Abstract
This study investigates the efficacy and safety of personalized cyclophosphamide (CY) dosing in 50 patients receiving CY with total body irradiation (TBI). Participants received CY 45 mg/kg with subsequent therapeutic drug monitoring with Bayesian parameter estimation to personalize the second CY dose to a target area under the curve for carboxyethylphosphoramide mustard (a reporter for CY-derived toxins) and for hydroxycyclophosphamide (to ensure engraftment). The mean second CY dose was 66 mg/kg; the total dose ranged from 45–145 mg/kg. After completion of this phase II study, we compared participants’ clinical outcomes to those of concurrent controls (N=100) who received TBI with standard CY doses of 120 mg/kg. Patients receiving personalized CY dosing had significantly lower post-conditioning peak total serum bilirubin (p=0.03); a 38% reduction in the hazard of acute kidney injury (p=0.03); and similar non-relapse and overall survival (p=0.70 and 0.63, respectively) despite lower doses of CY in most patients.
Keywords: Hematologic malignancy, cyclophosphamide, hydroxycyclophosphamide, carboxyethylphosphoramide mustard, pharmacokinetics, population pharmacokinetics, therapeutic drug monitoring, hematopoietic cell transplantation, liver toxicity, renal toxicity, mortality, outcomes
INTRODUCTION
For 40 years, cyclophosphamide has been an integral component of conditioning regimens for patients undergoing allogeneic hematopoietic cell transplant (HCT).1–3 One common conditioning regimen combines total body irradiation (TBI) with cyclophosphamide (CY) 120 mg/kg administered in divided doses on each of two consecutive days. The purpose of the TBI/CY conditioning regimen is two-fold: to minimize the risk of rejection of donor cells through its myeloablative effects, and, in patients with hematologic malignancy, to have an anti-tumor effect.3 TBI/CY conditioning, however, causes considerable regimen-related toxicity, particularly to hepatic sinusoids: destruction of sinusoidal endothelial cells, activation of stellate cells, deposition of extracellular matrix in sinusoids, and zone 3 hepatocyte necrosis (collectively known as sinusoidal obstruction syndrome or SOS).4 The clinical consequences of this liver injury include jaundice, hepatomegaly, portal hypertension, ascites, renal sodium retention, and acute kidney injury (AKI).4–8
Recent studies have elucidated the pathogenesis of SOS. Although generally not thought to be a liver toxin, there is now considerable evidence that CY is the primary cause of liver injury when used as part of myeloablative regimens for HCT.9,10 When CY is placed in co-culture with hepatocytes and sinusoidal endothelial cells, toxic metabolites generated within hepatocytes damage endothelial cells.11 A prospective study of 148 patients undergoing TBI/CY conditioning found that considerable interpatient variability in CY metabolism, and that the frequency of SOS, non-relapse mortality, and overall survival were related to how CY was metabolized.10 Carboxyethylphosphoramide mustard (CEPM), a metabolite of CY, was found to be a reporter molecule for hepatotoxins derived from CY; patients having the greatest exposure to CEPM (as measured by the area under the curve, or AUCCEPM) had the greatest frequency of severe liver injury and the worst outcomes.10 In our subsequent work, we developed a novel method for personalizing CY doses to greatly decrease the interpatient variability in AUCCEPM, with the long-range goal of lowering the liver toxicity of the TBI/CY regimen.12 In a phase I study of 20 patients undergoing TBI/CY conditioning, CY doses were personalized using therapeutic drug monitoring after the first CY infusion. This was done by measuring AUCCEPM from 0 to 16 hours, then adjusting the second CY dose to achieve a total exposure (AUC0–48 hr) to CEPM of 325±25 µM•h.12 We then demonstrated that using a population pharmacokinetic model provided a more accurate prediction of the target level of the metabolite CEPM, which was needed to achieve this personalized second CY dose.13
We now report the results of a phase II study of the TBI/CY conditioning regimen in 50 patients undergoing allogeneic HCT, in which we used therapeutic drug monitoring with Bayesian parameter estimation after the first CY dose to adjust the second CY dose. The aims of this study were to determine the frequency of liver and kidney injury, non-relapse mortality, relapse of malignancy, and survival among patients who received TBI 12 Gy plus personalized CY dosing. Upon completion of this phase II study, we compared the clinical outcomes of patients receiving personalized CY dosing to those of concurrent control patients who received TBI 12 Gy plus standard CY dosing at 120 mg/kg with identical supportive care (see Methods, Patient Selection).
