Abstract
Purpose
Sorafenib, a vascular endothelial growth factor receptor (VEGFR)-2 and RAF-kinase inhibitor, commonly causes skin toxicity. We retrospectively analyzed dermatologic toxicity in patients receiving combined anti-angiogenic therapy sorafenib and bevacizumab.
Experimental Design
Castration-resistant prostate cancer and metastatic non-small cell lung cancer patients were accrued to phase II studies, receiving sorafenib400mg BID. A phase I study explored sorafenib 200–400mg BID with bevacizumab 5–10mg/kg every 2 weeks in patients with advanced solid tumors. Probability of development of maximum grade of dermatologic toxicity as a function of the cumulative dose of sorafenib was determined. Additional analyses compared extent of toxicity, pharmacokinetics, and patient risk factors.
Results
Ninety-six patients were enrolled: 54 pts received sorafenib, 42 received bevacizumab/sorafenib. HFSR (hand-foot skin reaction) was observed in 50/96(52%) patients. Grade 2–3 HFSR developed in 16/54(30%) sorafenib patients and 24/42(57%) bevacizumab/sorafenib patients (p=0.012) and was associated with cumulative sorafenib exposure (p=0.0008). 24/42 phase I patients randomized to start with bevacizumab had increased risk of grade 2–3 HFSR than those starting with sorafenib (p=0.013) after adjusting for association between HFSR risk and hypertension (p=0.01), which was the only toxicity associated with HFSR. There was no association between HFSR and baseline history of neuropathy, prior taxane/platinum treatment, or systemic sorafenib levels.
Conclusions
Sorafenib-related HFSR is associated with increasing cumulative sorafenib dose. HFSR is increased in patients treated with bevacizumab/sorafenib combination anti-VEGF therapy, and this finding is not explained by pharmacokinetic interaction between the two agents. Our results suggest that the pathophysiology of HFSR may be related to VEGF inhibition.
Keywords: sorafenib, bevacizumab, hand-foot skin reaction, rash, angiogenesis inhibition
Introduction
Anti-angiogenesis therapy is an increasingly important category in the anticancer armamentarium. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, and sorafenib, a multi-kinase inhibitor that targets Raf-kinase, VEGF receptors (−1, −2, −3), platelet derived growth factor (PDGF) –α and -β, c-KIT, and RET, have been approved for use in various malignancies.5,6 Cutaneous side-effects are recognized adverse effects of many molecularly targeted therapies, including epidermal growth factor receptor (EGFR) inhibitors, and some cytotoxic agents (e.g. 5-fluorouracil and liposomal doxorubicin (1). The development of cutaneous signs may also have clinical implications, as in the case of cetuximab, where presence of rash is predictive of response (2).
Hand-foot skin reaction (HFSR; palmar-plantar dysesthesia; acral erythema) and rash have been described with the use of sorafenib, but have not been shown to be predictive of response to therapy (3, 4). Sorafenib-related non-HFSR skin eruptions include facial/scalp erythema and dysesthesias, alopecia, splinter hemorrhages, keratoacanthomas (5), leukocytoclastic vasculitis (6), and epidermal inclusion cysts. A single pooled analysis of four phase I trials showed an increased incidence of HFSR with higher starting doses of single agent sorafenib (7). However, HFSR incidence in relation to cumulative sorafenib dose has not been studied, nor has there been an exploration of dermatologic adverse events when sorafenib is combined with other targeted therapies. We report a significantly increased incidence of HFSR with combination sorafenib-based anti-VEGFA/EGFR treatment and a relationship between cumulative sorafenib dose and the development of HFSR. In addition, we analyzed multiple patient and treatment-associated risk factors for development of various cutaneous toxicities in sorafenib-treated patients.
Patients And Methods
Patient Eligibility
Three trials performed at the National Cancer Institute were included in this retrospective analysis. Two were phase II sorafenib monotherapy trials in metastatic castration-resistant prostate cancer (CRPC)(8) and non-small cell lung cancer (NSCLC). A phase I trial of the combination of sorafenib and bevacizumab in advanced solid tumors was also analyzed (9). All participants were required to have wellcontrolled blood pressure or cardiac disease, good end organ function, measurable disease, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 (the combination study required an ECOG PS of 0–1) at the time of enrollment, and no active intracranial disease. Patients had not previously received the experimental agent(s). All trials were approved by the Institutional Review Board of the National Cancer Institute and written informed consent was obtained before enrollment.
