Abstract
Blueberry fruits are known as a rich source of anthocyanin components. In this study we demonstrate that anthocyanins from blueberry have the potency to alleviate symptoms of hyperglycemia in diabetic C57b1/6J mice. The anti-diabetic activity of different anthocyanin-related extracts was evaluated using the pharmaceutically acceptable self-microemulsifying drug delivery system; Labrasol. Treatment by gavage (500 mg/kg body wt) with a phenolic-rich extract and an anthocyanin-enriched fraction formulated with Labrasol lowered elevated blood glucose levels by 33 and 51%, respectively. The hypoglycemic activities of these formulae were comparable to that of the known anti-diabetic drug metformin (Baily and Day, 2004; 27% at 300 mg/kg). The extracts were not significantly hypoglycemic when administered without Labrasol, demonstrating its bio-enhancing effect, most likely due to increasing the bioavailability of the administered preparations. The phenolic-rich extract contained 287.0 ± 9.7 mg/g anthocyanins, while the anthocyanin-enriched fraction contained 595 ± 20.0 mg/g (cyanidin-3-glucoside equivalents), as measured by HPLC and pH differential analysis methods. The greater hypoglycemic activity of the anthocyanin-enriched fraction compared to the initial phenolic-rich extract suggested that the activity was due to the anthocyanin components. Treatment by gavage (300 mg/kg) with the pure anthocyanins, delphinidin-3-O-glucoside and malvidin-3-O- glucoside, formulated with Labrasol, showed that malvidin-3-O- glucoside was significantly hypoglycemic while delphinidin-3-O-glucoside was not.
Keywords: Blueberry, Vaccinium angustifolium, Anthocyanins, Malvidin, Diabetes, Hyperglycemia, Hypoglycemia, Labrasol
Introduction
According to the International Diabetes Federation (IDF), type 2 diabetes currently affects 246 million people worldwide and is expected to increase to 380 million by 2025. Metabolic syndrome is a combination of related disorders, including impaired glucose tolerance, abdominal obesity, high cholesterol and high blood pressure, which increase the risk of cardiovascular disease and diabetes. Insulin resistance is a key pathophysiologic feature of metabolic syndrome and type 2 diabetes and is strongly associated with co-existing cardiovascular risk factors and accelerated atherosclerosis (Haffner, 1996). Strategies to improve insulin resistance by pharmacological means have been the most widely used approaches for clinical medicine (Davidson, 1998). In addition to conventional treatments relying on insulin injections or prescription medications, natural products, especially herbal preparations, have been utilized as alternative treatments for diabetes.
Blueberries and related fruits are particularly nutritious and have been shown to provide benefits for a wide variety of conditions. Most notably, blueberries contain powerful antioxidants that can neutralize free radicals that cause neurodegenerative disease, cardiovascular disease, and cancer (Kraft et al., 2005, Neto, 2007a, b, Shukitt-Hale et al., 2008). European blueberries and their relatives are also thought to improve vision and prevent macular degeneration (Trevithick and Mitton, 1999). In addition, blueberries are associated with improved digestion, reduction of colon inflammation and promotion of urinary tract health (PDR for Herbal Medicine). European and American members of the genus Vaccinium are known to possess anti-diabetic activity (Blumenthal, 1998; Chambers and Camire, 2003; Martineau et al., 2006) and have been used in traditional medicine, especially for the secondary complications of diabetes (Jellin et al., 2005). Some studies have shown that extracts from the leaves of wild blueberries (Vaccinium angustifolium) or bilberries (Vaccinium myrtillus) are useful for lowering blood glucose in animal models (Murray, 1997). The preparations, however, showed limited hypoglycemic activity in humans (Watson, 1928). Martineau et al. (2006) tested the anti-diabetic activity of ethanol extracts from roots, stems, leaves and fruits of wild blueberry in vitro, using a variety of cell-based bioassays. They found that different parts of the plant contain several active principles with insulin-like anti-diabetic properties The constituents of the root, leaf and stem extracts were very different, however, from the constituents of the fruit extracts. Only the fruit extract contained high concentrations of anthocyanins. The fruit extract increased 3H-thymidine incorporation into replicating β TC-tet cells, signifying the proliferation of pancreatic β cells, but no increase in insulin secretion was observed.
Despite the documented health benefits of blueberries, the use of blueberry preparations for enhancing insulin sensitivity and hypoglycemic activity has not been evaluated in vivo. The aim of the present work was to assess the effect of an orally administered phenolic-rich extract as well as a concentrated anthocyanin-enriched fraction prepared from V. angustifolium and formulated with a bio-enhancing agent (Labrasol) for lowering hyperglycemia associated with diabetes and metabolic syndrome in a rodent model. Also, the anti-hyperglycemic activity of pure malvidin and delphenidin glucosides, formulated with Labrasol, was evaluated in vivo and compared to the prepared blueberry anthocyanin extracts.
Materials and methods
The 3-O-β-glucosides of delphinidin and malvidin (anthocyanins standard, HPLC grade) were purchased from Polyphenols Laboratories AS (Sandnes, Norway). Amberlite XAD-7, Sephadex LH-20, trifluoroacetic acid (TFA), hydrochloric acid, potassium chloride, and sodium acetate were purchased from Sigma Chemical Co (St Louis, MO, USA). Labrasol was supplied by Gattefosse Corp. (Westwood, NJ, USA).
