Abstract
Objective
To compare the real-world clinical effectiveness and long-term clinical trajectory in patients with Alzheimer’s disease (AD) treated with combination (COMBO) therapy consisting of cholinesterase-inhibitor (CI) plus memantine (MEM) versus CI alone versus no treatment with either.
Methods
382 subjects with Probable AD underwent serial clinical evaluations at a memory disorders unit. Cognition was assessed by the Information-Memory-Concentration subscale of the Blessed Dementia Scale (BDS) and function was assessed by the Weintraub Activities of Daily Living Scale (ADL) at six-month intervals. 144 subjects received standard care without CI or MEM (NO-RX), 122 received CI-monotherapy (CI), and 116 received combination therapy (COMBO) with CI plus MEM. Mean follow-up was 30 months (4.1 visits) and mean cumulative medication treatment time was 22.5 months. Rates of declines were analyzed using mixed-effects regression models, and Cohen’s d effect sizes were calculated annually for years 1–4.
Results
Covarying for baseline scores, age, education and duration of illness, the COMBO group had significantly lower mean annualized rates of deterioration in BDS and ADL scores compared to the CI (p<0.001; Cohen’s dBDS=0.10–0.34 and dADL=0.23–0.46 at 1–2 years) and NO-RX groups (p<0.001; Cohen’s dBDS=0.56–0.73 and dADL=0.32–0.48 at 1–2 years). For the COMBO group, Cohen’s d effect sizes increased with treatment duration. Similar comparisons significantly favored the CI over the NO-RX group on the BDS.
Conclusions
Combination therapy slows cognitive and functional decline in AD compared to CI-monotherapy and no treatment. These benefits had small-to-medium effect sizes that increased with time on treatment and were sustained for years.
Keywords: treatment efficacy, modeling progression, cholinesterase inhibitor, memantine, memory, cognition and function in dementia
1. Introduction
Alzheimer’s disease (AD) is a devastating, costly and, to date, incurable neurodegenerative condition that affects over five million patients and accounts for over $148 billion of annual costs in the United States. [1, 2]. In the absence of prevention, AD is projected by 2050 to affect nearly 16 million Americans and to cause major strains on the national economy [2]. Current FDA- and EMA (European Agency for the Evaluation of Medical Products)-approved treatments for AD consist of cholinesterase-inhibitors (CI) and Memantine (MEM), which have modest symptomatic but not curative effects [3, 4]. Overall, monotherapy and combination therapy with these medications show highly variable individual clinical effects. Subjects with AD participating in short-term clinical trials and longer-term open-label extensions studies show transient improvement and stabilization or reduced deterioration on measures of cognition, behavior and activities of daily living [5–18]. While these trials may rigorously demonstrate the clinical safety and efficacy of drug versus placebo treatment in highly selected cohorts of subjects for short durations (usually 24–28 weeks in randomized, double-blinded, and placebo-controlled fashion, and in additional open-label extension studies), long-term, real-world clinical effectiveness data for monotherapy or combination therapy is lacking. A few short-term studies have assessed the effects of CI treatment in a “real-world” clinic setting [19–25], and a recent study from Japan assessed effects on patients clinically treated with donepezil for up to two years [26]. To our knowledge, there are no published studies that have assessed and compared the effects of CI monotherapy and CI-MEM combination therapy in a real-world setting for durations of greater than one year.
Clinical efficacy may not generalize to clinical effectiveness, since factors such as comorbid medical conditions, concurrent use of psychoactive medications, and adherence to treatment regimen that are important in clinical practice are minimized in formal clinical drug trials [27]. For this reason, clinical effectiveness of AD medications should be demonstrated in the setting of real-world clinical practice where patients are heterogeneous with respect to age, medical conditions, concurrent medications, prevailing symptoms and stages of illness [27–29].
Based on these considerations our objectives were 1) to assess whether combination therapy shows clinical effectiveness for cognition and functional benefits in a well-characterized prospective cohort of patients with AD treated over years in a memory disorders unit; 2) if so, then to determine the magnitude and duration of benefit; 3) to characterize the long-term clinical course of patients who receive combination therapy compared to those who were never treated with CI or MEM and those who only received CI monotherapy; and 4) to use modeling methods to make predictions about the effect sizes and clinical course in different treatment groups.
2. Methods
2.1. Subjects
A total of 382 subjects met inclusion criteria for this study. Data for consecutive eligible subjects clinically evaluated and treated for dementia at the MGH Memory Disorders Unit (MDU) from 1990–2005 and enrolled in the Massachusetts Alzheimer’s Disease Research Center (MADRC) Patient Registry Database (Registry) were prospectively collected, and subjects were selected according to the following criteria: 1) received a clinical diagnosis of Probable Alzheimer’s disease (PrAD) [30]; and 2) had at least three examinations in the MDU with completed assessments of cognitive and functional capacities. Subjects were assigned to the NO-RX group (n = 144) if their treatment did not include taking CI or MEM at any time. Subjects were assigned to the CI group (n = 122) if they were treated with a CI for any duration but were not treated with MEM. Subjects were assigned to the COMBO group (n = 116) if they were treated with a CI and MEM for any duration. Subjects in the NO-RX group were enrolled between 1990–1995, in an era when oral CI and MEM were not in routine clinical use, whereas, subjects in the CI and COMBO groups were enrolled after 1997, when widespread clinical use of oral CI began in the United States. The CIs in use in the MDU were donepezil (Aricept), glantamine (Reminyl/Razadyne) and rivastigmine (Exelon) - tacrine (Cognex), the first orally available CI, was not used due to a low benefit to risk ratio [31]. The subjects, on average, underwent their first clinical evaluation in the following years according to group: NO-RX 1993 (SD ± 2.0 years), CI 2000 (SD ± 1.4 years), and COMBO 2002 (SD ± 2.0 years). There was no overlap of subjects between the three study groups in our study; none of the NO-RX group subjects (from the early 90’s) received treatment with CI or MEM in our clinic at a later date, nor were any subjects in the CI or COMBO groups enrolled in the NO-RX epoch from the early 90’s. The mean time in the study for all subjects was 30 months (ie, 2.5 years), and for treatment with CI or COMBO was 22.5 months (i.e. 1.9 years).
All subjects underwent a comprehensive clinical dementia evaluation conducted by MDU staff neurologists according to a protocol in continuous use since 1985 when the MADRC was established. As part of the MADRC clinical protocol, all subjects were evaluated using standard instruments that included the Information-Memory-Concentration subscale of the Blessed Dementia scale (BDS) [32] and the Weintraub Activities of Daily Living Scale (ADL) scale [33]. The BDS score is a brief mental status test used to detect the presence and estimate the severity of cognitive impairments. In the MDU, the BDS is administered by the examining neurologist and it samples the domains of information, orientation, memory, and concentration; 0–3 mistakes are generally considered to be within the non-impaired range with a maximal score of 37 mistakes [34]. The ADL is a 31-item questionnaire of basic and instrumental ADL’s completed via interview with a knowledgeable informant (often a spouse or family caregiver); scores range from 0% (normal) to 100% dependency [33].
The duration of symptoms (illness) at the time of clinical evaluation was determined for each patient by the neurologist based on informant and collateral information. All subjects received standard care and treatment for AD during the course of the study. Among the 8 neurologists in the MDU from 1997, the consistent practice was to prescribe a CI at initial diagnosis of AD. Memantine was generally added on an individual basis. The study was approved by the Partners Healthcare-MGH Institutional Review Board.
