Abstract
Idiopathic ulmonary fibrosis (histopathology of usual interstitial pneumonia) is a progressive lung disease of unknown etiology. No treatment has been shown to improve the prognosis of the patients with this disease. Recent evidence, including the observations that the patients with idiopathic pulmonary fibrosis have higher levels of oxidant stress than control patients, and a recent multicenter European study examining the effect of the antioxidant N-acetylcysteine on the progression of idiopathic pulmonary fibrosis suggest that the cellular redox state may play a significant role in the progression of this disease. These complex mechanisms include activation of growth factors as well as regulation of matrix metalloproteinases and protease inhibitors. Potential future approaches for the therapy of interstitial pulmonary fibrosis may involve synthetic agents able to modulate cellular redox state. Investigation into therapeutic approaches to inhibit oxidant-mediated reactions in the initiation and progression of pulmonary fibrosis may provide hope for the future treatment of this disease.
Keywords: antioxidant, idiopathic pulmonary fibrosis, oxidant, radical
Idiopathic pulmonary fibrosis (IPF) is a prototype of idiopathic interstitial pneumonias, a chronic disease of the lung parenchyma that leads to diffuse scarring and end-stage tissue fibrosis (1). Typical features in this disease include dyspnea, diffuse interstitial infiltrates, progressive lung fibrosis, and poor prognosis. The pathologic changes in IPF include patchy fibrotic lesions that vary both in age and activity, and only weak inflammation. The focal zones of fibroblast proliferation are called “fibroblastic foci” and appear to occur at sites of recent alveolar injury (1, 2). The biochemical mechanisms in the pathogenesis of IPF are still poorly understood and medical therapies have thus far offered little if any benefit against the progression of this disease (1, 3, 4).
As the name implies, there is no known etiologic stimulus that initiates this disease. Current evidence suggests that both endogenous and exogenous stimuli may injure the alveolar epithelium. This is followed by an abnormal repair process in individuals unable to effectively heal the damage. The majority of the original articles and reviews on IPF have focused on the fibrotic process, importance of the fibroblastic foci in disease progression, and on the therapeutic regimens with antiinflammatory/antifibrotic drugs, such as corticosteroids, cytotoxic drugs, and IFN-γ (3, 5). However, there are a number of studies suggesting that the cellular redox state and the balance of oxidants/antioxidants play a significant role in the progression of pulmonary fibrosis in animal models and also possibly in human IPF. This article reviews the importance of oxidants and lung oxidant–antioxidant balance in human lung fibrogenesis. Although experimental models of lung fibrosis are difficult to extrapolate to human IPF due to differences in inflammation and the occurrence of fibroblastic foci, many similarities exist between them. Therefore, the key experimental studies examining potential mechanisms by which oxidative stress contributes to pulmonary fibrosis will also be emphasized.
MAJOR GENERATORS OF FREE RADICALS IN HUMAN LUNG
The lung is exposed to higher oxygen tension than other tissues. Exogenous oxidants and pollutants further increase oxidant production and activate inflammatory cells to generate free radicals. Many of these agents, including hyperoxia, cigarette smoke, asbestos fibers, drugs, and radiation, are also known to be associated with fibrotic interstitial lung reactions. Several important reactive oxygen species (ROS) are generated endogenously in these circumstances. They include the superoxide radical, hydrogen peroxide (H2O2), and the hydroxyl radical. The major enzymes/reaction pathways that can be activated to generate ROS in human lung include nicotinamide adenine dinucleotide phosphate oxidases, myeloperoxidase, eosinophil peroxidase, mitochondrial electron transport chain, and possibly xanthine oxidase (reviewed in References 6, 7). Microsomal and nuclear membrane cytochromes can also generate ROS, and these redox-cycling reactions may be linked to smoke- and drug-induced interstitial lung diseases. A number of reactive nitrogen species (RNS), such as peroxynitrite, can also be formed by reactions of superoxide with nitric oxide (NO). The majority of NO is produced by the inducible form of nitric oxide synthase (iNOS, NOS2), especially during inflammatory states in the lung. Constitutive forms of NOS are also expressed widely in human lung cells and have been shown to regulate cell signaling in normal cell homeostasis. Overall, a wide variety of oxidants are produced in response to injuries leading to pulmonary fibrosis. These oxidants can activate several genes related to cell growth, cell death, and fibroblast proliferation.
