Abstract
Compartmented neuronal cultures allow experimenters to establish separate fluid environments for neuronal axons and the soma from which they emanate. Physical isolation of cell bodies and axons is achieved by culturing neurons in tri-chambered Teflon rings. Dissociated ganglia are plated in one end compartment of the trichamber, and axonal growth is guided underneath watertight silicone grease barriers into a separate compartment. Since the axons and cell bodies are located in different compartments, they can be infected and assayed separately. We describe the assembly and use of compartmented neuronal cultures for in vitro study of directional infection of neurons by alpha herpesviruses. Selective application of viral inoculum to only one compartment ensures that the remainder of the neuron is not contaminated by input inoculum. This allows for quantification of viral spread, and unambiguous interpretation of immunofluorescence and electron microscopy images.
Keywords: compartmented neuron culture, Campenot chambers, tri-chamber, anterograde, retrograde, alpha herpesvirus, virus
INTRODUCTION
This unit describes the assembly and use of compartmented neuron cultures for the in vitro study of directional infection by alpha herpesviruses. The trichamber system we describe is based on the compartmented cultures developed by R.B. Campenot (Campenot, 1977, 1992). In the trichamber system, neuronal cell bodies and their axons are maintained in separate compartments, enabling the experimenter to establish unique fluid environments across the length of the neuron. Thus, a viral inoculum can be applied either to the compartment with neuronal cell bodies or to the compartment containing distal axons. If infection is initiated at axons, viral particle motion along microtubules is minus-end-directed and is termed retrograde transport. In contrast, viral particle motion that is plus-end-directed is termed anterograde. The efficiency of directional viral transport and subsequent spread can be determined by a combination of different techniques and assays including, but not limited to, viral titering, immunofluorescence imaging, and electron microscopy.
To study anterograde transport and neuron-to-cell transmission of infection, viral inoculum is applied to neuronal cell bodies, and the infection is transmitted via axons to detector cells plated in the axon compartment. Retrograde transport and axon-mediated infection of neurons are studied by applying the viral inoculum to the axonal compartment and titering the neuronal cell bodies at the appropriate time points after infection. Immunofluorescence and electron microscopy can be performed on infected samples after either type of infection. The trichamber system confers a major advantage for these experiments over non-compartmentalized cultures: the input inoculum is applied and restricted to only one compartment, and therefore does not confound measurements or imaging performed on the remainder of the sample; in addition, the direction of viral transport during infection is known.
NOTE: All incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
NOTE: Chamber assembly, dissection of ganglia, and all work with live virus and neurons should be performed under sterile conditions and aseptic technique should be used accordingly.
ASSEMBLING THE TRICHAMBER SYSTEM
BASIC PROTOCOL 1
Basic features of the trichamber system
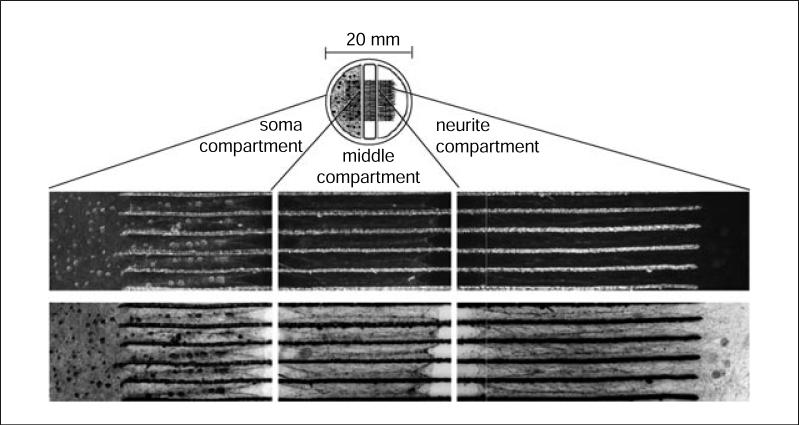
The trichamber system consists of three compartments: the soma (S)-compartment, where the neuronal cell bodies are cultured; the methocel (M)-compartment, where the axons first emerge after penetrating underneath the initial barrier; and the neurite (N)-compartment, where the axons emerge from the M-compartment (see Fig. 26.4.1 for a diagram of the trichamber). Spatially, the Teflon trichamber is symmetrical and the S- and N-compartments are identical. However, for nomenclature purposes, we designate the S-compartment as the location where the neuron cell bodies are plated.
Figure 26.4.1.
Diagram of trichamber setup, with images of neurons and axons passing through five inter-groove spaces. The trichamber is 20-mm across, and consists of a soma (S) compartment, middle (M) compartment, and neurite (N) compartment. These images show mouse SCG neurons after 3 weeks in trichamber culture. The Teflon trichamber has been removed to facilitate imaging. The top row of images is phase-contrast microscopy. The bottom row presents the same images, after inversion and contrast-adjustment in Photoshop, to better present the axon structure. The circular gray shadows visible in the N-compartment after image inversion are from silicone grease that floats on the surface of the medium after trichamber removal.
To assemble the trichamber system, we first apply a thin coat of silicone grease on one face of the trichamber and allow the chamber to form a watertight seal on a 35-mm plastic tissue culture dish. Silicone grease is viscous enough to prevent diffusion of liquids between compartments, but allows axons to penetrate underneath the chamber barrier. Once the chamber is affixed to the dish, freshly dissected and dissociated neurons are plated in the S-compartment. After 2 weeks, the axons emanating from the cell bodies in the S-compartment will have penetrated underneath the Teflon barriers, across the M-compartment, and into the N-compartment.
Preparing the trichamber components
Prior to dissecting neurons, the trichambers must be cleaned and sterilized. In addition, the tissue culture dishes must be coated with cell adhesion molecules to promote neuronal attachment and axonal growth. We regularly use poly-dl-ornithine and laminin as extracellular substrates. With respect to incubation times, this protocol must begin at least 1 day before neuronal dissection and plating is planned. This treatment may vary depending on the neuronal culture methods used in different laboratories or for different cell types.
Assemble chambers just before the dissections in a sterile environment; using a laminar flow hood for this step is highly recommended. We commonly culture rat or mouse superior cervical ganglia neurons in the S-compartment. Videos 1 and 2 provide visual demonstration of trichamber setup (see Videos 1 and 2 at http://www.currentprotocols.com).
Materials
Poly-dl-ornithine (Sigma) diluted in borate buffer
Tissue culture–grade water
Laminin (BD Biosciences)
HBSS without Ca2+ and Mg2+ (CMF-HBSS; HyClone)
Silicone high-vacuum grease, Dow Corning (VWR, cat. no. 59344−055)
70% ethanol
Neuronal medium containing 1% (v/v) methocel (see recipe)
Neuronal medium (see recipe)
Teflon trichambers (Tyler Research; see recipe)
Pin rake (homemade or Tyler Research)
35-mm plastic tissue culture dishes
15-cm non-tissue culture-treated dishes (optional)
Disposable 3-ml syringe
Machine-sawed 18-G hypodermic needle or truncated 200-μl pipet tip Autoclave
Hemostat (e.g., Roboz RS-7293)
Additional reagents and equipment for cleaning the Teflon chambers (Support Protocol 1)
Prepare the chamber
-
1
Clean the Teflon chambers thoroughly before assembly (see Support Protocol 1).
-
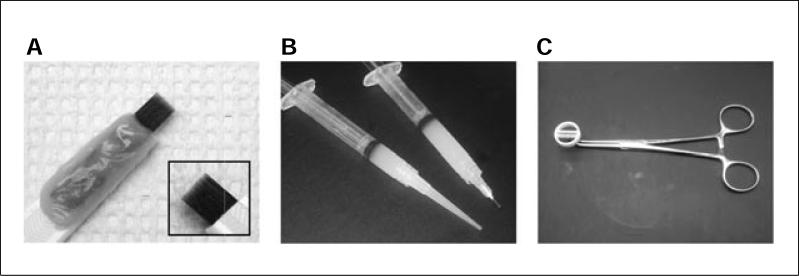
2Use a pin rake to etch 16 parallel, evenly spaced grooves in the middle of a 35-mm tissue culture dish. See Figure 26.4.2A for view of a pin rake.