Treatment Plan and Dose Modifications
Each cycle was 28 days. Sorafenib was administered at 400 mg BID in both sorafenib monotherapy studies. In the combination bevacizumab/sorafenib phase I study patients were treated with intravenous bevacizumab 5–10 mg/kg every 2 weeks and sorafenib 200–400 mg BID daily or days 1–5, according to dose level (Table 1) that consisted of a dose escalation cohort and a second group of patients. The latter was randomized to receive one month of bevacizumab or sorafenib monotherapy, followed by combination therapy for all subsequent cycles; doses administered to this group were dose level 1.
Table 1.
Dose levels for combination sorafenib+bevacizumab trial
| Dose Level | Number of Patients |
Sorafenib | Bevacizumab |
|---|---|---|---|
| 1 | 30 | 200 mg BID | 5 mg/kg q2wk |
| 2 | 6 | 200 mg BID | 10 mg/kg q2wk |
| 3 | Not accrued | 400 BID | 10 mg/kg q2wk |
| 4 | 4 | 200 mg BID 5 days each week |
5 mg/kg q2wk |
| 5A | 1 | 200 mg BID 5 days each week |
10 mg/kg q2wk |
| 5B | 1 | 400 BID 5 days each week |
5 mg/kg q2wk |
Toxicities were graded according to Common Terminology Criteria for Adverse Events (CTCAE v3); HFSR definition based on grade is listed in Table 2. Clinically significant HFSR was categorized as grade 2 and 3 for all analyses as this level of toxicity required modifications in patient management. Each study had a distinct algorithm for drug holiday and reductions (Table 3). The Dermatology Service was consulted for most grade 2 and all grade 3 cases of HFSR as needed.
Table 2.
Hand-foot skin reaction grading
| Grading for hand-foot skin reaction* | |
|---|---|
| Grade 1 | Minimal skin changes or dermatitis (e.g erythema) without pain |
| Grade 2 | Skin changes (e.g peeling, blisters, bleeding, edema) or pain not interfering with function |
| Grade 3 | Ulcerative dermatitis or skin changes with pain interfering with function |
| Grade 4 | None |
Based on NCI Common Terminology Criteria for Adverse Events version 3.0
Table 3.
Parameters for dose modifications of sorafenib*
| Toxicity | Sorafenib (prostate cancer) |
Sorafenib (lung cancer) |
Sorafenib plus bevacizumab |
|---|---|---|---|
| 1st grade 2 AE | No change† | 200 mg reduction |
Hold - no reduction |
| Recurrent grade 2 AE | No change† | 200 mg reduction |
50% reduction |
| Grade 3 AE | 200 mg reduction |
50% reduction |
50% reduction |
| Grade 4 AE | Discontinue | Discontinue** | Discontinue |
AE = Adverse event
Dosing continued as long as patient tolerated treatment
all reductions and holds require temporarily halting sorafenib until AE resolves to grade 1 or less then restarting
grade 4 non-hematologic toxicity
Patient Monitoring and Response Assessment
Pretreatment assessments were made within 1–2 weeks of therapy initiation and included history and physical examination, laboratory studies, EKG, chest radiograph, and noninvasive imaging to determine tumor burden. Patients were examined at least every 4 weeks. Re-assessment imaging was performed every 2 cycles and evaluated by a reference radiologist without knowledge of the patient’s clinical status. Results were characterized using Response Evaluation Criteria In Solid Tumors (RECIST) criteria (10). Prostate-specific antigen (PSA) PSA responses were recorded according to the PSA Working Group definition (11). PSA was used for determination of disease response in patients with prostate cancer for the first 22 patients (8); monthly CA125 levels were measured but not used for clinical decision making for patients with ovarian cancer (9, 12).