RESULTS
Demographics of the study cohorts
Table 1 shows patient characteristics by CY dosing method. Patients who received personalized CY dosing were, on average, older and less likely to have received 400 cGy testicular irradiation than those who received standard CY dosing of 120 mg/kg. Aside from transplant year, the groups were fairly well matched on the remaining factors.
Table 1.
Patient characteristics by CY dosing method.
| TBI 12 Gy + Standard CY dosing (120 mg/kg) (N = 100) N (%) |
TBI 12 Gy + Personalized CY dosing (N = 50) N (%) |
|
|---|---|---|
| Transplant year | ||
| 2003 – 2004 | 55 (55) | 0 |
| 2005 | 30 (30) | 15 (30) |
| 2006 – 2007 | 15 (15) | 35 (70) |
| Age (years) | ||
| 18 – 27 | 26 (26) | 11 (22) |
| 28 – 37 | 25 (25) | 14 (28) |
| 38 – 45 | 31 (31) | 6 (12) |
| ≥ 46 | 18 (18) | 19 (38) |
| Mean, 95% CI | 36, 34–38 | 39, 36–43 |
| Race | ||
| White | 79 (79) | 41 (82) |
| Non-white1 | 21 (21) | 9 (18) |
| Donor | ||
| Matched sibling | 40 (40) | 23 (46) |
| Mismatched relative | 5 (5) | 2 (4) |
| Unrelated | 55 (55) | 25 (50) |
| Sex: recipient – donor | ||
| Male – male | 35 (35) | 10 (20) |
| Female – male | 17 (17) | 8 (16) |
| Female – female | 18 (18) | 12 (24) |
| Male - female | 30 (30) | 20 (40) |
| Risk of relapse | ||
| Low | 28 (28) | 12 (24) |
| Lymphoid malignancies: | ||
| Acute lymphoblastic leukemia (ALL), first complete remission (CR) |
22 | 10 |
| Non-Hodgkin’s lymphoma (NHL), Burkitt's Disease or diffuse large cell, remission |
2 | 2 |
| NHL, Follicular, relapse | 3 | 0 |
| NHL, Mantle Cell, refractory relapse | 1 | 0 |
| Myeloid malignancies | 0 | 0 |
| Standard | 14(14) | 11(22) |
| Lymphoid malignancies: | ||
| Chronic lymphocytic leukemia | 0 | 1 |
| Myeloid malignancies: | ||
| Acute myeloid leukemia (AML), 1stor 2nd CR |
10 | 10 |
| Acute promyelocytic leukemia, 2nd CR | 1 | 0 |
| Chronic myeloid leukemia (CML), Chronic Phase |
3 | 0 |
| High | 58(58) | 27(54) |
| Lymphoid malignancies: | ||
| ALL, ≥1st relapse or primary refractory or refractory relapse |
11 | 4 |
| ALL, ≥2nd CR | 11 | 8 |
| NHL in relapse (Burkitt’s Disease, diffuse large cell, T cell) |
5 | 2 |
| NHL, T-cell, 2nd CR | 0 | 1 |
| Hodgkin’s Disease, refractory relapse | 1 | 0 |
| Myeloid malignancies: | ||
| AML ≥1st relapse or primary refractory | 14 | 7 |
| Secondary AML | 3 | 1 |
| Biphenotypic leukemia | 6 | 1 |
| CML, accelerated phase or blast crisis/remission |
7 | 3 |
| Testicular irradiation (400 cGy)2 | 24 (24) | 7 (14) |
Non-white includes the following categories: Asian, Black or African-American, Native Hawaiian/Pacific Islander, Multiple races, and Unknown.
Testicular irradiation was given during conditioning therapy to male patients with ALL (N=23), NHL (N=4), biphenotypic leukemia (N=3), and CML in remission following blast crisis (N=1).
