Abstract
In order to evaluate the effects of fatty acids on immune cell membrane structure and function, it is often necessary to maintain cells in culture. However, cell culture conditions typically reverse alterations in polyunsaturated fatty acid (PUFA) composition achieved by dietary lipid manipulation. Therefore, we hypothesized that T-cells from transgenic mice expressing the Caenorhabditis elegans n-3 desaturase (fat-1) gene would be resistant to the culture-induced loss of n-3 PUFA and, therefore, obviate the need to incorporate fatty acids or homologous serum into the medium. CD4+ T-cells were isolated from (i) control wild type (WT) mice fed a safflower oil-n-6 PUFA enriched diet (SAF) devoid of n-3 PUFA, (ii) fat-1 transgenic mice (enriched with endogenous n-3 PUFA) fed a SAF diet, or (iii) WT mice fed a fish oil (FO) based diet enriched in n-3 PUFA. T-cell phospholipids isolated from WT mice fed FO diet (enriched in n-3 PUFA) and fat-1 transgenic mice fed a SAF diet (enriched in n-6 PUFA) were both enriched in n-3 PUFA. As expected, the mol% levels of both n-3 and n-6 PUFA were decreased in cultures of CD4+ T-cells from FO-fed WT mice after 3 d in culture. In contrast, the expression of n-3 desaturase prevented the culture-induced decrease of n-3 PUFA in CD4+ T-cells from the transgenic mice. Carboxyfluorescein succinidyl ester (CFSE) -labeled CD4+ T-cells from fat-1/SAF vs. WT/SAF mice stimulated with anti-CD3 and anti-CD28 for 3 d, exhibited a reduced (P<0.05) number of cell divisions. We conclude that fat-1-containing CD4+ T-cells express a physiologically relevant, n-3 PUFA enriched, membrane fatty acid composition which is resistant to conventional cell culture-induced depletion.
Keywords: n-3 fatty acid desaturase, Fish oil, Phospholipids, Lymphocyte
1. Introduction
Several pathways contribute to the movement of long-chain polyunsaturated fatty acids (PUFA) through the plasma membrane into cells. These include: (i) diffusion through the phospholipid bilayer, (ii) transfer by membrane transport proteins, e.g., CD36 and caveolin-1, and in certain cell types (iii) the LDL receptor pathway [1,2]. In general, cell membranes become rapidly enriched with PUFA with significant changes in phospholipids achieved in 24 h [1,3]. Recently, it has been demonstrated that changes in membrane fatty acid composition brought about by dietary n-3 PUFA manipulation can disrupt the earliest steps of T-cell activation [4–9]. In order to study the mechanisms by which n-3 PUFA enrichment modulate plasma membrane organization and immune cell function, it is often necessary to incubate cells in culture for extended periods of time. However, previous research has shown that cell culture conditions have a significant influence on both T-cell bulk membrane and lipid raft fatty acid composition, reversing the alterations in PUFA composition achieved by dietary lipid manipulation [6,10]. To preclude the loss of diet-derived n-3 PUFA from membrane phospholipids, investigators have cultured T-cells in medium containing autologous or homologous serum, which typically exhibits a fatty acid composition resembling that of the diet [6,10,11]. These studies demonstrated the importance of adding autologous/homologous serum to long-term cultures in order to preserve the biological impact of diet on CD4+ T-cell function.
Mammals cannot synthesize n-3 PUFA, including eicosapentaenoic acid (EPA, 20:5Δ5,8,11,14,17) and docosahexaenoic acid (DHA, 22:6 Δ4,7,10,13,16,19), from the major n-6 PUFA found in the diet due to the lack of (n-3) Δ15-desaturase activity. Recently, the fat-1 gene encoding an n-3 fatty acid desaturase has been cloned from Caenorhabditis elegans and expressed in mammalian cells [12]. This enzyme can catalyze the conversion of n-6 PUFA to n-3 PUFA by introducing a double bond into fatty acyl chains. We have proposed that the activation of T-cells expressing n-3 desaturase transgene will not be accompanied by the culture-induced loss of n-3 PUFA, thus obviating the need to incorporate EPA or DHA into the medium. Therefore, we investigated the degree of maintenance of either dietary n-3 PUFA or genetic (fat-1) induced changes in CD4+ T-cell membrane phospholipids following activation and culture in medium containing fetal bovine serum, devoid of EPA and DHA.