PRIMARY ANTIOXIDANT PROTECTION OF THE LUNG
A balance between intracellular and extracellular oxidants and antioxidants is a prerequisite for normal lung homeostasis. The lung has highly specialized and compartmentalized antioxidant defenses to protect against ROS and RNS. These include the following: (1) small-molecular-weight antioxidants (e.g., glutathione, vitamins, uric acid), (2) mucins, (3) metal-binding proteins (transferrin, lactoferrin, metallothionein, etc.), (4) superoxide dismutases (SODs; e.g., mitochondrial manganese SOD [MnSOD], intracellular copper zinc SOD [CuZnSOD], and extracellular SOD [ECSOD]), (5) a group of enzymes that decomposes H2O2 (numerous glutathione-associated enzymes and catalase), (6) detoxification enzyme systems (e.g., glutathione-S-transferases), and (7) other redox regulatory thiol proteins (e.g., thioredoxin-peroxiredoxin system and glutaredoxins) (7–9). These enzymes are localized in specific cell types, such as bronchial and alveolar epithelial cells and alveolar macrophages, but can also exist extracellularly. Many of these enzymes are regulated by a redox-sensitive Nrf2 transcription factor that is essential to the induction of antioxidant enzymes and related proteins in the lung. Nrf2 may be of particular importance in IPF because Nrf2 deficiency significantly enhances bleomycin-induced pulmonary fibrosis in mice (10). Induction of these antioxidant enzymes and related proteins after pulmonary insults may protect the lung and promote normal repair. Conversely, impaired induction or inactivation/clearance of antioxidant enzymes may result in a sustained redox imbalance that may contribute to the progression of pulmonary fibrosis.
OXIDANT–ANTIOXIDANT IMBALANCE IN IPF
Several studies suggest that oxidant–antioxidant imbalances in the lower respiratory tract play a critical role in the pathogenesis of IPF. For example, pulmonary inflammatory cells of patients with IPF generate higher levels of oxidants than those in control patients (11). Bronchoalveolar lavage fluid of patients with IPF show elevated levels of myeloperoxidase and eosinophil cationic protein, suggesting a pathophysiologic role for neutrophils and possibly eosinophils in this disease (12). There is also evidence that increased myeloperoxidase is associated with epithelial injury in IPF (11). Mitochondrial generation of ROS has been suggested to be associated not only with increased cellular oxidative stress but also with apoptosis of alveolar epithelial cells (13, 14). Bronchoalveolar lavage fluid of patients with IPF contains higher levels of 8-isoprostane, a biomarker of oxidative stress, than that of control subjects (15). Patients with IPF have also been shown to have elevated levels of exhaled NO (reviewed in Reference 16). In addition, lung specimens of the patients with IPF show elevated expression of iNOS (17, 18). These findings suggest that patients with IPF have increases in both oxidative and nitrosative stress.
The presence of an oxidant–antioxidant imbalance in IPF is also indicated by the finding of altered levels of antioxidants in the lungs of affected patients. For example, patients with IPF have decreased levels of reduced glutathione in the epithelial lining fluid of their lungs (19–22). Several studies suggest that a number of antioxidant and detoxification enzymes are elevated in inflammatory and/or granulomatous interstitial lung diseases and in areas of epithelial regeneration; however, they are low/absent in the fibrotic lesions of IPF lungs. These enzymes include MnSOD, catalase, glutamate cysteine ligase (γ-glutamyl cysteine synthetase, the rate-limiting enzyme in glutathione synthesis), thioredoxin, glutaredoxin, and heme-oxygenase 1 (23–26). Although a decrease in antioxidant capacity likely increases oxidative stress, the observed elevations in antioxidants probably attempt to compensate for increased oxidative stress in the lung. It is, however, clear that there is a disruption of the normal redox balance in the lungs of patients with IPF.
ROS AS MODULATORS OF PROTEASE–ANTIPROTEASE BALANCE
Current evidence suggests that imbalance in proteases/antiproteases contributes to the pathogenesis of IPF. The importance of proteases in IPF was highlighted by the finding that matrilysin (matrix metalloproteinase 7 [MMP-7]) is significantly associated with pulmonary fibrosis in animal models, and is also overexpressed in human lung with IPF (27). Other MMPs are also upregulated in IPF (28). Importantly, both ROS and RNS may contribute to a protease–antiprotease imbalance because they can activate MMPs and inactivate protease inhibitors.