2.2. Timing of medications
The start and stop date for CI and MEM medications were documented based on the list of taken (and prescribed) medications recorded at each visit. For each subject, the assigned start date for CI or MEM medication was the date of the clinic visit when medication was prescribed. For subjects who at some point in the study discontinued CI or MEM medication for any duration (n = 28), the total time on medication was estimated by calculating the sum of all periods when the subject was verified to be on medication. For example, if the medication record indicated that a subject was on medication at visits 1 through N, was not on medication at visit N+1 and N+2, but was back on medication at visit N+3 through the last visit, the duration of time on medication was calculated to be the sum of the periods covered by visit 1 through N and visit N+3 through the last visit.
2.3. Statistical analysis
2.3.1. Descriptive statistics
Demographic data were analyzed using standard statistical tests (e.g., t-test, Fisher’s exact test, binomial test) according to two-tailed p-values. Statistical significance was a priori defined at the level of p ≤ 0.05.
2.3.2. Mixed fixed and random coefficients regression modeling of longitudinal data
To assess differences between medication groups (NO-RX, CI, COMBO) with respect to longitudinal change in mean BDS and ADL scores, mixed, linear and nonlinear, random and fixed coefficient regression modeling that covaried baseline BDS and ADL scores, age, education, and duration of illness, was employed. The random terms in the model were an intercept, years in the study and the square of same (the latter to assess curvilinear quadratic effects) with an unstructured covariance matrix. The fixed effect terms in the model were the main effect of medication group, the interaction of med group with years in study (linear and quadratic terms), age at baseline (i.e., at the first BDS or ADL assessment), duration of symptoms at baseline, and years of education. To adjust for baseline differences, the initial BDS and ADL score in the study for each patient and its interaction with linear and quadratic terms for years in the study were also included as fixed effects. Therefore, the dependent variables were scores assigned at the second and subsequent visits for each patient. The most non-significant terms (p > 0.05) were sequentially removed in an iterative backward elimination manner. As per convention, non-significant lower order terms (e.g., linear, main effects) corresponding to significant higher order terms (e.g., quadratic, interactions) were allowed to remain in the final model. Model estimation used restricted maximum likelihood, and the analyses were performed using the Mixed Procedure of Version 9.1.3 of the SAS/STAT software (SAS, Cary, NC; 2007; See Technical Appendix). The mathematical form and terms retained in the model, and their respective p-values, can be found in the Technical Appendix and Table A1.
Table A1.
Terms Retained in Final Model
| BDS | |
| Fixed Terms | P Value |
| Years in study | <0.0001 |
| Medication | 0.0146 |
| Yrs in study×medication | <0.0001 |
| First BDS | <0.0001 |
| Random Terms (variance) | |
| Intercept | <.0001 |
| Years in study | <.0001 |
| % Variance Accounted for in Dependent Variable | |
| All Fixed and Random Terms | 93% |
| All Fixed Terms | 49% |
| Only Terms Involving Med Groups | 10.2% |
| ADL | |
| Fixed Terms | P Value |
| Years in study | <0.0001 |
| Years in study Squared | 0.1413 |
| Medication | 0.5875 |
| Yrs in study×medication | 0.0002 |
| First ADL | <0.0001 |
| Yrs in study×1st ADL | 0.4965 |
| Yrs in study squared×1st ADL | 0.0117 |
| Random Terms (variance) | |
| Intercept | <0.0001 |
| Years in study | 0.0002 |
| Years in study squared | 0.0030 |
| % Variance Accounted for in Dependent Variable | |
| All Fixed and Random Terms | 95.1% |
| All Fixed Terms | 48.6% |
| Only Terms Involving Med Groups | 5.5% |
2.3.3. Estimation of effect size using Cohen’s d
Estimates of medication effect sizes were calculated using the Cohen’s d measure (d = difference in group means/error SDwithin) at one-year study intervals up to four years. Cohen’s d was chosen a priori as the effect size estimate, as opposed to other estimates such as standardized response means, in order to avoid liberal bias, i.e., to use the method that is most conservative in being least likely to show a medication treatment benefit. Cohen’s d is also the most appropriate method for the study sample. Whereas clinical trial samples, with their strict inclusion/exclusion criteria, produce artificially homogeneous samples with low variability, the clinic population sample in this study is comprised of patients longitudinally evaluated and treated at the MGH MDU who were unselected by any criteria other than the clinical diagnosis of PrAD. Cohen’s d was calculated as the difference between predicted means from the final fitted model for a given pair of medication groups at each year in the study divided by the estimated within group error standard deviation at that point in the study. The error standard deviation was based on a linear combination of the estimated variance in random intercepts, linear and quadratic coefficients, their pairwise covariances, and the residual variance for the fitted model.
2.3.4. Confirmatory analysis using Generalized Estimating Equations (GEE)
No method of longitudinal data analysis provides optimal results under all conditions [35]. Since mixed-effects models and Generalized Estimating Equations (GEE) [36] are the most widely used methods for analysis of longitudinal data of the kind collected in this study, we opted for the conservative approach of testing the validity of the results of the mixed-effects modeling analysis via a confirmatory analysis using GEE. GEE derives from an entirely different theoretical foundation and methodology than mixed-effects modeling [36]. For this confirmatory GEE analysis, we used the Genmod procedure in SAS (version 9.1.3), starting first with the fixed terms in the final derived model for the mixed-effects analyses, then estimating the structure of the correlations across time using different models in separate runs (e.g., first order autoregressive, compound symmetry, independence, etc.), and finally choosing the best fitting model according to the Quasi-likelihood Independence Criterion (QIC) developed by Pan [37].
3. Results
3.1. Descriptive statistics
Descriptive statistics for group demographics, clinical characteristics, and study parameters are shown in Table 1. The subjects in the COMBO group were slightly younger, more educated, and had lower BDS and ADL scores at baseline compared to the NO-RX and CI groups (most comparisons significant; p < 0.05), the latter two groups usually did not differ significantly from each other. Due to these minor differences between the three medication groups, longitudinal analysis, described below, adjusted for baseline differences and any interactions with time in study. The cumulative duration of medication treatment for subjects in the CI and COMBO groups was at least six months (0.50 yrs for CI and 0.48 yrs for COMBO), the median duration was 1.9 years for the CI group (mean 2.2 yrs) and 1.55 years for the COMBO group (mean 1.52 yrs), and about 90% of subjects in the CI and COMBO groups had a cumulative duration of medication use of approximately one or more years (1.05 yrs for CI and 0.98 yrs for COMBO). The percentage of subjects who “dropped-out” from each group (i.e. did not continue to have clinical follow-up past data inclusion) was 18 % for the NO-RX group, 34% for the CI group, and 25% for the COMBO group (p = 0.01 CI vs NO-RX, p > 0.28 COMBO vs NO-Rx and COMBO vs CI; Fisher’s Exact test).
Table 1.
Baseline demographics, characteristics of study subjects and other study parameters
| Characteristic, mean (SE) | NO-RX n = 144 |
CI n = 122 |
COMBO n = 116 |
ALL n = 382 |
|---|---|---|---|---|
| Age at baseline, y1 | 73.9 (0.6) | 75.5 (0.7) | 71.5 (0.9) | 73.7 (0.4) |
| Education, y2 | 13.1 (0.3) | 13.4 (0.3) | 14.7 (0.3) | 13.7 (0.2) |
| Women, n (%)3 | 92 (64) | 71 (58) | 62 (53) | 225 (59) |
| Duration symptoms at baseline, y3 | 3.2 (0.2) | 2.7 (0.1) | 2.9 (0.2) | 3.0 (0.1) |
| Time in study, y2 | 2.0 (0.1)^ | 2.2 (0.1) | 3.3 (0.2) | 2.5 (0.1) |
| Time Rx treatment started, y | NA | 0.2 (0.04) | 0.8 (0.05) | 0.5 (0.03) |
| Cumulative time on treatment, y | NA^ | 2.2 (0.1) | 1.5 (0.04) | 1.9 (0.1) |
| Number of study visits, n2 | 3.5 (0.1) | 3.4 (0.2) | 5.5 (0.3) | 4.1 (0.1) |
| BDS at baseline, errors4 | 12.8 (0.5) | 11.0 (0.4) | 8.8 (0.5) | 11.0 (0.3) |
| ADL at baseline, % dependent2 | 36.3 (1.5) | 31.7 (2.0) | 23.5 (1.7) | 31.2 (1.0) |
NA = Not Applicable, Rx treatment is defined by receiving CI or COMBO treatment
Combo significantly (p<0.05) different from CI.