Blueberry source
Individually quick-frozen, whole lowbush blueberries (Vaccinium angustifolium Aiton) were obtained from the Wild Blueberry Association of North America (Old Town, ME, USA). The blueberries were a composite of fruits from all major growing sites including Prince Edward Island, New Brunswick, Nova Scotia, and Maine. The composite was made in the fall 2006, frozen by Cherryfield Foods, Inc. at −15°C (Cherryfield, ME, USA), and subsequently stored at −80 °C in our lab at the University of Illinois at Urbana Champaign until use.
Preparation of the phenolic-rich extract
The whole frozen blueberry fruits (1 kg) were blended (Waring, Inc., Torrington, CT, USA) with methanol, acidified with 0.3% TFA (2 l/kg fruit), and filtered first through multiple layers of muslin sheets, and then on Whatman’s filter paper # 4 (Florham Park, NJ, USA) with the aid of suction. The collected hydro-alcoholic extract was evaporated to about 500 ml using a rotary evaporator at a temperature not exceeding 40°C. The obtained aqueous concentrated extract was partitioned against ethyl acetate (4 × 500 ml) to remove lipophilic material. After evaporation of remaining EtOAc, the aqueous layer (500 ml) was loaded on an Amberlite XAD-7 column (30 × 10 cm), preconditioned with acidified water (0.3% TFA). The resin was washed thoroughly with acidified water (0.3% TFA, ~ 3 l) to remove free sugars and phenolic acids. The polyphenolic mixture was then eluted with 1 liter of methanol (0.3% TFA), and the eluate was evaporated, and freeze-dried to yield 7.5 g of phenolic-rich extract.
Preparation of an anthocyanin-enriched fraction
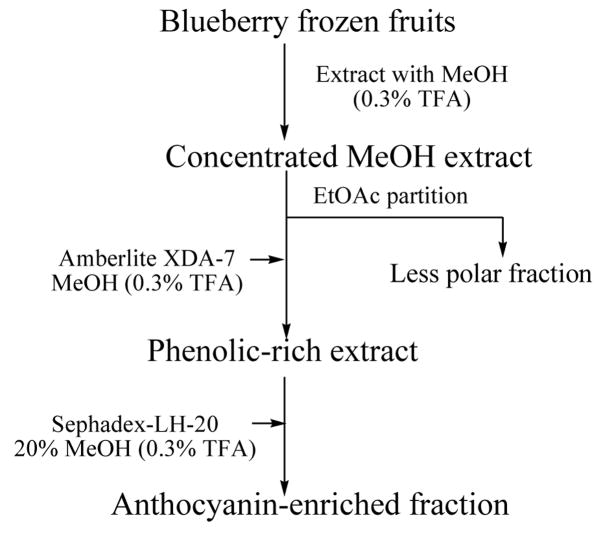
The phenolic-rich extract (5.0 g), obtained from the Amberlite XAD-7 column, was applied to a column packed with Sephadex LH-20 (30 × 3 cm) and preconditioned with H2O-MeOH 80:20 (0.3% TFA), with an isocratic elution using the same solvent ratio. Four fractions (1–4), each of 200 ml, were collected starting when the colored material began to elute from the column. The column was then washed with 70% acetone (fraction 5) to elute polymeric proanthocyanidins. Solvents were evaporated (< 40 °C) and the aqueous extract was immediately freeze-dried to afford 2.15, 0.26, 0.24, 0.20 g, and 1.75 g, respectively. Fraction 1 contained the highest anthocyanin concentration, thus it was designated as “anthocyanin-enriched fraction”. A flow chart illustrating the enrichment procedure is shown in Fig. 1.
Figure 1.
Flow chart showing extraction, clean-up and anthocyanin enrichment process from blueberry frozen fruits.
Total anthocyanins and phenolics
Total monomeric anthocyanins were estimated by a pH differential method (Cheng and Breen, 1991) using SpectraMax Plus spectrophotometer (Sunnyvale, CA, USA) and 1 cm path length cuvette. Absorbance was measured at 510 and 700 nm in buffers at pH 1.0 and 4.5, using A = (A510) − A700)pH1.0 − (A510 − A700) pH4.5. Anthocyanin content was calculated as milligrams of cyanidin-3-O-glucoside equivalent per gram dry weight, using a molar extinction coefficient of 26,900 and molecular weight of 449. Total soluble phenols were determined with Folin-Ciocalteu reagent by the method of Singleton et al. (1999). Results were expressed as milligrams of gallic acid equivalent (GAE) per gram dry weight.
HPLC-PDA analysis
The HPLC analyses were conducted using an 1100 HPLC (Agilent Technologies, Santa Clara, CA, USA) with a photodiode array (PDA) detector, and an autosampler with Chemstation software as a controller and for data processing. Anthocyanin separation was performed using a reversed phase Supelcosil-LC-18 column, 25 mm × 4.6 mm × 5 μm (Supelco, Bellefonte, PA). The mobile phase consisted of 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was constant during HPLC analysis at 1 ml/min with a step gradient of 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively. Samples were prepared by dissolving 5 mg in 1 ml methanol and filtering through 0.2 μm nylon filters (Fisher Scientific, Pittsburg, PA, USA) before injecting 15 μl onto the HPLC column with a constant temperature of 20°C. Three concentrations of cyanidin-3-O-glucoside were prepared at 1.0, 0.5, and 0.25 μg/ml where 15 μl was injected as an external standard. Quantification of anthocyanins was performed from the peak areas recorded at 520 nm with reference to the calibration curve obtained with cyanidin-3-O-glycoside. Blueberry extracts were analyzed in triplicates.
