regulated expression of survivin(T34A). YUSAC2/T34A-C4 cells expressing survivin(T34A) on Tet removal (Tet-off system) were analyzed for DNA content after a 3-day culture in the presence or absence of Tet (Tet+/Tet−). The percentages of cells with subG1 (apoptotic) DNA content are indicated. (Insets) Internucleosomal DNA fragmentation by TUNEL staining of Tet+ and Tet− YUSAC2/T34A-C4 cells. (D) Kinetics of apoptosis and cell cycle progression during Tet-regulated expression of survivin(T34A). Synchronized YUSAC2/T34A-C4 with or without Tet were analyzed for DNA content at the indicated time intervals after thymidine release. The percentages of apoptotic cells under the various conditions tested was 0 h, Tet+ 3.8%/Tet− 3.6%; 3 h, Tet+ 2.7%/Tet− 10%; 6 h, Tet+ 4.8%/Tet− 8.3%; 9 h, Tet+ 3.4%/Tet− 9%; 12 h, Tet+ 2.7%/Tet− 12.2%; 15 h, Tet+ 5%/Tet− 20%; 18 h, Tet+ 4.3%/Tet− 25%; 21 h, Tet+ 7%/Tet− 32%; 24 h, Tet+ 11%/Tet− 40%. (E) Western blotting of caspase-9 cleavage in synchronized Tet+ and Tet− YUSAC2/T34A-C4 cells. Arrows, position of ≈46-kDa proform caspase-9 and of ≈35-kDa and ≈37-kDa active caspase-9 subunits. TNFα, Extracts of apoptotic HeLa cells treated with 10 ng/ml TNFα plus 10 μg/ml cycloheximide. Data are representative of one experiment of three independent determinations.
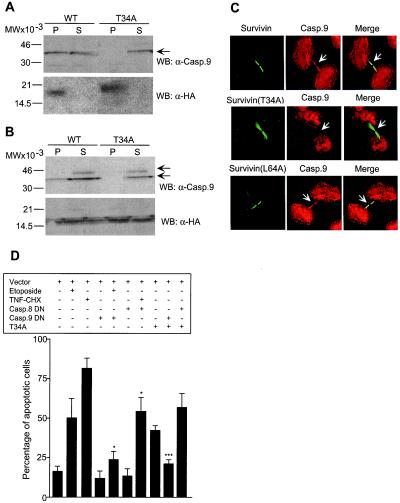
Next, we asked whether survivin phosphorylation on Thr34 was required to modulate a potential interaction with effector molecules of apoptosis, and we focused on the intrinsic initiator caspase-9 (18) for its association with survivin in affinity purification experiments (unpublished observations). Immunoprecipitates of HA-survivin from mitotic HeLa cells collected by mitotic shake-off revealed the presence of coassociated ≈35-kDa caspase-9, by immunoblotting (Fig. 5A). In contrast, HA-survivin(T34A) immmunoprecipitated from mitotic HeLa cells did not contain coassociated caspase-9 (Fig. 5A). Similar results were obtained in drug-synchronized cultures. HA-survivin immunoprecipitates from mitotic HeLa cells 12 h after thymidine release contained coassociated caspase-9 of ≈35 kDa, whereas no caspase-9 bands were coprecipitated with HA-survivin(T34A) from mitotic HeLa cells (Fig. 5B). By immunofluorescence and confocal microscopy, HA-survivin and HA-survivin(T34A) transfected in HeLa cells bound to mitotic spindle microtubules and accumulated at midbodies at telophase, indistinguishably from endogenous survivin (Fig. 5C), and in agreement with the data presented above. In HA-survivin transfectants, simultaneous labeling for caspase-9 revealed a diffuse cytoplasmic reactivity, and a prominent colocalization between survivin and caspase-9 at midbodies (Fig. 5C). In contrast, and consistent with the immunoprecipitation experiments described above, caspase-9 did not colocalize with HA-survivin(T34A) at midbodies (Fig. 5C). In control experiments, a survivin Leu64→Ala mutant did not cause apoptosis (7), and colocalized with caspase-9 at midbodies (Fig. 5C). Next, we asked whether cell death induced by survivin(T34A) was mediated by mislocalized, active caspase-9. Transfection of HeLa cells with a caspase-9(C287A) dominant negative mutant (19) inhibited nuclear fragmentation and chromatin condensation induced by the anti-cancer drug etoposide, and reversed HeLa cell apoptosis induced by survivin(T34A) (Fig. 5D). In contrast, cotransfection of HeLa cells with a caspase-8(C360S) dominant negative mutant did not affect apoptosis induced by survivin(T34A), whereas it completely inhibited cell death induced by TNFα + cycloheximide (20) (Fig. 5D).