2. Materials and methods
2.1. Diet and animals
All experimental procedures using laboratory animals were approved by the University Laboratory Animal Care Committee of Texas A&M University. Female pathogen-free weanling (12–14 g) C57BL/6 wild type (WT) mice (Frederick National Cancer Research Facility, Frederick, MD) were housed in autoclaved polycarbonate microisolator cages and were maintained at room temperature (~25 °C) on a 12 h light:dark cycle. C57BL/6 mice were fed standard non-purified diet (Teklad 9F Sterilizable Rodent diet) during a 1wk acclimation period and had free access to water and diet. Thereafter, animals were randomly assigned to one of two semi-purified diets (15–20 mice/diet group): safflower oil (SAF, n-6 PUFA) or an n-3 PUFA-enriched menhaden fish:corn oil (FO) mixture (4:1, w/w) at 100 g/kg diet for 14 d [6,13]. The purified diets met NRC requirements and varied only in lipid composition [13]. The diet composition, expressed in g/100 g of complete diet, was: 20 g casein, 37 g sucrose, 22 g cornstarch, 6 g cellulose, 3.5 g AIN-76 mineral mix, 1 g AIN-76 vitamin mix, 0.3 g dl-methionine, 0.2 g choline chloride, 0.02 g tertiary butyl hydroquinone, and 10 g oil. Diets were stored at −20 °C, provided ad libitum, and changed daily to prevent peroxidation. The fatty acid composition was assessed by gas chromatography as previously described [6]. The linoleic acid (18:2 n-6) content was 13.7% and 2.8% of total energy in the SAF and FO diets, respectively and, thus, met the minimum 1–2% essential fatty acid requirement for rodents. Vitamin A, D, and E levels were approximately equal and exceeded the minimum requirement [14]. SAF diet was obtained from Research Diets Inc. (New Brunswick, NJ). Corn oil was obtained from Dyets (Bethlehem, PA) and vacuum deordorized menhaden fish oil was provided by OmegaProtein (Houston, TX). There was no significant difference (P>0.05) in food intake between dietary groups, and weight gain was similar in all groups (data not shown).
fat-1 transgenic mice were generated and backcrossed onto a C57BL/6 background as previously described [12]. The colony of fat-1 mice used for this study was generated by breeding heterozygous mice. The genotype and phenotype of offspring of each animal were characterized using isolated DNA and total lipids from tail clips [15].
2.2. CD4+ T-cell isolation and stimulation
Mice were euthanized by CO2 asphyxiation. Spleens were placed in RPMI complete medium [(RPMI 1640 with 25 mmol/L HEPES (Irvine Scientific, Santa Ana, CA) supplemented with 5% heat-inactivated fetal bovine serum (Irvine Scientific), 1 × 105 U/L penicillin and 100 mg/L streptomycin (Irvine Scientific), 2 mmol/L l-glutamine, and 10 µmol/L 2-mercaptoethanol]. Fetal bovine serum typically contains very low levels of long-chain n-3 PUFA [6]. Spleens were dispersed with glass homogenizers and passed through a 149 µm wire mesh filter to create single-cell suspensions. CD4+ T lymphocytes were purified by positive selection using MACS CD4 microbeads (Miltenyi Biotec, Auburn, CA). The purity of the viable CD4+ T-cell population as analyzed by flow cytometry was 91.0±1.0%, n = 3. Cells were cultured in the presence of plate-bound hamster anti-mouse CD3e monoclonal antibody (1 µg/mL, BD Biosciences, San Diego, CA) with soluble hamster anti-mouse CD28 monoclonal antibody (5 µg/mL, BD Biosciences) as previously described [13]. Cells were incubated at 37 °C in an atmosphere of 5% CO2 for 3 d in complete medium (described above).
2.3. CD4+ T-cell proliferation assay
T-cell proliferation was evaluated by the carboxyfluoroscein succinimidyl ester (CFSE) profile assay. Purified CD4+ T-cells were labeled with 5 uM CFSE in PBS supplemented with 5% FBS. Cells (1 × 105) were seeded onto round-bottom 96-well plate at 200uL/well. Cells were incubated with either control RPMI medium or plate-bound anti-CD3 (1µg/mL) and soluble anti-CD28mAbs (5µg/mL). After 3 d incubation at 37 °C, CFSE was analyzed by flow cytometry (BD FACScalibur) as previously described [17]. Briefly, cells were harvested by centrifugation and resuspended in PBS. Viable lymphocytes were gated following the addition of propidium iodide and forward/side scatter plots by flow cytometry. CFSE profiles were analyzed by ModFit LT 3.0 (Verity Software House).
2.4. Analysis of phospholipid fatty acid composition
Total lipids were extracted from CD4+ T-cells by the method of Folch et al. [16]. Total phospholipids were separated by thin layer chromatography (TLC) on silica gel 60G plates using chloroform/methanol/ acetic acid/ water (90:8:1:0.8, v/v) as the developing solvent. Bands were detected under ultraviolet light after spraying with 0.1% 8-anilino-naphthalene-sulfonic acid. Total phospholipids were scraped from the plates, spiked with heptadecanoic acid (17:0) and transesterified in the presence of 6% methanolic HCl [4]. Fatty acid methyl esters were extracted using hexane and 0.1M potassium chloride and analyzed by capillary gas chromatography as previously described [4].