Personalized CY dosing method: Pharmacokinetics and dose adjustments
Patients participating in the phase II trial of personalized CY dosing received a first CY dose of 45 mg/kg. The mean second CY dose was 66 mg/kg (range, 0–100 mg/kg), and the mean total CY dose was 111 mg/kg (range, 45–145 mg/kg). There were 15 patients whose total CY dose exceeded 120 mg/kg. The mean AUCCEPM for the time from 0 to 48 hours was 302 µM•h (median, 301 µM•h; range, 136–442 µM•h); the target AUC for minimization of adverse effects was 325 ± 25 µM•h. The average percent deviation of the observed AUCCEPM for the time from 0 to 48 hours from our target of 325 µM•h was −7%, ranging from −58% to +36%. The mean AUCHCY for the time from 0 to 48 hours was 183 µM•h (median, 181 µM•h; range, 53–285 µM•h); our target had been greater than 50 µM•h. The mean AUCCY for the time from 0 to 48 hours was 3530 µM•h (median, 3496 µM•h; range, 915–5782 µM•h).
To confirm our previous observation that using a Bayesian pharmacokinetic method provided a more accurate and consistent method of dose adjustment,13 we compared the predicted AUC of each CY metabolite, based on either the regression equations or the Bayesian pharmacokinetic method, with the observed AUC. In comparison to the regression-based dosing method, the predicted AUCCEPM from the MAP Bayesian pharmacokinetic estimation was more closely correlated to the observed AUCCEPM. The mean percent error for the observed AUCCEPM from 0 to 48 hours was −13.9% (range, −60.4% to 33%) with the regression method and 11.8% (range, −16% to 39.1%) for the MAP pharmacokinetics method. The mean second CY dose that the regression-based method would have recommended was 62 mg/kg (range 9–184 mg/kg), compared to 66 mg/kg (range 0–100 mg/kg).
The systemic exposure of CY and its metabolites were not evaluated in the concurrent control patients.
Frequency of liver and renal injury in cases and controls
The maximum value for total serum bilirubin from the day of transplant to day 20 (a measure of the toxicity of the conditioning regimen4,8) was significantly lower among patients who received personalized CY dosing, compared to those who received 120 mg/kg (Table 2).
Table 2.
Patient outcomes by CY dosing method1
| TBI 12 Gy + Standard CY dosing (120 mg/kg) (N = 100) |
TBI 12 Gy + Personalized CY dosing (N = 50) |
HR (95% CI) | p-value | |
|---|---|---|---|---|
| Total serum bilirubin values: | ||||
| Days 0–20 | 1.7 mg/dL (0.4 – 18.3) |
1.3 mg/dL (0.7 – 8.8) |
0.03 | |
| Day of maximum serum bilirubin (day 0–20) |
Day 11 (0–20) |
Day 10 (1 – 20) |
||
| Days 21–100 | 1.4 mg/dL2 (0.5 – 35.0) |
1.1 mg/dL3 (0.5 – 34.3) |
0.47 | |
| Day of maximum serum bilirubin (day 21–100) |
Day 27 (21 – 99) |
Day 47 (21 – 99) |
||
| Acute Kidney Injury4 | ||||
| No | 23 (23) | 19 (38) | ||
| Yes | 77 (77) | 31 (62) | 0.62 (0.40 – 0.95) | 0.03 |
| Overall survival | ||||
| Alive | 44 (44) | 26 (52) | ||
| Died | 56 (56) | 24 (48) | 0.89 (0.55– 1.44) | 0.63 |
| Non-relapse mortality | ||||
| No | 71 (71) | 37 (74) | ||
| Yes | 29 (29) | 13 (26) | 0.88 (0.46 – 1.70) | 0.70 |
| Relapse | ||||
| No | 66 (66) | 37 (74) | ||
| Yes | 34 (34) | 13 (26) | 0.81 (0.42 – 1.54) | 0.52 |
| Low and standard risk disease | ||||
| Total | 42 | 23 | ||
| Alive | 25 (60) | 16 (70) | ||
| Died | 17 (40) | 7 (30) | 0.95 (0.38 – 2.35) | 0.91 |
| High risk disease | ||||
| Total | 58 | 27 | ||
| Alive | 19 (33) | 10 (37) | ||
| Died | 39 (67) | 17 (63) | 0.88 (0.50 – 1.56) | 0.67 |
| Lymphoid disease | ||||
| Total | 56 | 28 | ||
| Alive | 26 (46) | 12 (43) | ||
| Died | 30 (54) | 16 (57) | 1.14 (0.62 – 2.11) | 0.67 |
| Myeloid disease | ||||
| Total | 44 | 22 | ||
| Alive | 18 (41) | 14 (64) | ||
| Died | 26 (59) | 8 (36) | 0.60 (0.27 – 1.34) | 0.21 |
Data presented as median (range) or number of patients (% of relevant population)
Evaluable in 96 patients
Evaluable in 48 patients
Acute kidney injury is defined as a doubling of baseline serum creatinine.