MMPs share a common mode of activation termed the “cysteine switch” whereby disruption of the active site cysteine–zinc bond leads to autocatalytic cleavage of the prodomain and activation of the latent enzyme (reviewed in Reference 29). Modification of the cysteine switch by ROS or RNS can activate MMPs. Hypochlorous acid oxidizes and activates MMP-7 (30). H2O2, peroxynitrite, and oxidants produced by the xanthine/xanthine oxidase system can activate both MMP-2 and MMP-9 (29). The cysteine switch of MMP-9 can also be activated by NO (29). In addition, ROS have also been shown to directly induce MMP transcription (29). Importantly, ROS do not always activate proteases, but can inactivate them as well (30). Therefore, it is likely that local concentrations of ROS/RNS will determine whether MMPs are activated or inactivated. It has been suggested that the rapid activation and subsequent inactivation of MMPs by oxidative mechanisms could regulate powerful “quantum bursts” of proteolytic activity (31).
The primary antioxidant enzymes in the extracellular matrix and alveolar lining fluid that may inhibit oxidative activation or inactivation of MMPs are extracellular glutathione peroxidase (32) and ECSOD (9). Augmentation of glutathione levels with N-acetylcysteine (NAC) treatment has been shown to inhibit MMP activation (33). Mice lacking ECSOD show increased MMP activity in animal models of pulmonary fibrosis compared with wild-type mice (unpublished observation). ECSOD binds to the lung matrix via a matrix-binding domain that is itself sensitive to proteolysis (9, 34). In fact, there is enhanced proteolytic cleavage of ECSOD's matrix-binding domain and clearance of ECSOD from the matrix in various injury models that lead to pulmonary fibrosis (35, 36). Overall, oxidative and proteolytic processes can amplify each other and enhance injuries leading to pulmonary fibrosis.
In addition to protease activation, both ROS and RNS can inactivate protease inhibitors and further alter the protease–antiprotease balance. Some examples include tissue inhibitor of metalloproteinase (TIMP-1) inactivation by peroxynitrite (37) and ROS-mediated inactivation of α2-macroglobulin (38). Secretory leukoprotease inhibitor has been shown to ameliorate pulmonary fibrosis, but notably can be inactivated by oxidants (39). In addition, α1-proteinase inhibitor (α1PI) is sensitive to oxidation and inactivation by peroxynitrite (40) and oxidants produced by myeloperoxidase and xanthine oxidase. The absence of α1PI activity has been linked to the development of pulmonary fibrosis, and mice treated with α1PI (41) are resistant to pulmonary fibrosis. Collectively, this evidence suggests that oxidative inactivation of α1PI contributes to the pathogenesis of pulmonary fibrosis.
ROS AND TRANSFORMING GROWTH FACTOR β
Numerous cytokines and growth factors have been implicated as mediators in the pathogenesis of pulmonary fibrosis. In addition to directly injuring lung cells and matrix, oxidants may also contribute to the development of pulmonary fibrosis by their direct effects on cytokines and growth factors. One such mediator, transforming growth factor β (TGF-β), is a key regulator of both normal wound repair and the aberrant repair mechanisms characteristic of many fibrotic diseases, including pulmonary fibrosis.
Previous studies have shown that TGF-β1, if released soon after injury, acts primarily as a proinflammatory molecule because it has potent chemotactic effects on inflammatory cells (42). Later, TGF-β1 function switches to resolution of inflammation and initiation of repair. It has been hypothesized that the persistence of chronic fibrosis may be due to unabated continuation of repair processes after resolution of the inflammatory response. TGF-β therefore may be an important mediator of chronic but abnormal repair. For example, TGF-β is believed to be a central regulator of the recruitment, activation, and differentiation of myofibroblasts in early stages of tissue repair (43). The persistence of the myofibroblastic phenotype in the areas of active fibrosis is a characteristic finding in fibrotic lung disease (44). TGF-β itself can also stimulate accumulation of extracellular matrix through increased transcription of collagen mRNA (45). Therefore, consistently elevated levels of TGF-β in the lung may serve as a stimulus for myofibroblast activation and production of extracellular matrix.
