Abstract
The question of whether skeletal muscle fatigue is preserved or enhanced in older adults is a point of controversy. Disparate findings may be attributed to differences in subject population and study protocols, including contraction mode. The purpose of this study was to test the hypotheses that healthy older (65–80 years, n = 8M, 8F) adults who were matched to young adults (21–35; 8M, 8F) with similar physical activity levels would: 1) fatigue less during isometric knee extensor (KE) contractions, but 2) would show similar fatigue during dynamic KE contractions performed at 120 deg·s−1. Fatigue was induced with 4 min of intermittent, isometric or dynamic maximal voluntary contractions, performed on separate days. Electrically-stimulated contractions were used to evaluate central activation during both fatigue protocols. Older subjects maintained a higher percentage of baseline MVC torque than young subjects during isometric contractions (mean±SE of 71±3% and 57±3%, respectively, p<0.01). In contrast, there was no difference between age groups in torque maintenance during dynamic contractions (43±3% and 44±3%, respectively, p=0.86). For both groups, changes in electrically-stimulated and voluntary contractions followed similar trends, suggesting that central activation did not play a role in the age-related differences in fatigue. Fatigue during the isometric protocol was associated with fatigue during the dynamic protocol in the young group only (r=0.62, p=0.01), suggesting that distinct mechanisms influence fatigue during isometric and dynamic contractions in older adults.
Keywords: dynamic contraction, isometric contraction, central activation, aging, torque
INTRODUCTION
The decline in many aspects of skeletal muscle function that occur in old age is well documented. Maximum force-generating capacity is reduced in old compared to young adults at the whole muscle and single fiber levels 12,13,21. Lower contractile velocity 24,45 and power production 3,29 have also been observed in old muscle. Age-related changes in skeletal muscle fatigue, defined as the acute decline in force-producing capacity in response to repeated or sustained contractions, are less well understood.
An emerging body of literature suggests that the ability to resist muscle fatigue may increase with old age. While the mechanisms of this age-related fatigue resistance are not known, they could be due in part to morphological changes. In humans, old age generally is associated with a shift to a greater proportion of type I muscle fibers,32 apparently as a result of preferential atrophy or denervation of type II muscle fibers. The loss of motor neurons that innervate type II fibers is thought to be partially compensated by reinnervation of some fibers by nearby motor neurons associated with type I motor units.14,30 Thus, the shift toward a relatively greater type I fiber population could contribute to enhanced fatigue resistance in older muscle 31,43,44.
While many studies support the notion of age-related fatigue resistance, 8–10,17,23,28 other investigators report unchanged or diminished fatigue resistance in the elderly. 5,26,33 Disparate results in studies of age-related fatigue resistance may be due to a variety of causes, including variations in study population 26, muscle group 6 or the tasks used to induce fatigue 18,19,48. In particular, it has been suggested that the performance of dynamic contractions by older adults may suffer as a result of their slower contractile speeds. Indeed, knee extensor contraction velocity declined to a greater extent in older compared to young adults during a brief series of dynamic contractions 37, a result that may be explained in part by the leftward shift in the torque-velocity relationship in this population 29. Thus, while it is reasonable to expect that an age-related slowing of contraction speed could have a greater impact during dynamic contractions than during isometric contractions, this hypothesis has not been fully tested, particularly in the knee extensor muscles. In addition to changes in the torque-velocity relationship in older muscle, central activation failure may play a greater role in fatigue in older than young individuals, as has been reported during isometric contractions of the knee extensor muscles 41. Overall, the results to date suggest that both central and peripheral factors may be mechanisms by which age-related differences in fatigue resistance vary by contraction mode.
Given the muscle weakness that accompanies old age, any advantage in terms of an ability to resist fatigue has tremendous implications for an aging population that is facing mobility impairments. For example, an ability to prevent the fall of force during repetitive, dynamic contractions could partially offset baseline muscle weakness and allow an older person to remain above the minimum level of force needed to continue an activity. Thus, fatigue resistance would have a significant impact on functional mobility 38. In addition, identifying the extent and mechanisms of fatigue during isometric and dynamic contractions will provide insight for the design of interventions intended to maintain mobility and independence in the elderly. The aim of this study was to quantify muscle fatigue during intermittent maximal dynamic and isometric contractions of the knee extensors in young and older men and women. Age groups were matched for similar health and habitual physical activity levels. We hypothesized that: 1) older adults would fatigue less than young adults during repeated maximum voluntary isometric contractions (MVIC), but that 2) this age-related fatigue resistance would be abolished during repeated maximum voluntary dynamic contractions (MVDC), and young and older groups would fatigue to a similar extent. We anticipated that this contraction-mode dependence of fatigue would be a result of slower contraction speeds and greater central activation failure in older subjects during the dynamic fatigue protocol.
MATERIALS AND METHODS
Subjects
Sixteen young (21–35 years; 8 female) and 16 older (65–80 years; 8 female) individuals were recruited for the present study. Participants were healthy by self report. They were not taking any medications apart from low-dose aspirin and/or oral contraceptives. All participants were relatively sedentary (structured exercise < 20 minutes, twice per week), and they were excluded if they had cardiovascular disease, diabetes, stroke or any neurological impairment. Subjects were excluded if circulatory impairment was evident by an ankle-brachial index < 1 (systolic ankle pressure / systolic brachial pressure) as an indication of latent peripheral vascular disease 35. All older subjects supplied their physician’s written approval prior to participation. Subjects read and signed an informed consent document that was approved by the Human Subjects Review Board at the University of Massachusetts, Amherst. Experimental procedures were designed in accordance with the ethical principles defined in the Declaration of Helsinki for protection of human subjects.
Participants reported to the laboratory on 3 separate days, each no less than 5 days apart. On the first day, they were familiarized with equipment and study procedures, issued an accelerometer (ActiGraph, Pensacola, FL) for measuring physical activity level and instructed on its proper use. The second and third days were devoted to dynamic and isometric fatigue testing, the order of which was randomized.
Physical Activity
All participants wore a uni-axial accelerometer for at least 6 days to quantify habitual activity level. Accelerometers were worn during all waking hours, and subjects kept a physical activity diary in which they recorded sleep/wake cycles and activity behaviors. The sum of minute-by-minute acceleration data was used to quantify activity for each day, and it was expressed as average acceleration (counts) per day·1000−1. To characterize each subject’s overall physical activity, an average was taken for 5 days in which monitor use was consistent with instruction. Compliance was verified via subject interview and cross-examination with the physical activity diary.