LC-ESI-MS analysis
The HPLC separations were carried out on Waters Alliance 2795 (Waters Corporation, Beverly, MA, USA) with a C-18 reversed-phase column (150 mm, 2.1 mm i.d., particle size 5 μm, 90 Å) (VYDAC, Western Analytical, Murrieta, CA, USA). The analysis was carried out using mobile phase consisting of 95:5 acetonitrile-water (0.1% formic acid) (A), and 5:95 acetonitrile-H2O (0.1% formic acid) (B), with a step gradient of 5%, 30%, 60%, 90%, 90%, 5% and 5% of solvent B at 0, 40, 45, 50, 55, 60, 70 min, respectively. A constant flow rate of 200 μl/min and an injection volume of 10 μl were employed. The column temperature and the samples were kept at 20°C. The HPLC system was connected to a Waters 996 PDA detector set to acquire data every second from 275 to 530 nm with a resolution of 1.2 nm, and subsequently to a Q-TOF Ultima mass spectrometer (Waters Corporation). An ESI source working in the positive ion mode was used for all MS analyses. Acquisition of LC-PDA-MS data was performed under MassLynx 4.0 (Waters Corporation). Standard references (2 mg) and samples (5 mg) were dissolved in 1 ml 100% MeOH and filtered (0.22 μm PTFE) before injection.
Evaluation of anti-diabetic activity in mice
Five-week-old male C57bl/6J mice (10–20 g) were purchased from Jackson Labs (Bar Harbor, ME, USA) and were acclimatized for 1 week before being randomly assigned into the experimental groups. The animals were housed four to a cage with free access to water in a room with a 12:12 h light–dark cycle (7:00 am–7:00 pm), a temperature of 24±1°C, and the animals were weighed weekly. During the acclimatization period, each animal was fed a regular diet ad libitum. At 6-weeks old, the mice were randomly divided into two groups: low fat or very high fat fed groups. The mice continued to receive either a low fat diet (LFD) or very high-fat diet (VHFD) for a 12-week period. The low fat and high-fat diet food were purchased from Research Diets Inc. (New Brunswick, NJ, USA). The nutritional content of very high-fat diet food was similar to that of the low fat diet food with the exceptions of lower carbohydrates (70 kcal% vs. 20 kcal%) and higher fat from lard (10 kcal% vs. 60 kcal%). Body weights were measured weekly, and at every other week blood was collected for blood glucose analysis. Animals fed with VHFD were fasted for 4 hours and fed orally (gavaged) with plant extracts (500 mg/kg), pure anthocyanin compounds (delphinidin-3-glucoside and malvidin-3-glucoside, 300 mg/kg), or vehicle (Labrasol, 66% w/v) to test efficacy of plant extracts. Animals fed with LFD were also fasted for 4 hours before treatment. All treatments were provided by gavage with a vehicle of 66% Labrasol. Blood glucose readings were made at 0, 3, and 6 hours after treatment (animals were fasted during the testing period). As a positive control, metformin was administered to a group of animals at a dose of 300 mg/kg (MET300) using the same vehicle. The experimental protocols were approved by Rutgers University Institutional Care and Use Committee and followed federal and state laws.
Results
Analysis of blueberry anthocyanins
Blueberry extracts were prepared from frozen fruits using a successive enrichment method to create related extracts with different levels of anthocyanins, while having the same relative composition of anthocyanin components. Anthocyanins are commonly extracted from plant material with methanol that contains small amounts of acid (TFA). The acid lowers the pH of the solution and prevents degradation of anthocyanin pigments. After the blueberry frozen fruits had been extracted with acidified MeOH, and partitioned with EtOAc, the aqueous layer was loaded over an Amberlite XDA-7 column. Anthocyanin molecules, along with other polyphenolics were adsorbed by the resin, while the phenolic acids and free sugars were washed away with acidified water. The polyphenolic compounds were then recovered from the resin, by elution with MeOH (0.3% TFA), to afford the phenolic-rich extract. This extract was then loaded onto a column of Sephadex LH-20, and eluted with 20% MeOH in water (0.3% TFA), to produce the anthocyanin-enriched fraction.
Results of the rapid quantification of total anthocyanins utilizing the pH differential analysis method (Cheng and Breen, 1991) showed that the phenolic-rich extract contained 287 ± 9.7 mg/g total anthocyanins (calculated as cyanidin-3-O-glucoside equivalents). Total phenolics in this extract were 702.0 ± 19.24 mg/g GAE (estimated by Folin Ciocalteu). The anthocyanin-enriched fraction (fraction 1 from the Sephadex LH-20 column) had a higher anthocyanin content than the parent phenolic-rich extract that reached 595 ± 20 mg/g, while the total phenolics were found to be in the same range (685.0 ± 16.60 mg/g). The other fractions eluted from the Sephadex LH-20 column (2–5) showed decreasing levels of total anthocyanin concentrations and were not used in the animal studies.