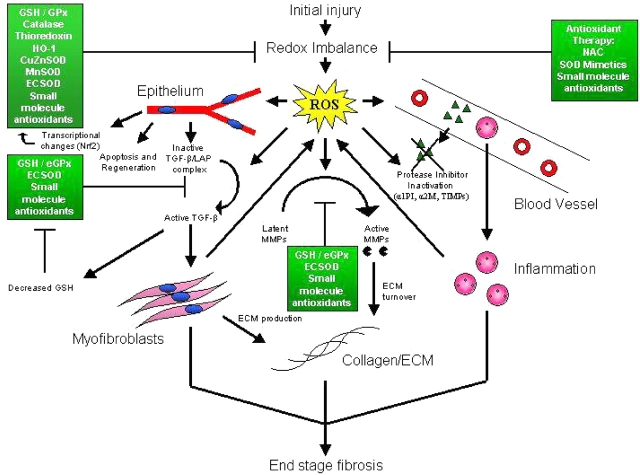
There are several potential interactions between TGF-β and oxidants/antioxidants in the lung. For example, TGF-β–differentiated myofibroblasts can themselves serve as a source of oxidant production (46). Furthermore, in vitro studies have shown that ROS increase the release of TGF-β from pulmonary epithelial cells (47) and can directly activate TGF-β by disrupting its interaction with latency-associated peptide (48), suggesting a positive feedback mechanism within the myofibroblast microenvironment. TGF-β has been shown to activate NADPH oxidase in human fibroblasts, leading to increased production of ROS (49). Furthermore, TGF-β has been shown to downregulate glutamate cysteine ligase mRNA synthesis, the rate-limiting enzyme in the production of the antioxidant molecule glutathione (50), which is known to be decreased in IPF (see above). Glutathione synthesis is decreased in TGF-β–overexpressing mice, and these mice are also more susceptible to oxidant-induced lung injury (51). Thus, oxidants and TGF-β may interact to enhance the fibrotic response in patients with IPF (Figure 1).
Figure 1.
Potential roles of reactive oxygen species (ROS) in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Exogenous and endogenous irritants in IPF create a redox imbalance, resulting in the production of ROS. Widespread effects on epithelium, myofibroblasts, growth factors (e.g., transforming growth factor β [TGF-β]), inflammatory cells, proteases (e.g., matrix metalloproteinases [MMPs]), protease inhibitors, and the extracellular matrix (ECM) may ultimately contribute to the development of end-stage fibrosis. Shown also are endogenous antioxidants (green boxes) and the steps at which they can protect the lungs from the effects of ROS. Processes outside the cell, such as the activation of TGF-β and MMPs, would be primarily affected by the major extracellular antioxidants, including glutathione (GSH), extracellular superoxide dismutase (ECSOD), and other small molecules, such as ascorbate. Exogenous antioxidants, such as N-acetylcysteine (NAC) and SOD mimetics can augment antioxidant defenses and thus serve as potential therapies for IPF. α1PI = α1-proteinase inhibitor; α2M = α2-macroglobulin; CuZnSOD = copper/zinc superoxide dismutase; ECSOD = extracellular superoxide dismutase; eGPx = extracellular glutathione peroxidase; GPx = glutathione peroxidase; HO-1 = heme-oxygenase 1; MnSOD = manganese superoxide dismutase; TIMP = tissue inhibitor of metalloproteinases.
EXOGENOUS ANTIOXIDANTS AND ANTIOXIDANT MIMETICS IN PULMONARY FIBROSIS
Experimental Models of Lung Fibrosis
It is well known that exogenous agents, including asbestos, radiation, and drugs, can cause pulmonary fibrosis through production of ROS/RNS in animal models (52). Studies examining these models have shown not only increased oxidant burden on exposure to these agents but also that exogenous treatment with antioxidants can protect the lung in vivo. The most widely investigated antioxidants in these models include glutathione, NAC, and SODs. Glutathione, however, crosses cell membranes poorly and can cause several side effects, including bronchoconstriction (53, 54). As an alternative, NAC has been shown to improve glutathione homeostasis by increasing cysteine levels, the rate-limiting substrate in glutathione synthesis. Notably, NAC significantly decreases primary inflammatory reactions, collagen deposition, and the progression of bleomycin-induced lung fibrosis (55–57). NAC, however, also has prooxidant characteristics, and there are studies suggesting that NAC does not necessarily improve lung glutathione homeostasis (53, 54). Other compounds with NAC-like activity, including glutathione esters and glutathione precursors, have been tested in many laboratories, but the effects of these compounds in fibrotic lung disorders is unknown (54).