Torque Measures
A Biodex System 3 dynamometer (Biodex Medical, Shirley, NY) was used to measure concentric and isometric knee extension torque. Analog signals corresponding to torque were sampled and digitized using custom Labview software (National Instruments, Austin TX). These signals were sampled at 2500 Hz during baseline measurements and 1000 Hz during the fatigue protocols. Subjects were seated in the dynamometer with the shank, hips and shoulders securely strapped. The left leg was tested in all individuals with the exception of two older women, who reported mild discomfort in that leg. As a result, their right legs were tested. A resting knee angle of 105° was used in each contraction mode (180° = full extension), and hips were flexed at 90°. Biodex hardware was slightly modified during isometric testing. Force was measured via a transducer mounted in line with the point of force application to the lever arm. A tare load was placed on the lever using a spring aligned with the transducer. This reduced compliance in the lever arm of the dynamometer and yielded an improved signal-to-noise ratio when compared to the standard Biodex arrangement.
Prior to muscle strength measurements, subjects performed 5 minutes of light cycling exercise on a recumbent stationary cycle ergometer (Schwinn, Nautilus Inc, Vancouver WA) followed by stretches of the knee flexor muscle group. Stretching of the knee flexors was used to minimize inhibition of knee extensor torque from tight knee flexor muscles. During all measures, subjects received visual feedback of force production from a custom-built LED box. To assess baseline isometric torque, 3–5 MVICs were performed, each separated by at least one minute of rest, until the subject had performed 3 contractions within 10% of each other.
Following isometric strength measures, a series of 3 concentric MVDCs was performed. Angular velocity during extension was restricted to 120 deg·s−1, and contractions were performed through a 60° range of motion. An extension velocity of 120 deg·s−1 was selected, because it is relatively rapid but still reliably attainable by both young and older subjects in the unfatigued knee extensor muscles.29 For this measure, MVDCs were cued every 3 s, and the greatest peak torque produced during any one of these 3 contractions was recorded as peak MVDC. Multiple contractions were performed to account for the increase in torque that can occur during repeated dynamic contractions 4,15.
Following baseline torque measures, one of two fatiguing protocols was initiated. The isometric contraction protocol consisted of 4 minutes of intermittent MVICs lasting 5 s with a 5 s rest between contractions (50% duty cycle). Torque produced in these contractions was quantified as a time tension integral (TTI, Nm·s), which was calculated as the average of all torque values produced for the duration of the contraction multiplied by the duration of the contraction. Fatigue was defined as the average TTI for the final 2 contractions (MVICend) divided by an average of the highest TTI from the baseline measures and the highest MVIC torque achieved during performance of the fatigue protocol (MVICbaseline) expressed as a percent [(MVICend /MVICbaseline)·100]. Quantifying fatigue in this manner minimized the contribution of between-contraction variability.
The dynamic contraction protocol was also performed for 4 minutes and consisted of MVDCs performed every 2 s. Because knee extension velocity was constrained to 120 deg·s−1, and range of motion was limited to 60 deg, the time to full extension was ~0.5 s. The resulting contraction (0.5 s), and the time between contractions (2 s) resulted in a 25% duty cycle. Passive flexion for this protocol was limited to 500 deg·s−1. Subjects practiced dynamic contractions at 120 deg·s−1 at sub-maximal intensities until they could demonstrate reliable coordination of contraction and relaxation within the 2 s interval. Although it was difficult to assess whether additional flexion torque was produced by the subject during the passive return to the starting position following a maximal knee extension, the relatively high angular velocity used in this phase (500 deg·s−1) ensured that any torque produced was minimal. All subjects, both young and old, reliably demonstrated the ability to produce the contractions within the allotted time frame and experienced similar duty cycles. Several minutes passed between this practice and initiation of the fatigue protocol. Dynamic contractions were quantified via peak torque. Following similar logic used in assessment of the isometric protocol, dynamic fatigue was defined as the average of peak torque produced in the final 5 contractions divided by an average of the highest baseline torque and the highest torque achieved during performance of the dynamic fatigue protocol.
Electrical Stimulation
Electrical stimulation was used to assess tetanic torque production and rates of torque development and relaxation. The quadriceps muscle group was stimulated using a pair of 3”×5”, self adhesive electrodes (FastStart, Vision Quest Industries, Irvine, CA). The cathode was placed transversely, ~5 cm distal to the inguinal crease, and the anode was placed ~3 cm proximal to the proximal border of the patella. Electrical stimulation was delivered via a constant current stimulator (Digitimer DS7A, Hertforshire UK). Isometric, tetanic contractions (200 µs, 50 Hz, 480 ms) were initiated at low current (2.0 milliamps) in all subjects ~ 5 min after determination of baseline MVIC. The current was progressively increased until isometric tetanic torque (TT) was equivalent to 50% of baseline MVIC, which is a level that minimizes discomfort but still elicits a substantial force production. Once the appropriate current was established for each subject, it was used to produce either isometric or dynamic TT before, every 30 s during, and immediately after the isometric and dynamic protocols, respectively. The contraction mode for TT measurement matched the contraction mode of the fatigue protocol being performed, i.e., TT was measured dynamically during the MVDC protocol and isometrically during the MVIC protocol. Because the rapid pace of the dynamic fatigue protocol did not allow enough time to measure TT between successive contractions, the dynamic TT measure was substituted for every 15th MVDC. The time between contractions in the isometric protocol (5 s) was sufficient to accurately measure isometric TT without interruption of voluntary contractions.
The maximum rate of torque development (RTD) and the half-time (T1/2) of torque relaxation were determined from the isometric TT. For each TT, RTD was measured as the maximum rate achieved during torque development and expressed as % peak torque•ms−1. The half-time of torque relaxation (ms) was taken as the time from the last stimulus in the tetanic train to the time at which torque had decreased by half. Time points for stimulation were determined from electromyographic (EMG) recordings acquired concurrently with torque.
Voluntary activation of the quadriceps was assessed using the central activation ratio (CAR) measure, in which a 50 Hz, 480 ms stimulation was superimposed when torque had plateaued during a 3–5 s MVIC. The point of torque plateau was determined by visual inspection of the real-time torque tracing. Additional torque produced during the superimposed stimulation was considered to indicate incomplete central activation and was quantified by dividing the greatest voluntary torque obtained prior to stimulation by the greatest torque obtained during the period of superimposed stimulation 22. Comparing the relative decline in MVC (dynamic and isometric) and TT during each protocol provided an additional means of quantifying central fatigue. Greater declines in MVC torque than TT indicate central activation failure 7 and were quantified by subtracting end-exercise TT (% initial) from MVICend (% initial). This measure is noted as TT-MVC.
