Abstract
Work in functional neuroimaging has mapped interference resolution processing onto left inferior frontal regions for both verbal working memory and a variety of semantic processing tasks. The proximity of the identified regions from these different tasks suggests the existence of a common, domain-general interference resolution mechanism. The current research specifically tests this idea in a within-subject design using fMRI to assess the activation associated with variable selection requirements in a semantic retrieval task (verb generation) and a verbal working memory task with a trial-specific proactive interference manipulation (recent-probes). High interference trials on both tasks were associated with activity in the midventrolateral region of the left inferior frontal gyrus, and the regions activated in each task strongly overlapped. The results indicate that an elemental component of executive control associated with interference resolution during retrieval from working memory and from semantic memory can be mapped to a common portion of the left inferior frontal gyrus.
Keywords: Prefrontal cortex, Cognitive control, Magnetic resonance imaging, Working memory, Semantic retrieval, Interference, Selection
1. Introduction
One of the major problems of contemporary cognitive neuroscience is understanding how cognitive control is implemented in the brain, particularly with respect to the organization of function within prefrontal cortex (PFC). Several important and influential theories have been proposed which map general functions to large sections of cortex. For example, Duncan and Owen (2000) propose a frontal lobe network of dorsal anterior cingulate, mid-dorsolateral PFC, and mid-ventrolateral PFC that is generally recruited across many behavioral tasks in response to increased cognitive demands. Other work has examined the particular contribution of one or more of these individual areas; for example, O'Reilly, et al., (1999) argue that dorsal anterior cingulate cortex mediates conflict detection, especially response conflict, while lateral cortex mediates goal or context maintenance (see also Botvinick, et al., 2001; Braver and Cohen, 2001).
However, the variety of neuroanatomical connections and cytoarchitecture of lateral PFC (Petrides, 2002) suggests that sub-areas of differing functionality may exist within these larger functionally defined regions, even if existing large scale characterizations of function are correct. The present work explores the possibility of mapping function at a finer level of organization in ventrolateral cortex (e.g., Badre & Wagner, 2007).
Research using two very different tasks, one a short term letter or word recognition task (i.e. verbal working memory task), and the other a semantic retrieval task, suggests that they may share a cognitive operation that resolves interference by selecting among competing representations. Evidence from independent studies of each task has linked their selection requirements to midventrolateral prefrontal cortex, in a portion of the left inferior frontal gyrus (IFG) within cytoarchitectonically defined Brodmann's Area (BA) 45. In the present investigation, our goal was to obtain further evidence that the selection/interference resolution processes entailed in these tasks share a neural substrate, using fMRI to identify the neural correlates of each task within the same individuals. If these tasks do not recruit overlapping regions of cortex, the hypothesis that they entail a common executive process would be seriously challenged. Alternatively, identifying a neural substrate that is common to both tasks is consistent with the hypothesis that these tasks invoke a common executive process, and would represent progress toward clarifying the neural organization of cognitive control. What follows is an overview of the tasks under investigation, and then a summary of the existing evidence linking their selection demands to the left IFG.
The working memory task is the “recent-probes task”, in which on certain trials subjects must deal with a form of proactive interference. In the version of the task used by Jonides et al (1998, based on Monsell, 1978), subjects are required to retain a set of four letters in memory briefly, and then to respond to a probe letter that on half of the trials matches one of the members of the current test set (positive probes) and on half of the trials does not (negative probes). In a high-recency condition, half of the negative probes were members of the test set on the immediately preceding trial; in a low recency condition, no negative probe was a member of the preceding test set. Jonides et al. found an interference effect of about 50ms on response times for high-recency negative probes. PET measurements indicated significantly more activation in left Brodmann's area (BA) 45, located in the left IFG, in the high-recency condition compared to the low-recency condition. This was the only significantly different region of activation between the two conditions, and activity in this region has been found consistently in subsequent task variations using other stimuli such as words or objects (e.g., Badre &Wagner, 2005; Jonides & Nee, 2006). Converging evidence from patients with focal left hemisphere lesions further suggests that left IFG involvement is necessary for proper interference resolution on the recent-probes task (Thompson-Schill, et al., 2002, see also Hamilton and Martin, 2005).
It is hypothesized that two sources of information are potentially used to produce a response in the recent-probes task: 1) familiarity of the probe, and 2) an explicit contextual or temporal tag that marks a probe item as belonging to a list (such as proposed by Monsell, 1978). We have proposed that left BA45 is recruited to meet the increased selection demands and to resolve the interference produced by this competing information for recent probes (e.g., Nelson et al., 2003).