Another widely used group of antioxidants include the SODs and their derivatives. Initially, SODs, encapsulated SODs, liposomal SOD preparations, and recombinant MnSOD have been shown to offer significant protection in animal models that lead to fibrosis (reviewed in Reference 7). Because these compounds were later shown to produce immunogenic complications, synthetic small-molecular-weight SOD mimetics have been developed (58, 59). These agents include salen compounds, macrocyclics (e.g., M404903), and metalloporphyrins (e.g., MnTBAP, AEOL 10,150) (58, 59). These agents are promising, because they have been shown to decrease oxidative stress, lung inflammation, and significantly protect the lung in a wide range of animal models, including bleomycin-, asbestos-, and radiation-induced pulmonary fibrosis (7, 59–63). These compounds have, however, not yet been tested in human lung fibrosis.
Human IPF
There has been much hope that new therapeutic agents, such as IFN-γ and antifibrotic agents, can offer clinical benefits to patients with IPF. Unfortunately these agents have not shown significant benefits so far (3, 4). Given the apparent oxidant burden and disturbance of glutathione homeostasis in fibrotic human lung diseases, a number of studies have already been conducted with small-molecular-weight antioxidants in an attempt to prevent the progression of IPF (20, 22, 64–66). NAC has been tested in a variety of conditions and treatment schedules, being used orally, by inhalation, and intravenously (54). Most human studies, although of relatively short duration, suggest that NAC is safe and beneficial in human interstitial lung diseases where oxidant stress is elevated. Treatment with NAC (600 mg three times daily for 12 weeks) has been shown to decrease markers of oxidant injury and improve both total and reduced glutathione levels in the epithelial lining fluid of patients with IPF (66). Conversely, other studies have shown that the effects of NAC on glutathione homeostasis are not so robust (54).
Recently, the multicentered European Idiopathic Pulmonary Fibrosis International Group Exploring NAC I Annual (IFIGENIA) study on IPF evaluated the effects of oral NAC (1800 mg/day) in IPF. The primary endpoints at 12 months showed that NAC significantly improves vital capacity (8%) and diffusion capacity (24%; measured values) when compared with the prednisolon-azatioprine group of patients (155 patients) (67). Similarly, there was a trend for the improvement of other markers of disease activity, such as dyspnea (68). NAC in combination with prednisolone plus azatioprine did not show any significant effect on patient survival (mean follow-up, 1.1.years); however, this trial was not powered to detect a survival effect (68). Overall, the above preliminary results suggest that NAC and/or other redox modulatory therapies, either alone or in combination with immunosuppressive therapy, may have beneficial effects in patients with IPF.
It is very likely that the cellular redox balance has critical effects on gene expression and the synthesis of numerous compounds associated with pulmonary fibrosis. Synthetic antioxidants might therefore be useful in suppressing this process in the human lung. However, more studies will be needed not only to examine these compounds in the context of the various stages of IPF but also to evaluate several classes of the new synthetic antioxidants (e.g., SOD mimetics), alone or in combination with other treatment interventions in IPF. Studies will also be required to test the safety of the new synthetic antioxidants, because it is also unknown whether these powerful redox modulators have adverse effects on cell survival and proliferation in general. It is also unknown whether the new redox modulators might be more effective when delivered locally, because inhalation therapy is superior to systemic therapies in many other lung diseases and causes very few side effects when compared with systemic therapies. Overall, continued investigation into therapeutic approaches to inhibit ROS-mediated reactions in the initiation and progression of lung fibrosis may provide hope for the future treatment of IPF.
This and the original studies of V.L.K. have been partly funded by the Finnish Antituberculosis Association Foundation, the Juselius Foundation, and the Foundation of Helsinki University Hospital. This work is also supported by National Institutes of Health grants R01 HL63700, R01 HL73745, 1F30ES013621, and the American Heart Association Established Investigator Award (T.D.O).