Combo significantly different from CI and from No-Rx.
No significant differences among groups. Significantly more women than men in All (n = 382) subjects (p < 0.001).
All groups significantly different from each other.
by definition subjects in NO-RX group started and received standard-of-care treatment (excluding CI or MEM) throughout the duration of the study
3.2. Longitudinal analyses of BDS and ADL scores in medication groups
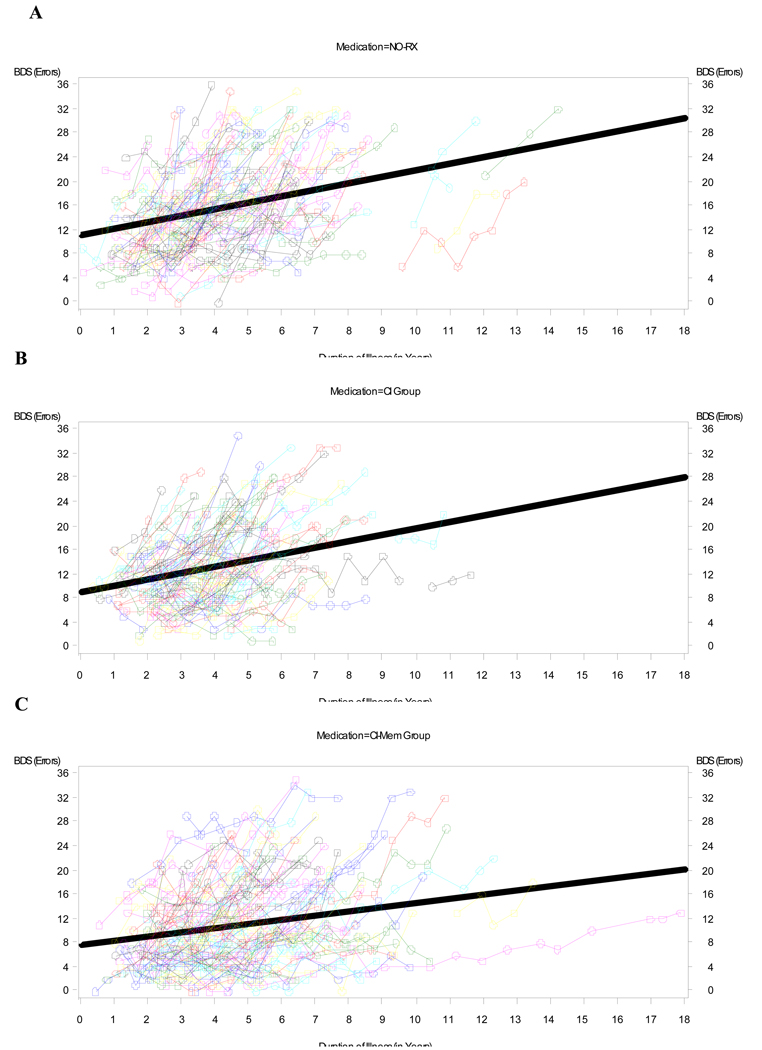
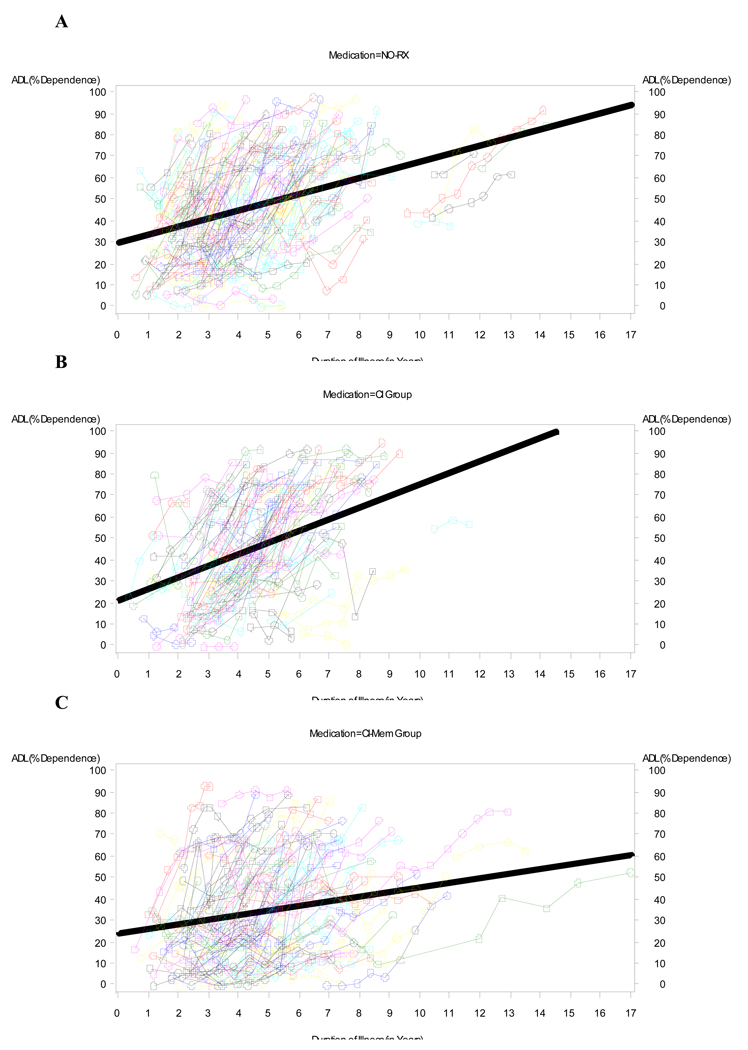
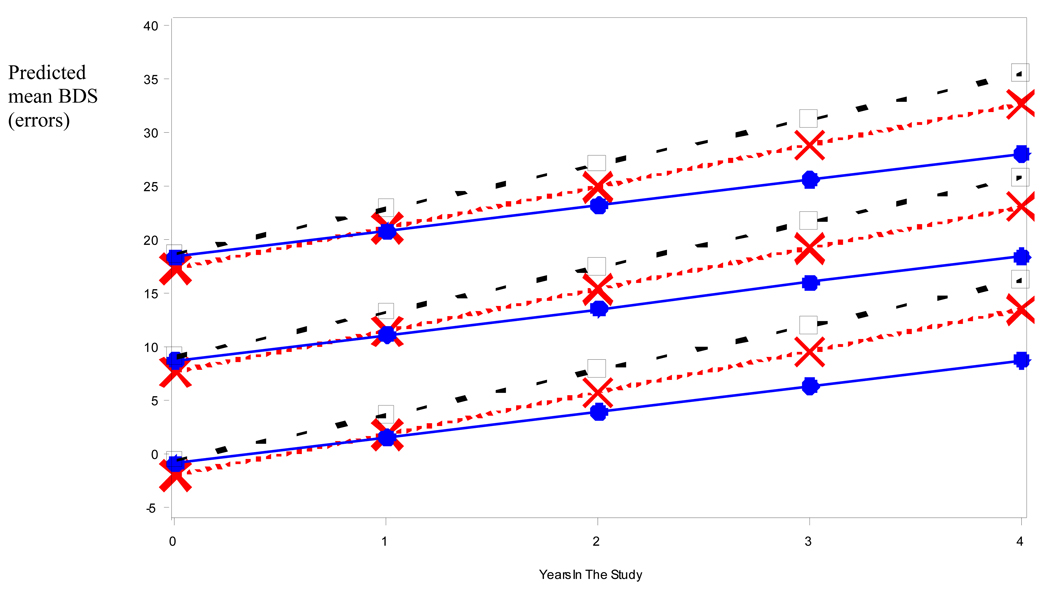
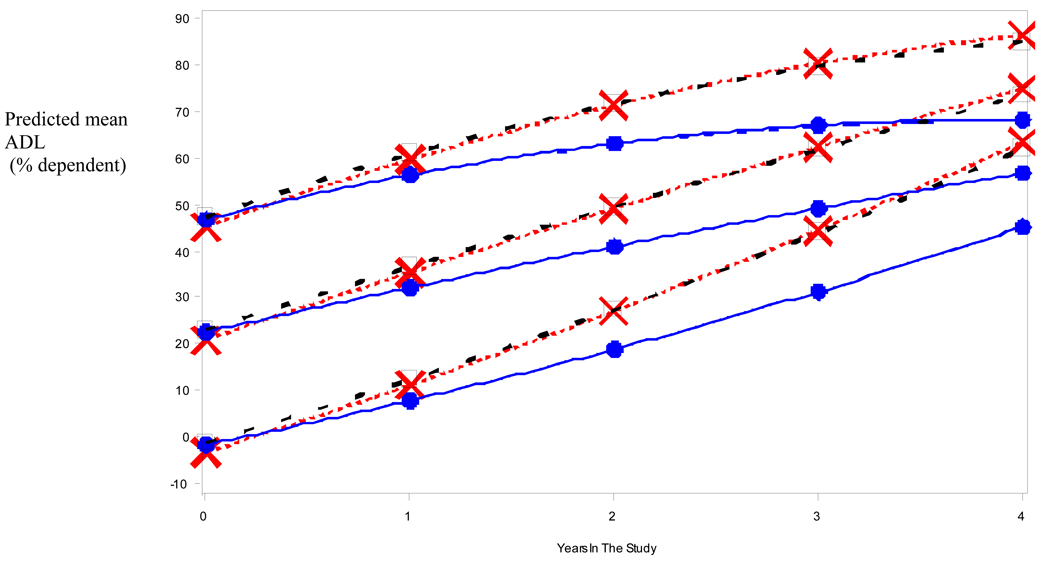
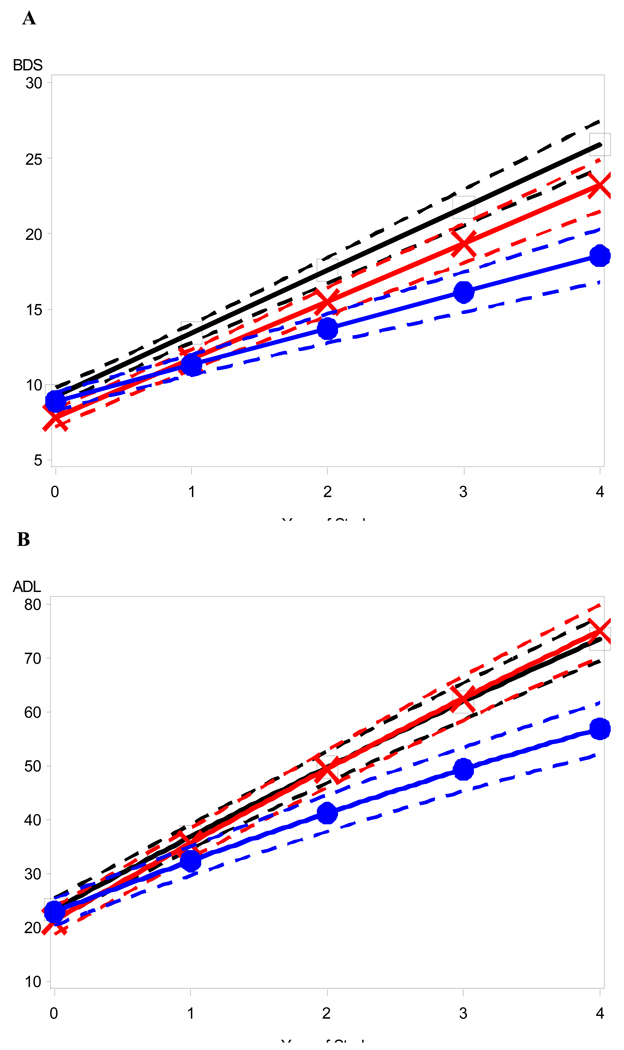
Figure 1 and Figure 2 depict the raw data for BDS (Fig 1) and ADL (Fig 2) scores versus duration of illness (symptoms) for each medication group (NO-RX, CI, and COMBO), as well as the best fitting straight (ordinary least square, OLS) regression line (thick lines) for the data. Unlike the mixed-effects analyses, the OLS lines are blind to within- versus between-subject distinctions and somewhat underestimate the within-subject slope. However, the shallower slope of the OLS regression line for the COMBO group compared to the other groups (NO-RX and CI) is evident and is consistent with a relatively slower rate of cognitive and functional decline in the COMBO group. Mixed fixed and random effects linear and nonlinear regression models that adjusted for differences in baseline BDS (or ADL) scores, age, education level, duration of illness, and interactions of BDS (or ADL) scores with time in the study, were fitted to the raw data to quantify separate effects of medication group on BDS (or ADL) scores. Figure 3 and Figure 4 show model predictions for progression in BDS and ADL scores, respectively, at three different starting points (i.e. scores at initial visit) (see also Fig. 5). Cohen’s d effect size estimates were then calculated for each fitted model at annual time points (Table 2 and Table 3).