Pharmacokinetics
Samples for pharmacokinetic analysis were drawn and evaluated as previously described (13) at baseline, 0.25, 0.5, 1,2,4, 6, 8, 12 and 24 hrs after administration of initial doses of sorafenib in all three trials (8, 9). The PK parameters AUC0–12, Cmax and tmax were calculated using the WinNonlin professional software v5.0. AUC0–12 was calculated for each patient as the area under the curve from time 0 to 12, using the linear trapezoidal method.
Statistical analysis
The data for the single agent sorafenib studies were pooled, as the same dose and general modification scheme was used in both studies. Comparisons were made to combination anti-VEGFA/EGFR therapy. Cumulative sorafenib dose for each cycle was used for the analyses to minimize the variations in sorafenib dosing strategies (i.e. dose reductions, dose levels) between the studies and among patients. The analyses comparing toxicity grades or treatment characteristics among or between the trials or to patient risk factors were performed using categorical or non-parametric tests, including Fisher's exact test, Mehta's modification to Fisher's exact test for generalized r × c tables (14), a Cochran-Armitage test for trend (15), Jonckeere-Terpstra test for trend (16), a Kruskal-Wallis test, or a Wilcoxon rank sum test.The probability ofdevelopment of the maximum grade of toxicity as a function of the cumulative dose of drug administered was determined by the Kaplan-Meier method, with the statistical significance of the difference between curves determined by the log-rank test. Logistic regression analysis for ordered categories was used to assess the relationship between increasing grade of HFSR toxicity and prognostic factors when considered jointly, after verifying that there were proportional odds. All p-values are two-tailed and presented without adjustment for multiple comparisons.
Results
Patient Accrual
Ninety-six patients were accrued to the three studies between September 2004 and August 2007. Forty-one patients were treated on the prostate cancer study, 13 patients on the NSCLC study, and 42 patients on the two-drug combination phase I study. Tumor types and demographics are included in Table 4.
Table 4.
Demographics
| Sorafenib alone in prostate cancer |
Sorafenib alone in lung cancer |
Combination sorafenib and bevacizumab |
|
|---|---|---|---|