Figure 5.
Modulation of survivin-caspase-9 complex by survivin phosphorylation on Thr34. (A) Immunoprecipitation from nonadherent cells. HeLa cells transfected with HA-survivin (WT) or HA-survivin(T34A) (T34A) were harvested after mitotic shake off, immunoprecipitated with anti-HA followed by Western blotting (WB) with an antibody to caspase-9 (casp.9). (B) Immunoprecipitation from synchronized cells. HeLa cells were transfected with HA-survivin (WT) or HA-survivin(T34A) (T34A), immunoprecipitated with anti-HA 12-h after thymidine release, and analyzed for coassociated caspase-9 by Western blotting. Arrows, position of 46-kDa proform caspase-9 and ≈35-kDa active caspase-9. P, pellet; S, supernatant. (A and B) Blots were sequentially immunoblotted with anti-HA. (C) Mislocalization of caspase-9 from midbodies in survivin(T34A)-expressing cells. HeLa cells transfected with HA-survivin (Survivin), survivin(T34A), or survivin(L64A) were labeled for survivin (FITC, green) with a mAb to HA or mAb 8E2 (Bottom), and caspase-9 (Casp.9, TR, red), and analyzed by confocal microscopy. Image-merging analysis is shown on the right. Arrows, differential localization of wild-type survivin, survivin(T34A), or survivin(L64A) with caspase-9 at midbodies. Experiments were repeated at least four times with comparable results. (D) Apoptosis in survivin(T34A)-expressing cells is mediated by caspase-9. HeLa cells transfected with the various indicated combinations of GFP-constructs, with or without etoposide (10 μg/ml) or TNFα (TNF, 10 ng/ml) plus cycloheximide (CHX, 10 μg/ml), were morphologically scored for nuclear fragmentation by DAPI staining. DN, dominant negative. *, P < 0.05; ***, P < 0.0005. Data are the mean ± SEM of four independent experiments.
In summary, these data identify survivin as a mitotic substrate of p34cdc2-cyclin B1 (21), and suggest that survivin phosphorylation on Thr34 may regulate apoptosis at cell division via an interaction with caspase-9 (2). Although a role of unscheduled p34cdc2 activity in apoptosis has been debated (22, 23), the data presented here are consistent with a cytoprotective role of p34cdc2, previously suggested from genetic inactivation (24), or pharmacologic inhibition of the kinase (25). Similar to other mitotic substrates (26, 27), phosphorylation by p34cdc2 may increase the affinity of survivin for active caspase-9, or, alternatively, stabilize survivin or a survivin-caspase-9 anti-apoptotic complex at midbodies during cell division. Based on the survivin crystal structure, Thr34 is ideally positioned to influence survivin function(s) potentially mediated by the baculovirus IAP repeat (BIR) (28). Conversely, loss of survivin phosphorylation on Thr34 resulted in dissociation of a survivin-caspase-9 complex at midbodies and caspase-9-depedent apoptosis at cell division. Although survivin clearly plays an evolutionary conserved role (9, 10) in regulation of mitotic progression and cytokinesis (8), cells expressing survivin(T34A) progressed through the cell cycle and did not exhibit a G2/M arrest, as typically seen after interference with regulators of cytokinesis (29, 30). Taken together with the identification of a Drosophila survivin homolog, Deterin, which acts as a genuine apoptosis inhibitor (31), these data suggest that survivin may have integrated a primordial role in cell division (9, 10) with a more recent function in apoptosis control at G2/M. Because of the over-expression of survivin in cancer but not in normal tissues (5, 11), selective antagonists of survivin phosphorylation by p34cdc2 may trigger apoptosis of transformed cells and facilitate their elimination at mitosis.