Fig 1.
Raw data for BDS versus Duration of Illness (symptoms) for A) NO-RX group (no medications), B) CI group, and C) COMBO group of subjects. Thin lines connect data for an individual (subject), while the thick line is the best fitting ordinary least square (OLS) regression line for the data. There is progressive shallowing of the slope for the OLS regression lines going from NO-RX to CI to COMBO that is consistent with a respective slower rate of cognitive decline with use of medication(s).
Fig 2.
Raw data for ADL versus Duration of Illness (symptoms) for A) NO-RX group (no medications), B) CI group, and C) COMBO group of subjects. Thin lines connect data for an individual (subject), while the thick line is the best fitting OLS regression line for the data. There is shallowing of the slope for the OLS regression lines going from NO-RX and CI to COMBO that is consistent with a slower rate of functional decline in the COMBO group.
Fig 3.
Illustrative mean BDS values predicted by best fitting longitudinal model for patients in the different medication regimens. Baseline scores of 0, 10 and 20 are given as arbitrary examples. (Square = No Meds; X = CI Only; dot = COMBO).
Fig 4.
Illustrative mean ADL values predicted by best fitting longitudinal model for patients in the different medication regimens. Baseline scores of 0, 25% and 50% dependent are given as arbitrary examples. (Square = No Meds; X = CI Only; dot = COMBO).
Fig 5.