| Age | |||
| Range (Median) | 48–88 (66) | 35–84 (64) | 30–76 (58) |
| Sex | |||
| Male | 41 | 8 | 14 |
| Female | 0 | 5 | 28 |
| Patients treated with prior | |||
| Taxanes | |||
| Liposomal doxorubicin | 31 | 11 | 26 |
| 0 | 0 | 12 | |
| Number of prior treatments | 1–8(4) | 1–7(1) | 1–15(6) |
| Tumor type | Prostate 41 | NSCLC 13 | EOC 15 Melanoma 7 Sarcoma 5 Breast 3 Renal 3 Colorectal 2 Others* 7 |
| Cycles administered† | |||
| Range (median) | 1 – 10 (2) | 1 – 7 (3) | 1 – 26+ (median 4) |
| Cumulative sorafenib dose in mg†† | |||
| Range (median) |
4,800 – 200,000 (44,000) |
16,800 – 95,600 (44,800) |
4600 – 174,200 (32,900) |
NSCLC = Non-small cell lung cancer EOC = Epithelial ovarian cancer
Uterine adenocarcinoma (1), endometrial carcinoma (1), thyroid (1), testicular (1), adrenal (1), basal cell carcinoma (1), and cervix (1)
p=0.0002
p = 0.19
Dermatological toxicity
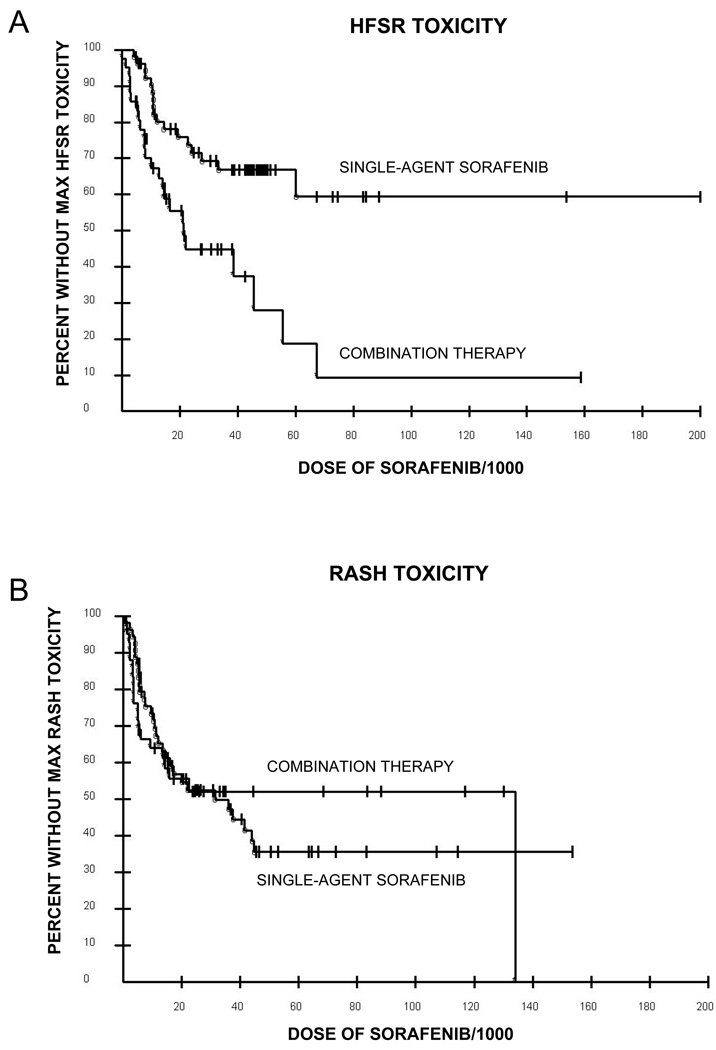
Grade 1–3 HFSR was observed in 50/96 (52%) patients in the three trials. Thirtyone percent of patients (17/54) on the single agent sorafenib studies had grade 1–3 HFSR whereas 79% (33/42) of patients who received combination bevacizumab and sorafenib had HFSR. Clinically important HFSR was defined as grades 2–3 (Figure 1) and was noted in 16/54 (30%) sorafenib alone patients and 24/42 (57%) sorafenib/bevacizumab patients (p=0.012). Once initial diagnosis of HFSR was made, patients had a cyclical waxing/waning course of recurrent grade 2 toxicity for the duration of the treatment (ranges 1 to 30+cycles). Four of 41 (10%) and 4/13 (31%) of patients on the prostate cancer and lung cancer studies were dose reduced for HFSR toxicity, respectively; 9/42 (21%) patients on the combination study were dose reduced for HFSR. Holds in sorafenib for grade 2 HFSR at a median of once every 2 cycles were required in patients after maximal dose reduction to sorafenib 200 mg QD in patients on the combination therapy trial. Management interventions included emollients, cushioning, pyridoxine therapy (maximum dose 800mg daily) and paring of calluses with varying degrees of benefit. Combination therapy resulted in higher grade HFSR (p=0.0006), as well as a lower cumulative sorafenib dose at which the highest grade toxicity was noted (21,117 mg in the combination trial versus not reached in the single agent trial; p=0.0008; Figure 2A).