Acknowledgments
We thank Drs. J. C. Reed for discussion, D. Schatz for pTA-Neo plasmid, V. Dixit for caspase-3, -9, and caspase-8(C360A) cDNAs, and K. Weber and M. Osborn for mAb 20C6. This work was supported by National Institutes of Health Grants CA78810, HL54131 (to D.C.A.) and CA72878 (to H.Z.), and Associazione Italiana per la Ricerca sul Cancro and Telehon (to P.C.M.). D.G. was supported by National Institutes of Health Dermatology Training Grant 5T32AR07016 and by a Dermatologist Investigator Research Fellowship from the Dermatology Foundation.
Abbreviations
- IAP
inhibitor of apoptosis
- BIR
baculovirus IAP repeat
- HA
hemagglutinin epitope
- GST
glutathione S-transferase
- GFP
green fluorescent protein
- DAPI
4′,6-diamidino-2-phenylindole
- Tet
tetracycline
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240390697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240390697
References
- 1.Vaux D L, Korsmeyer S J. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Green D R. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 3.Evan G, Littlewood T. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 4.Pines J. Nat Cell Biol. 1999;1:E73–E79. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosini G, Adida C, Altieri D C. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 6.Deveraux Q L, Reed J C. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Ambrosini G, Chu E Y, Plescia J, Tognin S, Marchisio P C, Altieri D C. Nature (London) 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Ackermann E J, Bennett C F, Rothermel A L, Plescia J, Tognin S, Villa A, Marchisio P C, Altieri D C. Nat Cell Biol. 1999;1:461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 9.Fraser A G, James C, Evan G I, Hengartner M O. Curr Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 10.Uren A G, Beilharz T, O'Connell M J, Bugg S J, van Driel R, Vaux D L, Lithgow T. Proc Natl Acad Sci USA. 1999;96:10170–10175. doi: 10.1073/pnas.96.18.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velculescu V E, Madden S L, Zhang L, Lash A E, Yu J, Rago C, Lal A, Wang C J, Beaudry G A, Ciriello K M, et al. Nat Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 12.Grossman D, McNiff J M, Li F, Altieri D C. J Invest Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 13.van den Heuvel S, Harlow E. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 14.Holmes J K, Solomon M J. J Biol Chem. 1996;271:25240–25246. doi: 10.1074/jbc.271.41.25240. [DOI] [PubMed] [Google Scholar]
- 15.Ookata K, Hisanaga S, Bulinski J C, Murofushi H, Aizawa H, Itoh T J, Otani H, Okumura E, Tachibana K, Kishimoto T. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shockett P, Difilippantonio M, Hellman N, Schatz D G. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan T A, Hermeking H, Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 18.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 19.Duan H, Orth K, Chinnaiyan A M, Poirier G G, Froelich C J, He W-W, Dixit V M. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 20.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 21.Nurse P. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 22.Shi F, Nishioka W K, Th'Ng J, Bradbury E M, Litchfield D W, Greenberg A H. Science. 1994;263:1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- 23.Martin S J, McGahon A J, Nishioka W K, La Face D, Guo X, Th'Ng J, Bradbury E M, Green D R. Science. 1995;269:106–107. doi: 10.1126/science.7604270. [DOI] [PubMed] [Google Scholar]
- 24.Itzhaki J E, Gilbert C S, Porter A C G. Nat Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- 25.Patel V, Senderowicz A M, Pinto D, Jr, Igishi T, Raffeld M, Quintanilla-Martinez L, Ensley J F, Sausville E A, Gutkind J S. J Clin Invest. 1998;102:1674–1681. doi: 10.1172/JCI3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blangy A, Arnaud L, Nigg E A. J Biol Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- 27.Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jamsa E, Rahman D, Pappin D J C, Warren G. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 28.Muchmore S W, Chen J, Jakob C, Zakula D, Matayoshi E D, Wu W, Zhang H, Li F, Ng S-C, Altieri D C. Mol Cell. 2000;6:173–182. [PubMed] [Google Scholar]
- 29.Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Nature (London) 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 31.Jones G, Jones D, Zhou L, Steller H, Chu Y. J Biol Chem. 2000;275:22157–22165. doi: 10.1074/jbc.M000369200. [DOI] [PubMed] [Google Scholar]