Statistics
Subject characteristics (mass, height, physical activity, baseline MVIC, MVDC, T½), and measures of fatigue were compared between young and old using 2-factor (age, sex) ANOVA. Due to the skewed distribution (assessed by Kolmogorov Smirnov test) for baseline CAR, differences between groups were assessed using Mann-Whitney U tests. Two-factor (age, sex) ANOVA was used to detect changes in CAR (CARbaseline-CARend) and TTend-MVCend during each protocol. With the exception of baseline MVIC and MVDC, which are presented separately by sex, there were no age-by-sex interactions for any measures so all other data are reported by age group. Linear regression analyses were used to examine the associations between: 1) baseline isometric and dynamic torque, 2) fatigue during the isometric and dynamic protocols, and 3) baseline torque and fatigue. Data are presented as means ± SE; precise p values and 95% confidence intervals for the difference in means are reported, as appropriate. A value of p ≤ 0.05 was considered to indicate statistical significance.
RESULTS
Subject Characteristics
The average age of each group was 26.1 ± 0.9 and 70.9 ± 1.1 years for young and old, respectively. Young and older groups had similar mass (79.4 ± 4.8 and 70.4 ± 2.4 kg, respectively; p = 0.09) and height (1.70 ± 0.02 and 1.71 ± 0.02 m, respectively; p = 0.68), although body mass index (BMI) was higher in young than in old (27.4 ± 1.4 and 24.1 ± 0.6 kg·m−2, respectively; p = 0.04). Habitual physical activity levels were similar across groups (212.0 ± 18.6 and 200.1 ± 17.3 counts per day·1000−1, Y and O respectively; p = 0.60).
Torque and Fatigue
Baseline muscle characteristics are summarized in Table 1. As expected, older subjects produced significantly less torque during MVDC and MVIC than young. Of note, the difference in torque between age groups was more apparent for the dynamic contraction than for the isometric contraction (Figure 1), with the result that the ratio of MVDC:MVIC was greater in young than old (0.82 ± 0.03 and 0.68 ± 0.01, respectively, p < 0.01). Baseline isometric and dynamic torques were well-correlated in both young (r = 0.86, p < 0.001;) and older (r = 0.90, p < 0.001) groups. As intended, TT was ~50% of MVIC and MVDC for evoked isometric and dynamic contractions, respectively, for both groups. Isometric TT was 51.2% and 50.5% of MVIC for young and old, respectively. Dynamic TT was 47.7% and 49.4% of MVDC for young and old, respectively. Older subjects had similar rates of torque development (% peak·ms−1) compared to young during isometric TT. In contrast, there was a tendency for slowed force relaxation in the older group when compared with the young group at baseline (p = 0.08).
Table 1. Baseline muscle characteristics.
Baseline maximum voluntary isometric (MVIC) and dynamic (MVDC) torques, stimulated isometric and dynamic tetanic torques (TT), rate of torque development (RTD) during isometric TT, and the half-time for isometric torque relaxation (T1/2) are provided. Older subjects produced less torque than young, had similar RTD (%·ms−1), but tended to have slower torque relaxation. Stimulated TT was close to the target of 50% MVC for both protocols. Y, young; O, old; CI, confidence interval for difference in means. Values are mean ± SE.
| Y | O | 95% CI | P | |||
|---|---|---|---|---|---|---|
| MVIC (Nm) | 184.5 ± 14.4 | 136.6 ± 10.3 | −84.6, −11.2 | 0.01 | ||
| ♂ | ♀ | ♂ | ♀ | |||
| 224.5 ± 18.7 | 144.4 ± 9.2 | 162.0 ± 16.3 | 114.4 ± 7.1 | <0.01 | ||
| MVDC (Nm) | 147.5 ± 9.8 | 93.2 ± 7.6 | −79.7, −28.9 | < 0.01 | ||
| ♂ | ♀ | ♂ | ♀ | |||
| 175.2 ± 10.5 | 119.7 ± 9.1 | 114.2 ± 10.3 | 72.1 ± 4.1 | < 0.01 | ||
| Isometric TT (Nm) | 93.6 ± 6.9 | 69.1 ± 5.8 | −43.1, −5.8 | 0.01 | ||
| Isovelocity TT (Nm) | 69.3 ± 4.8 | 46.2 ± 4.6 | −36.9, −9.4 | < 0.01 | ||
| RTD (%peak·ms−1) | 0.77 ± 0.04 | 0.82 ± 0.03 | −0.05, 0.17 | 0.28 | ||
| T1/2 (ms) | 115 ± 5 | 127 ± 4 | −2, 27 | 0.08 | ||
Figure 1. Isometric and dynamic torque in young and old.
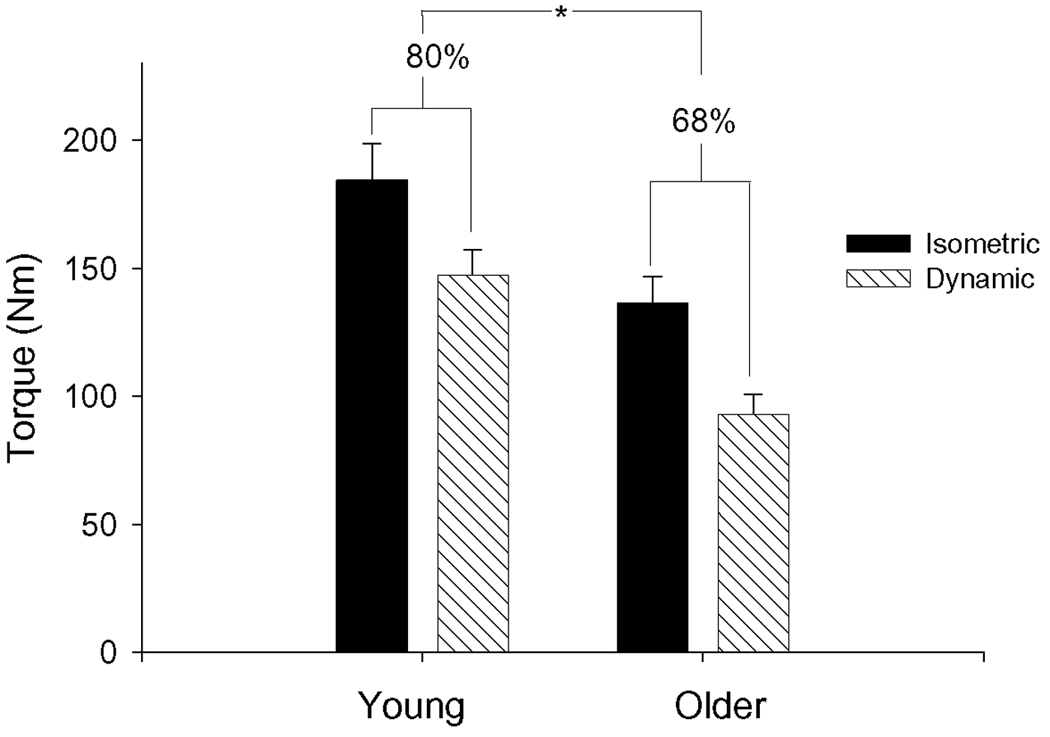
Baseline torque (Nm) produced by young and older groups for isometric (filled) and isovelocity (hatched) contractions at 120 deg•s−1. Young produced more torque than old for both contraction modes. Both groups produced less torque during the dynamic contraction than during the isometric contraction. The ratio of torque produced during isometric contractions to that produced during dynamic contractions was greater for young than old (see text).