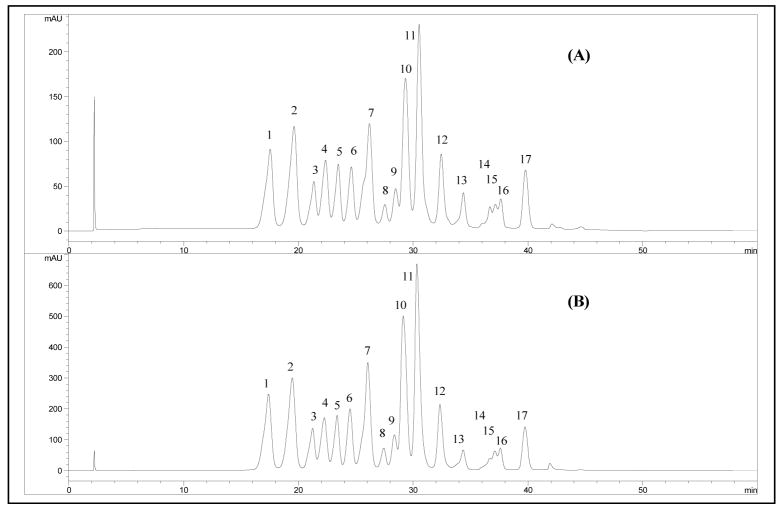
HPLC and LC-MS analysis of both the phenolic-rich extract and the anthocyanin-enriched fraction showed HPLC profiles typical for lowbush blueberries (Gao and Mazza, 1994; Wu and Prior, 2005). The HPLC chromatograms revealed that both extracts had nearly the same relative distribution of 17 discrete components, when measured at 520 nm (maximum absorption of anthocyanins) (Figs. 2A and B). The total anthocyanin content of the anthocyanin-enriched fraction was 636.8 mg/g as calculated from the sum of total anthocyanin peak areas measured by HPLC. The phenolic-rich extract contained only 261.2 mg/g total anthocyanin content measured as cyanidin-3-O-glucoside equivalents. The concentrations of individual anthocyanin compounds in the anthocyanin-enriched fraction are presented in Table 2. Identification and peak assignment of the anthocyanins were based on matching their accurate mass signals with those reported for blueberry anthocyanins (Gao and Mazza, 1994; Wu and Prior, 2005), MS/MS spectral information, and comparison to reference standards.
Figure 2.
RP-HPLC-PDA chromatograms of blueberry phenolic-rich extract (A), and anthocyanin-enriched fraction (B) showing relative absorbance of 17 individual anthocyanins at 520nm. For peak identification, see Table 2.
Table 2.
Identification and content of anthocyanins in the anthocyanin-enriched fraction from lowbush blueberry fruits (Vaccinium angustifolium Aiton) using HPLC and ESI-MS at 520 nm.
| Peak No | RT (min) | Anthocyanin | m/z MS/MS | mg/g anthocyanin |
|---|---|---|---|---|
| 1 | 17.40 | delphinidin-3-galactoside | 465/303 | 55.41 |
| 2 | 19.46 | delphinidin-3-glucoside | 465/303 | 68.34 |
| 3 | 21.24 | cyanidin-3-galactoside | 449/287 | 24.45 |
| 4 | 22.25 | delphinidin-3-arabinoside | 435/303 | 32.69 |
| 5 | 23.37 | cyanidin-3-galactoside | 449/287 | 29.10 |
| 6 | 24.49 | cyanidin-3-arabinoside | 419/287 | 33.66 |
| 7 | 26.04 | petunidin-3-glucoside | 479/317 | 71.01 |
| 8 | 27.43 | peonidin-3-galactoside | 463/301 | 11.38 |
| 9 | 28.37 | Petunidin-3-arabinoside | 449/317 | 17.40 |
| 10 | 29.135 | malvidin-3-galactoside | 493/331 | 90.85 |
| 11 | 30.33 | malvidin-3-glucoside | 493/331 | 110.30 |
| 12 | 32.33 | malvidin-3-arabinoside | 463/331 | 35.12 |
| 13 | 34.35 | delphenidin-6-acetyl-3-glucoside | 507/303 | 12.08 |
| 14 | 36.688 | cyanidin-6-acetyl-3-glucoside | 491/287 | 5.84 |
| 15 | 37.11 | malvidin-6-acetyl-3-galactoside | 535/331 | 8.06 |
| 16 | 37.59 | Petunidin-6-acetyl-3-glucoside | 521/317 | 8.60 |
| 17 | 39.74 | Malvidin-6-acetyl-3-glucoside | 535/331 | 22.15 |
| Total 636.52 mg/g |
Effect of blueberry preparations on blood glucose levels in food restricted C57B6 mice
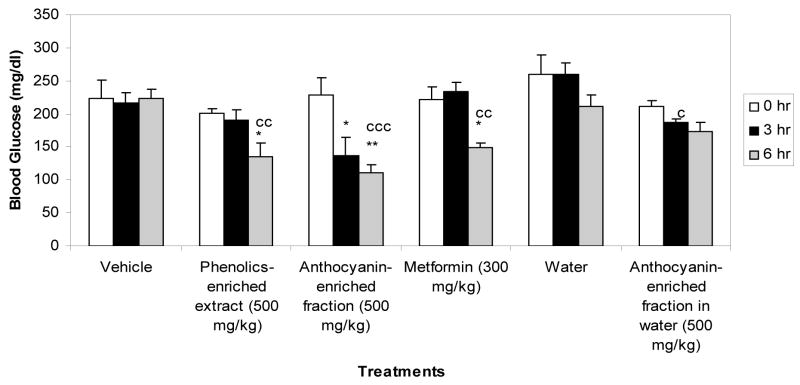
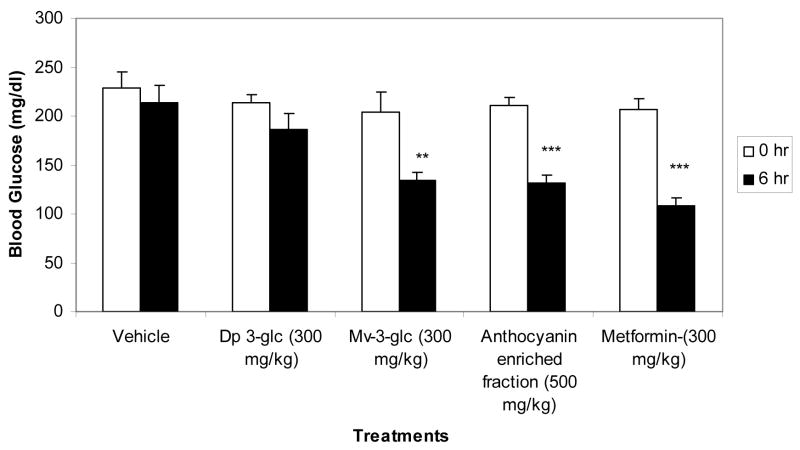
Blueberry phenolic-rich extract and anthocyanin-enriched fraction formulated with Labrasol effectively lowered blood glucose levels in diabetic C57B6 mice (Fig. 3). The anthocyanin-enriched fraction exhibited greater hypoglycemic activity (51% decrease in blood glucose level) compared to the phenolic-rich extract (33% decrease in blood glucose level). Both extracts formulated with Labrasol showed higher potency in lowering blood glucose levels compared to the positive control drug metformin (32% decrease in blood glucose level). The anthocyanin-enriched fraction was not significantly active, however, when administered without Labrasol (Fig. 3). Since malvidin glycosides are the predominant anthocyanins of the lowbush blueberry extracts, we