2.5. Reverse transcriptase-PCR
CD4+ T-cell total RNA was isolated using the RNAqueous-4 PCR kit from Ambion (Austin, TX). RNA was treated with DNase to remove contaminating DNA and reverse-transcribed to cDNA using SuperScript II (Gibco BRL). Real-time PCR was performed using an ABI 7900 (Applied Biosystems, Foster City, CA) system and primer pairs for the fat-1 transgene; 5′-TGTTCATGCCTTCTTCTTTTTCC-3′ (forward) and 5′-GCGACCATACCTCAAACTTGGA-3′ (reverse).
2.6. Statistical analysis
Membrane lipid data were analyzed using one-way ANOVA. T/F tests were used to examine differences between treatment groups. For cell proliferation data, the significance of the difference between treatment and control groups was assessed by using a permutation test [18]. The test statistic was obtained by calculating the difference between the treatment and control means for each batch and then averaging over all batches. A reference null distribution was generated by calculating the values of the test statistic under random permutation of the labels (i.e., ab stimulation or control) of the experimental units (i.e., mice). P-values were determined by comparing the observed test statistic with this reference distribution. To remove the nuisance batch effect from the analysis, experiment units were permuted within each batch. Analyses were performed using statistical package R (version 2.6.2, R Foundation for Statistical Computing). A 95% level of probability was accepted as being statistically significant.
3. Results
3.1. Effect of fat-1 transgene expression on CD4+ T-cell membrane fatty acid composition
The expression of fat-1n-3 desaturase in CD4+ T-cells was confirmed by RT-PCR (data not shown). A comparison of relative differences between n-3 PUFA (dietary) and fat-1 induced (genetic) changes in fatty acid composition immediately following T-cell isolation is shown in Table 1. As expected, CD4+ T-cell total phospholipids isolated from WT mice fed FO (WT/FO) and fat-1 transgenic mice fed an n-6 PUFA-containing SAF diet (Fat-1/SAF) were both enriched in n-3 PUFA (20:5n-3, 22:5n-3 and 22:6n-3). Overall, fat-1/SAF and WT/FO derived T-cells exhibited a similar n-3/n-6 PUFA ratio. These data indicate that the fat-1CD4+ T-cells express a membrane enrichment of n-3 PUFA which approximate that obtained by feeding a FO-enriched diet to WT mice.
Table 1.
Effect of transgene expression on freshly-isolated CD4+ T-cell fatty acid profiles
| WT/SAF | WT/FO | fat-1/SAF | |
|---|---|---|---|
| 14:0 | 1.22 ± 0.14a | 2.42 ± 0.28b | 1.22 ± 0.18a |
| 14:1 | 0.65 ± 0.23 | 0.90 ± 0.26 | 0.72 ± 0.23 |
| 16:0 | 31.24 ± 0.23 | 37.15 ± 2.32 | 32.46 ± 2.85 |
| 16:1n-7 | 1.17 ± 0.03a | 2.17 ± 0.19b | 1.32 ± 0.11a |
| 18:0 | 21.73 ± 0.52 | 21.36 ± 0.76 | 22.14 ± 0.99 |
| 18:1n-9 | 4.61 ± 0.32 | 5.03 ± 0.53 | 5.06 ± 0.28 |
| 18:1n-7 | 1.94 ± 0.30a | 3.31 ± 0.63b | 2.17 ± 0.33ab |
| 18:2n-6 | 9.26 ± 0.17b | 7.37 ± 0.36a | 10.23 ± 0.86b |
| 18:3n-3 | nd | nd | nd |
| 20:3n-6 | 1.39 ± 0.24 | 1.47 ± 0.27 | 1.44 ± 0.40 |
| 20:4n-6 | 20.70 ± 2.08b | 11.41 ± 1.65a | 15.77 ± 1.76ab |
| 20:3n-3 | 0.59 ± 0.17 | 0.49 ± 0.20 | 0.55 ± 0.19 |
| 20:5n-3 | nda | 1.60 ± 0.39b | 1.03 ± 0.35b |
| 22:4n-6 | 2.87 ± 0.76b | 0.27 ± 0.11a | 1.17 ± 0.38a |
| 22:5n-6 | 2.23 ± 0.61b | 0.34 ± 0.14a | 0.30 ± 0.19a |
| 22:5n-3 | nda | 1.47 ± 0.53 b | 1.77 ± 0.56b |
| 22:6n-3 | 0.40 ± 0.13a | 3.25 ± 1.15b | 2.65 ± 0.85ab |
| (n-6) PUFA | 36.46 ± 3.66b | 20.86 ± 2.29a | 28.90 ± 2.37ab |
| (n-3) PUFA | 0.98 ± 0.07a | 6.81 ± 1.89b | 6.00 ± 1.54b |
| (n-3)/(n-6) ratio | 0.03 ± 0.01a | 0.30 ± 0.07b | 0.20 ± 0.05b |
Values represent mol% means±SEM; n = 5, nd, not detected (<0.1mol%). Splenic CD4+ T-cells were isolated from wild type mice fed either an n-6 PUFA enriched control safflower oil based diet (WT/SAF), n-3 PUFA enriched fish oil based diet (WT/FO), or fat-1 transgenic mice fed an n-6 PUFA enriched safflower oil based diet (Fat-1/SAF) for 2wks. Immediately following T-cell isolation (basal, 0 d), total phospholipids were isolated and fatty acid composition was analyzed by gas chromatography. Values with different letters indicate significant differences (P<0.05) between treatments.