The torque traces from the isometric and dynamic contraction protocols for one 73-year old male are shown in Figure 2. Group means for the voluntary contraction protocols are provided in Table 2. The older group (n = 15) fatigued less than the young group (n = 16) during the isometric fatigue protocol (p < 0.01, Figure 3A), and isometric TT declined less in old than young (p = 0.05, Figure 3B). Isometric fatigue from one older man is absent as he did not complete the study.
Figure 2. Representative torque traces for isometric and dynamic fatigue protocols.
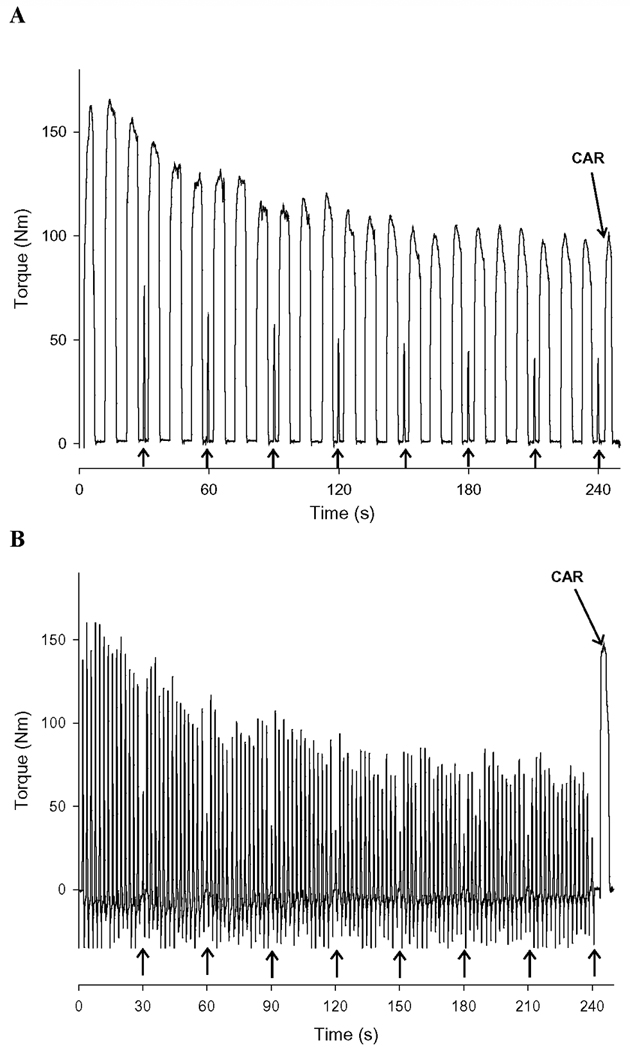
Sample torque traces from one older male subject during the isometric (A) and dynamic (B) fatigue protocols. Arrows at the bottom of the x-axis represent time points at which electrically-stimulated contractions (TT) were applied. Each protocol concluded with the assessment of the isometric central activation ratio (CAR). Resolution in the figure is 100 Hz, but data were acquired and analyzed at 1000 Hz.
Table 2. Fatigue and central activation.
Changes in voluntary and stimulated torque, expressed relative to baseline, in response to isometric and dynamic fatigue protocols. Older subjects fatigued less than young during the isometric protocol (p < 0.01), but both groups fatigued to a similar level during the dynamic protocol (p = 0.86). There were no differences between groups in the change in CAR or the difference in stimulated and voluntary fatigue (TTend-MVCend) for either protocol. CI, confidence interval for difference in means. Values are mean ± SE for young (Y) and older (O) groups.
| Isometric Protocol | Y | O | 95% CI | P |
|---|---|---|---|---|
| MVICend/MVICbaseline | 0.57 ± 0.03 | 0.71 ± 0.03 | 0.03, 0.21 | < 0.01 |
| TTend/TTbaseline | 0.53 ± 0.03 | 0.62 ± 0.03 | −0.004, 0.18 | 0.05 |
| Baseline CAR | 0.98 ± 0.01 | 1.00 ± 0.00 | na | 0.03 |
| TTend - MVCend | −0.04 ± 0.03 | −0.06 ± 0.03 | −11.7, 6.3 | 0.54 |
| ΔCAR(baseline-end) | 0.03 ± 0.02 | 0.01 ± 0.01 | −0.06, 0.03 | 0.41 |
|
Dynamic Protocol | ||||
| MVICend/MVICbaseline | 0.43 ± 0.03 | 0.44 ± 0.03 | −0.08, 0.10 | 0.86 |
| TTend/TTbaseline | 0.51 ± 0.02 | 0.54 ± 0.03 | −0.04, 0.11 | 0.40 |
| Baseline CAR | 0.98 ± 0.01 | 0.99 ± 0.002 | na | 0.38 |
| TTend - MVCend | 0.08 ± 0.03 | 0.09 ± 0.05 | −0.11, 0.13 | 0.82 |
| ΔCAR(baseline-end) | 0.00 ± 0.01 | 0.02 ± 0.01 | −0.01, 0.04 | 0.27 |
Figure 3. Isometric fatigue protocol.