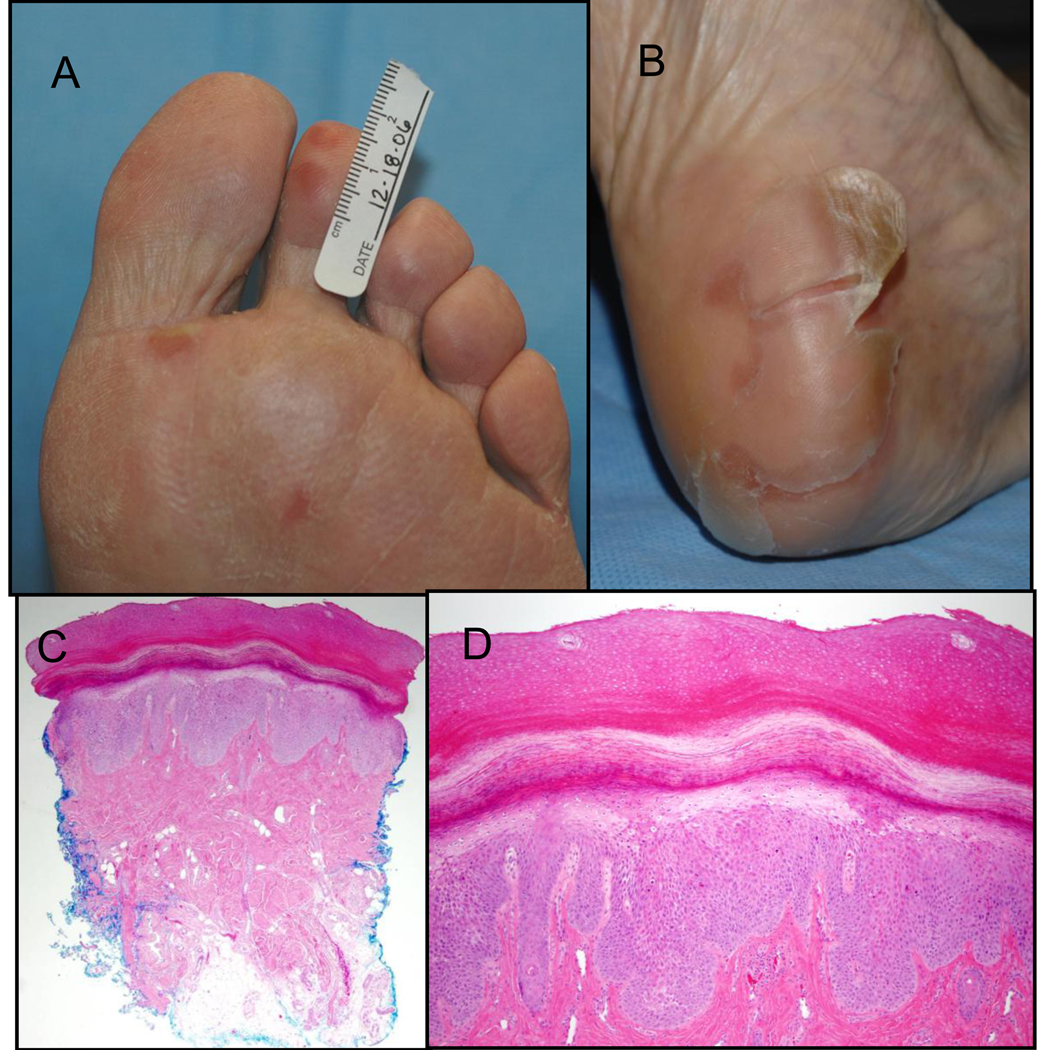
Figure 1.
A) Grade 2 hand-foot skin reaction showing early tender erythematous plaques at pressure points; B) Grade 2 hand-foot skin reaction demonstrating large sheets of desquamating skin overlying a tender erythematous plaque on the heel; C) Histology of an early HFSR lesion from Figure 1A shows epidermal thickening, reactive epithelial changes in the basal layer of the epidermis and in eccrine sweat ducts, mild perivascular infiltrate, and mild vascular dilatation (40X); D) Higher magnification of HFSR histology from Figure 1A (100X)
Figure 2.
A) Kaplan-Meier curves for the development of HFSR toxicity with single agent sorafenib (°) versus combination therapy (*), p=0.0008. Median dose of sorafenib to noted HFSR toxicity for combination therapy was 21,117 mg versus not reached for single agent. B) Kaplan-Meier curves for the development of rash with single agent sorafenib (°) versus combination therapy (*), p=0.99. Median dose of sorafenib to noted rash for combination therapy was 26,870 mg versus 31000 mg for single agent.
Skin lesions other than HFSR were observed in 52% of patients. Several types of rash were observed including diffuse maculopapular eruptions, facial and scalp erythema, keratoacanthomas, and epidermal inclusion cysts were seen. The variety and grade distributions of these skin findings were similar in all three studies, affecting 30/54 (56%) patients on sorafenib alone treatment and 20/42 (48%) patients treated with a combination of bevacizumab and sorafenib. The dose of sorafenib at which the rashes were noted did not differ between the two groups (Figure 2B). The frequency of grade 2–3 non-HFSR skin reactions did not differ between single agent (10/54; 19%) and combination therapy (6/42; 14%).