3.2. Effect of culture conditions on CD4+ T-cell membrane fatty acid composition
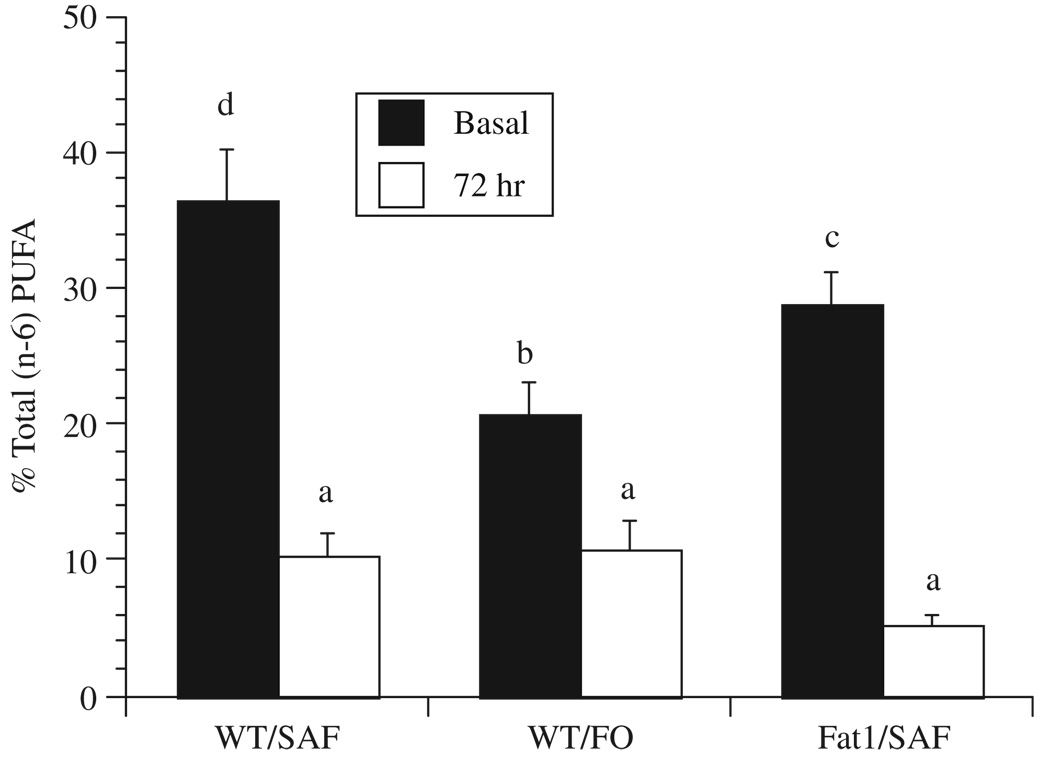
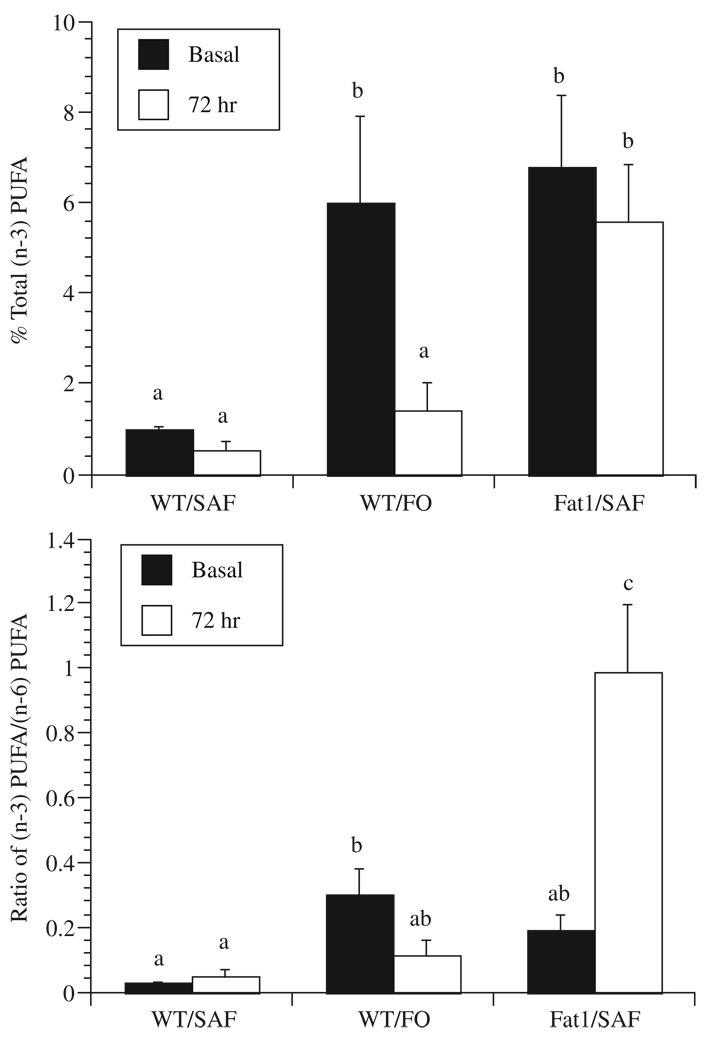
In order to clarify how dietary n-3 PUFA membrane enrichment is influenced by the 3 d culture period often used for T-cell activation, we compared CD4+ T-cell fatty acid composition from membrane total phospholipids immediately following isolation (0 d) and after cell culture (3 d) in the presence of a mitogenic (anti-CD3 and anti-CD28) stimulus. Gas chromatographic analysis of the fatty acid composition of cell phospholipids (Table 2) showed that, compared to the initial (0 d) time point, the amount of both n-6 and n-3 PUFA was decreased in cultures of stimulated CD4+ T-cells from FO-fed WT mice 3 d after culture (Fig. 1 and Fig 2). In contrast, the expression of fat-1 n-3 desaturase in CD4+ T-cells prevented the culture-induced decrease in n-3 PUFA content (Fig. 2A). Examination of the n-6 PUFA mol% content (18:2n-6, 20:3n-6, 20:4n-6, 22:4n-6, 22:5n-6) of both SAF and FO-fed WT mouse CD4+ T-cells (Table 1 and Table 2) revealed the significant depletion of this class of fatty acids. Similarly, stimulated CD4+ T-cells from SAF-fed fat-1 mice exhibited a reduction in n-6 PUFA.
Table 2.
Splenic CD4+ T-cell fatty acid profiles following mitogenic stimulation for 72 h in vitro