The semantic retrieval task is a version of the Verb Generation task (Petersen, et al., 1989) developed by Thompson-Schill et al. (1997). Thompson-Schill and colleagues specifically varied the selection requirements in three different semantic tasks, two of which commonly activate inferior frontal regions in neuroimaging experiments (verb generation and object classification) and one type of task that does not (noun comparison). The authors reported greater activation in left IFG for high-selection versus low-selection versions of all three tasks. The authors proposed “activation of the left IFG during semantic tasks is the result not of semantic retrieval per se, but of the need to select some relevant feature of semantic knowledge from a set of competing alternatives.” (p. 14792). An increased interference effect was found in another study with a group of patients with left IFG lesions (Thompson-Schill, et al., 1998). Control patients whose lesions did not include left IFG did not show an exaggerated interference effect.
In that both the recency manipulation of the recent-probes task and the selection manipulation of the semantic task are hypothesized to involve selection between or among competing mnemonic representations, it is possible that they may both be tapping a common component of cognitive control. The reported activations associated with high-interference conditions were very similar and overlapping, although the peak activations straddled the borders of Brodmann's area 45 (Jonides, et al 1998) and Brodmann's area 44 (Thompson-Schill, et al. 1997). The seemingly similar anatomical locations of the activations from these two tasks should be interpreted with caution, however. The findings are from two different groups of subjects measured with two different imaging techniques (PET vs. functional MRI), so the overlapping but non-identical activations may arise from distinct but adjacent cortical regions.
Because inter-study variability can make fine distinctions difficult, testing for neural overlap between different tasks in a single study within a single set of subjects is preferable to comparing activations across studies. In the present study, we examine the patterns of activation associated with interference resolution in a verbal working memory task (a version of the recent-probes task) and also a semantic retrieval task (verb-generation) in a group of subjects who performed both tasks during a single fMRI session. This approach provides a sensitive measure of the degree of overlap between the respective patterns of activation, and potentially allows mapping a particular component of cognitive control shared by both tasks to a specific region of frontal cortex. Similar studies have been done either within a task domain (e.g., semantic retrieval, Thompson-Schill et al., 1997, Badre, et al., 2005) or across task domains in an exploratory fashion (e.g., LaBar, et al., 1999; Nyberg, et al., 2003). The current study is narrowly focused on a single functional component, but across different task domains.
2. Results
Behavioral results
The behavioral results are summarized in Table 1 and replicate numerous published reports of these tasks. The key comparison in the recent-probes task is between the non-recent and recent non-match trials, where we expected longer and less accurate responses for recent probes. Likewise in the verb generate task, we expected longer response times in the many than few condition. These predicted behavioral effects were evaluated using one-tailed t tests.
Table 1.
Behavioral performance on the modified recent-probe task and verb generation task.
| Recent-probe task | Match | Non-match | |
|---|---|---|---|
| Non-Recent | Recent | ||
| Reaction Time | 845 ms | 843 | 953 |
| (Standard error) | (39.2) | (43.9) | (47.7) |
| Accuracy | 90.4% | 96.6 | 89.5 |
| (s.e.) | (2.35) | (1.65) | (2.03) |
| Verb Generation | Read | Generate | |
| Few | Many | ||
| Reaction Time | 647 ms | 1176 | 1346 |
| (s.e.) | (90.0) | (95.3) | (103.1) |
The expected interference effects were found for both tasks, and were of comparable magnitudes to effects reported previously from our lab and others (Badre & Wagner, 2005; Jonides et al, 2000; Nelson et al., 2003; Persson et al., 2004; Thompson-Schill et al., 1997). In the recent-probes task, subjects took 109ms longer to make a response, (t(16)=6.50, one-tailed P<.001) and were 7.0% less accurate (t(16)=5.73, one-tailed P <.001) on recent negative probes than non-recent negative probes. In the verb generation task, subjects were 170ms slower (t (16) =2.15, one-tailed P=0.046) to respond to noun probes with many alternative responses than noun probes with few alternative responses.