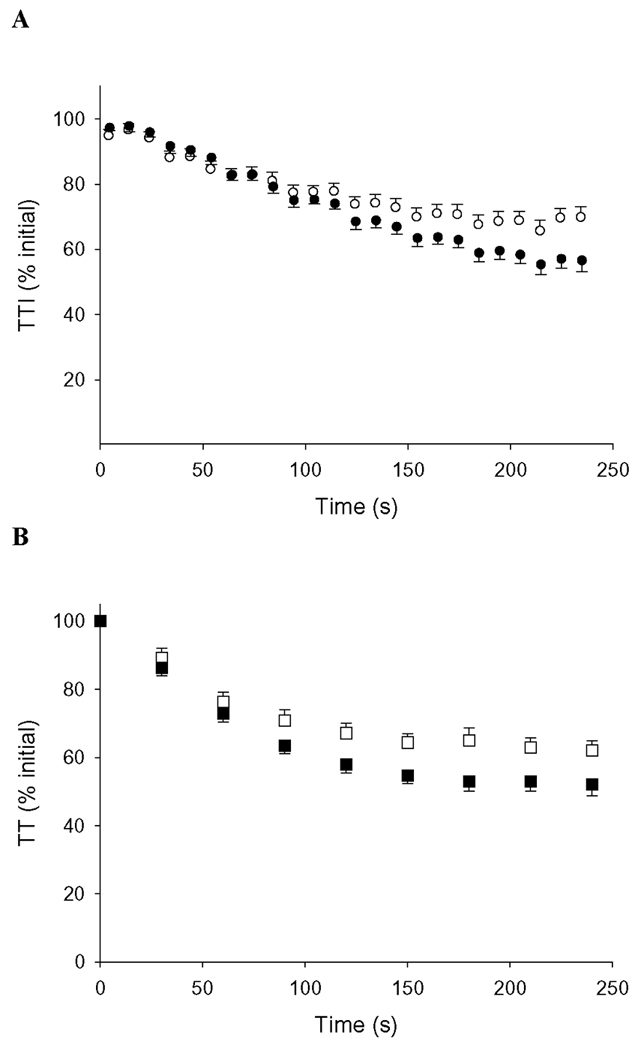
A) Voluntary torque during the isometric protocol, normalized to peak baseline torque. Younger subjects (closed symbols) fatigued more than older subjects (open symbols), p <0.01. B) Isometric TT during isometric protocol, normalized to baseline TT. During fatigue, TT fell less in older compared to young subjects (p = 0.05). Error bars are SE.
Young (n = 16) and old (n = 13) groups fatigued to nearly identical levels during 4 min of MVDCs (p = 0.86, Figure 4A). Dynamic fatigue data from 2 older women are missing because they withdrew from the study prior to its completion, and 1 older man’s dynamic fatigue data were excluded from statistical analysis due to his inability to reliably perform the contractions. As with the voluntary contractions, dynamic TT fatigue was not different between age groups (p = 0.40, Figure 4B). As shown in Figure 5, linear regression analyses indicated a significant correlation between isometric and dynamic fatigue in the young (r = 0.62, p = 0.01), but not in the older subjects (r = 0.01, p = 0.99). In a pooled analysis including both groups, baseline MVIC and isometric fatigue were significantly correlated (r = 0.49, p < 0.01). However, when the groups were separated by age, there was no significant correlation in either group between these variables (young: p = 0.18; older: p = 0.19). There were no significant correlations between MVDC and fatigue during the dynamic protocol (r = 0.14, p = 0.44).
Figure 4. Dynamic fatigue protocol.
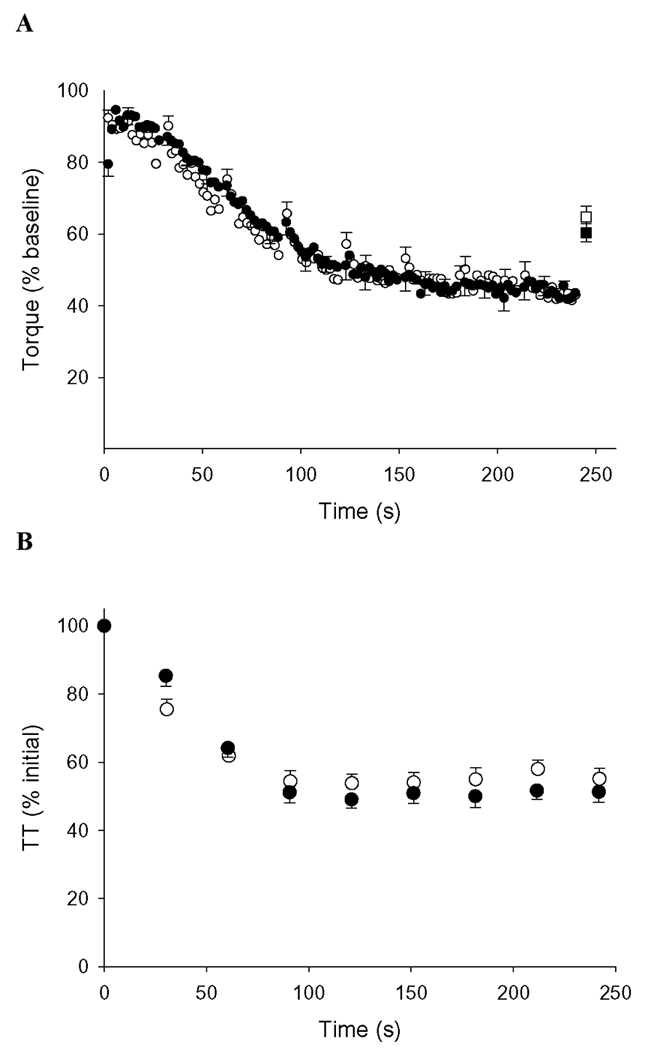
A) Voluntary torque during the dynamic protocol, normalized to peak baseline torque. Fatigue was similar in younger (closed symbols) and older (open symbols) groups (p = 0.86). Square symbols at time = 245 s represent torque produced during a maximum voluntary isometric contraction (MVIC) performed at the end of the fatigue protocol and expressed relative to baseline MVIC. This was not different between groups (p=0.39). B) Dynamic TT during the protocol, normalized to baseline TT. The fall of TT did not differ between groups during this protocol (p = 0.40). Error bars are SE.
Figure 5. Associations between isometric and dynamic fatigue.