| WT/SAF | WT/FO | fat-1/SAF | |
|---|---|---|---|
| 14:0 | 4.86 ± 0.34 | 4.80 ± 0.21 | 4.70 ± 0.27 |
| 14:1 | 1.16 ± 0.31 | 1.42 ± 0.15 | 1.32 ± 0.05 |
| 16:0 | 37.43 ± 2.10 | 36.82 ± 2.25 | 34.17 ± 1.58 |
| 16:1n-7 | 6.78 ± 1.15 | 7.05 ± 0.88 | 7.70 ± 0.41 |
| 18:0 | 17.61 ± 0.92 | 16.95 ± 0.98 | 16.65 ± 0.81 |
| 18:1n-9 | 13.93 ± 1.88 | 13.63 ± 1.99 | 16.15 ± 0.95 |
| 18:1n-7 | 7.30 ± 1.60 | 7.20 ± 1.83 | 8.38 ± 1.65 |
| 18:2n-6 | 3.97 ± 0.30b | 3.65 ± 0.33ab | 3.04 ± 0.13a |
| 18:3n-3 | nd | nd | nd |
| 20:3n-6 | 0.46 ± 0.19ab | 0.78 ± 0.21b | nda |
| 20:4n-6 | 5.67 ± 1.04b | 5.94 ± 1.47b | 2.30 ± 0.37a |
| 20:3n-3 | 0.31 ± 0.19 | 0.12 ± 0.12 | nd |
| 20:5n-3 | nda | 0.04 ± 0.04a | 3.02 ± 0.59b |
| 22:4n-6 | 0.21 ± 0.14 | 0.25 ± 0.15 | nd |
| 22:5n-6 | nd | 0.11 ± 0.11 | 0.05 ± 0.05 |
| 22:5n-3 | nda | 0.44 ± 0.22a | 1.12 ± 0.29b |
| 22:6n-3 | 0.25 ± 0.18a | 0.80 ± 0.38ab | 1.46 ± 0.39b |
| (n-6) PUFA | 10.37 ± 1.59b | 10.73 ± 2.05b | 5.34 ± 0.49a |
| (n-3) PUFA | 0.56 ± 0.17a | 1.40 ± 0.61a | 5.59 ± 1.25b |
| (n-3)/(n-6) ratio | 0.05 ± 0.02a | 0.11 ± 0.05a | 0.99 ± 0.21b |
Refer to Table 1 for legend details. Splenic CD4+ T-cells from the different dietary treatment groups were cultured in the presence of 5% fetal bovine serum complete medium for 3 d, after which total phospholipids were isolated and fatty acid composition was analyzed by gas chromatography.