- This homemade pin rake is constructed from 16 insect pins (size 00) arranged in a row such that sharp ends of the pins are aligned. Truncate the blunt ends of the pins with a wire cutter and fix the pins to a plastic holder with epoxy resin. A microcentrifuge tube opener can be used as the plastic holder. Always sterilize the pin rake with 70% ethanol (never autoclave). See Campenot (1992) for additional instructions on making a pin rake. Alternatively, a stainless-steel pin rake can be purchased from Tyler Research Corporation.
-
3Make a notation at the side of the dish with a marker to determine the relative orientation of the dish with regards to the trichamber.
- When preparing multiple 35-mm dishes at once, it is useful to hold eight at a time by placing them within a 15-cm petri dish
-
4
Add 1 ml of 500 μg/ml of poly-dl-ornithine diluted in borate buffer to the dish. Incubate the dish for at least 6 hr.
-
5
Remove the dish from the incubator, aspirate poly-dl-ornithine and wash three times, each time with 1 ml tissue culture–grade water.
-
6
Add 1 ml of 10 μg/ml laminin in CMF-HBSS to the dish and incubate overnight.
-
7Load silicone grease in a 3-ml disposable syringe. Fill the barrel of the syringe ⅔ of the way through with silicone grease. Attach a truncated 18-G hypodermic needle, or a truncated 200-μl pipet tip, onto the syringe. Autoclave the silicone grease–loaded syringe, the trichambers, and tools for dissection. See Figure 26.4.2B for a view of grease-loaded syringes.
- The purpose of truncating the needle or the pipet tip is to dispense the silicone grease easily and evenly. If truncating a pipet tip, aim for a diameter equivalent to that of an 18-G needle.
Figure 26.4.2.
Tools for trichamber assembly. (A) Close-up of pin rake for making grooves in the trichamber dish. The pin rake is assembled from insect pins, epoxy, and a plastic handle. Inset shows close-up view of pins from the reverse side. (B) Two types of syringes for dispensing grease. The inner diameter of the needle or plastic tip determines the width of the grease strip. (C) Curved hemostat clamped onto trichamber, prior to addition of silicone grease.
Assemble the trichamber system
-
8Aspirate laminin from dishes, wash twice with 1 ml CMF-HBSS and air-dry briefly.
- To facilitate drying, keep the dishes tilted. Do not over-dry laminin-coated plates because the protein may degrade.
-
9
As the dishes are drying, remove the Teflon chambers and silicone grease–loaded syringe from the autoclave. Cool briefly and sterilize syringe tip with 70% ethanol. Eject a small amount of silicone grease from the syringe to remove any air pockets formed at the tip of the needle during autoclaving.
-
10
Use a sterile hemostat to clamp one of the two central barriers of a Teflon trichamber. Flip the hemostat over and place it flat, so that the chamber is facing upward. See Figure 26.4.2C for a view of the hemostat and trichamber clamped together and see Video 1 at http://www.currentprotocols.com.
-
11Apply a thin strip of silicone grease around the entire chamber, starting with the two central barriers followed by the outer circular ring. See Video 1 at http://www.currentprotocol.com.
- It is crucial to maintain the continuity of the strip of silicone grease, particularly in the central barriers, since a break in the strip will cause leakage between compartments.
-
12Set the clamped hemostat aside. Add a 50-μl drop of neuronal medium with 1% methocel in the middle of the laminin-coated dish, on top of the etched grooves (see Video 1 at http://www.currentprotocols.com).
- The drop of methocel provides moisture for the seal between the chamber and the dish. Without methocel, the barrier is devoid of liquid, which deters axon penetration.
-
13Flip the dish over and gently place it on the Teflon chamber, centering the grooves directly over and perpendicular to the chamber barriers. Lift the hemostat and view the trichamber assembly through the bottom-side of the dish. Tilt the hemostat slightly to allow light to hit the dish at different angles.
- This will reveal whether or not a seal has started to form between the chamber and the dish. If the seal has formed at very few locations, touch the edges of the dish very lightly until seal formation is observed.
- The seal does not need to be continuous at this point. It is only necessary for sufficient adhesion to ensure that the dish will not fall free of the hemostat when it is inverted in the next step.
-
14Flip the hemostat over and gently release the clamp close to the surface of the bench, thus freeing the dish.
- Avoid letting the assembled trichamber and dish come in contact with the bench prior to releasing the clamp, since this may inadvertently disrupt the silicone grease strip and cause a breach in the barrier.
-
15Wait for the seal to become continuous; while waiting, proceed with the assembly of the remaining chambers. Check for seal formation by lifting each 35-mm dish, inverting it, and allowing light to hit the bottom surface at different angles. If after 10 min the seal has not yet formed, apply gentle pressure to the chamber to complete the seal.
- If too much pressure is applied, axon penetration underneath the barriers will be hindered.
-
16Add 200 μl of neuron medium to the M-compartment and wait 1 min; note whether the medium leaks from this compartment. If no leaks are detected, apply 300 μl of medium to the S-compartment and 400 μl to the N-compartment.
- By adding only 300 μl to the S-compartment, up to 100 μl volume of dissociated neuron suspension can be added (see below).
- Approximately 300 to 400 μl volume is sufficient for the S- and N-compartments, and 200 μl volume for the M-compartment.
PLATING AND MAINTAINING DISSOCIATED NEURONS IN THE TRICHAMBER SYSTEM
BASIC PROTOCOL 2
We routinely culture and plate peripheral neurons from the sympathetic ganglia (SCG) of embryonic rats and mice in the trichamber system. In this protocol, we provide a streamlined method for culturing mouse embryonic SCG neurons in the trichamber system. These dissociation and medium conditions work equally well for rat SCG neurons. Protocols for dissection of embryonic rat SCG neurons can be found in Ch'ng et al. (2005).
While different types of neurons can be cultured in the trichamber system, the success of this system depends on the robust outgrowth of the axonal or neuritic projections from the cell bodies. In general, embryonic neurons work better in the trichamber system than older neurons, and peripheral nervous system neurons display more robust outgrowth than central nervous system neurons. The trichamber system can be optimized for different neuronal cell types by modifying the cell adhesion substrates, medium, or other cell culture parameters.
Materials
Pregnant mouse, E14 or E15
HBSS (HyClone)
HBSS without Ca2+ and Mg2+ (CMF-HBSS; HyClone)
- 2.5 mg/ml trypsin in HBSS (see recipe)
- 1 mg/ml DNase in neurobasal medium (see recipe)
- 2 mg/ml soybean trypsin inhibitor (SBTI) in neurobasal medium (see recipe)
- Neuronal medium (see recipe)
- Cytosine β-d-arabinofuranoside hydrochloride (AraC; see recipe)
- 15-ml conical tubes
- Water bath at 37°C
- Flame-polished glass Pasteur pipet
- Hemacytometer
- Teflon trichambers (Tyler Research; see recipe)
- Additional reagents and equipment for counting cells using a hemacytometer (unit 1.1)
Prepare neurons
-
1
Dissect ganglia in standard HBSS from embryonic day 14 (E14) or E15 mouse embryos, using the approaches previously described for rat superior cervical ganglion neurons (Johnson, 2001; Ch'ng et al., 2005).
-
2Transfer dissected ganglia to a 15-ml conical tube and dilute with 5 ml CMF-HBSS. Allow the ganglia to settle, and then remove all but 800 μl of the buffer.
- CMF-HBSS is preferable at this step for optimal trypsin activity.
-
3
To digest the ganglia, add 100 μl of 2.5 mg/ml trypsin in HBSS and 100 μl of 1 mg/ml DNase in neurobasal medium, and incubate the tube for 15 min in a 37°C water bath. Swirl the tube once during this time.
-
4During this time, flame-polish a glass Pasteur pipet by holding the open end in a flame for ∼5 to 10 sec.
- The goal is to smooth the jagged edges of the glass pipet opening to minimize cell damage.
- We also coat the inner glass surface of the Pasteur pipet with a sterile 2% BSA in PBS solution. This prevents neurons from adhering to the glass surface.
-
5After 15 min, add an additional 100 μl DNase in neurobasal medium, and 650 μl SBTI in neurobasal medium. Incubate the tube for an additional 5 min in a 37°C water bath.
- The enzyme solutions are optimized to give the desired final concentration in this volume. The final concentrations are: trypsin, 0.25 mg/ml; DNase, 0.2 mg/ml; and SBTI, 0.8 mg/ml.
- DNase aids in trituration by digesting genomic DNA released from lysed cells.