Neuroimaging results
The primary question is whether the interference-related activity associated with our two cognitive tasks is localized to the same region of the left inferior frontal gyrus. To address this question we defined our region of interest as the entire left IFG defined anatomically using the preset anatomical regions (Tzourio-Mazoyer, et al.2002) from the WFU-PickAtlas utility (Maldjian, et al. 2003). For the verb generation task, the “Many” – “Few” contrast revealed a cluster of activation (69 voxels, FWE p<.05) with two primary peaks of activation at x = −52.5, y=22.5, z=20, one on the superior surface of the left IFG, pars triangularis, in BA 45 and the other at -56.25, 11.25, 20, near the border of BA 44 (See Figure 4.1, top row). Additionally, a third, weaker sub-peak was found at the extreme border of the ROI (at coordinates -45.0, 0.0, 35); however, this “sub-peak” resides in the outskirts of the middle frontal gyrus in area 6. A whole brain analysis indicates that this is actually the edge of an additional area of activation outside of the ROI. [footnote 3]
For the recent-probes task, using the left IFG ROI, a recent – non-recent contrast revealed a 9-voxel activation with a peak activation at -52.5, 18.75, 25.0, (FWE p < .05, peak T = 5.94). Critically, the main peak for the recent-probes task contrast is immediately adjacent to the peak for the Verb Generation contrast, with the recent-probes peak being only one voxel posterior and one voxel superior to the verb generation peak. Furthermore, the activation from the recent-probes task is entirely contained within the area defined by the many/few conflict activation from the verb generation task. Note however, the blocked design of the verb generation task typically has greater statistical power than the event- related design used in the recent-probes task. Therefore, we may be underestimating the extent of the relevant region for the Recent-probes task, and omitting relevant but non-overlapping voxels. To address this issue, we present results from the recent-probes task at a more liberal threshold of p < .005, uncorrected for multiple comparisons. At this more liberal threshold, the recent-probes activation expands to a size of 39 voxels (See Figure 4.1, bottom row) 33 of which are contained within the area of activation defined by the many/few contrast from the verb generation task.
Individual activations in IFG
To confirm that the high degree of overlap in interference-related activation between the two tasks was not an artifact of averaging across the entire group of subjects, we also examined individual results. As in the group analysis we present results from the recent-probes task at a more liberal threshold of p < .005, uncorrected for multiple comparisons (individual isolated voxels were discounted.)
Ten of the seventeen subjects showed significant left IFG activation (high-interference compared to low-interference trials) in both tasks (Figure 4.2). In 7 out of these 10 cases, these task activations overlapped, and furthermore these individually defined regions of overlap also coincided with the group-defined region of overlap (figure 3a-g). In one case (h) the task activations overlapped, but this region was immediately adjacent to the group-defined overlap region. In the final two cases (i and j) the task related activations did not overlap but were closely interleaved.
3. Discussion
We found that the region of activation correlated with interference resolution in a verbal working memory task is superimposed on the cortical region recruited by increased selection demands in semantic retrieval, with peak activations located in adjacent voxels. Although each region of activation derives from divergent task types that draw on different memory domains, the high degree of overlapping cortical activity suggests that the two different tasks recruit a shared selective process.
These neuroimaging results converge nicely with recent behavioral work (Persson, et al. 2007; Persson & Reuter-Lorenz, 2008) demonstrating cognitive overlap between these two tasks. In the study by Persson et al. (2007) subjects complete two sessions of a verb generation task, a pre-test and a post-test, with either a recent probes task or item-recognition without recent probes intervening. The interference effect on response time in the Many compared to the Few condition was significantly increased at post-testing when a recent-probes task intervened. When the pre- and post-test task was replaced with an episodic memory task featuring proactive interference (thought to activate left BA45 as well; Henson, et al., 2002), a similar increase in interference was also observed due to the intervening recent-probes task. In contrast, when a stop-signal task (known to involve right rather than left inferior frontal cortex, Aron, et al., 2003, 2004), was substituted as the intervening task, there was no modulation of the Many-Few effect. The authors interpreted this pattern of results as indicating process-specific “fatigue” (Wickens, 1984), in which cognitive processes are resource limited, and tasks that use a common neuro-cognitive component may deplete these common resources resulting in decreased functional efficiency. In contrast to these short-term negative transfer effects demonstrated within a single test session, more recent work shows that two weeks of training on variants of the recent-probes task can lead to more efficient verb generation under high selection demands, and reduced proactive interference in episodic memory (Persson and Reuter-Lorenz, 2008)