Fig. 1.
Membrane n-6 polyunsaturated fatty acid content decreases with time in culture in CD4+ T-cells from both WT and fat-1 mice. Refer to Table 1 for legend details. Values represent mean mol% ± SEM (n = 5). Values not sharing the same superscript are significantly different (P<0.05).
Fig. 2.
CD4+ T-cells from fat-1, but not WT mice, maintain the n-3 polyunsaturated fatty acid content of membrane phospholipids. (A) Mol% of total n-3 PUFA in CD4+ T-cells at the time of isolation (0 d) or after mitogenic stimulation in the presence of 5% fetal bovine serum in complete medium for 3 d. (B) Ratio of n-3 PUFA/n-6 PUFA mol%. Values represent mean mol% ± SEM (n = 5). Values not sharing the same superscript are significantly different (P<0.05).
3.3. fat-1 expression suppresses CD4+ T-cell mitogen-induced proliferation
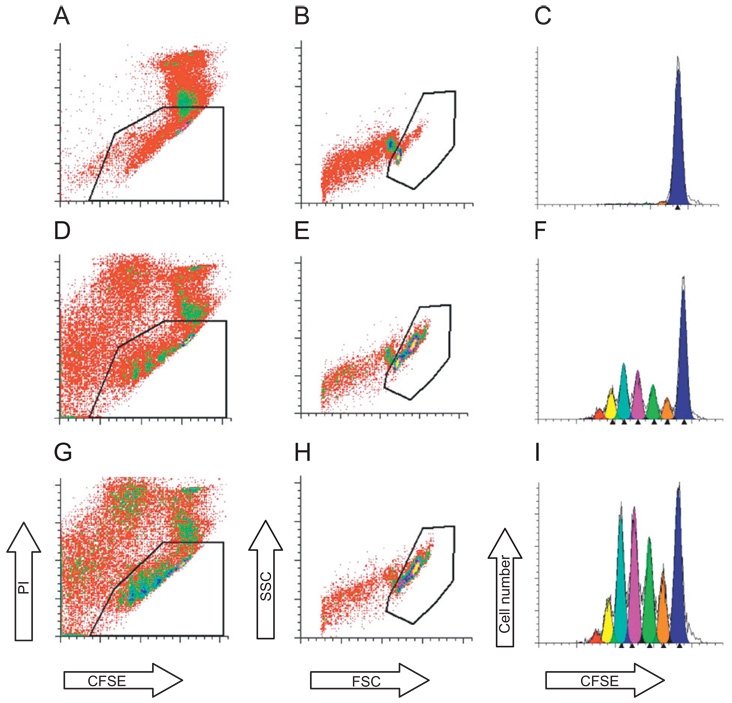
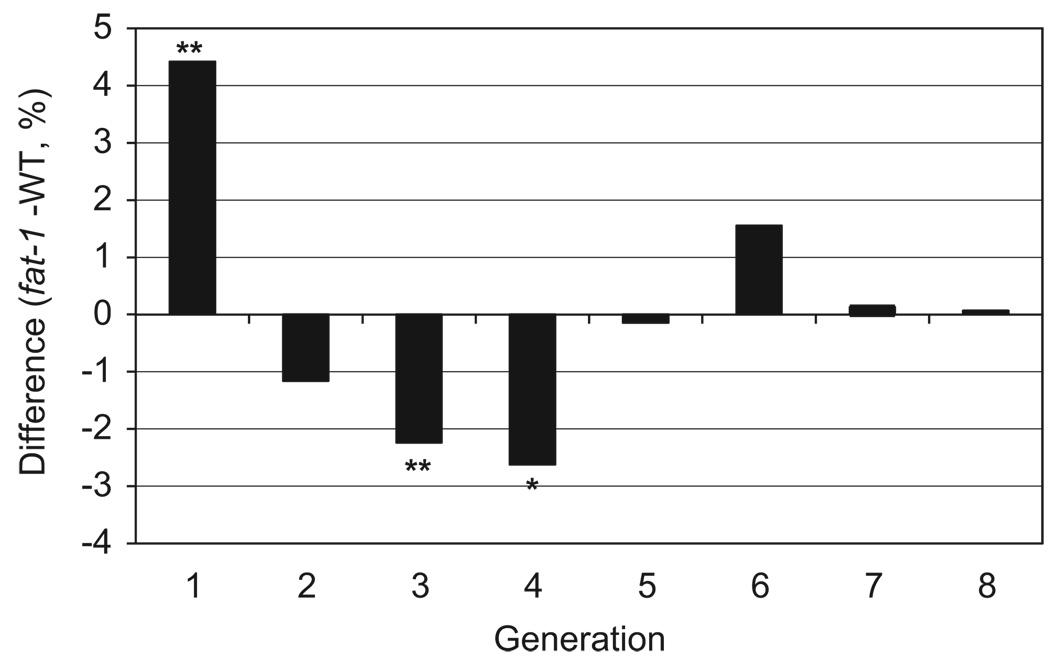
To determine the effect of n-3 PUFA derived from an endogenous (genetic) source on CD4+ T-cell proliferation, CFSE labeled CD4+ T-cells from both fat-1 and WT mice fed a SAF diet were cultured for 3 d in the presence of a mitogenic stimulus, anti-CD3 and anti-CD28 mAbs, as described in the Methods. As expected, CFSE profiles from stimulated cells exhibited a distinctive peak shift to the left (Fig. 3) compared to non-stimulated control cultures, indicating additional rounds of division. Examination of the number of rounds of cell division (expressed as generation number) revealed that CD4+ T-cells from fat-1/SAF mice (Fig. 3F) exhibited a higher (P = 0.05) percentage of early generation cells compared to WT/SAF mice (Fig. 3I and Fig 4). In contrast, percentages of cells in daughter generations 3 (P = 0.06) and 4 (P = 0.03) were reduced in fat-1/SAF vs. WT/SAF mice (Fig. 4). These data indicate that fat-1 derived D4+ T-cells were arrested in their progression through the proliferation cycle.
Fig. 3.