-
6
Dilute the ganglia-enzyme mix with additional CMF-HBSS to 10 ml. Centrifuge 1 min at 3000 × g in a tabletop centrifuge, room temperature. Remove all but 200 μl volume, then add 1.5 ml of neuronal medium.
-
7
Use trituration to mechanically dissociate the ganglia. Pipet the resuspended ganglia up and down with the flame-polished glass pipet until the neuronal suspension is free of clumps.
Plate neurons
-
8Count a small aliquot of the resulting neuronal suspension in a hemacytometer (unit 1.1). Plate 20,000 to 25,000 mouse superior cervical ganglion cells in each trichamber S-compartment. Place the cultures in a 37°C, 5% CO2 humidified incubator.
- We plate approximately the same number of neurons in the S-compartment whether using mouse SCG neurons or rat SCG neurons.
- 20,000 to 25,000 cells is approximately a whole ganglion obtained from an E14-E15 mouse embryo, or half of a superior cervical ganglion obtained from an E15.5 rat embryo.
- Counting SCG neurons before plating is complicated by the presence of non-neuronal cells (satellite cells and/or fibroblasts from neighboring tissue). These cells will die during subsequent treatment with AraC. In addition, SCG neurons will divide for a short time in culture (DiCicco-Bloom and Black, 1988; DiCicco-Bloom et al., 1990). We have empirically observed that the neuron number at 2 weeks post-plating is ∼¼ the number of plated cells. Thus, plating 20,000 cells will yield ∼5,000 mature neurons by the time of experimental infection.
- Note that the S- and N-compartments each hold a maximum of 400 μl of medium, so the amount of dissociated neuron suspension added must not exceed this capacity.
-
9
Allow the neuronal cultures to grow for 2 days before adding an antimitotic drug to the S-compartment.
-
10
After 2 days, add AraC to 1 μM in the existing S-compartment medium to eliminate any mitotic, non-neuronal cells. Incubate overnight.
-
11Remove AraC-containing medium after overnight incubation and replace with fresh, warm neuronal medium.
- Fluid volumes are 300 to 400 μl for the S- and N-compartments and 200 μl for the M-compartment.
- Always change medium with slow and gentle pipetting. Do not remove all of the medium and let the cells dry; instead, always leave a small volume at the bottom of the dish.
- We recommend selecting one edge of the chambers (for instance, the top edge) from which to always add or remove medium. The force of fluid motion is enough to rip or lift the neurons from the dish. The top edge is far from the grooves and it will thus minimize effects on axon penetration through the compartments. In addition, by consistently choosing the same spot, the neuronal disruption is contained to only one part of the chamber.
- If non-neuronal cells are still abundant 2 to 3 days after AraC treatment, repeat the treatment on the evening before a scheduled medium change.
-
12Change neuronal medium in all three compartments every 2 to 3 days.
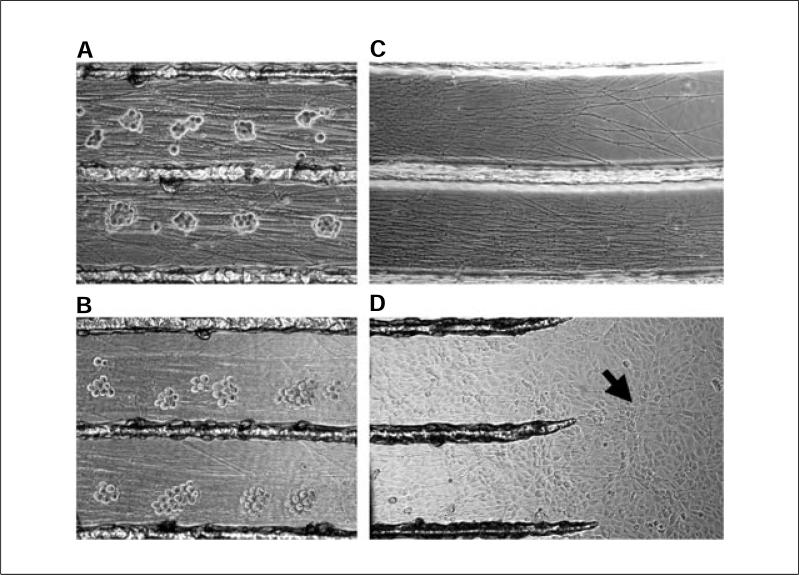
- Figure 26.4.3 shows mature neuron cell bodies and axon outgrowth in the trichamber setup after 3 weeks of growth. Figure 26.4.3A,B provides a close-up view of neuron cell bodies in the S-compartment, and Figure 26.4.3C of neurites in the N-compartment.
- In general, older cultures require more frequent medium changes. Observe the phenol red indicator found in most media formulations (including Neurobasal). The pH of the medium can fluctuate rapidly in small volumes, and the health of the neuronal cultures can drop dramatically if the medium becomes too acidic.
- It is important not to bump or put pressure on the trichamber setup during medium changes, since this can tear the neurites or compress the silicone grease seal.
Figure 26.4.3.
Highlights of trichamber culture features. (A) Enlarged view of mouse SCG neurons in the S compartment of a 3-week-old culture. The clusters of cell bodies are tightly packed. (B) At 24 hr after alpha herpesvirus infection (e.g., PRV or HSV-1), the clusters of SCG neurons are less tightly packed, and axons become grainy. (C) Enlarged view of dense axon growth in the N-compartment of a 2-week-old culture of rat SCG neurons. (D) View of the N-compartment 24 hr after plating of a monolayer of PK15 detector cells. Arrow highlights mouse SCG axons visible among the PK15 cells.
DISASSEMBLING AND CLEANING THE TRICHAMBERS: ROUTINE CLEANING
SUPPORT PROTOCOL 1
The Teflon trichambers are durable and can be cleaned and reused. However, the silicone vacuum grease must be removed entirely before assembling new cultures. With proper care, these chambers can last for many experiments. Traditionally, the chambers are soaked in concentrated sulfuric acid to remove residual silicone grease (Campenot, 1992). We currently use ethanol as a gentler solvent, reserving sulfuric acid for occasional strong cleaning. This protocol describes cleaning the trichambers with ethanol.
Materials
Distilled water
200-proof (absolute) ethanol
Trichambers that need cleaning
Blunt forceps
Paper towels
Kimwipes
Autoclave
10-cm glass petri dish (optional)
- Upon the conclusion of an experiment, aspirate all residual medium from the compartments. Disassemble the trichamber setup by lifting the chamber from the tissue culture dish using a pair of blunt forceps.
- Using sharp forceps will damage the Teflon.
- Blot each trichamber onto a paper towel: apply gentle pressure and then lift again to leave additional grease behind on the paper towel. Remove any residual silicone grease by carefully wiping the chamber with Kimwipes.
- It is crucial to remove most of the silicone grease before further treatment.
Rinse the chambers in distilled water and blot dry with paper towels.
Soak the chambers in 200 proof ethanol for 24 hr or longer. Swirl or shake the solution occasionally to aid in dissolution of any grease residue on the chambers.
Rinse the chambers with distilled water several times, then boil them in distilled water for 30 min.
- Air dry and autoclave the chambers.
- We use a 10-cm glass petri dish to hold the trichambers for autoclaving and storage.
DISASSEMBLING AND CLEANING THE TRICHAMBERS: OCCASIONAL CLEANING
SUPPORT PROTOCOL 2
Periodically (after 3 to 4 uses), the trichambers should be cleaned with stronger reagents to thoroughly clean it. This protocol describes the use of concentrated sulfuric acid to remove residual silicone grease (Campenot, 1992).
Additional Materials (also see Support Protocol 1)
Concentrated sulfuric acid
Disassemble trichambers and remove grease as described in Support Protocol 1 (steps 1 to 3).
- Soak the chambers in concentrated sulfuric acid for 24 hr. Swirl the acid solution occasionally.
- Longer incubation will not result in damage.
- Rinse the chambers with distilled water several times before boiling them in distilled water for 30 min.
- Boiling the chambers should remove any residual sulfuric acid trapped within the Teflon.