Risk factors for HFSR
Patient data were analyzed from the prostate cancer single-agent sorafenib study (41 patients) and the sorafenib/bevacizumab study (42 patients) to identify possible risk factors for HFSR. There was no association between HFSR and prior taxane exposure, age, baseline neuropathy, or development of rash while on study drugs in either study (Table 4). No association of HFSR and prior platinum or liposomal doxorubicin treatment was seen in the combination sorafenib/bevacizumab trial. Analysis of the association between HFSR and the other most common sorafenib toxicities (fatigue, diarrhea, hypertension) on the combination study revealed that only hypertension was associated with the development of HFSR, with 7/13 (54%) patients without HTN developing HFSR, compared with 26/29 who developed or worsened pre-existing HTN (90%; p=0.02). Both cumulative sorafenib or bevacizumab dose were associated with increasing grade of HFSR toxicity (p=0.009 and p<0.0001, respectively) in the cohort of patients randomized to one cycle of single drug therapy. In the same cohort of patients, those who received bevacizumab first had a greater risk of being associated with increasing grade of HFSR compared to those who received sorafenib first (p=0.013; odds ratio 5.85, 95% confidence interval: 1.44–23.8) after adjusting for development of hypertension on study, which also was associated with an increased risk of HFSR (p=0.01; odds ratio =6.13; 95% confidence interval: 1.54–24.31). There were inadequate data to assess the relationship between clinical response and HFSR due to the paucity of responses (10 partial responses overall by RECIST; 7 in the combination therapy group, 1 in the single-agent prostate group, 2 in the single-agent NSCLC group).
Pharmacokinetics
The dose-normalized AUC0–12 and Cmax were highly correlated (r=0.94) and AUC0–12 was used for subsequent analyses. There was no statistically significant association between HFSR or dry skin and sorafenib exposure as measured by AUC0–12 in the pooled analysis of the three studies. However, an association was found between increasing rash grade and increasing AUC0–12 (p=0.02). We have previously reported that there was no significant change in PK measurements with combination sorafenib and bevacizumab therapy compared to single agent sorafenib (9).
Discussion
Single agent sorafenib therapy at standard doses of 400 mg twice daily has been shown to be well-tolerated, with a total incidence of HFSR in approximately 25–30% of patients (7, 17); the use of bevacizumab alone has not been associated with development of HFSR. The approval of sorafenib for renal cell carcinoma and hepatocellular carcinoma and its use in combination therapies for other cancers, make it important to identify the mechanisms and predictors of the development of sorafenib-associated HFSR. We hypothesized that combination therapy targeting the VEGF pathway might result in augmentation of sorafenib HFSR. Comparison of patients receiving single agent sorafenib versus reduced dose sorafenib in combination with bevacizumab confirmed that hypothesis. Expected potential risk factors such as prior taxane exposure, pre-existing peripheral neuropathy, or development of rash were not associated with HFSR. However, an increase in the incidence and severity of HFSR was demonstrated with combination sorafenib/bevacizumab therapy compared to single agent sorafenib at any given cumulative dose of sorafenib (Figure 2), suggesting that bevacizumab’s anti-VEGF effects potentiates sorafenib-related HFSR symptoms.
Sorafenib is associated with a variety of different dermatologic side effects, including facial/scalp erythema and dysesthesias, alopecia, splinter hemorrhages, keratoacanthomas (5), leukocytoclastic vasculitis (6), and epidermal inclusion cysts. HFSR is the sorafenib dermatologic toxicity with the greatest frequency and the greatest morbidity. HFSR associated with sorafenib therapy affects friction and weight-bearing acral surfaces more focally than the classic hand-foot syndrome that has been reported with traditional chemotherapeutic agents such as cytarabine, fluorouracil, and methotrexate (Table 2) (18, 19).