Representative flow cytometry analysis of fat-1/SAF and WT/SAF CD4+ T-cell cultures. (A–C) Unstimulated CD4+ T-cell cultures; (D–F) stimulated fat-1/SAF cultures; (G–I) stimulated WT/SAF cultures. Live T-cells were gated by propidium iodide staining (A, D & G) and forward (FSC) and side scatter (SSC) plots (B, E & H), respectively. The undivided parental generation was established using unstimulated (quiescent) cultures (C). Daughter cell generation was analyzed using the Proliferation Wizard in ModFitLT as described in the Methods (C, F and I). Each peak shift to the left indicates consecutive daughter cell generation.
Fig. 4.
fat-1 gene expression suppresses T-cell clonal expansion. Values represent data sets derived from multiple generations and were plotted by expressing the percentage of fat-1/SAF cells subtracted from the percentage of WT/SAF cells. The data shown were compiled from a total of six mice for each animal group from three separate experiments. Values above 0 indicate that in the fat-1/SAF group, the % of cells in a specific generation exceeds the WT/SAF group. Likewise, values below 0 indicate that in the WT/SAF group, the % of cells in a specific generation exceeds the fat-1/SAF group. P-values were calculated using a permutation test, (*P<0.05, **P<0.1).
4. Discussion
With respect to the immunosuppressive effects of n-3 PUFA, EPA and DHA are unique fatty acids in part because they significantly alter the biophysical properties of T-cell membranes and differentially modulate resident protein function [4,5,7–9,19,20]. In order to elucidate the mechanisms by which n-3 PUFA alter the balance of positive and negative regulatory signals, it is often necessary to maintain cells in culture for several days. Consistent with previous research [10,11], extended culturing induced an equilibration of n-3 and n-6 PUFA from CD4+ T-cell membrane phospholipids isolated from WT mice. In order to avoid the dilution of membrane n-3 PUFA in cultured lymphocytes, several investigators have utilized autologous serum containing a fatty acid composition closely resembling that of the diet. This is significant because culturing in autologous or homologous serum had a demonstrable effect on T-cell function [6,11,19].