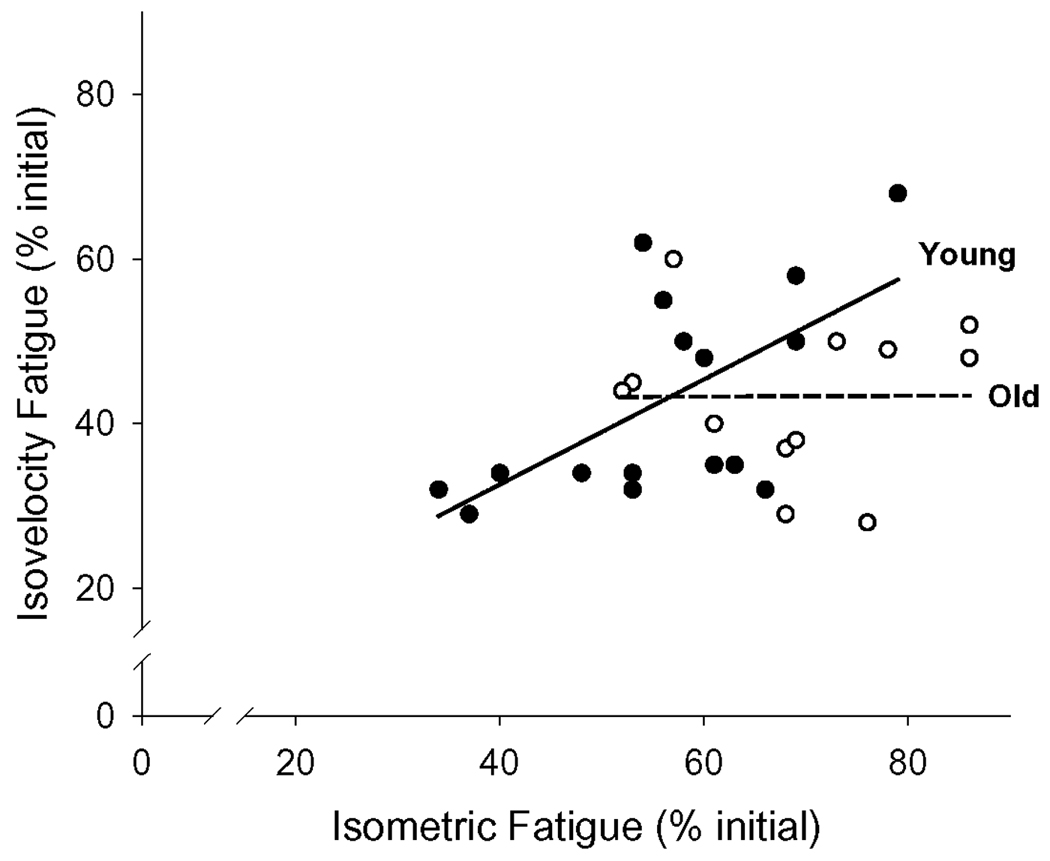
Linear regressions between dynamic and isometric fatigue for young (closed symbols) and old (open symbols) individuals. A positive correlation was observed between fatigue in the two protocols for young (dark solid line, r = 0.62; p = 0.01), but not for old (dashed line, r = 0.02; p = 0.99).
Central Activation
Prior to the isometric protocol, CAR was lower in young compared to old (p = 0.03, Table 2), but there was no difference between groups prior to the dynamic protocol (p = 0.38). The small difference (~2%) between groups in CAR during the baseline MVIC is not considered to be physiologically significant. Due to technical issues, CAR values were not obtained for one older man and two older women. There were no differences between age groups in the change in CAR (measured isometrically), nor in the difference between stimulated and voluntary fatigue (TTend-MVCend) for either protocol (Table 2), suggesting no effect of age on central activation failure during these fatigue protocols.
DISCUSSION
In support of our first hypothesis, the knee extensors of healthy men and women aged ~71 years fatigued less during intermittent MVICs than did those of young men and women aged ~26 years who had similar levels of habitual physical activity. The TT data suggest this was also the case for isometric contractions induced by electrical stimulation. In contrast, there were no differences between age groups in the fatigue of either voluntary (MVDC) or stimulated (TT) dynamic contractions, which supports our second hypothesis. Our measures indicated no age-related difference in the loss of central activation during these protocols. Thus, it appears that the abolition of fatigue resistance in the older group during dynamic KE contractions was a result of changes in the periphery.
Contraction Mode
We report the first study of isometric and dynamic fatigue characteristics of young and old men and women who were matched on an important behavioral variable (physical activity level), with the important finding of a loss of fatigue resistance only during dynamic knee extension exercise in healthy elders. The age-related fatigue resistance observed during isometric contractions in this study is consistent with previous results from our laboratory and others 8,17,23,27,34. The loss of this fatigue resistance in the same subjects during dynamic contractions agrees with and extends several previous reports 26,31,33. In particular, our results are consistent with those of McNeil and Rice 36, in which young and older (64.3 years) men had similar fatigue during very rapid dynamic dorsiflexor contractions. Notably, in the same study, a group of “very old” men (83.7 years) fatigued more than the young group, suggesting that the age of the subjects may be important in studies of fatigue. Our data conflict with those of Baudry et al, who reported greater fatigue in older than young subjects during concentric dorsiflexion contractions that were pre-loaded by isometric contractions 5. In addition to some methodological differences, habitual physical activity level was not quantified or matched between groups in the Baudry, et al. study 5, an important distinction that may explain a portion of the differences in fatigue resistance across age groups 26. Also noteworthy is the somewhat greater age of subjects in their cohort (77.2 years) compared to the older subjects in the present study (70.9 years).
The recent study from McNeil and Rice illustrates the importance of relatively small differences in age in adults beyond ~65 years 36, as differences in fatigue in response to rapid, isotonic dorsiflexion contractions were observed only in the “very old” group of men. The results from our investigation represent a middle ground between those of McNeil and Rice 36 and Baudry, et al.5 with respect to subject age, and they may explain a portion of the divergent results with respect to fatigue in the elderly. As with the present study, Baudry, et al.5 assessed central activation failure using maximal isometric contractions, while McNeil and Rice36 compared pre and post EMG values to infer changes in central activation. Although it is difficult to accurately quantify central activation failure during dynamic contractions (see below), it is important to note that none of these studies identified central activation as a mediator of the age-related differences in fatigue resistance.
While our results reinforce those studies that have shown age-related fatigue resistance during isometric contractions in healthy older adults, they conflict with a previous investigation conducted in our laboratory that also found greater fatigue resistance in older compared to young subjects during intermittent maximum voluntary dynamic dorsiflexion contractions 28. In addition to highlighting the muscle-specific nature of the fatigue response in older adults, this discrepancy also suggests the potential importance of angular velocity in studies of fatigue. In unfatigued muscle, we found greater impairment of torque production in older compared to younger adults during dorsiflexion performed at 90°·s−1 29. However, the difference between the same groups in torque produced during knee extensions at 120°·s−1 was proportionally greater. In fact, the age-related deficit in power production increased with increasing velocities in the knee extensors, but not in the dorsiflexors 29.