Confidence bands around illustrative mean (A) BDS and (B) ADL trajectories. Confidence bands (dashed lines) corresponding to 95% confidence interval around the predicted model trajectories (solid lines) for patients in different medication regimens who start out in the study with mild-to-moderate stage dementia severity corresponding to a baseline BDS score of 10 and a baseline ADL score of 25% dependent. (Square = No Meds; X = CI Only; dot = COMBO).
Table 2.
Effect size estimates for BDS favor CI over NO-RX, and COMBO over both NO-RX and CI. Also displayed for each group are the predicted mean BDS scores and 95% confidence intervals at one-year intervals.
| BDS, predicted mean errors (95% confidence interval) |
Cohen’s dBDS | |||||
|---|---|---|---|---|---|---|
| Years in Study |
NO–RX | CI | COMBO | CI vs NO–RX |
COMBO vs NO–RX |
COMBO vs CI |
| 1 | 13.4 (12.8–14.0) |
11.7 (11.0–12.3) |
11.3 (10.6–12.0) |
0.47*** | 0.56*** | 0.10 |
| 2 | 17.6 (16.7–18.4) |
15.5 (14.6–16.4) |
13.7 (12.7–14.7) |
0.39** | 0.73*** | 0.34** |
| 3 | 21.7 (20.5–22.9) |
19.3 (18.0–20.7) |
16.1 (14.8–17.5) |
0.32** | 0.76*** | 0.44*** |
| 4 | 25.9 (24.3–27.5) |
23.2 (21.5–24.9) |
18.5 (16.8–20.3) |
0.28* | 0.77*** | 0.49*** |
significantly different from zero; p < 0.05
p < 0.01
p < 0.001
Table 3.
Effect size estimates for ADL favor COMBO treatment over NO-RX and over CI alone. Also displayed for each group are the predicted mean ADL scores and 95% confidence intervals at one-year intervals.
| ADL, model predicted mean %-dependent (95% confidence interval) |
Cohen’s dADL | |||||
|---|---|---|---|---|---|---|
| Years in Study |
NO-RX | CI | COMBO | CI vs NO-RX |
COMBO vs NO-RX |
COMBO vs CI |
| 1 | 36.9 (34.6–39.2) |
35.7 (32.9–38.5) |
32.4 (29.7–35.1) |
0.08 | 0.32* | 0.23 |
| 2 | 49.7 (46.9–52.6) |
49.5 (46.0–53.0) |
41.2 (37.8–44.6) |
0.02 | 0.48*** | 0.46*** |
| 3 | 62.0 (58.5–65.4) |
62.6 (58.5–66.7) |
49.4 (45.3–53.4) |
−0.03 | 0.60*** | 0.62*** |
| 4 | 73.6 (69.5–77.6) |
75.0 (70.2–80.0) |
56.9 (52.1–61.7) |
−0.06 | 0.67*** | 0.73*** |
significantly different from zero; p < 0.05
p < 0.01
p < 0.001
3.3. Longitudinal changes in BDS scores
Figure 3 displays mean BDS values predicted by the fitted model for representative initial BDS values (0, 10, 20 errors) for the three medication groups across four years of study. BDS results indicated a positive linear relation with years in the study that significantly interacted with medication groups (p < 0.0001) such that the mean linear increase of approximately 4 points/year (i.e. 4 errors/year) for the NO-RX group is lowered by 0.3 points/year in the case of the CI group and by 1.76 points/year in the COMBO group. The slopes for the three medication groups decrease in a significantly (p < 0.0001) linear fashion in going from the NO-RX group to the CI group to the COMBO group, and are estimated at just under a one point per year reduction in each step. The COMBO Group showed a significantly slower increase across years of the study than each of the other two medication groups (p < 0.001 for each comparison). As expected, the covariate BDS score at baseline showed a significant (p < 0.0001) main effect with a regression coefficient near one (0.96) though it does not interact with years in the study. Consistent with a good model fit and adherence to normality assumptions, the model residuals were bell-shaped across time.
Table 2 displays Cohen’s d effect size estimates for the BDS (Cohen’s dBDS) for all three pair-wise comparisons of medication groups (i.e. CI vs NO-RX, COMBO vs NO-RX, and COMBO vs CI) at 1, 2, 3 and 4 years into the study. The dBDS values for COMBO versus NO-RX were statistically significant with p < 0.001 for all years in the study, increasing across time from 0.56 to 0.77 where predicted means differed by over 75% of the within-group error standard deviation. The dBDS values for COMBO versus CI were statistically significant with p < 0.01 at year 2 with a magnitude of 0.34, and were significant at p < 0.001 at years 3 and 4 with values of 0.44 and 0.49 respectively (Table 2). Since the error standard deviation increases across years in the study (because varying participants’ regression lines “flare out” across time), larger mean differences are required to obtain the same dBDS as time increases.
3.4. Longitudinal changes in ADL scores
Figure 4 displays mean ADL values predicted by the fitted model for representative initial ADL values (0, 25, 50 %-dependent) for the three medication groups across four years of study. Results for the ADL showed a curvilinear, quadratic relation of the ADL to years in the study. The initial ADL score (at baseline visit) significantly (p = 0.01) interacted with this relation such that subjects with low initial ADL scores (i.e. those who are more independent on ADLs) tended to accelerate across years in the study, whereas subjects with initially high ADL scores (i.e. those who are less independent) tended to decelerate, as if constrained by a ceiling effect. Superimposed on these effects was a significant (p = 0.0002) interaction of medication group with the linear term for years in the study such that the COMBO group showed a significantly (p = 0.001) slower increase across years of the study than each of the other two groups. These two groups, NO-RX and CI, did not differ significantly from each other with respect to progression of ADL scores across the four-year study period. Note in Figure 4, that although change is curvilinear, rate of deterioration for the NO-RX group is initially about 10 points per year higher, with the rate increasing or decreasing slightly (depending on baseline level) as time goes on. Consistent with a good model fit and adherence to normality assumptions, the model residuals were bell-shaped across time.
Table 3 displays Cohen’s d effect size estimates on the ADL score (Cohen’s dADL) for all three pair-wise comparisons of medication groups at one to four years into the study separately. The dADL values for COMBO vs. NO-RX were statistically significant with p < 0.05 at year-1 and had a value of 0.32, were significant at p < 0.001 at years 2–4 with values that increased from 0.48 to 0.67. The dADL values for COMBO vs. CI were statistically significant with p < 0.001 at years 2–4 increasing from 0.46 to 0.73.
3.5. Confirmatory analysis utilizing GEE methods
The results of the GEE analyses on the BDS and ADL data confirmed the important findings from the mixed effects analyses. The same patterns of statistically significant (p < 0.0001) effects favoring the COMBO group over CI and NO-RX groups on progression of BDS and ADL scores, and for CI over NO-RX on progression of BDS (but not ADL) scores were found using GEE analyses. Parameter estimates from the GEE analyses were very similar to the corresponding fixed parameter estimates from the random effects runs, and the predicted means from the GEE analyses looked nearly identical to those displayed in Figure 3 and Figure 4 for all three groups. Also as expected, best fitting correlation structures showed declining positive correlations with increasing time intervals.
4. Discussion
Cognitive and functional deterioration in subjects with AD receiving clinical care at a memory disorders unit were significantly different across a four-year span when comparing a group never treated with cholinesterase-inhibitor or memantine (NO-RX group), a group clinically treated with cholinesterase-inhibitor monotherapy (CI group) and a group clinically treated with combination therapy consisting of memantine added onto a cholinesterase-inhibitor (COMBO group). Adjusting for baseline differences between the three groups on BDS and ADL scores, age, education, duration of illness, and interactions of baseline scores with years in the study, we found significant incremental decreases in the rate of progression of cognitive impairment going from the NO-RX to the CI to the COMBO groups as reflected by the annual rate of increase in the number of mistakes (errors) made on the BDS scale. Thus, for a measure of cognition, CI was superior to NO-RX, and COMBO was superior to both CI and NO-RX. In the domain of daily function, we found that the COMBO group also had a significantly lower rate of decline on the ADL than the NO-RX and the CI groups, which did not significantly differ from each other.