examined the hypoglycemic activity of mavidin-3-glucoside relative to the blueberry extracts. Also, delphinidin-3-glucoside was tested since it is a major component of the blueberry extracts and is reported to be the active hypoglycemic compound from the leaves of Vaccinium species (Murray, 1997). Our results showed that malvidin-3-glucoside had significant hypoglycemic activity at a dose of 300 mg/kg that was comparable to the hypoglycemic activity of the phenolic-rich extract at a dose of 500 mg/kg (Fig. 4). The delphinidin-3-glucoside, however, did not significantly show hypoglycemic activity.
Figure 3.
Hypoglycemic effect of blueberry phenolic-rich and anthocyanin-enriched extracts relative to metformin in insulin resistant C57bl/6J mice. The animals were maintained on a high fat diet and food restricted 4 hours prior to treatment by gavage. The extracts and metformin were formulated with 66% Labrasol as the delivery vehicle. The anthocyanin-enriched extract was also used with only water as the vehicle and compared to water and the other treatments.
*:p<0.05; **:p<0.01; ***:p<0.001 vs. 0 hr. c:p<0.05; cc:p<0.01;ccc:p<0.001vs vehicle
Figure 4.
Hypoglycemic effect of delphinidin-3-glucoside (Dp 3-glc), malvidin-3-glucoside (Mv-3-glc) relative to blueberry anthocyanin-enriched extracts and metformin in insulin resistant C57bl/6J mice. The animals were maintained on a high fat diet and food restricted 4 hours prior to treatment by gavage. The extracts and metformin were formulated with 66% Labrasol as the delivery vehicle
*:p<0.05; **:p<0.01; ***:p<0.001 vs. 0 hr.
Discussion
The phenolic-rich extract contained 287 ± 9.7 mg/g total anthocyanins, as measured using pH differential analysis, and 702.0 ± 19.24 mg/g total phenolics using the Folin Ciocalteu method. The freeze-dried fruits contained 23.2 ± 0.26 mg/g anthocyanins and 30.4 ± 2.13 mg/g total phenolics (Table 1). These results indicated that the first cleanup procedure, using Amberlite XDA-7 resin, increased the anthocyanin content by approximately 12-fold, and total phenolics by 23-fold, on a dry weight basis. However, the anthocyanin-enriched fraction, obtained from the second clean-up procedure on Sephadex LH-20, contained 595 ± 20.0 mg/g total anthocyanins which was more than 20-fold greater than the freeze-dried fruit powder. Although the total phenolic content of both the phenolic-rich extract and its anthocyanin-enriched fraction were very close (702 and 685 mg/g, respectively), the anthocyanin content of the anthocyanin-enriched fraction was more than a 2-fold increase over the phenolic-rich extract. This increase in anthocyanin content was reflected in the anthocyanin/total phenolic ratio which increased from 0.41 to 0.86 (Table 1). The overall increase in anthocyanin and total phenolic content of the anthocyanin-enriched fraction compared to fresh fruit weight was greater than 150-fold. The total anthocyanin content of the extracts determined by HPLC analysis (Table 2), confirmed the measurement made by the pH differential method (calculated as cyanidin-3-O-glucose equivalents, Table 1). HPLC chromatograms of the phenolic-rich extract, measured at 280 nm, showed peaks for phenolic-non anthocyanin components that were diminished in the HPLC profile of the anthocyanin-enriched fraction, indicating their removal in the second clean-up step over Sephadex LH-20. HPLC analysis also confirmed that the clean-up processes did not change the relative distribution of the anthocyanin components in the extracts (Fig. 2).
Table 1.