The current neuroimaging results and this behavioral work provide converging evidence for a functional component of cognitive control that is neurally mediated by a discrete region of the inferior frontal gyrus in the left hemisphere. This component of cognitive control is potentially applicable to a wider range of tasks than the two used in the current study. This same general region has been implicated in interference resolution in other tasks such as directed forgetting (Zhang, et al., 2003, Nee, & Jonides,2008), n-back tasks with lures in non-n positions (Gray et al., 2003), resolving proactive interference generally due to accumulating trials (Postle and Brush, 2004), resolving proactive interference in a cued-recall task, (Henson, et al., 2002) as well as semantic tasks such as the classification and comparison tasks (Badre et al., 2005; Thompson-Schill, et al., 1997). This putative control process which we and others have linked to left midventrolateral IFG seems to be recruited for selection among competing memory representations, and thus differs from other forms of attentional selection and inhibition that have been associated with executive control. For example, we have shown that when response competition is manipulated in the recent probes task, an additional medial region of prefrontal cortex in area 6/32 is activated (Nelson et al., 2003; see also Milham et al., 2001). Similarly, other variants of the item recognition task have dissociated left IFG activation associated with selection from memory representations from parietal activations associated with perceptual selection (Nee and Jonides, 2008). Thus additional control demands in the item recognition task activate regions outside of left IFG, consistent with their functional separability. Furthermore, attentional selection demands associated with tasks involving incongruent, competing stimulus dimensions, such as Stroop and flanker-type tasks, typically activate regions of left dorsolateral cortex (e.g., Milham et al, 2001; Liu et al., 2006) and in some cases regions along the inferior frontal sulcus (Brass and Von Cramon, 2004) that are superior to the selection-related activations in left ventrolateral IFG.[footnote 4] Future work using an overlap approach such as we report here, along with tests for behavioral interactions among tasks can further determine the functional separability of various forms of cognitive control, and thereby further delineate the components of executive processing (e.g, Miyake et al, 2000).
Several specific mechanisms have been proposed in the literature on a task-by-task basis for this area of activation. The challenge is to specify a process that may provide a unifying explanation across task domains. The recent-probes task activation has been attributed to selecting between competing familiarity-based and explicit contextual representations (Jonides, et al., 2000), while the role of this region in semantic tasks such as verb generation has been characterized as the selection of semantic features from among competing alternatives (Thompson-Schill et al. 1997). Both of these explanations were developed to explain results within working memory or semantic retrieval. However Thompson-Schill and colleagues (Kan & Thompson-Schill, 2004, Thompson-Schill, et al., 2005) have subsequently re-characterized their proposed mechanism as a more domain-general conceptual selection process, analogous to biased competition models of perceptual selection (Desimone & Duncan, 1995). This mechanism is flexibly applicable to a variety tasks in that it can add weight or bias to contextually appropriate competitors and so is of sufficient generality to apply to a variety of tasks, including the recent probes task. This model is attractive in that it builds upon a well-established model already shown to be useful for perceptual selection. It also provides a mechanistic account for both patterns of interference on recent negative probes, and facilitation on recent positive probes respectively (e.g., Badre and Wagner, 2005).
Kan and Thompson-Schill suggest that domain specificity arising from patterns of connections to posterior regions may play an organizing role in which portions of PFC are involved in different tasks. By this principle, left BA45 may be specific to verbal or auditory stimuli and not involved with purely visual or spatial stimuli. While some reports are consistent with this (Mecklinger, et al., 2003, Leung and Zhang, 2004), there is also evidence for domain generality (Postle et al., 2004, Badre and Wagner, 2005).
Other possible mechanisms corresponding to left BA45 activation have been proposed (for recent reviews, see Jonides and Nee, 2006; Badre and Wagner, 2007). However, one possibility should be addressed here, and that is that the present site of activation does not reflect a particular mechanism but only a response to general difficulty, or time on task. For example, both recent negative probes and “Many” verb generation trials are more difficult than baseline trials. However, recent positive probes, in which a positive probe was also a member of the preceding trial, are equally difficult or even less difficult than baseline probes, but are still associated with increased left BA45 activation (Badre and Wagner, 2005). This is inconsistent with attributing the activation generally to task difficulty rather than to a specific process. Also inconsistent with a “general difficulty” role, Feredoes et al. (2006), using repetitive transcranial magnetic stimulation of the left IFG during the probe phase of a recent probes task, showed a selective disruption of accuracy (but not reaction time) on recent negative trials only (see Jonides and Nee, 2006 for additional discussion of the general difficulty hypothesis).
The present results suggest that executive functions may be mapped in PFC as separable neural components, as opposed to a unitary organization of cognitive control. One might be tempted to view this conclusion as promoting a highly modular view of the functional organization of PFC that stands in opposition to theories of general-purpose networks, or accounts that assign functions to large sections of PFC. However, we view this approach as complementary, rather than in direct contrast to theories of large-scale organization.