4.1 Effects of combination therapy on the rate of cognitive decline
Our study’s results support the current use of drugs in treating AD in that both COMBO and CI produced better outcomes than NO-RX. The results are also consistent with a recent study that compared CI (donepezil)-treated and untreated patients in an outpatient clinical setting in Japan and found less cognitive deterioration on the MMSE with treatment, and benefits that lasted at least two years [26].
Combination therapy is often prescribed for patients with moderate or severe stage AD, and usually takes the form of memantine add-on therapy to a cholinesterase-inhibitor started months or years earlier. Prior to this report, the strongest evidence supporting the superiority of COMBO Rx therapy over CI-monotherapy was from a 24-week pivotal phase III clinical trial of memantine add-on therapy to chronically stable donepezil treatment in highly selected subjects with moderate to severe AD [38]. Based on approximately 149 patient-years of completers data, this study showed significantly better outcomes for memantine-add-on combination therapy than placebo-add-on donepezil monotherapy with respect to measures of cognition, daily function, behavior and global outcome [38].
These results go beyond findings from short-term clinical trials and provide evidence that combination therapy with cholinesterase-inhibitor and memantine has real-world clinical effectiveness in the treatment of patients with AD. Our findings suggest that COMBO Rx is superior to no Rx and CI monotherapy. Further, the clinical benefits of COMBO are sustained for at least two years. As seen in Table 2 and Figure 3, there is an additional and clinically significant benefit for COMBO Rx, which appears to decrease the rate of cognitive decline in patients with AD. The mild to moderate effect size estimates for this superiority (Table 2) are consistent with those found in meta-analyses of short-term clinical trials of CI monotherapy that suggest Cohen’s d estimates in the 0.1–0.3 range [6, 9]. Although there is considerable intra- and inter-subject, and time-dependent variability, for untreated patients, the expected mean rate of deterioration on the BDS is in the range of 3–4 errors per year [34]. Our results predict that, on average, CI monotherapy decreases this deterioration by about one error per year and that COMBO Rx decreases it by about two errors per year. Effects of this magnitude were statistically robust (p < 0.001) and also likely to be noticed clinically [6].
4.2. Effects of combination therapy on the rate of functional decline
Our findings reinforce conclusions from other clinical trials [38, 39] showing that COMBO Rx is significantly superior to placebo and CI monotherapy: COMBO decreased the rate of functional decline on the ADL scale compared to NO-RX and CI. The mild to moderate effect size estimates for COMBO (Table 3) are similar to the modest benefits reported in shorter-term clinical trials [38, 39]. Contrary to expectation, CI alone did not affect the slope of decline on the ADL, whereas the slope was approximately halved during COMBO treatment. Further, benefits become more evident at intermediate impairment levels (e.g. ADLs around 50% dependent) and increased with time in study. For the COMBO group, at higher functional impairment, after steady decline, there appeared to be some leveling off during a plateau period. One can exclude simple ceiling effects on the ADL scale by noting that the ADL instrument is able to detect higher functional dependency for the NO-RX and CI groups which continue to increase with little or no leveling. One potential explanation for these results is discussed below in the context of a possible signal for disease-modifying effects in the combination therapy group. Regardless of the reasons, this result was statistically robust (p < 0.001) and would also provide clinical significance with respect to slowing deterioration on ADLs.
4.3. Clinical course of symptom progression with combination therapy
Another aim of this study was to assess the long-term clinical course in study subjects based on exposure and non-exposure to CI and COMBO therapy. There was clear superiority in both cognitive and functional domains for COMBO Rx over CI monotherapy and NO-RX. There was also superiority for CI montherapy over NO-RX with respect to cognitive functioning. Further, the long-term course of subjects in the COMBO group showed divergence from the other two groups with increasing effect size benefits over time for both cognitive and functional domains. One possible explanation for this long-term benefit is pharmacological, i.e. COMBO potentiates the individual symptomatic improvements due to CI and MEM alone. An alternative possibility is even more intriguing: COMBO treatment may have mild neuroprotective or disease-modifying benefits as well as symptomatic effects.
Support for neuroprotection or disease-modification comes from our model predictions that the beneficial effects of combination therapy in limiting the symptomatic decline in ADLs are greater in years 3–4 than in years 0–2. This effect cannot be simply explained by a ceiling effect on the ADL questionnaire in those with greater functional impairment because the mean ADL scores continued to increase in both the NO-RX and CI groups while those for the COMBO group did not. This finding appears to be a medication effect, and raises the possibility that combination therapy may have potential synergistic or disease-modifying effects that become increasingly evident over time. This observation does not reveal the underlying mechanisms of potential neuroprotective or disease-modifying effects, but does prompt speculation about possible biologic actions. Such mechanisms range from suggestions that CI may help reduce levels and deposition of cortical and vascular amyloid-β, and disrupt tau aggregation, to increase cerebral blood flow [40] and slow the rate of hippocampal atrophy [41, 42]. Meta-analyses of long-term clinical trials and open-label extension studies suggest a lower rate of decline with CI monotherapy than would be expected from historical cohorts or predicted by the Stern Equation [43, 44] (see [40] for a review of similar data). Yet interpretation of these data to support disease-modification effects for CI monotherapy is fraught with the pitfalls of interpreting open-label clinical trials data, coupled with seemingly contradictory evidence from several long-term trials of CI monotherapy in mild cognitive impairment that failed to show evidence consistent with disease-modification [40, 45].
The presumed pharmacological action of memantine in modulating glutamate-induced calcium excitotoxicity offers a potential explanation for a neuroprotective effect. There are also preclinical data that memantine has direct effects on the amyloidogenic cascade that could be disease-modifying in AD [46]. Clinical data on this point are contradictory. Data from a phase III clinical trial hinted at a possible difference in slope for decline at 24-weeks in cognition and function favoring combination therapy (MEM add-on to donepezil) over stable CI monotherapy with donepezil [38]. However, data from an open-label study suggested that memantine monotherapy had only symptomatic effects, as there were no significant differences at 52-weeks between groups of patients on memantine for the duration of the study versus the group on placebo during the initial 28-week randomized, double-blinded, placebo-control phase [39]. While it is possible that only COMBO therapy, as opposed to CI or memantine monotherapy, may have disease-modifying effects, it is more likely that these previous clinical trials could not reveal such potential effects due to their short durations and that trials lasting years are necessary [47].
We used analysis of slope and effect size estimates to assess the long-term course of drug effects on cognitive and functional decline. These approaches are well-suited to detect potential disease-modifying effects, which would be expected to show increasing effect sizes with time on treatment [47–50]. Analysis of slope also offers the advantage of being considered the least complicated method and is particularly suitable to assess data of two or more years in duration [50]. While our study is not able to distinguish between COMBO therapy having a sustained symptomatic benefit, a true neuroprotective benefit, a disease-modifying effect, or a combination thereof, what our study does demonstrate is that COMBO therapy has disease-course modifying effects [48] in which cognitive decline and loss of functional independence were slowed.