4. Air dry and autoclave the chambers.
STUDYING THE SPREAD OF VIRAL INFECTION: ANTEROGRADE TRANSPORT AND NEURON-TO-CELL TRANSMISSION OF INFECTION
BASIC PROTOCOL 3
This assay was developed to study the spread of viral infection initiated in the neuron cell body (S-compartment) to axonally contacted epithelial detector cells plated in the N-compartment. Inoculum is applied to the S-compartment, and the titer of infectious virus in the N-compartment is indicative of viral spread from the soma, through axons, to the detector cells, which amplify viral titer. Because diffusion of viral particles is prohibited by the methocel-containing middle compartment and two watertight silicone grease seals, neuron-to-cell transmission of infection is mediated exclusively via neuronal axons that span all three compartments. By co-plating different cell types in the N-compartment, various axon-cell interactions can be established. For instance, if a standard tissue culture cell line that is permissive for viral infection is plated in the N-compartment, these cells serve as sensitive detectors and amplifiers for the axonal transmission of infection. Alternatively, if neurons are co-plated in the N-compartment, then the spread of infection from pre- to post-synaptic neurons can be studied. In this protocol, we describe the plating of a porcine kidney cell line (PK15), a commonly used detector and amplifier of Pseudorabies virus (PRV) infection.
Materials
Two-week-old neuron cultures in the trichamber system (Basic Protocol 2)
A confluent monolayer of tissue culture cells (e.g., PK15 cells)
Trypsin (Invitrogen)
Dulbecco's Modified Eagle Medium (DMEM; Invitrogen)
Fetal bovine serum (HyClone)
Neuronal medium containing 1% (v/v) methocel (see recipe)
Viral stock to be studied (e.g., 107 to 108 PFU/ml PRV)
Disposable protein gel-loading tip (Rainin)
−80°C freezer
- Culture dissociated neurons in the S-compartment for ∼2 weeks prior to the actual experiment.
- After 2 weeks, axons are easily visible in the N-compartment.
- Score the axonal densities in the N-compartment from 1 to 4, with chambers scoring a 4 having the densest network in the N-compartment, and those with a score of 1 having little to no axonal penetration.
- An alternative method to score the N-compartment is by counting how many of the inter-groove spaces (there are 15 of these made by a 16-pin rake) are filled by penetrating axons. Another criterion is length of penetration into the N-compartment.
- Only chambers with a score of 3 or 4 are used in experiments; these chambers are evenly distributed among the different experimental variables.
- One the day prior to infection, plate the detector cells (e.g., tissue culture cells such as PK15s) in the N-compartment.
- These cells settle on top of and in contact with the axons in the N-compartment (see Fig. 26.4.3D).
- For example, dissociate a 90% confluent monolayer of tissue culture cells with 1 ml of trypsin and bring the total volume of the dissociated cells up to 10 ml with neuronal medium. Add ∼66 μl of the trypsinized cells into the N-compartment. Add fetal bovine serum to a final concentration of 1% in the N-compartment to facilitate cell attachment and growth.
- The goal is to plate enough tissue culture cells to form a confluent monolayer ∼24 hr post-plating. Examples of detector cells for other viruses (e.g., Herpes simplex virus 1 or West Nile virus) can be found in Ch'ng et al. (2007) and Samuel et al. (2007). We plate ∼6.6 × 103 PK15 cells per N-compartment.
- Neurons can also be plated in the N-compartment 2 to 3 days before their intended use (Feierbach et al., 2007; Samuel et al., 2007). This short time frame prevents axon growth from the newly plated neurons into the M-compartment.
- On the day of infection, replace existing medium in the M-compartment with 200 μl of neuronal medium with 1% methocel. Return the dish to the incubator for 30 min to allow the methocel to settle to the bottom of the dish.
- The viscous, methocel-containing neuronal medium serves as an additional barrier to diffusion between the trichamber-compartments.
- Dilute the viral inoculum in neuronal medium, to a final volume of 50 μl. Remove medium from the S-compartment, and add the viral inoculum.
- Keeping the volume of infection to only 50 μl is important for efficient viral adsorption.
- When preparing to use this system in your laboratory, it is important to characterize the amount of virus needed to synchronously infect all cells in the S-compartment. This can be empirically determined by applying a titration series of inoculum amounts to several trichambers. Assay infection by immunofluorescence at an early point in infection. Choose an inoculum concentration that adequately infects all cells.
- For PRV strains in our laboratory, we generally add 105 plaque-forming units (PFU) into the S-compartment, which is approximately a multiplicity of infection (MOI) of 20.
Incubate the trichambers with viral inoculum for 1 hr.
Gently remove the viral inoculum from the S-compartment with a pipet and replace it with 300 to 400 μl of neuronal medium. Place the chamber back in the incubator.
- At the appropriate time post-infection, remove the trichamber dish from the incubator, and separately scrape and harvest the contents of the S- and N-compartments for later titering on the appropriate cell types.
- Use a sterile object to scrape the cells in the compartments for titering; we use a disposable protein gel-loading pipet tip for this purpose. The neurons should peel off easily as a sheet. The detector cells often adhere tightly and they must be scraped thoroughly.
- In anterograde-mediated infections (where inoculum is added to the S-compartment), both the S- and N-compartments are typically harvested for titering. Collecting the contents of the S-compartment also informs the experimenter about the titer of input inoculum and replication in neurons.
Freeze all harvested samples at −80°C before titering.
STUDYING THE SPREAD OF VIRAL INFECTION: RETROGRADE TRANSPORT AND AXON-MEDIATED INFECTION OF NEURONS
ALTERNATE PROTOCOL 1
Some neuroinvasive viruses, such as rabies virus and alpha herpesviruses, enter neurons at axon termini or growth cones and undergo retrograde axonal transport toward the cell body, where replication occurs. The trichamber system can be used to study this form of entry, and subsequent infection of neuron cell bodies. In addition, because not all neurons in the S-compartment send axons into the M- and N-compartments, this assay can be used to study secondary spread among S-compartment neurons (Curanović et al., in preparation). If the S-compartment is assayed 24 hr post-infection of the N-compartment with PRV, for instance, the titer obtained comes from both the initially infected neurons (primary infection), and also from secondary spread to additional neurons in the S-compartment (secondary infection/spread).
Materials
Two-week-old neuron cultures in the trichamber system
Neuronal medium containing 1% methocel (see recipe)
Viral stock to be studied (e.g., PRV)
Disposable protein gel-loading pipet tip (Rainin)
−80°C freezer
Grow dissociated neurons in the S-compartment for at least 2 weeks prior to any infection.
- Score the axonal densities in the N-compartment (see Basic Protocol 3, step 2).
- Since viral particles are entering neurons via distal axons, it is critical that the axonal density in the N-compartment is robust and comparable among all chambers.
On the day of infection, replace medium in the M-compartment with 200 μl of neuronal medium containing 1% methocel. Return the dish to the incubator for 30 min, to allow the methocel to settle to the bottom of the dish.
- Dilute the viral inoculum in neuronal medium, to a final volume of 50 μl. Remove medium from the N-compartment, and add the viral inoculum.
- Keeping the volume of infection to only 50 μl is important for efficient viral adsorption
- We use the same PFU for retrograde-mediated infection as for anterograde-mediated infections. See above for description of how to determine the appropriate amount of viral inoculum for your system.
- The terminal field densities and the number of infectious virions placed in the N-compartment are critical. Reproducible results are obtained only when inoculum titer is carefully controlled.
After 1 hr of incubation, gently remove the inoculum with a pipet and replace with 300 to 400 μl of neuronal medium. Place the chamber back in the incubator.
- At the appropriate time post-infection, scrape and harvest the infected neuronal cell bodies in the S-compartment.
- We routinely use a sterile, disposable protein gel-loading pipet tip to scrape the neuron cell bodies.
- In retrograde-mediated infections, the goal is to measure the ability of a viral strain to undergo transport to the cell soma, replicate, and/or spread among neurons in the S-compartment. There is no local virus production in the axon; thus, only the S-compartment is harvested.
Freeze the harvested samples at −80°C until ready to titer.
VISUALIZING INFECTION IN THE TRICHAMBER SYSTEM: IMMUNOFLUORESCENCE
BASIC PROTOCOL 4
Visualizing neurons grown in the trichamber system via immunofluorescence or transmission electron microscopy requires slight modification of existing protocols. The trichambers are assembled on a plastic surface called Aclar. This flexible, thermoplastic fluoropolymer film is biochemically inert and exhibits no detectable autofluorescence; it can be molded or cut into any shape desired, and treated like a coverslip. However, unlike a glass coverslip, Aclar can be etched to produce grooves that are required for guiding axonal penetration into the N-compartment.