Total monomeric anthocyanins and total phenolics in blueberry fruits and prepared extracts.
| Sample | Total anthocyaninsb | Total phenolicsc | Anthocyanins/total phenolics |
|---|---|---|---|
| Fresh fruitsa | 3.27± 0.25 | 4.26 ± 0.3 | 0.76 |
| Freeze-dried fruits | 23.2 ± 0.26 | 30.4 ± 2.13 | 0.76 |
| Phenolic-rich extract | 287.0 ± 19.7 | 702.0 ± 19.2 | 0.41 |
| Anthocyanin-enriched fraction | 595.0 ± 20.0 | 685.0 ± 16.6 | 0.83 |
Data from freeze-dried samples was converted to a fresh weight basis based upon dry matter content of samples,
mg/g of cyanidin-3-glucoside equivalent using pH differential analysis method,
mg/g of gallic acid equivalent estimated by Folin Ciocalteu.
The hypolipidemic effect of berry fruits or leaves in animal models is well documented in the literature (Cignarella et al., 1996;Jankowski et al., 2000, Nagao et al 2008; Valcheva-Kuzmanova et al., 2007), but hypoglycemic activity was only reported for blueberry leaf infusions (Cignaella et al., 1996) and chokeberry fruit juice (Valcheva-Kuzmanova et al., 2007). While the exact mechanism of the hypoglycemic effect was not well established, some studies (Jayaprakasam et al 2005) demonstrated the ability of anthocyanins to stimulate insulin secretion from rodent pancreatic β cells, in vitro. In addition, the consumption of fruits and vegetables rich in polyphenols was reported to decrease the incidence of type 2 diabetes through an antioxidant protective effect on pancreatic β cells (Anderson and Polansky 2002; Landrault et al., 2003).
Anthocyanins are distinguished from other flavonoids in that they undergo molecular rearrangements in response to the pH of the environment. In aqueous solution, they occur as an equilibrium of four related molecular structures; namely the flavylium cation, quinoidal base, hemiacetal and chalcone forms. The relative abundance of the four forms depends on the pH of the solution. The flavylium cation is the most stable and exists only in very acidic solutions (pH 1–3). Nearly neutral pH leads to the hydration of the flavylium cation to give the colorless hemiacetal. Tautomerization of the hemiacetal form is responsible for the ring opening which results in the formation of cis-chalcones then undergoing further isomerization to trans-chalcones (pH 2–7). Trans-chalcones are the ultimate end product of this reaction and the more stabilized form at nearly neutral pH (Pina et al., 1998; Brouillard et al., 1982). During the passage of anthocyanins through the GIT, they are exposed to different pH environments and therefore might exist as different forms. The anthocyanin forms present in the different regions of the body are not known with certainty. It is likely that the flavylium cation will exist in the stomach due to low pH and that other forms will predominate lower down the GIT and in the plasma, if absorbed (McGhie et al., 2003; Matuschek et al., 2006). Studies involving the absorption and metabolism of anthocyanins showed that they were detected in blood and urine of humans and animals as intact glycosides (Matsumoto et al., 2002; Wu et al., 2001). Since anthocyanins undergo chemical transformations with changes in pH, the neutral pH of the blood might favor the hemiacetal or chalcone forms. Many biological activities have been reported for chalcones including induction of apoptosis, anti-proliferative action in various cancer cell types, inhibition of pro-inflammatory mediators, antiplatelet activity and potential antimalarial activity (Frolich et al., 2005). In addition, chalcones may be modulators of carbohydrate metabolism, especially glucose (Alberton et al., 2008). Based on the documented activity of chalcones, we might attribute the hypoglycemic effect of the anthocyanins to their chalcone and/or hemiacetal transformation products.
In order for anthocyanins to achieve a pharmacological effect in vivo, the compounds must be bioavailable, i.e. effectively absorbed from the gut, dispersed into circulation and delivered to the responsive site in tissues or organs. While oral administration of anthocyanins has confirmed an increased antioxidant status in serum (Matsumoto et al., 2002, Bitsch et al., 2004), the uptake of the anthocyanins was very low (<1% of dose in the serum) as were the levels of urinary excretion of the intact or conjugated forms (Cooney et al., 2004; McGhie et al., 2003; Wu et al.,, 2002).
Because anthocyanins are known to be poorly bioavailable, potential bioenhancement with the use of a commercial microemulsifying agent was evaluated in hyperglycemic mice. Microemulsions are defined in general as thermodynamically stable, isotropically clear dispersions (Constantinides, 1995). While emulsifying agents are most commonly used with hydrophobic agents because they enhance solubility by emulsification, some are also effective with very polar agents, such as the hydrophilic drug gentamycin sulfate (Koga et al., 2006). One of the advantages of a self-microemulsion system is that the mixture of drug and emulsifier can form fine microemulsion with only a gentle agitation such as that which occurs when the formulation disperses into intestinal fluid. This property makes self-microemulsifiers good vehicles for the oral delivery of poorly absorbable drugs. Labrasol (caprylocaproyl macrogol-8 glyceride) was chosen as a safe, nonionic self emulsifying agent to improve the oral bioavailability of anthocyanins because it was an effective bioenhancer of the hydrophilic drug gentimycin sulfate (Koga et al., 2006). Labrasol also shows high tolerance and low toxicity in animals (Hu et al., 2001).