The observation in the present study that the older group was 26% weaker than the young group during the baseline MVIC, but 37% weaker during the baseline MVDC demonstrates a velocity-dependent weakness in the older group prior to fatigue (Figure 2). The difference across age groups in the MVDC:MVIC illustrates the concept that weakness in the older group varied by contraction mode. Given the age-related shift in the torque velocity curve in the knee extensor muscles, 29 any further shift that accompanies fatigue 40 would have a proportionally greater effect in older compared to young muscle. It is possible that the greater age-related disparity in torque-velocity curves in the knee extensors compared to the dorsiflexor muscles 29 could explain the abolition of age-related fatigue resistance in dynamic contractions by the knee extensors, but not the dorsiflexors. Future studies that examine fatigue resistance across a range of velocities could be designed to explore this possibility.
The maximal rate of torque development during the isometric TT was similar between our study groups. While this result differs from a previous report in young and older quadriceps muscles 39, it is important to note that we express RTD relative to the maximum torque for the contraction to account for the large differences in peak torque between individuals and age groups. Although the similar RTD across groups indicates that our older volunteers were not disadvantaged in the rate of isometric torque development compared to the young group, it is likely that this RTD measure does not capture the subjects’ ability to produce torque during a voluntary contraction at 120°· −1. Future studies would benefit from the inclusion of measures of velocity during dynamic contractions.
The significant correlation between isometric and dynamic fatigue in the young but not the older subjects (Figure 5) supports the concept that the mechanisms controlling fatigue in the elderly during isometric contractions are not the same as those that influence fatigue during dynamic contractions. As discussed above, a likely source of this difference may be age-related changes in the torque-velocity relationship. The precise mechanisms of this effect are not known at this time, but it is possible that a decreased ability to rapidly and repeatedly modulate motor unit recruitment and discharge rates, coupled with a slowing of contractile properties, limits velocity-dependent contractions in the knee extensors of healthy older adults.
Consistent with previous studies 9,17, there was a moderate correlation between baseline MVIC and isometric fatigue, suggesting that a portion of the fatigue during the isometric protocol was influenced by muscle strength. However, the lack of association between these variables when the groups were assessed separately suggests that this effect was modest. In contrast, there was no association between MVDC and fatigue during dynamic contractions, again suggesting that differing factors regulate fatigue in the elderly during isometric vs. dynamic contractions. It is notable that, in the dynamic fatigue protocol, the age-related fatigue resistance was abolished regardless of the contraction mode used to measure fatigue. A single isometric contraction performed immediately following the final contraction of the dynamic protocol also indicated that fatigue following dynamic contractions was not different between groups (Figure 4A).
Central Activation
We used two approaches to evaluate central fatigue in this study- the change in isometric CAR and the difference in the fall of TT and voluntary torque at the end of each protocol. There was no change in CAR in either age group for either contraction protocol (Table 2), suggesting no obvious failure of voluntary muscle activation as a result of fatiguing isometric or dynamic contractions. It is noted that, while there were no significant changes in the isometric CAR measure upon completion of the dynamic fatigue protocol, it is possible that changes in central activation failure may be specific to contraction mode 2. Likewise, the ability to detect central activation failure can vary by muscle group 20. Overall, the assessment of the completeness of central activation during dynamic contractions using the CAR, which is an isometric measure, has its limitations.
At the end of the isometric contraction protocol, isometric TT was 4–6% lower than MVIC for both age groups. To be a valid measure of peripheral fatigue, sub-maximal, electrically-stimulated contractions should activate a consistent population of muscle fibers that represent the whole muscle with respect to fiber type and associated contractile characteristics 16. If these conditions are met, the decline in TT should not exceed that of maximal voluntary contractions during fatigue. It is possible that electrical stimulation preferentially recruits more fatigable type II muscle fibers due to differences in the stimulation thresholds between motor neuron types 47. However, during motor point stimulation of the quadriceps, recruitment preference is more likely based on proximity to the point of stimulation than variations in stimulation thresholds 1,46. Other investigators have found minimal, non-systematic reversal of motor unit recruitment during electrically-stimulated contractions when compared with voluntary activation 11,21, further diminishing the likelihood that biased recruitment patterns resulted in a greater fall of TT than MVIC in this protocol. Finally, an increase in recruitment threshold could occur in some motor units during fatigue25, with the result that fewer motor units would contribute to torque production during the sub-maximal stimuli. For these reasons, use of sub-maximal motor point stimulation to rule out central activation failure in the knee extensor muscles should be viewed with some caution.
Given the possibility that motor point stimulation of the knee extensors may have underestimated central activation failure, the better preservation of dynamic TT compared with MVDC during the dynamic fatigue protocol becomes more striking. The fall of MVDC was 8–9% greater than the fall of dynamic TT, suggesting some central activation failure in both groups for this contraction mode. It is important to note, however, that neither the change in CAR nor the TT-MVC measure differed by age group, indicating that differences in central activation do not explain the contraction-mode specificity of fatigue that we observed in the present study.
Methodological Considerations
A potential criticism of our study design is the use of isovelocity contractions to investigate the fatigue characteristics of dynamic contractions. Although isotonic contractions, such as those provided by an isokinetic dynamometer, have been suggested to be more comparable to contractions performed in activities of daily living, this viewpoint is debatable. While few movements in a free-living environment can be described as isokinetic, neither can they be described as strictly isotonic in the way they are modulated by an electronically-braked dynamometer. During movement of a constant mass, applied torque will change in accordance with the inertial properties of the mass in motion, as opposed to the near-constant resistance provided by an electrically-braked dynamometer. In the present study, using the isovelocity mode of the dynamometer facilitated appropriate timing of stimulus trains for TTs and ensured a consistent duty cycle between subjects and within protocols. This consistency is an important consideration given the fact that greater duty cycles will promote greater fatigue 19,42, which could bias age-group comparisons if older individuals have slower baseline contractile properties and demonstrate greater contractile slowing during fatigue 37.
Conclusions
Although consensus in the literature has not been reached regarding age-related differences in fatigue resistance, the results of this study clearly demonstrate the important role of contraction mode in the development of fatigue in the knee extensor muscles. Despite better maintenance of torque production in older adults compared to young during maximal isometric contractions, this advantage was abolished during maximal dynamic contractions in a manner consistent with a leftward shift in the torque-velocity curve. Measures of central activation, although not without limitations, suggest that central fatigue did not explain the loss of greater fatigue resistance in the older subjects during dynamic contractions. Studies designed to examine the extent and bases of the velocity dependence in the muscle fatigue response of older adults are needed. The functional consequences of contraction-mode effects on knee extensor fatigue may be critical in the elderly.