The basic steps of any protocol for fixation and antibody incubation can be adapted to the trichamber system. Here, we describe our preferred particular fixation and antibody incubation times, along with notes about handling that are particular to Aclar and the trichamber setup. Users are encouraged to adapt their own immunofluorescence staining protocols to this system.
Materials
Poly-dl-ornithine (Sigma)
Laminin (BD Biosciences)
Phosphate-buffered saline (PBS; HyClone, cat. no. SH30028.03)
3.2% (w/v) paraformaldehyde in PBS (store up to 2 weeks, in the dark, at 4°C)
PBS/BSA: 3% (w/v) bovine serum albumin (BSA) in PBS (HyClone store solution up to 6 months at 4°C)
PBS/BSA/SAP: PBS (HyClone)/3% (w/v) bovine serum albumin (BSA; Roche)/0.5% (v/v) saponin solution (Sigma; store solution up to 6 months at 4°C)
Primary antibody
Fluorophore-conjugated secondary antibody
Tissue culture–grade water
Mounting medium (e.g., Aqua Poly/Mount from Polysciences)
Aclar (EM Sciences)
UV source for sterilization
Pin rake (Tyler Research or homemade)
35-mm tissue culture dish
Blunt forceps
Sharp forceps
Spatula
Dissection scissors, optional
Microscope slide
Microscope coverslip
Additional reagents and equipment for assembling the trichamber (Basic Protocol 1) and plating dissociated neurons, maintaining the cultures, scoring axon penetration, and subsequent infection assays (Basic Protocol 2 and 3 and Alternate Protocol 1)
-
1UV-sterilize a sheet of Aclar in the tissue culture hood. Cut a circular piece of Aclar that is slightly larger than the trichamber but smaller than a 35-mm tissue culture dish.
- The size of a standard United States quarter works well.
-
2Etch 16 parallel grooves in the middle of the Aclar strip using a pin rake.
- Since Aclar is transparent, it can be challenging to determine which side of Aclar contains the etched grooves. Make a small mark or notch on the Aclar strip to determine orientation.
-
3Place the Aclar in a 35-mm tissue culture dish and assemble the trichamber (Basic Protocol 1).
- Aclar is very hydrophobic. It is necessary to increase the volume of poly-dl-ornithine to 3 ml, and that of laminin to 2 ml per dish to ensure that the Aclar is completely submerged.
- Similarly, increase the volume of washing solutions to 2 to 3 ml. Aclar remains adhered to the tissue culture dish owing to a thin liquid film generated between the surfaces during poly-dl-ornithine and laminin incubations and wash steps.
-
4
Carry out all steps of plating dissociated neurons, maintaining the cultures, scoring axon penetration, and subsequent infection assays (Basic Protocols 2 and 3 and Alternate Protocol 1) in the same way for trichambers on Aclar as for the usual trichamber setup.
Fix cells
-
5At the desired time point after infection, wash all compartments once with 300 to 400 μl PBS per compartment and fix the cells with 300 to 400 μl of 3.2% paraformaldehyde in PBS for 10 min at room temperature.
- As with medium changes, use the same edge of the trichambers for all fluid changes in this protocol. This will minimize neuronal damage during the washes.
-
6Remove the fixative from all compartments and wash the samples three times, each time with 1 ml PBS containing 3% BSA (PBS/BSA). After the final wash, flood the entire 35-mm dish with 3 ml of PBS-BSA.
- Be gentle with the multiple rinses described in this protocol. Use a pipet to slowly flow solutions down the sides of the chamber. If the neuronal network starts to peel off due to washes, wash twice instead of three times. Infected neurons are especially likely to peel off during processing.
Remove the chamber
-
7Using a pair of serrated, blunt forceps, grasp the central barrier and gently lift the trichamber upwards. To anchor the Aclar on the surface of the dish, apply the same downward force on the Aclar in the M-compartment with a pair of sharp forceps.
- Using the sharp end of the forceps in the M-compartment will minimize damage done to the sample. Avoid applying pressure sideways when lifting the chambers from the Aclar as this will disrupt the neuronal network. If the correct force is applied during this process, the majority of the silicone grease will come off together with the trichamber.
-
8Remove excess silicone grease on the surface of Aclar.
- We find that spatulas or pipet tips work well to remove the excess silicone grease, which prevents the Aclar from floating to the surface of the antibody solution. An uneven Aclar surface will also hinder proper mounting on a coverslip.
-
9
Optional: Trim and discard the excess Aclar surrounding the chamber using dissection scissors.
Permeabilize the cells
-
10To permeabilize the sample, add 1 ml of PBS containing 3% bovine serum albumin and 0.5% saponin (PBS/BSA/SAP) in the well. Incubate the sample 10 min at room temperature.
- Preparation and treatment of samples for immunofluorescence can vary. We often use 0.1% (v/v) Triton X-100 instead of 0.5% saponin.
- To perform immunofluorescence on nonpermeabilized samples, omit detergents from all solutions.
- In place of bovine serum albumin, 10% goat serum can be used as a blocking reagent.
Stain the cells for immunofluorescence
-
11Remove PBS/BSA/SAP and replace with 1 ml of primary antibody solution diluted in PBS/BSA/SAP. Incubate 1 hr at room temperature or overnight at 4°C.
- Some laboratories prefer to use the drop method by inverting the Aclar and placing it on a drop of antibody solution; however, this may not be as gentle on the axons.
- Dilutions vary and they should be optimized for each antibody.
-
12
After the appropriate time, remove the primary antibody solution and rinse the well three times, each time with 1 ml PBS/BSA/SAP.
-
13Add 1 ml of fluorophore-conjugated secondary antibody solution diluted in PBS/BSA/SAP and incubate 1 hr at room temperature.
- If the secondary antibody is fluorescent, be sure to carry out this incubation in the dark.
-
14
After the appropriate time, remove the secondary antibody and rinse the well three times, each time with 1 ml PBS and once with 1 ml distilled water.
Mount the samples
-
15
Using a pair of forceps, carefully pick up the Aclar and blot away the excess liquid by touching the edge of the Aclar to a Kimwipe.
-
16Mount the Aclar on a microscope slide, cell side up, and place a coverslip over the sample.
- We routinely use Aqua Poly/Mount as the mounting medium. Place a drop of Aqua Poly/Mount on the cover slide and place the Aclar cell-side up. Make sure that that the Aclar is firmly in place. Next, place a drop of Aqua Poly/Mount on the Aclar and place a coverslip over the sample.
- If the Aclar is mounted cell-side down, the thickness of the Aclar may hinder proper viewing of the sample when using certain oil objectives that have a shorter focal plane.
-
17
Allow the samples to set overnight before doing any microscopy.
VISUALIZING INFECTION IN THE TRICHAMBER SYSTEM: ELECTRON MICROSCOPY OF TRICHAMBER SYSTEM SAMPLES
ALTERNATE PROTOCOL 2
One of the more challenging aspects of electron microscopy is preserving the ultrastructure of the sample, particularly the fragile neuronal network, during separation of the polymerized resin from the substrate. Often, the resin is bound tightly to the surface of the dish and the sample is damaged during separation. Aclar is an excellent substrate to use for electron microscopy because it is more hydrophobic than glass or plastic—hence, the resin tends to bind less tightly to Aclar (Kingsley and Cole, 1988). These protocols for transmission electron microscopy (TEM) were initiated and developed by Margaret Bisher, of the Princeton Confocal and Electron Microscopy Core Facility, and Lisa Pomeranz (Pomeranz et al., unpub. observ.).
Materials
Two-week-old cultures in the trichamber system, set up on Aclar (Basic Protocol 4)
Phosphate-buffered saline (PBS; HyClone)
2% (v/v) glutaraldehyde in sodium cacodylate buffer (see recipe)
0.2 M sodium cacodylate buffer, pH 7.2 (see recipe)
1% (w/v) osmium tetraoxide in sodium veronal buffer (see recipe)
Sodium veronal buffer (see recipe)
0.25% (w/v) toluidine blue in sodium cacodylate buffer (see recipe)
0.05 M sodium maleate buffer, pH 5.1 (see recipe)
2% (w/v) uranyl acetate in sodium maleate buffer (see recipe)
Ethanol solutions of 30%, 50%, 70%, 90%, 95%, and 100%
Epon resin (Embed812; see recipe)
BEEM capsules
Fine-tipped forceps
60°C oven
Ultramicrotome (e.g., Leica UC6)
200-mesh hexagonal copper grid (EM Sciences)
Zeiss 912AB TEM with an Omega Energy Filter
Fix samples
-
1At the desired time post-infection, wash all compartments twice, each time with 300 to 400 μl PBS per compartment and fix with 300 to 400 μl 2% glutaraldehyde solution 4 hr at room temperature.