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society: international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 2.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP III. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 2004;64:405–430. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KR, Toews GB, Travis WD, Colby TV, Kazerooni EA, Gross BH, Jain A, Strawderman RL III, Paine R, Flint A, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275–283. [DOI] [PubMed] [Google Scholar]
- 6.Kinnula VL, Crapo JD, Raivio KO. Generation and disposal of reactive oxygen metabolites in the lung. Lab Invest 1995;73:3–19. [PubMed] [Google Scholar]
- 7.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003;167:1600–1619. [DOI] [PubMed] [Google Scholar]
- 8.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med 2000;29:312–322. [DOI] [PubMed] [Google Scholar]
- 9.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 2003;35:236–256. [DOI] [PubMed] [Google Scholar]
- 10.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 2004;18:1258–1260. [DOI] [PubMed] [Google Scholar]
- 11.Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest 1987;79:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallgren R, Bjermer L, Lundgren R, Venge P. The eosinophil component of the alveolitis in idiopathic pulmonary fibrosis: signs of eosinophil activation in the lung are related to impaired lung function. Am Rev Respir Dis 1989;139:373–377. [DOI] [PubMed] [Google Scholar]
- 13.Kuwano K, Hagimoto N, Maeyama T, Fujita M, Yoshimi M, Inoshima I, Nakashima N, Hamada N, Watanabe K, Hara N. Mitochondria-mediated apoptosis of lung epithelial cells in idiopathic interstitial pneumonias. Lab Invest 2002;82:1695–1706. [DOI] [PubMed] [Google Scholar]
- 14.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J 2003;21:232–240. [DOI] [PubMed] [Google Scholar]
- 15.Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 1998;158:1524–1527. [DOI] [PubMed] [Google Scholar]
- 16.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med 2001;163:1693–1722. [DOI] [PubMed] [Google Scholar]
- 17.Lakari E, Soini Y, Saily M, Koistinen P, Paakko P, Kinnula VL. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol 2002;117:132–142. [DOI] [PubMed] [Google Scholar]
- 18.Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155:1763–1769. [DOI] [PubMed] [Google Scholar]
- 19.Behr J, Degenkolb B, Maier K, Braun B, Beinert T, Krombach F, Vogelmeier C, Fruhmann G. Increased oxidation of extracellular glutathione by bronchoalveolar inflammatory cells in diffuse fibrosing alveolitis. Eur Respir J 1995;8:1286–1292. [DOI] [PubMed] [Google Scholar]
- 20.Behr J, Degenkolb B, Krombach F, Vogelmeier C. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J 2002;19:906–911. [DOI] [PubMed] [Google Scholar]
- 21.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1989;139:370–372. [DOI] [PubMed] [Google Scholar]
- 22.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 1991;338:215–216. [DOI] [PubMed] [Google Scholar]
- 23.Tiitto L, Kaarteenaho-Wiik R, Sormunen R, Holmgren A, Paakko P, Soini Y, Kinnula VL. Expression of the thioredoxin system in interstitial lung disease. J Pathol 2003;201:363–370. [DOI] [PubMed] [Google Scholar]
- 24.Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 2004;35:1000–1007. [DOI] [PubMed] [Google Scholar]
- 25.Lakari E, Paakko P, Pietarinen-Runtti P, Kinnula VL. Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung. Am J Respir Crit Care Med 2000;161:615–621. [DOI] [PubMed] [Google Scholar]
- 26.Lakari E, Pylkas P, Pietarinen-Runtti P, Paakko P, Soini Y, Kinnula VL. Expression and regulation of hemeoxygenase 1 in healthy human lung and interstitial lung disorders. Hum Pathol 2001;32:1257–1263. [DOI] [PubMed] [Google Scholar]
- 27.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2000;162:1949–1956. [DOI] [PubMed] [Google Scholar]
- 29.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 2004;37:768–784. [DOI] [PubMed] [Google Scholar]
- 30.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem 2003;278:28403–28409. [DOI] [PubMed] [Google Scholar]
- 31.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 32.Avissar N, Finkelstein JN, Horowitz S, Willey JC, Coy E, Frampton MW, Watkins RH, Khullar P, Xu YL, Cohen HJ. Extracellular glutathione peroxidase in human lung epithelial lining fluid and in lung cells. Am J Physiol 1996;270:L173–L182. [DOI] [PubMed] [Google Scholar]
- 33.Lois M, Brown LA, Moss IM, Roman J, Guidot DM. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med 1999;160:1354–1360. [DOI] [PubMed] [Google Scholar]
- 34.Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, et al. Extracellular superoxide dismutase (ECSOD) binds to type I collagen and protects against oxidative fragmentation. J Biol Chem 2004;279:13705–13710. [DOI] [PubMed] [Google Scholar]