The precise characterization of neurally-instantiated cognitive functions in general and executive processes in particular is an abiding goal of cognitive neuroscience. The identification of specific processing demands from two different tasks with a focal region of neural tissue constitutes an important step toward achieving this goal. Further research to clarify the boundary conditions for recruitment of this left IFG process holds the potential to specify a basic component of cognitive control, and by contrast to better define the role of other, closely related frontal subsystems.
4. Experimental Procedure
Participants
The participants were 17 young adults (18-30 years old, 10 female). Participants were recruited from the University of Michigan community through newspaper and posted advertisements. All participants were right-handed and had no history of neurological or psychiatric illness. None of the participants was taking medication or had a medical condition that could affect blood-oxygen levels (e.g. high blood pressure). All participants gave informed consent, and the study was approved by the University of Michigan Institutional Review board. All were native English speakers and had normal vision or wore contact lenses. Ten of these participants were also members of the sample of an earlier report on the verbal working memory task (Nelson, et al., 2003).
Tasks and Stimuli
Participants performed six total experimental blocks. Blocks 1 and 4 were the verb generation task, while the remaining blocks were a modified recent-probes task. After initial instruction, participants also received a 10-trial practice session of the recent probes task and a full block of the verb generation task before entering the scanner.
Modified recent-probes task
Participants performed 192 trials of a recent-probes task, divided into four runs of 48 trials each. Trials were pseudorandomized within each run (truly random trial orders were not feasible in order to maintain the proper relations between each trial's probes and previous probes and targets; see below for a full explanation of trial-types). Half of the participants received runs 1 and 2 of the pseudorandom list first, and half received runs 3 and 4 first.
At the start of a trial, four lowercase letters (consonants only, excluding lowercase “L”) and a central fixation cross were presented in a square pattern for 1,500 ms. After a 3,000-ms delay, a 1,500-ms probe appeared, which consisted of a single uppercase letter. On 50% of the trials, this probe was a member of the current trial's set of four target letters (positive trials), and on 50% of the trials it was not (negative trials). Disregarding case, participants responded with a “yes” for a positive probe, with their right index finger, or with a “no” for a negative probe, with their right middle finger. A variable length inter-trial interval (ITI) followed (96 ITIs of 1.5 sec, 48 ITIs of 3 sec, 24 ITIs of 4.5 sec, 16 ITIs of 6 sec, 4 ITIs of 7.5 sec, and 4 ITIs of 9 sec). Participants never received more than two positive or two negative trials in a row.
The target sets were constructed so that each contained one letter in common with the preceding trial's target set, but with no other letter in common with the previous two trials. The probe letter for positive trials was a member of the current target set, but was not a letter in common with either the preceding or subsequent trial's target set. The probe for a non-recent negative trial was a letter neither in the current target set nor the target set of the previous two trials.
In addition, there were three types of recent trial: familiar, highly familiar, and response conflict trials. The probe for familiar negative trials was a letter in the immediately preceding target set. The probe for highly familiar negative trials was a letter in the previous two trials. The probe for response-conflict trials was a member of the previous target set, and was also a positive probe on the previous trial. For negative trials, each of four types of negative trials was represented equally. For the current analyses, we will group familiar and highly familiar trials as “recent” probes, and compare them to non-recent probes. Response conflict trials are excluded from subsequent analyses. These modifications to the standard recent-probes task were developed for analyses in Nelson, et al., (2003). For this report we do not consider the quantitative differences in activation between highly familiar and familiar trials, or the qualitatively different activations arising from familiarity-based conflict and response-based conflict; instead see Nelson, et al. (2003).
Verb generation
The stimuli were nouns that varied in length from 3 to 8 letters (median = 4) and in Kucera-Francis frequency from 0 to 591 (median = 32); high selection nouns were defined by a response strength ratio of 1.0 - 3.0, and low selection nouns by a response strength ratio of 5.0 - 50.0 (Thompson-Schill et al., 1997).