Previous studies have reported a dose-dependent relationship between the starting dose of sorafenib and the incidence of HFSR. Our study adds to this data by demonstrating the increased incidence of HFSR with increasing cumulative sorafenib dose. This was observed both in our single agent sorafenib studies, as well as in the combination sorafenib/bevacizumab study. The association with cumulative dose cautions providers to maintain a high index of suspicion for HFSR the longer patients receive sorafenib. The impact of dose on the development of HFSR is not welldelineated, but based on clinical experience, dose-reduction and discontinuation of sorafenib reduces the severity of HFSR. Although no other association was found between risk of HFSR and number and type of prior therapies including exposure to pegylated liposomal doxorubicin, previous toxicities, baseline neuropathy, or dermatologic toxicity from prior treatment, an additional confounder that was not evaluated may have contributed to our findings. Development of HFSR was not demonstrated to be a harbinger of clinical benefit as suggested by rash in response to EGFR inhibitors (2).
There are no data to date addressing the role of the VEGF pathway in the pathophysiology of HFSR. However, several findings in this study suggest that the VEGF pathway is important in the causation of HFSR: 1) the frequency and severity of HFSR was increased when sorafenib is paired with bevacizumab; 2) the predilection of HFSR for traumatic foci suggests that VEGF inhibition may retard tissue repair from minor trauma; 3) the increased incidence of HFSR in patients randomized to initiate treatment with one cycle of single agent bevacizumab prior to combination therapy illustrates the potentiating effects of bevacizumab on sorafenib-related HFSR; 4) the association of HFSR and the development of or worsened pre-existing hypertension suggests a vascular effect; and 5) the cumulative bevacizumab dose was also directly associated with incidence of HFSR (P<0.0001), further illustrating the impact of the antiVEGF property of bevacizumab on this sorafenib-associated toxicity. Apart from this study, it is known that bevacizumab treatment results in poor wound healing (20).
Sorafenib causes an array of non-HFSR dermatologic toxicities (21, 22). Approximately half of the patients in the three studies developed skin rash, with no variance between the studies. Why this incidence was higher than previously reported (18–40%) is unclear; our patients had a high frequency of dermatologic consultations, and thus had a higher number of recognized rashes (7, 17). Several of the dermatologic findings (de novo keratosis pilaris, epidermal inclusion cysts, and keratoacanthomas) are characterized by keratinocyte proliferation and focal apoptosis histologically. The MAPK, MSK1, and VEGF pathways play important role in normal keratinocyte function and inhibition of these pathways by sorafenib may result in the toxicity observed (23). This hypothesis should be explored in future studies.
Development of non-HFSR skin toxicities was associated with circulating sorafenib concentration. This suggests that rash may herald higher circulating concentration and thus higher sorafenib concentration in skin. Preclinical sorafenib organ distribution studies demonstrated that the half-life of sorafenib in skin is longer (72.8 hrs) than in other organs (20–36 hrs).7 Other hypotheses regarding the etiology of sorafenib-associated HFSR have been posited. These include 1) accumulation of potentially toxic local concentrations in eccrine sweat glands that present in greatest number or density in the palms and soles; 2) damaged vascular integrity due to sorafenib’s dual VEGFR-2 and PDGF-β inhibition; and 3) keratinocyte injury from sorafenib inhibition of c-kit or RAF-kinase (24–26). The histology of skin biopsies of early sorafenib-related HFSR lesions demonstrated focal epithelial damage with dyskeratotic keratinocytes, reactive epithelial changes in the basal layer of the epidermis and in eccrine sweat ducts, and lack of obvious vascular damage (Figure 1C).
In summary, sorafenib-related dermatologic manifestations are varied. HFSR and rash are the most common dermatologic toxicities associated with sorafenib, and their etiology remains uncertain. We report a direct association between cumulative sorafenib and bevacizumab doses and incidence of HFSR as well as increased HFSR in patients treated with combination anti-VEGF/VEGFR therapy. Our results suggest that sorafenib’s inhibition of the VEGF pathway may be an important factor in HFSR pathogenesis.
Acknowledgements
This work was supported by the Intramural Research Program of the National Cancer Institute. We would like to thank our data managers Ms. C. Graves and S. Tiwari for their support, Dr. C. R. Lee for providing histology images, the research nurses and fellows in their care of our patients, and our patients.
Footnotes
Authors’ Disclosures: The authors indicated no potential conflicts of interest.