- As with medium changes described in Basic Protocol 2, use the same edge of the trichambers for all fluid changes in this protocol. This will minimize neuronal damage during the washes.
-
2
After 4 hr, remove the trichambers and clean the Aclar as described in Basic Protocol 4 (steps 7 and 8). Do not trim the Aclar.
-
3
Remove the fixative and wash twice, each time with 3 ml 0.2 M sodium cacodylate buffer. Post-fix cells with 4 ml 1% osmium tetraoxide solution for 1 hr on ice.
Stain sample
-
4Rinse the sample four times, each time with 4 ml sodium veronal buffer. Incubate sample with 4 ml of 0.25% toluidine blue solution 1 hr at room temperature.
- Toluidine blue is used to visualize the sample and identify areas of interest.
-
5
Remove the toluidine blue and rinse the sample four times, each time with 4 ml sodium veronal buffer.
-
6
Rinse the sample four times, each time with 4 ml of 0.05 M sodium maleate buffer.
-
7
Incubate the sample overnight in the dark with 4 ml of 2% uranyl acetate solution.
-
8
Rinse the sample four times, each time with 4 ml sodium maleate buffer.
Dehydrate sample
-
9
Dehydrate the sample by incubating in 4 ml of 30%, 50%, 70%, 90%, 95%, and two treatments of 100% ethanol, for 10 min each.
Embed sample
-
10
Replace the 100% ethanol with a 2:1 dilution of ethanol:resin for 2 hr, then 1:1 ethanol: resin overnight at room temperature.
-
11
The next day, replace with 100% resin and incubate for most of the day.
-
12Meanwhile, fill BEEM capsules with 100% resin and allow the resin to slightly overfill the capsule. Make sure that there are no bubbles in the resin.
- It is a good idea to fill the capsules early in the day and allow them to degas for a few hours
-
13
Using a pair of fine-tipped forceps, gently pull the Aclar sample, sitting in resin, out of the well. Prop it on its edge at a slight angle, and allow most of the resin to slide off the Aclar.
-
14Holding on the sample with the forceps, flip it onto the slightly overfilled top of the BEEM capsule (cell-side down, into the resin) to form a seal.
- Sections can be obtained separately from all three compartments of the chamber, by using a pair of fine-tipped scissors to cut the sample into pieces.
-
15
Place the BEEM capsule, with the Aclar top, into the 60°C oven and allow polymerizing for about 24 hr.
-
16The next day, let the resin cool to room temperature, and then use a pair of forceps to pull the Aclar off the top of the BEEM capsule.
- The cells should be in the resin, away from the Aclar.
Section and view the sample
-
17
Obtain 70-μm sections using a Leica UC6 ultramicrotome and mount on a 200-mesh hexagonal copper grid.
-
18Image using a Zeiss 912AB TEM with an Omega Energy Filter.
- To enhance contrast of cellular membranes, sectioned samples can be post-stained with lead citrate for 2 min, washed twice with water, and incubated another 2 min in 2% uranyl acetate.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Cytosine β-d-arabinofuranoside hydrochloride (AraC)
Prepare AraC (from powder; Sigma) as a 5 mM stock in DMEM. Dilute this to a 500 μM working stock in DMEM. Protect AraC stocks from light. Store up to 1 year, in the dark, at 4°C.
DNase, 1 mg/ml in Neurobasal medium
Dissolve DNase (Sigma) in Neurobasal medium (Invitrogen) at 10 mg/ml concentration. Filter-sterilize using a 0.2-μm filter. Excess stock can be stored (−80°C) at this high concentration. Dilute stock to 1 mg/ml in Neurobasal medium.%Divide into 200-μl aliquots and store frozen up to 1 year at −80°C.
Epon Resin (Embed812)
| Part A: | 5 ml EMbed812 (EM Sciences) |
| 8 ml dodecenyl succinic anhydride (DDSA; EM Sciences | |
| Part B: | 8 ml EMbed812 |
| 7 ml nadic methyl anhydride (NMA EM Sciences) | |
| Part C: | 0.47 ml tris(dimethylaminomethyl)phenol (DMP30; EM Sciences) |
| Part A and Part B can be prepared in advance and stored at 4°C. Bring both parts to room temperature before use. Mix Part A with Part B first, then add Part C. Mix well on a gentle rocker for ∼1 hr. | |
| Do not store mixed resin. Aliquots of Parts A/B/C can be stored for up to 6 months at 4°C. | |
Glutaraldehyde, 2% (v/v), in sodium cacodylate buffer
10 ml ampoule of 10% glutaraldehyde (EM Sciences, cat. no. 16120)
Prepare fresh, by diluting 10% glutaraldehyde 1:5 with 0.2 M sodium cacodylate buffer (see recipe)
Prepare fresh
Methylcellulose (methocel), 2% (w/v), in DMEM
4 g methylcellulose
100 ml distilled water
100 ml 2× DMEM (make from powder using tissue culture–grade water; Invitrogen)
Weigh out 4 g methylcellulose, add to glass bottle, then add 100 ml distilled water (powder will remain clumpy).
NOTE: This initial solution is 4% (w/v) methocel.
Autoclave on liquid setting. Remove from autoclave, add a sterile stir bar, and stir overnight at 4°C. Add 100 ml 2× DMEM, for a final solution that is 2% methocel in 1$ DMEM. If clumps remain, stir overnight again. Store up to 1 year at 4°C.
Neuronal medium
Neurobasal medium (Invitrogen)
1× glutamine/penicillin/streptomycin solution (Invitrogen)
1× B-27 medium supplement (Invitrogen)
NGF to 100 ng/ml (Invitrogen, cat. no. 13257−019)
Mix all components except NGF, and filter-sterilize using a 0.2-μm filter
Add NGF last
Store up to 10 days at 4°C
Neurobasal medium with B-27 supplement provides essentially the same medium formulation previously used by our laboratory for rat superior cervical ganglia cultures (Brewer et al., 1993; Ch'ng et al., 2005). This straightforward formulation works equally well for rat and mouse SCG cultures. The final concentration of glutamine is 2 mM, with 100 U/ml penicillin and 100 μg/ml streptomycin.
Neuronal medium containing 1% methocel
Prepare appropriate volume (e.g., 10 ml) of Neurobasal medium (Invitrogen) with 2× glutamine/penicillin/streptomycin (Invitrogen) and 2× B-27 medium supplement (Invitrogen). Filter-sterilize using a 0.2-μm filter. Add NGF (Invitrogen, cat. no. 13257−019) to 200 ng/ml.
Combine this 2× Neurobasal mix with an equal volume (e.g., 10 ml) of 2% methocel in DMEM (see recipe). Mix by pipetting or inverting tube several times.
Store up to 10 days at 4°C.
Osmium tetraoxide (OsO4), 1%, in sodium veronal buffer
4% aqueous solution of OsO4 (EM Sciences, cat. no. 19170)
Dilute OsO4 with freshly made sodium veronal buffer (see recipe) to make a 1% solution
Prepare fresh; do not store
Sodium veronal buffer is recommended for superior membrane preservation with OsO4 (Hayat, 2000).
Sodium cacodylate buffer (pH 7.2), 0.2 M
42.80 g sodium cacodylate
1000 ml distilled water
Mix all ingredients until dissolved
Adjust pH to 7.2 using 0.1 N HCl
Store up to 6 months at 4°C
Check for clarity before using.
CAUTION: Cacodylate contains arsenic. Avoid skin contact and inhalation. Avoid strong reducing agents such as aluminum, zinc, sodium borohydride, sulfur dioxide.