In the generation task, participants were asked to generate a verb related to a visually presented noun. For each of the words, the participants responded by pressing a button with their right index finger when they had generated a word. Participants first practiced with experimenter supervision while saying the generated noun out loud, but generated the noun silently while in the scanner. In the condition with high selection demands (the “MANY” condition), items were nouns with many appropriate associated responses (e.g. BALL – THROW, KICK, BOUNCE etc.) without any clear dominant response. In the low selection condition (the “FEW” condition), items were nouns with few associated responses or with a clear dominant response (e.g. SCISSORS – CUT). A separate pilot study was conducted to establish that the latency differences between the two selection conditions (MANY/FEW) was replicated with overt verbal responses and with button press responses (cf. Persson, et al, 2004). Two control tasks were also included; (i) participants were asked to silently read words (nouns) that were presented visually for 4 s each and to respond by pressing the response button (READ), and (ii) a low-level baseline (rest). Six nouns were presented in each block (except for baseline), and each word was presented for 4 s. The study was divided into two runs, each with 8 MANY, 8 FEW, 8 READ, and 4 Baseline blocks.
fMRI Methods
Images were acquired using a 3T whole-body MRI scanner (General Electric) equipped with a standard quadrature headcoil. Functional T2* blood oxygenation level-dependent (BOLD) images were acquired using a spiral sequence with 25 contiguous axial 5 mm slices [repetition time (TR) = 1500 ms, echo time (TE) = 25 ms, flip angle = 90°, and field of view (FOV) = 24 cm]. A T1-weighted gradient echo (GRE) anatomical image was also acquired by using the same FOV and slices as were used in the functional scans (TR = 275 ms, TE = 35 ms, and flip angle 90°). In addition, a 60-slice high-resolution set of anatomical images was acquired by using spoiled gradient-recalled acquisition in steady state (SPGR) imaging (TR = 35 ms, TE = 3 ms, flip angle = 35°, and FOV = 24 cm, 2.5 mm slice thickness). The T1 GRE images were acquired at the start of the scanning session, and the SPGR images were acquired at the end of the scanning session. Experimental tasks were presented using E-Prime (Psychology Software Tools, Pittsburgh) and the IFIS 9.0 system (MRI Devices, Waukesha, WI) and responses were collected using a 10-button response pad. Head movement was minimized with foam padding, as well as a restraint that was strapped across the participants' foreheads. Images were corrected for slice-timing differences using a local 17-point sinc interpolation program (Oppenheim et al., 1999). Head movement was adjusted by using the Automated Image Registration (AIR) software (Woods et al., 1998). Subsequent preprocessing and analyses were done using SPM99 (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm/). SPGR images were corrected from signal inhomogeneity using a toolbox developed by G. Glover and K. Christhoff (www-psych.stanford.edu/∼kalina/SPM99/Tools/vol_homocor.html), and coregistered to the T1 images. The skull was removed from the SPGR images using the Brain Extraction Tool from FSL (Smith, 2002), and normalized to a T1 template in the Montreal Neurological Institute (MNI) space. The same normalization procedure was used for the functional images. After spatial normalization, the functional images were smoothed using an 8-mm full-width at half-maximum Gaussian filter. All images were high-pass filtered and scaled to a global mean intensity of 100.
All subsequent analyses of the functional images were performed using the general linear model implemented in SPM99. For the verb generation task, all conditions (READ, FEW, and MANY) were modeled as a fixed response (box-car) waveform of 24 seconds duration convolved with the hemodynamic response function. For the recent-probes task, event-onset times for the probes of the five trial types (positive probes, and four kinds of negative probe types: non-familiar, familiar, highly familiar, and response-conflict) were convolved with the canonical hemodynamic response function. High pass filter periodicity was computed by SPM99 and was 66, 63, 63, and 57 seconds for runs 1 to 4 of the recent-probes task, and 88 seconds for both blocks of the verb generation task. Statistical parametric maps (were generated using t-statistics to identify regions activated according to the model. Statistical models were fit for each participant, and contrasts of interest were estimated. Group data were analyzed using a random-effects model. Portions of runs with fMRI spike artifacts were omitted from model fitting. Localization of activations to specific Brodmann's Areas was achieved after converting the coordinates to Talairach space (using a transform developed by Matthew Brett, Medical Research Council Cognition and Brain Sciences Unit, Cambridge, U.K., which can be accessed at http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
Figure 1.