A new experimental tool to determine the molecular mechanisms by which changes in T-cell fatty acid composition modulate intracellular signaling cascades was developed by Kang et al. [12], who cloned the n-3 fatty acid desaturase (fat-1 gene) into mice in an attempt to endogenously produce high tissue levels of n-3 PUFA. This novel development is relevant because mammals cannot produce n-3 PUFA from the major n-6 PUFA found in the diet due to the lack of Δ15-desaturase activity. Therefore, it has been necessary to enrich the diet with EPA and/or DHA in order to assess their biological properties in vivo. The n-3 fatty acid desaturase encoded by the fat-1 gene catalyzes the conversion of n-6 PUFA to n-3 PUFA by introducing a double bond into fatty acyl chains. The generation of transgenic mice expressing fat-1have allowed us for the first time to investigate the biological properties of n-3 PUFA without having to incorporate EPA and/or DHA in the diet. We have used this model to determine whether CD4+ T-cells from fat-1 transgenic mice obviate the need to include autologous/homologous serum into long-term cultures in order to preclude the loss of diet-derived n-3 PUFA. In this study, we have shown for the first time that fat-1transgenic CD4+ T-cells maintain membrane phospholipid n-3 PUFA levels in comparison to WT cells for at least 3 d following stimulation despite a fundamental depletion in n-6 PUFA levels. In addition, we demonstrate that fat-1 expression suppresses CD4+ T-cell proliferation. These data are consistent with earlier dietary studies indicating that n-3 PUFA have immunosuppressive properties [5,9,15,17].
Taken together, these data indicate that fat-1-containing CD4+ T-cells express a physiologically relevant membrane fatty acid composition which is resistant to culture-induced depletion. We propose that this model has great utility in determining how n-3 PUFA modulate immune function.
Acknowledgements
We greatfully acknowledge Dr. Jing X. Kang, Department of Medicine, Harvard University, for providing fat-1 breeder mice, and OmegaProtein Inc. for donation of the vacuum deodorized fish oil.
Abbreviations
- CFSE
carboxyfluorescein succinidyl ester
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FO
fish oil
- PUFA
polyunsaturated fatty acids
- SAF
safflower oil
- WT
wild type
Footnotes
This work was supported in part by NIH grants DK71707, CA59034, CA129444, P30ES09106 and USDA 2008-34402-19195, “Designing Foods for Health”, Vegetable & Fruit Improvement Center.
Conflict of interest
None.
References
- 1.Edwards IJ, Berquin IM, Sun H, et al. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clin. Cancer Res. 2004;10:8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. ProstLeuk. Essential Fatty Acids. 2007;77:355–361. doi: 10.1016/j.plefa.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Collett ED, Davidson LA, Fan YY, Lupton JR, Chapkin RS. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic Ras activation in colonocytes. Am. J. Physiol. Cell. Physiol. 2001;280:C1066–C1075. doi: 10.1152/ajpcell.2001.280.5.C1066. [DOI] [PubMed] [Google Scholar]
- 4.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 5.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T-cell protein kinase C-theta lipid raft recruitment and interleukin-2 production. J. Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 6.Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in the Th1-polarized murine CD4+ T-cells. J. Lipid Res. 2004;45:1482–1492. doi: 10.1194/jlr.M400028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeyda M, Stulnig TM. Lipid Rafts & Co.: an integrated model of membrane organization in T cell activation. Prog. Lipid Res. 2006;45:187–202. doi: 10.1016/j.plipres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr. Opin. Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 9.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J. Nutr. 2007;137:200S–204S. doi: 10.1093/jn/137.1.200S. [DOI] [PubMed] [Google Scholar]
- 10.Yaqoob P, Newsholme EA, Calder PC. Influence of cell culture conditions on diet-induced changes in lymphocyte fatty acid composition. Biochim. Biophys. Acta. 1995;1255:333–340. doi: 10.1016/0005-2760(94)00251-s. [DOI] [PubMed] [Google Scholar]
- 11.Pompos LJ, Fritsche KL. Antigen-driven murine CD4+ T lymphocyte proliferation and interleukin-2 production are diminished by dietary (n-3) polyunsaturated fatty acids. J. Nutr. 2002;132:3293–3300. doi: 10.1093/jn/132.11.3293. [DOI] [PubMed] [Google Scholar]
- 12.Kang JX, Wang J, Wu L, Kang ZB. Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 13.Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin. Exp. Immunol. 2001;125:499–507. doi: 10.1046/j.1365-2249.2001.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Research Council. Nutrient Requirements of Laboratory Animals. fourth ed. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 15.Jia Q, Lupton JR, Smith R, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Zhang P, Kim W, Zhou L, et al. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J. Nutr. 2006;136:2391–2398. doi: 10.1093/jn/136.9.2391. [DOI] [PubMed] [Google Scholar]
- 18.Good PI. Permutation, Parametric and Bootstrap Tests of Hypotheses. third ed. Berlin: Springer; 2005. [Google Scholar]
- 19.Ly LH, Smith R, Switzer KC, Chapkin RS, McMurray DN. Dietary eicosapentaenoic acid modulates CTLA-4 expression in murine CD4+ T-cells. Prost. Leuk. Essent. Fatty Acids. 2006;74:29–37. doi: 10.1016/j.plefa.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ cells by affecting membrane raft formation. J. Immunol. 2008 doi: 10.4049/jimmunol.181.9.6236. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]