STATEMENT OF TRANSLATIONAL RELEVANCE
Sorafenib inhibits xmultiple kinases including VEGFR2. Hand-foot skin reaction (HFSR) is currently emerging as a major toxicity of sorafenib treatment requiring clinical management and dose modifications, though the mechanism underlying HFSR is not clearly understood. The dose level of sorafenib as a single agent has been associated with the development of HFSR, but the relationship between cumulative dose of sorafenib and development of HFSR has not been explored. We report the first correlation between cumulative sorafenib dose and HFSR for both single agent sorafenib and combined anti-VEGF therapy. In addition, this is the first study to examine dermatologic toxicities of combination anti-VEGF therapies involving sorafenib and bevacizumab. We find that the frequency of adverse events is greater with combination anti-VEGF therapy than with sorafenib alone. This study supports the hypothesis that the anti-VEGF properties of sorafenib may cause HFSR. This finding has important clinical relevance regarding monitoring and treatment of patients on sorafenib and other anti-VEGF therapy.
REFERENCES
- 1.Lokich JJ, Moore C. Chemotherapy-associated palmar-plantar erythrodysesthesia syndrome. Ann Intern Med. 1984;101:798–799. doi: 10.7326/0003-4819-101-6-798. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 3.Lai SE, Kuzel T, Lacouture ME. Hand-foot and stump syndrome to sorafenib. J Clin Oncol. 2007;25:341–343. doi: 10.1200/JCO.2006.08.9565. [DOI] [PubMed] [Google Scholar]
- 4.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 5.Kong HH, Cowen EW, Azad NS, Dahut W, Gutierrez M, Turner ML. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–172. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung NM, Gutierrez M, Turner ML. Leukocytoclastic vasculitis masquerading as handfoot syndrome in a patient treated with sorafenib. Arch Dermatol. 2006;142:1510–1511. doi: 10.1001/archderm.142.11.1510. [DOI] [PubMed] [Google Scholar]
- 7.Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43–9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer. 2006;42:548–556. doi: 10.1016/j.ejca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Dahut WL, Scripture C, Posadas E, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 9.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 12.Azad NS, Annunziata CM, Steinberg SM, et al. Lack of reliability of CA125 response criteria with anti-VEGF molecularly targeted therapy. Cancer. 2008 doi: 10.1002/cncr.23374. [DOI] [PubMed] [Google Scholar]
- 13.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal. 2008;46:362–367. doi: 10.1016/j.jpba.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta CR, Patel NR. A network algorithm for performing Fisher's exact test in r x c contigency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. New York: John Wiley and Sons, Inc; 1990. pp. 79–129. [Google Scholar]
- 16.Hollander M, Wolfe DA. Non-parametric Statistical Methods. 2nd edition. New York, NY: John Wiley & Sons, Inc; 1999. pp. 189–269. [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Crider MK, Jansen J, Norins AL, McHale MS. Chemotherapy-induced acral erythema in patients receiving bone marrow transplantation. Arch Dermatol. 1986;122:1023–1027. [PubMed] [Google Scholar]
- 19.Valks R, Fraga J, Porras-Luque J, Figuera A, Garcia-Diez A, Fernandez-Herrera J. Chemotherapy-induced eccrine squamous syringometaplasia. A distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133:873–878. doi: 10.1001/archderm.133.7.873. [DOI] [PubMed] [Google Scholar]
- 20.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 21.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 23.Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora's vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sumtimb. Clin Exp Med. 2007;7:127–134. doi: 10.1007/s10238-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 24.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 25.Tsai KY, Yang CH, Kuo TT, Hong HS, Chang JW. Hand-foot syndrome and seborrheic dermatitis-like rash induced by sunitinib in a patient with advanced renal cell carcinoma. J Clin Oncol. 2006;24:5786–5788. doi: 10.1200/JCO.2006.08.6868. [DOI] [PubMed] [Google Scholar]
- 26.Morita E, Lee DG, Sugiyama M, Yamamoto S. Expression of c-kit ligand in human keratinocytes. Arch Dermatol Res. 1994;286:273–277. doi: 10.1007/BF00387600. [DOI] [PubMed] [Google Scholar]