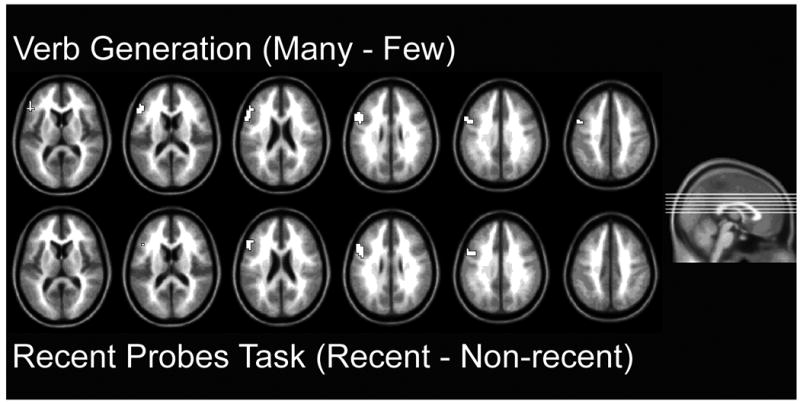
Comparison of activations from recent-probe and verb generation tasks (Group analysis). Activity in left inferior frontal gyrus in the verb generation interference resolution contrast (top row) using a family-wise error of p<.05 with a left inferior frontal gyrus region of interest analysis, and the recent-probe conflict condition (bottom row) at a lower threshold of p=.005, uncorrected (see text for details).
Figure 2.
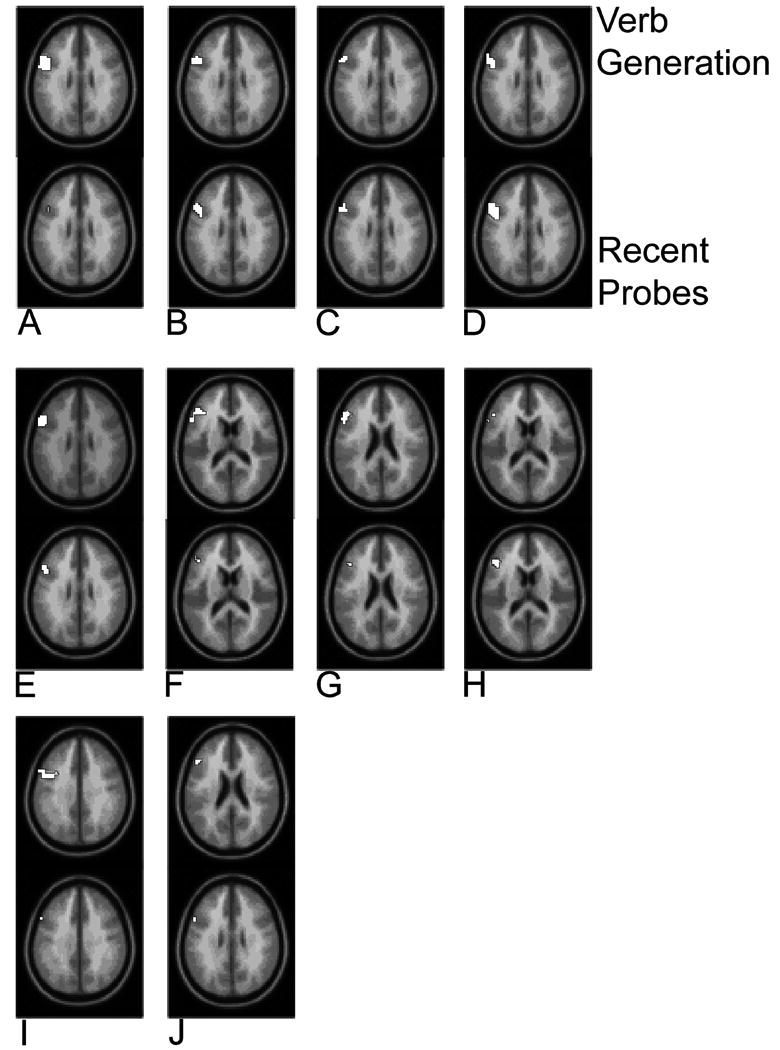
Comparison of activations from recent-probe and verb generation tasks (individual subjects). Activity in left inferior frontal gyrus in the verb generation interference resolution contrast (top row) using a family-wise error of p<.05 with a left inferior frontal gyrus region of interest analysis, and the recent-probe conflict condition (bottom row) at a lower threshold of p=.005, uncorrected (see text for details).
Acknowledgments
We thank Kathryn Welsh for her assistance with this project. This project was supported by grant NIH AG18286 to PARL and grants MH 60655 and a grant from NSF to JJ.