Treatment by gavage (500 mg/kg body wt) with both the blueberry phenolic-rich extract and the anthocyanin-enriched fraction lowered elevated blood glucose levels by 33 and 51%, respectively, when formulated with Labrasol. This hypoglycemic activity was comparable to that of the known anti-diabetic drug metformin (27% at 300 mg/kg). The anthocyanin-enriched fraction did not promote any significant hypoglycemic effects when administered orally to the mice with a vehicle of water only, thus indicating bioenhancement of the anthocyanin preparations with the Labrasol. The greater activity of the anthocyanin-enriched fraction compared to the parent phenolic-rich extract suggested that the hypoglycemic activity of the extracts is specifically attributable to their anthocyanin components. Pure anthocyanins formulated with Labrasol were then tested in the animal model for hypoglycemic activity. Malvidin-3-O-glucoside treatment (300 mg/kg) by gavage with Labrasol lowered blood glucose levels in the mice by 34%, while the same treatment with delphinidin-3-O-glucoside did not have significant hypoglycemic activity. The hypoglycemic activity of malvidin glucoside was comparable to the metformin positive control at 300 mg/kg and the anthocyanin extracts extract at 500 mg/kg. The malvidin glucoside concentration in the anthocyanin-enriched extract was only about 10%, thus providing an effective dose of 50 mg/kg for the malvidin glucoside. Therefore, if the bioavailability is proportional for both the pure malvidin glucoside and the malvidin glucoside within the anthocyanin-enriched fraction, then the hypoglycemic activity observed for the anthocyanin-enriched extract is likely to result from the activity of additional components acting together with the malvidin-3-O- glucoside.
It is likely that the delphinidin-3-O-glucoside is contributing significantly less to the hypoglycemic activity of the anthocyanin-enriched extract since it is not active when delivered as a pure compound. This observation is not consistent with early reports naming myrtillin, another name for delphinidin-3-O-glucoside, as the hypoglycemic compound from the leaves of Vaccinium species (Murray, 1997). Since the early reports were conducted in a chronic manner, however, it is possible that delphinidin-3-O-glucoside was simply not active acutely or in the specific animal model used for these studies. Any animal model is inherently influenced by genetic background, epigenetic factors and dietary effects such as the calorie source and fed or fasted status.
Anti-diabetic properties were previously reported for lowbush blueberry extracts in vitro (Martineau et. al., 2006). Some increases in glucose uptake were observed in cell cultures treated with extracts from the root, stem or leaf, which could theoretically promote a hypoglycemic effect in vivo. Since the composition of these extracts is completely different from the composition of the fruit extracts, different compounds (not anthocyanins), must be associated with the response. The fruit extract was reported to increase β cell proliferation in vitro, which may predict a protective effect for a diabetic pancreas, but this effect could be purely anti-oxidative. However, the fruit extract had no effect on glucose uptake or insulin secretion in vitro and would not be expected to have any hypoglycemic effects, in vivo, in contrast to the anthocyanin enriched extract reported here. The hypoglycemic compounds from the blueberries of our studies may not have promoted any activity associated with hypoglycemia in the cell culture assays previously reported (Martineau et. al., 2006), because the compounds may not act in those cell types used for the assays or because the in vivo mechanism may be different for a variety of reasons.
In summary, the hypoglycemic activity of the anthocyanin-enriched fraction from lowbush blueberry was demonstrated in an acute mouse model for type 2 diabetes when formulated with Labrasol. In addition, the primary anthocyanin contained in the anthocyanin-enriched extract, malvidin-3-O- glucoside, was an active hypoglycemic agent when administered as a pure compound while another abundant anthocyanin from the extract, delphinidin-3-O-glucoside, did not show significant bioactivity. Therefore, we concluded that the hypoglycemic activity of the blueberry extract was largely anthocyanin specific. Other components of the extract may contribute to the over all effect observed in vivo. In vivo metabolic and bioavailability studies with anthocyanin preparations may be used to ultimately relate specific anthocyanins to a specific bioactivity.
Acknowledgments
Research supported by the NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, grant # 1-P50 AT002776-01; Fogarty International Center of the NIH under U01 TW006674 for the International Cooperative Biodiversity Groups. We are thankful to the Wild Blueberry Association of North America (WBANA) for the providing the blueberries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberton EH, Damazio RG, Cazarolli LH, Chiaradia LD, Leal PC, Nunes RJ, Yunes RA, Silvaa FRM. Influence of chalcone analogues on serum glucose levels in hyperglycemic rats. Chemico-Biological Interactions. 2008;171:355–362. doi: 10.1016/j.cbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002;50:7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- Baily CJ, Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21:115–117. [Google Scholar]
- Bitsch I, Janssen M, Netzel M, Strass G, Frank T. Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int J Clin Pharmacol Ther. 2004;42:293–300. doi: 10.5414/cpp42293. [DOI] [PubMed] [Google Scholar]
- Blumenthal M. The Complete German Commission E Monographs – Therapeutic Guide to Herbal Medicines. American Botanical Council; Austin, TX: 1998. [Google Scholar]