4.4. Strengths of the study
There are several important strengths of this study. First, there is the large number of well-characterized AD subjects who were prospectively examined by the same measures, in the same subspecialty memory disorders unit, in the same hospital, by the same group of clinicians over multiple years with good long-term follow-up. In contrast to most AD clinical trial and open-label extension studies that have typically included data on the order of 12–52 week spans and 100–300 patient-years [5, 9, 13–17, 39, 51, 52], this study includes data collected over years totaling 955 patient-years, with all subjects cumulatively treated with medications for at least six months, and about 90% of subjects cumulatively treated with medications for greater than one year. Also, the percentage of subjects who discontinued clinical follow-up prior to the end of the study epochs, which may be considered to be analogous to “dropping-out”, ranged from 18–34% and was not significantly different between the CI and COMBO groups. These percentages are similar to discontinuation/drop-out rates reported in short-term (12–52 weeks) [5, 9, 13–18, 39, 51, 52] AD clinical drug trials utilizing placebo, CI and combination treatments, and may be superior when viewed in the context of the substantially longer average duration of clinical follow-up in our study (2.5 years). Further, our study examined a broad spectrum of AD patients and avoided the limitations of most AD clinical trials designed to demonstrate drug efficacy. These studies often have stringent inclusion and exclusion criteria (including age, disease severity, concomitant medications, and medical conditions) that are intended to select a homogeneous and highly-leveraged sub-population with AD in order to maximize the likelihood of finding significant drug treatment effects and to minimize the potential for adverse effects [27–29]. Subjects participating in such clinical trials are not representative of the AD patient population as a whole [27–29] due to exclusion of those who have multiple or less stable medical conditions, take multiple medications with potential adverse interactions, and who do not have the motivation, ability or resources to participate due to the rigorous demands of clinical trials. For these reasons, the unselected cohort of subjects and results from this study reflect real-world clinical practice. In methodological terminology, our study gave priority to “external validity” to complement previous studies that stressed “internal validity” at the expense of being externally representative.
Second, the collected data for the treatment group comparisons are unique. To replicate our findings to satisfy the strongest grade of evidence to assess clinical effectiveness of CI and MEM in the treatment of AD in a prospective study would be difficult if not impossible. Such a study design would require a real-world clinical practice setting that employs randomization, blinding, and use of placebo-control. Implementing such trials would be impractical due to recruitment barriers and high dropout rates [53] and also pose strong ethical challenges given that MEM and CI medications are standard-of-care for stage-appropriate treatment of AD. In the absence of data meeting this grade of evidence, analyzing longitudinal clinical data and developing models similar to those employed in this study constitute the strongest way to determine long-term drug effects, and can also be used to test the validity of the results and conclusions of our study.
Third, the conservative bias of the methods and analyses employed towards minimizing potential drug treatment benefits is another strength for the study. For example, the study combined exposure-to-treatment and all-observations-included (observed case) analyses to assess clinical course of cognitive and functional status. In this approach, the totality of each subject’s data were included in only one of the three medication groups, and all data for each subject were used to calculate progression (deterioration) rates for BDS and ADL scores regardless of whether a subject discontinued drug for any reason or for any length of time. In this way, in order to assess the overall course, those subjects who underwent combination therapy but then stopped one or both medications due to any reason, including lack of response, adverse effects, high cost, inconvenience, or other illness and hospitalization, had all their data-points analyzed as part of the COMBO group, even after discontinuation of medication. This approach decreases the potential for drug-responder bias to elevate drug treatment benefits falsely by giving greater weight to subjects who continue drug treatment due to more favorable responses or other characteristics.
4.5. Limitations of the study
The major limitation of the study is a potential cohort confound when group comparisons are made to the NO-RX group: the NO-RX group is composed of subjects enrolled from 1991–1995 when neither of the current two classes of AD medications were in routine clinical use, the CI group enrolled from 1998–2002, and the COMBO group enrolled from 2000–2004. It is likely that patients are being diagnosed at earlier stages of AD than they were in the past. This may partially account for the differences in initial visit scores between the groups. Our analyses attempt to adjust for the differences in baseline severity and their interactions with time in the study. It is also possible that over the past 15 years improvements in the standard of general medical and supportive care provided to elderly and AD patients may have contributed to the relative slowing of cognitive or functional decline observed in the CI or COMBO groups. Such improvements include a greater emphasis on maintaining optimal lipid, glucose, and blood pressure control, widespread prescription of medications such as statins and aspirin, and more attention to diet, body weight and exercise. However, as yet, there is no compelling evidence from prospective clinical trials that any of these “interventions” have significant beneficial effects on performance measures of cognition or daily function in patients with AD. Further, changes in general health considerations would not be expected to account for the difference between the CI and COMBO groups, as these were the most recent groups. Finally, it should be noted that, the NO-RX group is not a typical historical cohort as they were treated in the same unit by the same neurologists who prescribed CIs and MEM as per standard of care.
Another potential limitation of this study is the possibility that the results may less accurately estimate the long-term effects of CI monotherapy on cognition or function since subjects who started CI and subsequently had memantine added to their regimen moved from the CI to the COMBO group for purposes of these analyses. Conversely, the long-term effects of COMBO therapy may have been underestimated (or overestimated) for the same reason. We believe that these considerations have limited importance as each treatment group had over 100 subjects who were followed for more than two years. This cohort size and duration of treatment were sufficient to detect superiority [47] of CI and COMBO over NO-RX, and COMBO over both.
Because memantine was always an add-on therapy to CI, we cannot assess the clinical effectiveness of memantine monotherapy, nor of combination therapy that consists of adding a CI to existing memantine treatment. However, due to FDA-label indications for CIs and memantine, it is unlikely a different order of drug administration will be tested in a clinical practice setting or for such an ordered combination to produce significantly different results from those found in the COMBO group.
4.6. Conclusion
In summary, COMBO was superior to NO-RX and CI monotherapy in slowing progression of cognitive and functional decline in this large, well-characterized and prospectively assessed cohort of patients with AD who received clinical care at a memory disorders unit. In addition, with respect to cognition, CI monotherapy was superior to NO-RX. The benefits of COMBO therapy were significant, with small-to-medium effect sizes that were sustained for years. The results also raise the intriguing possibility that COMBO therapy modestly modifies the long-term clinical course of AD, although sustained symptomatic pharmacologic effects that vary in strength with time are equally possible.
Acknowledgments
The study was supported by the National Institutes of Aging through grants 1K23 AG27171-01 (Atri) and 5P50 AG05134 (Dr. John H. Growdon and Dr. Bradley T. Hyman; Massachusetts Alzheimer’s Disease Research Center [MADRC]), and the NIH Loan Repayment Program (Atri). We would like to acknowledge Dr. Liang Yap for assistance with database queries, Dr. Joseph Tang for assistance with data processing, and Dr. Reisa A. Sperling and Dr. Bradley T. Hyman for their valuable suggestions and support. Finally, we would like to acknowledge our great debt to the patients and families involved with research in the MGH Memory Disorders Unit and MADRC without whom this research would not be possible.
Technical Appendix
The initial Mixed Model employed (prior to backward elimination) was:
BDSij or ADLij = β0 + β1 · timeij + β2 · timeij2 + β3 · base_BDS(ADL)i + β4 · timeij · base_BDS(ADL)i + β5 · timeij2 · base_BDS(ADL)i + β6 · med_groupi + β7 · timeij · med_groupi + β8 · timeij2 · med_groupi + [other fixed effects] + bi0 + bi1 · timeij + bi2 · timeij2 + εij
where:
BDSij or ADLij = BDS or ADL of subject i at time j;
timeij = years in the study for subject i at time j;
base_BDS(ADL)i = baseline BDS (or ADL) for subject i;
med_groupi = medication group membership (NO-RX, CI, or COMBO) (coded as a set of dummy indexing variables); and
other fixed effects are: age at baseline, duration of illness at baseline, years of education
β = fixed effect coefficient
-
bi = random effect coefficient ~ N(0,G):
bi0 = random intercept for subject i
bi1 = random linear time coefficient for subject i
bi2 = random quadratic time coefficient for subject i
G = variance-covariance matrix for bi0, bi1, bi2
εi = errors ~ N(0,sε2I)
The terms retained in the final model, along with their p-value are shown in the Table A1.