Sodium maleate buffer (pH 5.1), 0.05 M
36 ml 0.1 N NaOH
125 ml 0.2 M maleic acid
339 ml distilled water
Mix solution
Adjust pH to 5.15 using 0.1 N NaOH
Store up to 6 months at room temperature
Sodium veronal buffer (veronal acetate buffer)
Stock solution
2.94 g sodium veronal (sodium 5,5-diethylbarbiturate)
1.94 g sodium acetate
3.40 g sodium chloride
100 ml distilled water stock solution can be stored up to 6 months at room temperature
Working solution
5 ml stock solution (see recipe)
13 ml distilled water
0.25 ml 1.0 M CaCl2
∼7 ml 0.1 M HCl
Add the HCl dropwise until the required pH (between 4.2 and 5.2) is obtained Prepare the buffer fresh every time; do not store
Soybean trypsin inhibitor, 2 mg/ml in Neurobasal medium
Dissolve soybean trypsin inhibitor (SBTI; Sigma) in Neurobasal medium (Invitrogen) at a concentration of 10 mg/ml. Pass through a 0.45-μm filter, then a 0.2-μm filter. Excess stock can be stored (−80°C) at this high concentration. Dilute stock to 2 mg/ml in Neurobasal medium. Divide into 650-μl aliquots and store frozen up to 1 year at −80°C.
Teflon trichambers
Tyler Research Corporation- Edmonton, Canada.
The trichambers are called Camp3A, which is essentially the catalog Camp3 chamber, scaled down to 20-mm diameter (www.tylerresearch.com).
Toluidine blue, 0.25 (w/v), in sodium cacodylate buffer
1 g of toluidine blue
400 ml 0.2 M sodium cacodylate buffer (see recipe)
Mix all ingredients well
Pass solution through a 0.22-μm syringe filter
Store up to 6 months at room temperature
Trypsin, 2.5 mg/ml in HBSS
Dissolve trypsin (Sigma) in Hank's balanced salt solution (HBSS; HyClone) at 10 mg/ml concentration. Pass solution through a 0.2-μm filter. Excess stock can be stored (−80°C) at this high concentration. Dilute stock to 2.5 mg/ml in HBSS. Divide into 100-μl aliquots and store frozen up to 1 year at −80°C.
This method of freezing highly concentrated enzyme stocks, while more dilute enzyme stocks are frozen in single-use aliquots, is both economical and prevents enzyme degradation during multiple freeze-thaw cycles.
Uranyl acetate, 2% (w/v), in sodium maleate buffer
0.5 g of uranyl acetate
25 ml 0.05 M sodium maleate buffer (see recipe)
Mix thoroughly to dissolve
Pass through 0.22-μm syringe filter
Store up to 6 months at room temperature, in the dark
Check clarity of solution before use
COMMENTARY
Background Information
Alpha herpesviruses are neuroinvasive pathogens that spread in a bidirectional, transneuronal fashion through the nervous system of the infected host. When viral particles are moving via plus-end directed microtubule transport in the axons of infected neurons, we term this motion anterograde transport; movement in the opposite direction is termed retrograde. When viral transport leads to viral movement from a presynaptic to a post-synaptic neuron, we call this anterograde spread.
Viral spread has been studied extensively in vivo: inoculum is injected into specific structures of the peripheral or central nervous system of an animal, and the extent of spread is determined by detecting viral antigen in anatomical sections. Because alpha herpesviruses spread between synaptically connected neurons, such experiments are valuable for elucidating neural circuit architecture and connectivity. However, for the purpose of studying large numbers of viral mutants, or defining the exact timing of viral transport within and between cells, it is much easier and less tedious to have a facile in vitro system.
Robert Campenot established a system for compartmentalization of neurons for studying neurite development after modulation of somatic or axonal media environments (Campenot, 1977). These chambers have since been modified, and their application extended to a variety of model systems, including other models of alpha herpesvirus infection (Ziegler and Herman, 1980; Lycke et al., 1984; Penfold et al., 1994; Mikloska and Cunningham, 2001; Karten et al., 2002, 2005). The trichamber cultures we describe differ structurally from the original Campenot chambers: in the original setup, the middle compartment is open to the environment outside the chamber; neurons are plated here, and axonal outgrowth is directed into both side-compartments. In the modified version, the middle compartment is not open to the external environment; dissociated ganglia are plated in one side compartment, from which axons first emerge into the middle, and then the opposite side compartment. Plating neurons in this fashion puts a greater distance between soma and axonal termini; furthermore, placing methocel-containing medium into the middle compartment serves to ensure that no appreciable fluid exchange can occur between the soma- and neurite-containing compartments.
For these reasons, the modified Campenot culture system offers an ideal in vitro setup for examining the efficiency of directional transport and the spread of viral infection. By co-plating epithelial cells with axons, the efficiency of neuron-to-cell transmission of infection can be investigated. Conversely, retrograde-directed infection can be studied by applying the inoculum to axons, and measuring viral content of the neuronal cell bodies. Because only one-half to one ganglion per chamber (∼5000 mature cell bodies) is required to achieve dense, experimentally viable neuronal cultures, the system yields more data in less time than in vivo animal infection experiments.
Performing immunofluorescence and electron microscopy on infected neurons can yield information regarding the mechanisms of directional infection. When imaging experiments are performed on infections of standard dissociated neuronal cultures, the site being observed by microscopy may also contain viral particles from the original inoculum. These conditions complicate data interpretation. Compartmentalization of neurons provides a solution, since the input inoculum is confined to a single-chamber compartment. Furthermore, the direction of viral transport is defined, enabling investigators to confidently describe anterograde-bound or retrograde-bound particles.
Critical Parameters
Not all of the neurons plated in the S-compartment extend axons that reach the N-compartment; additionally, the efficiency of axonal penetration underneath the Teflon barriers will vary. Therefore, if treatment is applied to the neurite compartment, only a sub-population of cells in the soma compartment will respond to it directly. For this reason, it is crucial to plate the same number of cells in each S-compartment, and to score the efficiency of axonal penetration for each trichamber. After designating axonal density scores, the trichambers must be distributed evenly among the variables being tested.
It is also critical to establish ahead of time the amount of viral inoculum required to infect all cells in the S-compartment synchronously. This can be done with a titration series and immunofluorescence assays, and can be applied to all subsequent experiments involving the same virus and cells. If anterograde-mediated infection is incomplete or asynchronous, it will confound interpretation of the data.
When detector cells are plated in the neu-rite compartment, it is critical to supplement the cells with 1% fetal bovine serum. In the absence of serum, these cultured cells will not adhere to the bottom of the compartment. While the neuronal medium overall is serum-free, it should be noted that the N-compartment axons are necessarily exposed to serum whenever detector cells are plated there.
Troubleshooting
Axons hit the first barrier, turn away, and never penetrate into the M-compartment
Grooves etched into the bottom of the 35-mm tissue culture dish must be deep enough to generate rough, physical barriers that axons cannot cross. If the grooves are too shallow and smooth, they will not provide sufficient guidance for axonal penetration; the solution is to etch deeper grooves.
During assembly of the trichambers, do not apply too much force on the trichambers to create the watertight seal. Also, take care not to hit the chambers during subsequent medium changes. Either of these mistakes may inhibit axon penetration.
Alternatively, the type of neurons being cultured may not exhibit the robust growth necessary for barrier penetration. Neurons that have been successfully cultured in trichambers include embryonic and newborn rat superior cervical ganglia (SCG; Campenot, 1977; Ch'ng et al., 2005), embryonic mouse SCG neurons (Szpara and Enquist, unpub. observ.), and adult rat dorsal root ganglia (DRG; Kimpinski et al., 1997). Neuronal cultures grown in the original Campenot chambers, where axonal penetration under only one barrier is required, include newborn mouse SCG (Manning et al., 1987), rat DRG (Bi et al., 2006), and rat retinal ganglion cells (Hayashi et al., 2004). A modified Campenot chamber has also been used on cultured hippocampal neurons to study local neurite degeneration (Ivins et al., 1998). To our knowledge, neurites extended by neuron-like cell lines (e.g., PC12 cells) do not robustly penetrate the Teflon barriers in the trichamber system.
Neurons die 2 to 3 days after being plated
Optimization of adhesion substrates and/or neuronal medium is required. It is also possible that the incorrect ganglia are being isolated during dissection; for an example, it can be difficult to distinguish between the embryonic rat nodose ganglion and the superior cervical ganglion due to their physical proximity and morphological similarities. Low neuronal viability can result from treating the ganglia too harshly during dissociation and trituration. Test the plate-coating, dissection, dissociation, and culturing methods by culturing neurons in 35-mm dishes without the trichamber setup. Make sure that neurons grow successfully in noncompartmented, dissociated cultures before moving on to trichambers.