- 35.Fattman CL, Chu CT, Kulich SM, Enghild JJ, Oury TD. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med 2001;31:1198–1207. [DOI] [PubMed] [Google Scholar]
- 36.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol 2004;97:2006–2013. [DOI] [PubMed] [Google Scholar]
- 37.Frears ER, Zhang Z, Blake DR, O'Connell JP, Winyard PG. Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett 1996;381:21–24. [DOI] [PubMed] [Google Scholar]
- 38.Wu SM, Pizzo SV. Mechanism of hypochlorite-mediated inactivation of proteinase inhibition by alpha 2-macroglobulin. Biochemistry 1999;38:13983–13990. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuhashi H, Asano S, Nonaka T, Hamamura I, Masuda K, Kiyoki M. Administration of truncated secretory leukoprotease inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am J Respir Crit Care Med 1996;153:369–374. [DOI] [PubMed] [Google Scholar]
- 40.Moreno JJ, Pryor WA. Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol 1992;5:425–431. [DOI] [PubMed] [Google Scholar]
- 41.Nagai A, Aoshiba K, Ishihara Y, Inano H, Sakamoto K, Yamaguchi E, Kagawa J, Takizawa T. Administration of alpha 1-proteinase inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am Rev Respir Dis 1992;145:651–656. [DOI] [PubMed] [Google Scholar]
- 42.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 1987;84:5788–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 2003;278:12384–12389. [DOI] [PubMed] [Google Scholar]
- 44.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Shi W, Wang YL, Chen H, Bringas P Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L585–L593. [DOI] [PubMed] [Google Scholar]
- 46.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 47.Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C, Cadranel J, Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol 1999;21:128–136. [DOI] [PubMed] [Google Scholar]
- 48.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 1996;10:1077–1083. [DOI] [PubMed] [Google Scholar]
- 49.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 1995;270:30334–30338. [DOI] [PubMed] [Google Scholar]
- 50.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol 1997;17:599–607. [DOI] [PubMed] [Google Scholar]
- 51.Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS. Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 1998;273:15846–15853. [DOI] [PubMed] [Google Scholar]
- 52.Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest 1993;69:261–274. [PubMed] [Google Scholar]
- 53.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol 2002;64:1019–1026. [DOI] [PubMed] [Google Scholar]
- 54.Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med 2000;28:1405–1420. [DOI] [PubMed] [Google Scholar]
- 55.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 2000;162:225–231. [DOI] [PubMed] [Google Scholar]
- 56.Mata M, Ruiz A, Cerda M, Martinez-Losa M, Cortijo J, Santangelo F, Serrano-Mollar A, Llombart-Bosch A, Morcillo EJ. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J 2003;22:900–905. [DOI] [PubMed] [Google Scholar]
- 57.Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol 2003;138:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov 2002;1:367–374. [DOI] [PubMed] [Google Scholar]
- 59.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today 2004;9:557–566. [DOI] [PubMed] [Google Scholar]
- 60.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol 2001;25:164–169. [DOI] [PubMed] [Google Scholar]
- 61.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 2003;35:763–771. [DOI] [PubMed] [Google Scholar]
- 62.Bowler RP, Nicks M, Warnick K, Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2002;282:L719–L726. [DOI] [PubMed] [Google Scholar]
- 63.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med 2002;33:857–863. [DOI] [PubMed] [Google Scholar]
- 64.Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J 1994;7:431–436. [DOI] [PubMed] [Google Scholar]
- 65.Meyer A, Buhl R, Kampf S, Magnussen H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am J Respir Crit Care Med 1995;152:1055–1060. [DOI] [PubMed] [Google Scholar]
- 66.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med 1997;156:1897–1901. [DOI] [PubMed] [Google Scholar]
- 67.Demedts M, Behr J, Buhl R, Convasce G, Costabel U, Dekhuijzen R, Jansen HM, Lankhorst I, Koyama A, MacNee W, et al. IFIGENIA: effects of N-acetylcysteine (NAC) on primary end points VC and Dlco [abstract]. Eur Respir J 2004;24:668s. [Google Scholar]
- 68.Demedts M, Behr J, Buhl R, Costabel U, Jansen HM, Thomeer M, Wallaert B, Lankhorst I, Sardina M, Corvasce G, et al. IFIGENIA-study in IPF: effects of N-acetylcysteine (NAC) on CRP, dyspnea, exercise test and HRCT [abstract]. Eur Respir J 2004;24:668s. [Google Scholar]