Footnotes
Disclosure: Dr. Atri has received remuneration for lectures or consulting from Eisai, Forest, Merck and Pfizer, and Investigator-Initiated research grant support from Forest. He has also participated in conducting clinical trials in association with the Alzheimer’s Disease Cooperative Study (ADCS), Elan, Neurochem and Wyeth. There was no sponsorship or involvement by the pharmaceutical industry, including the motivation, design, execution, interpretation, presentation and costs associated with any part of this study. Ms. Shaughnessy, Dr. Locascio and Dr. Growdon have no potential conflicts to disclose.
References
- 1.Hebert LE, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's-Association. Alzheimer's disease prevalence rates rise to more than five million in the United States. 2007 [Google Scholar]
- 3.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 4.Burns A, et al. Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J Psychopharmacol. 2006;20(6):732–755. doi: 10.1177/0269881106068299. [DOI] [PubMed] [Google Scholar]
- 5.Burns A, et al. The effects of donepezil in Alzheimer's disease - results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10(3):237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75(5):677–685. doi: 10.1136/jnnp.2003.029074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winblad B, et al. Memantine in Moderate to Severe Alzheimer's Disease: a Meta-Analysis of Randomised Clinical Trials. Dement Geriatr Cogn Disord. 2007;24(1):20–27. doi: 10.1159/000102568. [DOI] [PubMed] [Google Scholar]
- 8.Kaduszkiewicz H, et al. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. Bmj. 2005;331(7512):321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006 Jan;:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006 Feb;:CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 11.Grimmer T, Kurz A. Effects of cholinesterase inhibitors on behavioural disturbances in Alzheimer's disease: a systematic review. Drugs Aging. 2006;23(12):957–967. doi: 10.2165/00002512-200623120-00003. [DOI] [PubMed] [Google Scholar]
- 12.Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2007 doi: 10.1002/gps.1746. [DOI] [PubMed] [Google Scholar]
- 13.Raskind MA, et al. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 14.Farlow M, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer's disease. Eur Neurol. 2000;44(4):236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 15.Rogers SL, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50(1):136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 16.Tariot PN, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54(12):2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 17.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. Bmj. 2000;321(7274):1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doody RS, et al. Meta-analysis of six-month memantine trials in Alzheimer's disease. Alzheimer's & Dementia. 2007;3(1):7–17. doi: 10.1016/j.jalz.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Brodaty H, et al. Naturalistic treatment of Alzheimer's disease with galantamine: 12-month follow-up from the NATURE study. CNS Drugs. 2007;21(4):335–336. doi: 10.2165/00023210-200721040-00006. [DOI] [PubMed] [Google Scholar]
- 20.Aguglia E, et al. An open-label, comparative study of rivastigmine, donepezil and galantamine in a real-world setting. Curr Med Res Opin. 2004;20(11):1747–1752. doi: 10.1185/030079904X6273. [DOI] [PubMed] [Google Scholar]
- 21.Bellelli G, et al. Results of a multi-level therapeutic approach for Alzheimer's disease subjects in the "real world" (CRONOS project): a 36-week follow-up study. Aging Clin Exp Res. 2005;17(1):54–61. doi: 10.1007/BF03337721. [DOI] [PubMed] [Google Scholar]
- 22.Mossello E, et al. Effectiveness and safety of cholinesterase inhibitors in elderly subjects with Alzheimer's disease: a "real world" study. Arch Gerontol Geriatr Suppl. 2004;(9):297–307. doi: 10.1016/j.archger.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Brodaty H, et al. A naturalistic study of galantamine for Alzheimer's disease. CNS Drugs. 2006;20(11):935–943. doi: 10.2165/00023210-200620110-00006. [DOI] [PubMed] [Google Scholar]
- 24.Hansen RA, et al. Functional outcomes of drug treatment in Alzheimer's disease: A systematic review and meta-analysis. Drugs Aging. 2007;24(2):155–167. doi: 10.2165/00002512-200724020-00007. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, et al. Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol. 2000;57(1):94–99. doi: 10.1001/archneur.57.1.94. [DOI] [PubMed] [Google Scholar]
- 26.Tomita N, et al. Long-term cognitive benefits of donepezil in Alzheimer’s disease: A retrospective comparison between 1994–1999 and 2000–2004. Geriatrics & Gerontology International. 2007;7(1):41–47. [Google Scholar]
- 27.Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 28.Godwin M, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gartlehner G, et al. A simple and valid tool distinguished efficacy from effectiveness studies. Journal of Clinical Epidemiology. 2006;59(10):1040. doi: 10.1016/j.jclinepi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Growdon J. Treatment for Alzheimer's disease? N Engl J Med. 1992;327(18):1306–1308. doi: 10.1056/NEJM199210293271810. [DOI] [PubMed] [Google Scholar]
- 32.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub S. The record of independent daily living: an informant completed measure of activities of daily living and behavior in elderly patients with cognitive impairment. Am J Alzheimer Care. 1986;1:35–39. [Google Scholar]
- 34.Locascio JJ, Growdon JH, Corkin S. Cognitive test performance in detecting, staging, and tracking Alzheimer's disease. Arch Neurol. 1995;52(11):1087–1099. doi: 10.1001/archneur.1995.00540350081020. [DOI] [PubMed] [Google Scholar]
- 35.Diggle PJ, et al. Analysis of Longitudinal Data. 2002 [Google Scholar]
- 36.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, Florida: Chapman & Hall/CRC; 2003. [Google Scholar]
- 37.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 38.Tariot PN, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 39.Reisberg B, et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63(1):49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Sabbagh MN, et al. Do cholinergic therapies have disease-modifying effects in Alzheimer's disease? Alzheimer's & Dementia. 2006;2(2):118–125. doi: 10.1016/j.jalz.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan KR, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. Am J Psychiatry. 2003;160(11):2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto M, et al. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 2005;162(4):676–682. doi: 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- 43.Stern RG, et al. Deterioration on the Blessed test in Alzheimer's disease: longitudinal data and their implications for clinical trials and identification of subtypes. Psychiatry Res. 1992;42(2):101–110. doi: 10.1016/0165-1781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 44.Stern RG, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151(3):390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 46.Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18 Suppl 1:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- 47.Mohs RC, Kawas C, Carrillo MC. Optimal design of clinical trials for drugs designed to slow the course of Alzheimer's disease. Alzheimer's & Dementia. 2006;2(3):131–139. doi: 10.1016/j.jalz.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Cummings JL. Challenges to demonstrating disease-modifying effects in Alzheimer's disease clinical trials. Alzheimer's & Dementia. 2006;2(4):263–271. doi: 10.1016/j.jalz.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aisen PS. Commentary on "Challenges to demonstrating disease-modifying effects in Alzheimer's disease clinical trials". Alzheimer's & Dementia. 2006;2(4):272–274. doi: 10.1016/j.jalz.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Knopman D. Finding potent drugs for Alzheimer's disease is more important than proving the drugs are disease modifying. Alzheimer's & Dementia. 2006;2(3):147–149. doi: 10.1016/j.jalz.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Feldman H, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology. 2001;57(4):613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 52.Reisberg B, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 53.Courtney C, et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 2004;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]