Footnotes
For the verb generation task, a whole brain analysis of the “Many-Few” contrast showed large regions of cortical activation in medial and lateral frontal cortices (with a corrected false-discovery rate [FDR] set at p<.05). The medial frontal activation was a 112-voxel volume with two sub-peaks at x=-7.5, y=18.75, z =40 (T=5.88, left anterior cingulate gyrus, Brodmann's Area 32, all coordinates are MNI coordinates) and 0, 11.25, 50 (T=5.49, medial frontal gyrus, Supplementary Motor Area/BA 6). The lateral frontal activation was a 167-voxel volume in dorso- and ventro-lateral PFC with sub-peaks of activation at -45, 0, 50 (T=6.87, left middle frontal gyrus, lateral BA 6), -52.5, 22.5, 20 (T=6.76, left inferior frontal gyrus, BA45), and -56.25, 11.25, 20 (T=5.97, left inferior frontal gyrus, BA44). In the recent-probes task, no areas of activation were found on a “recent – non-recent” contrast in the whole brain analysis (FDR p<.05).
One reviewer suggested, extrapolating from figure 1, that the identified region in the “recent probes” task may in fact overlap with the inferior frontal sulcus region identified in the Milham, et al., Liu, et al, and Brass and Von Cramon studies referenced in the text. A closer inspection of the data is suggestive but inconclusive. The published peak activations of these areas are outside the area of overlap found in the present results, and either on the extreme edge (Liu, et al) or outside the area of activation found in the recent-probes task, even at an uncorrected statistical threshold of p<.005 and without any region-of-interest mask. However, this sort of cross-study comparison is inherently less reliable and less precise than a direct, within-subject comparison between tasks such as used in the present paper. While the general pattern of findings and our favored hypotheses regarding the cognitive nature of the relevant processes suggests to us that these areas are likely to be anatomically distinct regions, there may well be partial overlap in the areas identified. Given the variability that exists between neuroimaging studies, this question of relatively fine-scale overlap and non-overlap is best addressed by the analytical approach and within-subject analysis utilized in the current paper. The characterization of the process that is shared between the verb generation and recent probes task will be more accurately characterized when the convergence and dissociation with cognitively similar and anatomically proximal processes has been more comprehensively documented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon Y. Selection for cognitive control: A functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24(40):8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Working memory, cognitive control, and the prefrontal cortex: Computational and empirical studies. Cogn Process. 2001;2:25–55. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proc Natl Acad Sci U S A. 2006;103:19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: A single-case study. Cogn Affect Behav Neurosci. 2005;5:1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Josephs O, Dolan NJ. Functional magnetic resonance imaging of proactive interference during spoken cued recall. Neuroimage. 2002;17:543–558. [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neurosci. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neurosci. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA. Inhibition in verbal-working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cogn Affect Behav Neurosci. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam MM. Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Leung HC, Zhang JX. Interference resolution in spatial working memory. Neuroimage. 2004;23:1013–1019. doi: 10.1016/j.neuroimage.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: Response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW. Dissociable brain mechanisms for inhibitory control: effects of interference content and working memory capacity. Cogn Brain Res. 2003;18:28–38. doi: 10.1016/j.cogbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Recency, immediate recognition memory, and reaction time. Cognit Psychol. 1978;10:465–501. [Google Scholar]
- Nee D, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. NeuroImage. 2008;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CYC, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proc Natl Acad Sci U S A. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete Time Signal Processing. Prentice-Hall; Englewood Cliffs, NJ: 1999. [Google Scholar]
- O'Reilly RC, Braver TS, Cohen JD. A biologically-based computational model of working memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; New York: 1999. [Google Scholar]
- Persson J, Reuter-Lorenz PA. Gaining control: Training executive function and far transfer of the ability to resolve interference. Psychological Science. 2008;19:881–888. doi: 10.1111/j.1467-9280.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester CYC, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Persson J, Welsh KM, Jonides J, Reuter-Lorenz PA. Cognitive fatigue of executive processes: Interaction between interference resolution tasks. Neuropsychologia. 2007;45:1571–1579. doi: 10.1016/j.neuropsychologia.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2002;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Brush LN. The neural bases of the effects of item-nonspecific proactive interference in working memory. Cogn Affect Behav Neurosci. 2004;4:379–392. doi: 10.3758/cabn.4.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Brush LN, Nick AM. Prefrontal cortex and the mediation of proactive interference in working memory. Cogn Affect Behav Neurosci. 2004;4:600–608. doi: 10.3758/cabn.4.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D'Espositio M, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wickens CD. Processing resources in attention. In: Parasuraman R, editor. Varieties of Attention. Academic Press; Florida: 1984. [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:141–154. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Leung HC, Johnson MK. Frontal activations associated with accessing and evaluating information in working memory: an fMRI study. Neuroimage. 2003;20:1531–1539. doi: 10.1016/j.neuroimage.2003.07.016. [DOI] [PubMed] [Google Scholar]