Infection yields low titer
Infections of either the soma or neurons that produce low titers may result if the axonal density of cultures used in the experiment is low. One solution is to maintain the trichamber cultures several days longer to achieve denser axonal penetration into the N-compartment. Check your conditions by performing the experiment with a viral strain (e.g., wild-type PRV) that has been shown to infect cultured neurons efficiently. Another important control is to check the titer of the S-compartment cell bodies after S-compartment infection.
Neurons are fragile when processed for immunofluorescence
Standard immunofluorescence protocol options apply to the trichamber system. Wash steps and antibody incubations can be lengthened to increase sensitivity and/or decrease background. Care must always be taken to wash gently so that the network of neurons does not peel away. This is especially true late in infection by alpha herpesviruses. While removing the excess silicone grease, work carefully to avoid disrupting the neuronal networks, but swiftly to avoid drying out the sample.
No sample seen in EM
The neuronal network is both fragile and highly interconnected. While the edges may tear during wash steps, they can also lift off and eventually pull the entire neuronal network off the Aclar. Be cautious in wash steps and check that the sample is present throughout all incubations and washes, up to embedding in resin.
If imaging axons in the N-compartment, it may be difficult to find areas of axonal growth dense enough to capture via electron microscopy. The area closest to the Teflon barrier generally contains denser axonal networks.
Anticipated Results
The in vitro development of neurons isolated from embryonic rat or mouse superior cervical ganglia is consistent and predictable. Neurite outgrowth is detectable 1 day after the dissociated neurons are plated. Axons are visible in the M-compartment 3 to 4 days post-plating; they reach the N-compartment 10 to 12 days post-plating. High axonal density in the N-compartment can be achieved by 14 to 21 days. Under these growth conditions, the mature neurons will have only axons in the neurite compartment and not dendrites, which can be confirmed by immunofluorescence. For instance, the axonal marker Tau should be visible throughout cell bodies and all neurites, where the somatodendritic marker microtubule-associated protein 2 (Map2) should only be visible in the neuron soma.
When studying neuron-to-cell spread of wild-type PRV with PK15 detector cells in the N-compartment, the yield in the N-compartment will be ∼107 PFU/ml at 24 hr post-infection. Infection of axons, which leads to retrograde transport and virus production by neuron cell bodies in the S-compartment, typically yields 105 PFU/ml in the S-compartment at 24 hr post-infection. Virus yield depends on the type of virus used in the study [e.g., PRV, herpes simplex virus 1 (HSV-1), etc.], degree of spread, and duration of infection (e.g., higher titers with more time for replication and/or spread).
Time Considerations
Setting up the trichamber system requires extensive planning and preparation. The average time from set up to initiating an experiment is 2 weeks. The intensive set up phase begins with coating the plates early on the day before a dissection is planned. Dissection tools and silicone grease are autoclaved late in the day or early the next morning. Trichambers are greased and assembled on the morning of a dissection. Neurons are dissected, dissociated, and plated later in the same day. To gain some flexibility in the schedule, plates can be coated with poly-ornithine and/or laminin for longer periods of time (overnight or over several nights). However, trichambers should be assembled no earlier than the evening before a dissection.
During the maintenance phase, the trichamber cultures are treated with an anti-mitotic agent (AraC) 2 days after dissection, and the medium changed for the first time on day 3. Thereafter, medium must be changed every 2 to 3 days. It is advisable to change medium in all the compartments even prior to axonal penetration to the M- and N-compartments. Axonal penetration to the middle compartment is visible within days, although the fine neuronal processes can be difficult to observe by phase-contrast microscopy. Axons typically reach the second barrier and begin to penetrate into the third compartment around 1 week. By 2 weeks, the axonal penetration in the neurite compartment will likely be sufficient for use; cultures may be maintained as long as 4 weeks if more time for axon growth is needed. However, in some cases, neuronal viability may start to decline after more than 3 weeks in culture.
It is important to assemble extra trichambers, since success of axon penetration is variable, and some chambers will not have adequate penetration even at 2 to 3 weeks post-plating. It is common for novice users to have 50% or less of their trichambers succeed in having good axonal penetration.
Neuronal infections may take several hours or days, depending on the virus, time points examined, and other experimental parameters. Absorption of viral inoculum to the cells or axons is typically performed for 1 hr. Although one should be meticulous when scraping the S- or N-compartment for titering, collecting the contents of one compartment typically takes <1 min.
Immunofluorescence experiments require several wash steps and incubations, 10-min fixation and permeabilization steps, and sequential 1-hr incubations with primary and secondary antibodies. With the required wash steps, the entire procedure for a single sample can take 3 hr. Because processing samples for electron microscopy requires more than one overnight incubation, the procedure can require 3 or more days.
Literature Cited
- Bi J, Tsai NP, Lin YP, Loh HH, Wei LN. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19919–19924. doi: 10.1073/pnas.0607394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Compartmented culture analysis of nerve growth. In: Stevenson RB, Gallin WJ, Paul DL, editors. Cell-Cell Interactions: A Practical Approach. IRL Press; Oxford: 1992. pp. 275–298. [Google Scholar]
- Ch'ng TH, Flood EA, Enquist LW. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Molec. Biol. 2005;292:299–316. doi: 10.1385/1-59259-848-x:299. [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Spear PG, Struyf F, Enquist LW. Glycoprotein D-independent spread of pseudorabies virus infection in cultured peripheral nervous system neurons in a compartmented system. J. Virol. 2007;81:10742–10757. doi: 10.1128/JVI.00981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanović D, Lyman M, Bisher M, Enquist LW. Mutations in UL21 affect the efficiency of retrograde transneuronal infection by PRV-Bartha. Manuscript in preparation.
- DiCicco-Bloom E, Black IB. Insulin growth factors regulate the mitotic cycle in cultured rat sympathetic neuroblasts. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4066–4070. doi: 10.1073/pnas.85.11.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Townes-Anderson E, Black IB. Neuroblast mitosis in dissociated culture: regulation and relationship to differentiation. J. Cell Biol. 1990;110:2073–2086. doi: 10.1083/jcb.110.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Bisher M, Goodhouse J, Enquist LW. In vitro analysis of transneuronal spread of an alpha herpesvirus infection in peripheral nervous system neurons. J. Virol. 2007;81:6846–6857. doi: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Hayat MA. Principles and Techniques of Electron Microscopy: Biological Applications. Cambridge University Press; 2000. [Google Scholar]
- Ivins KJ, Bui ET, Cotman CW. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol. Dis. 1998;5:365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- Johnson MI. Primary cultures of sympathetic ganglia. Humana Press Inc.; Totowa, N.J.: 2001. [Google Scholar]
- Karten B, MacInnis BL, Eng H, Azumaya Y, Martin G, Lund K, Watts RC, Vance JE, Vance DE, Campenot RB. Apoptosis Techniques and Protocols. Vol. 37. Humana Press; 2002. Analytical approaches for investigating apoptosis and other biochemical events in compartmented cultures of sympathetic neurons. pp. 163–175. [Google Scholar]
- Karten B, Hayashi H, Campenot RB, Vance DE, Vance JE. Neuronal models for studying lipid metabolism and transport. Methods. 2005;36:117–128. doi: 10.1016/j.ymeth.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Campenot RB, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J. Neurobiol. 1997;33:395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kingsley RE, Cole NL. Preparation of cultured mammalian cells for transmission and scanning electron microscopy using Aclar film. J. Electron Microsc. Tech. 1988;10:77–85. doi: 10.1002/jemt.1060100110. [DOI] [PubMed] [Google Scholar]
- Lycke E, Kristensson K, Svennerholm B, Vahlne A, Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 1984;65:55–64. doi: 10.1099/0022-1317-65-1-55. [DOI] [PubMed] [Google Scholar]
- Manning PT, Johnson EM, Jr., Wilcox CL, Palmatier MA, Russell JH. MHC-specific cytotoxic T lymphocyte killing of dissociated sympathetic neuronal cultures. Am. J. Pathol. 1987;128:395–409. [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 2001;75:11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: Evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Herman RE. Peripheral infection in culture of rat sensory neurons by herpes simplex virus. Infect. Immun. 1980;28:620–623. doi: 10.1128/iai.28.2.620-623.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]