- Brouillard R, Iacobucci GA, Sweeny JG. Chemistry of anthocyanin pigments. 9 UV-visible spectrophotometric determination of the acidity. J Amer Chem Soc. 1982;104:7585–7590. [Google Scholar]
- Chambers B, Camire M. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care. 2003;26:2695–2696. doi: 10.2337/diacare.26.9.2695-a. [DOI] [PubMed] [Google Scholar]
- Cheng GW, Breen PJ. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci. 1991;116:865–869. [Google Scholar]
- Cignarella A, Nastasi M, Cavalli E, Puglisi L. Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic. Thromb Res. 1996;84:311–22. doi: 10.1016/s0049-3848(96)00195-8. [DOI] [PubMed] [Google Scholar]
- Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–1572. doi: 10.1023/a:1016268311867. [DOI] [PubMed] [Google Scholar]
- Cooney JM, Jensen DJ, McGhie TK. LC-MS identification of anthocyanins in boysenberry extract and anthocyanin metabolites in human urine following dosing. J Sci Food Agric. 2004;84:237–245. [Google Scholar]
- Davidson MB. Diabetes Mellitus: Diagnosis and Treatment. 4. W.B. Saunders Company; Philadelphia.: 1998. [Google Scholar]
- Frolich S, Schubert C, Bienzle U, Jenett-Siems K. In vitro antiplasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. J Antimicrob Chemotherapy. 2005;55:883–887. doi: 10.1093/jac/dki099. [DOI] [PubMed] [Google Scholar]
- Gao L, Mazza G. Quantitation and distribution of simple and acylated anthocyanins and other phenolics in blueberries. J Food Sci. 1994;59:1057–1059. [Google Scholar]
- Haffner SM. The insulin resistance syndrome revisited. Diabetes Care. 1996;19:275–277. doi: 10.2337/diacare.19.3.275. [DOI] [PubMed] [Google Scholar]
- Hu Z, Tawab R, Konishia T, Shibataa N, Takada K. A novel emulsifier, Labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci. 2001;69:2899–2910. doi: 10.1016/s0024-3205(01)01375-3. [DOI] [PubMed] [Google Scholar]
- Jankowski A, Jankowska B, Niedworok J. The effect of anthocyanin dye from grapes on experimental diabetes. Folia medica Cracoviensia. 2000;41:5–15. [PubMed] [Google Scholar]
- Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- Jellin JM, Gregory PJ, Batz F, Hitchens K. Pharmacist’s Letter/Prescriber’s Letter. Therapeutic Research Faculty; Stockton, CA: 2005. Natural medicines comprehensive database; p. 2239. [Google Scholar]
- Koga K, Kusawake Y, Ito Y, Sugioka N, Shibata N, Takada K. Enhancing mechanism of Labrasol on intestinal membrane permeability of the hydrophilic drug gentamicin sulfate. Eur J Pharm Biopharm. 2006;64:82–91. doi: 10.1016/j.ejpb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kraft TF, Schmidt BM, Yousef GG, Knight CTG, Cuendet M, Kang Y, Pezzuto JM, Seigler DS, Lila MA. Chemopreventive potential of wild lowbush blueberry fruits in multiple stages of carcinogenesis. J Food Sci. 2005;70:159–166. [Google Scholar]
- Landrault N, Poucheret P, Azay J, Krosniak M, Krosniak FM, Gasc F, Jenin C, Cros G, Teissedre P. Effect of a polyphenols-enriched chardonnay white wine in diabetic rats. J of Agric Food Chem. 2003;51:311–318. doi: 10.1021/jf020219s. [DOI] [PubMed] [Google Scholar]
- Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, Le PM, Prentki M, Bennett SA, Arnason JT, Haddad PS. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13:612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Nakamura Y, Hirayama M, Yoshiki Y, Okubo K. Antioxidant activity of blackcurrant anthocyanin aglycones and their glycones measured by chemiluminescence in a neutral pH region and in human plasma. J Agric Food Chem. 2002;50:5034–5037. doi: 10.1021/jf020292i. [DOI] [PubMed] [Google Scholar]
- Matuschek MC, Hendriks WH, McGhie TK, Reynolds GW. The jejunum is the main site of absorption for anthocyanins in mice. J Nutr Biochem. 2006;17:31–36. doi: 10.1016/j.jnutbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolised by both humans and rats. J Agric Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- Murray MT. Bilberry (Vaccinium myrtillus) AmJ Nat Med. 1997;4:17–21. [Google Scholar]
- Nagao K, Higa K, Shirouchi B, Nomura S, Inoue N, Inafuku M, Yanagita T. Effect of Vaccinium ashei Reade leaves on lipid metabolism in Otsuka Long-Evans Tokushima fatty rats. Biosci Biotechnol Biochem. 2008;72:1619–1622. doi: 10.1271/bbb.80036. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007a;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007b;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- PDR for Herbal Medicine. 3. Thomson PDR publisher; Montvale NJ: 2004. [Google Scholar]
- Pina F, Roque A, Melo MJ, Maestri M, Belladelli L, Balzani V. Multistate/Multifunctional Molecular-Level Systems: Light and pH switching between the various forms of a synthetic flavylium salt. Chem-A Eur J. 1998;4:1184 – 1191. [Google Scholar]
- Shukitt-Hale B, Lau FC, Carey AN, Galli RL, Spangler EL, Ingram DK, Joseph JA. Blueberry polyphenols attenuate kainic acid -induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci. 2008;11:172–82. doi: 10.1179/147683008X301487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Trevithick JR, Mitton KP. Antioxidants and diseases of the eye. In: Papus AM, editor. Antioxidant Status, Diet, Nutrition, and Health. New York: CRC Press; 1999. pp. 545–566. [Google Scholar]
- Valcheva-Kuzmanova S, Kuzmanov K, Mihova V, Krasnalev I, Borisova P, Belcheva A. Antihyperlipidemic Effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant Foods for Human Nutrition. 2007;62:19–24. doi: 10.1007/s11130-006-0036-2. [DOI] [PubMed] [Google Scholar]
- Watson Some observations on the effect of blueberry leaf extract in diabetes mellitus, The Can. Med Association J. 1928;19:166–171. [PMC free article] [PubMed] [Google Scholar]
- Wu X, Prior RL. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J Agric Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- Wu X, Cao G, Prior RL. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132:1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]