Acknowledgements
We thank the study participants and members of the Muscle Physiology Laboratory for their assistance with this project. The contributions of Professors Graham Caldwell and John Staudenmeyer are likewise appreciated. This project was supported by NIH/NIA R01 AG21094 and K02 AG023582.
Abbreviations
- CAR:
Central Activation Ratio
- F:
Female
- EMG:
Electromyogram
- LED:
Light-emitting diode
- KE:
Knee Extensors
- M:
Male
- MVC:
Maximum Voluntary Contraction
- MVDC:
Maximum Voluntary Dynamic Contraction
- MVIC:
Maximum Voluntary Isometric Contraction
- O:
Older group
- RTD:
Rate of Torque Development
- T1/2:
Half-Time of Torque Relaxation
- TT:
Tetanic Torque
- TTI:
Time Tension Integral
- Y:
Young group
Reference List
- 1.Adams GR, Harris RT, Woodard D, Dudley GA. Mapping of electrical muscle stimulation using MRI. J Appl Physiol. 1993;74:532–537. doi: 10.1152/jappl.1993.74.2.532. [DOI] [PubMed] [Google Scholar]
- 2.Babault N, Pousson M, Ballay Y, Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- 3.Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 4.Baudry S, Duchateau J. Postactivation potentiation in a human muscle: effect on the load-velocity relation of tetanic and voluntary shortening contractions. J Appl Physiol. 2007;103:1318–1325. doi: 10.1152/japplphysiol.00403.2007. [DOI] [PubMed] [Google Scholar]
- 5.Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 6.Bemben MG, Massey BH, Bemben DA, Misner JE, Boileau RA. Isometric intermittent endurance of four muscle groups in men aged 20–74 yr. Med Sci Sports Exerc. 1996;28:145–154. doi: 10.1097/00005768-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RH. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54:609–614. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- 8.Chan KM, Raja AJ, Strohschein FJ, Lechelt K. Age-related changes in muscle fatigue resistance in humans. Can J Neurol Sci. 2000;27:220–228. doi: 10.1017/s0317167100000858. [DOI] [PubMed] [Google Scholar]
- 9.Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol. 2007;103:1628–1635. doi: 10.1152/japplphysiol.00320.2007. [DOI] [PubMed] [Google Scholar]
- 10.Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. [PubMed] [Google Scholar]
- 11.Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res. 1997;114:117–123. doi: 10.1007/pl00005610. [DOI] [PubMed] [Google Scholar]
- 12.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279:C611–C618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 14.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. 2004;26:174–185. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- 15.Grange RW, Vandenboom R, Xeni J, Houston ME. Potentiation of in vitro concentric work in mouse fast muscle. J Appl Physiol. 1998;84:236–243. doi: 10.1152/jappl.1998.84.1.236. [DOI] [PubMed] [Google Scholar]
- 16.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–364. [PubMed] [Google Scholar]
- 17.Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol. 2005;99:890–897. doi: 10.1152/japplphysiol.00243.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve. 2005;31:730–740. doi: 10.1002/mus.20325. [DOI] [PubMed] [Google Scholar]
- 19.Iridiastadi H, Nussbaum MA. Muscular fatigue and endurance during intermittent static efforts: effects of contraction level, duty cycle, and cycle time. Hum Factors. 2006;48:710–720. doi: 10.1518/001872006779166389. [DOI] [PubMed] [Google Scholar]
- 20.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–462. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- 21.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch. 1997;434:246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- 22.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 24.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–455. doi: 10.1097/00002060-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara S, Lin CS, Mogyoros I, Cappelen-Smith C, Burke D. Voluntary contraction impairs the refractory period of transmission in healthy human axons. J Physiol. 2001;531:265–275. doi: 10.1111/j.1469-7793.2001.0265j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laforest S, St Pierre DM, Cyr J, Gayton D. Effects of age and regular exercise on muscle strength and endurance. Eur J Appl Physiol Occup Physiol. 1990;60:104–111. doi: 10.1007/BF00846029. [DOI] [PubMed] [Google Scholar]
- 27.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- 29.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 30.Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- 31.Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–136. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 32.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom B, Lexell J, Gerdle B, Downham D. Skeletal muscle fatigue and endurance in young and old men and women. J Gerontol A Biol Sci Med Sci. 1997;52:B59–B66. doi: 10.1093/gerona/52a.1.b59. [DOI] [PubMed] [Google Scholar]
- 34.Mademli L, Arampatzis A. Effect of voluntary activation on age-related muscle fatigue resistance. J Biomech. 2008;41:1229–1235. doi: 10.1016/j.jbiomech.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 35.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 36.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- 37.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol. 2005;98:211–220. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 38.Rantanen T, Guralnik JM, Izmirlian G, Williamson JD, Simonsick EM, Ferrucci L, Fried LP. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Ruiter CJ, Didden WJ, Jones DA, Haan AD. The force-velocity relationship of human adductor pollicis muscle during stretch and the effects of fatigue. J Physiol. 2000;526 Pt:671–681. doi: 10.1111/j.1469-7793.2000.00671.x. 3:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- 42.Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- 43.Tesch PA, Wright JE, Vogel JA, Daniels WL, Sharp DS, Sjodin B. The influence of muscle metabolic characteristics on physical performance. Eur J Appl Physiol Occup Physiol. 1985;54:237–243. doi: 10.1007/BF00426139. [DOI] [PubMed] [Google Scholar]
- 44.Thorstensson A, Karlsson J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol Scand. 1976;98:318–322. doi: 10.1111/j.1748-1716.1976.tb10316.x. [DOI] [PubMed] [Google Scholar]
- 45.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderthommen M, Depresseux JC, Dauchat L, Degueldre C, Croisier JL, Crielaard JM. Spatial distribution of blood flow in electrically stimulated human muscle: a positron emission tomography study. Muscle Nerve. 2000;23:482–489. doi: 10.1002/(sici)1097-4598(200004)23:4<482::aid-mus5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 47.Vanderthommen M, Duteil S, Wary C, Raynaud JS, Leroy-Willig A, Crielaard JM, Carlier PG. A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-MRS in humans. J Appl Physiol. 2003;94:1012–1024. doi: 10.1152/japplphysiol.00887.2001. [DOI] [PubMed] [Google Scholar]
- 48.Yue GH, Bilodeau M, Hardy PA, Enoka RM. Task-dependent effect of limb immobilization on the fatigability of the elbow flexor muscles in humans. Exp Physiol. 1997;82:567–592. doi: 10.1113/expphysiol.1997.sp004048. [DOI] [PubMed] [Google Scholar]