Acute kidney injury (AKI) developed in 77% of patients receiving standard CY dosing, compared to 62% of those receiving personalized CY dosing—a reduction of 38% in the hazard of AKI (p=0.03) (Table 2, Figure 1). Patients with AKI were more likely to have had high maximum total serum bilirubin values during days 0 to 20 than those without AKI (Table 3). This association was stronger in those who received standard CY than in those who received personalized CY dosing, although the difference between groups was not statistically significant.
Figure 1.
Time to development of acute kidney injury by CY dosing method.
Table 3.
Maximum total serum bilirubin values (mg/dL) during days 0 – 20 by acute kidney injury (AKI).
| No AKI | AKI | p-value | |
|---|---|---|---|
| All patients | |||
| Median (range) | 1.3 (0.6 – 5.9) | 1.8 (0.4 – 18.3) | |
| Total serum bilirubin | |||
| ≤ 1.5 mg/dL: N (%) | 29 (69) | 45(42) | 0.003 |
| > 1.5 mg/dL | 13(31) | 68(58) | |
| Standard CY dosing | |||
| Total serum bilirubin | |||
| ≤ 1.5 mg/dL | 16 (70) | 28 (36) | |
| > 1.5 mg/dL | 7 (30) | 49 (64) | 0.007 |
| Personalized CY dosing | |||
| Total serum bilirubin | |||
| ≤ 1.5 mg/dL | 13 (68) | 17 (55) | |
| > 1.5 mg/dL | 6 (32) | 14 (45) | 0.34 |
Non-relapse mortality, relapse, and overall survival in cases and controls
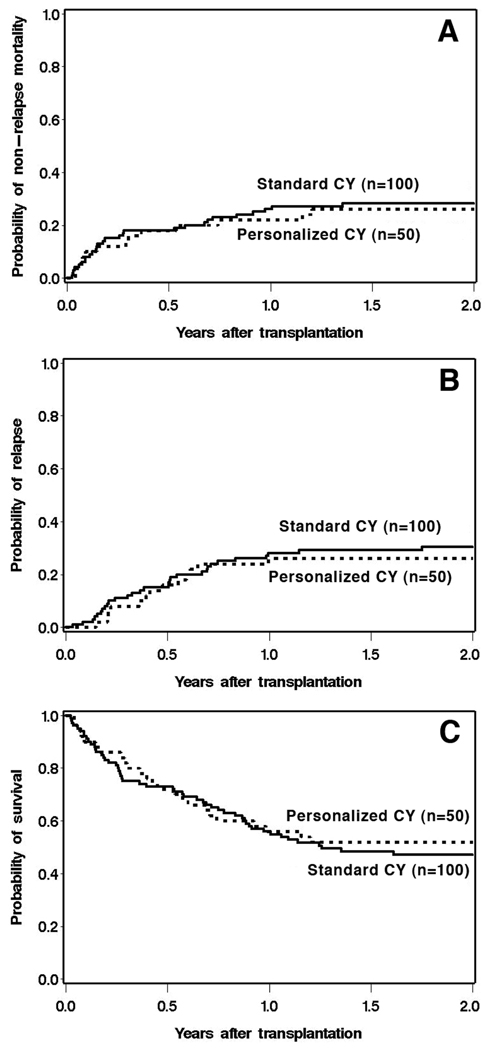
Table 2 shows analyses of time-to-event outcomes for cases and controls, with regard to these outcomes. The corresponding cumulative incidence plots are shown in Figure 2. Despite an overall reduction in total CY dose for most patients, the use of personalized CY dosing was not associated with worse outcomes in terms of relapse or overall survival when compared with standard CY dosing. There was also no evidence of lower non-relapse mortality with personalized CY dosing.
Figure 2.
Non-relapse mortality (A), relapse (B), and overall survival (C) by CY dosing method.
Subgroup analysis of outcomes by type of malignancy and by risk of relapse after transplant
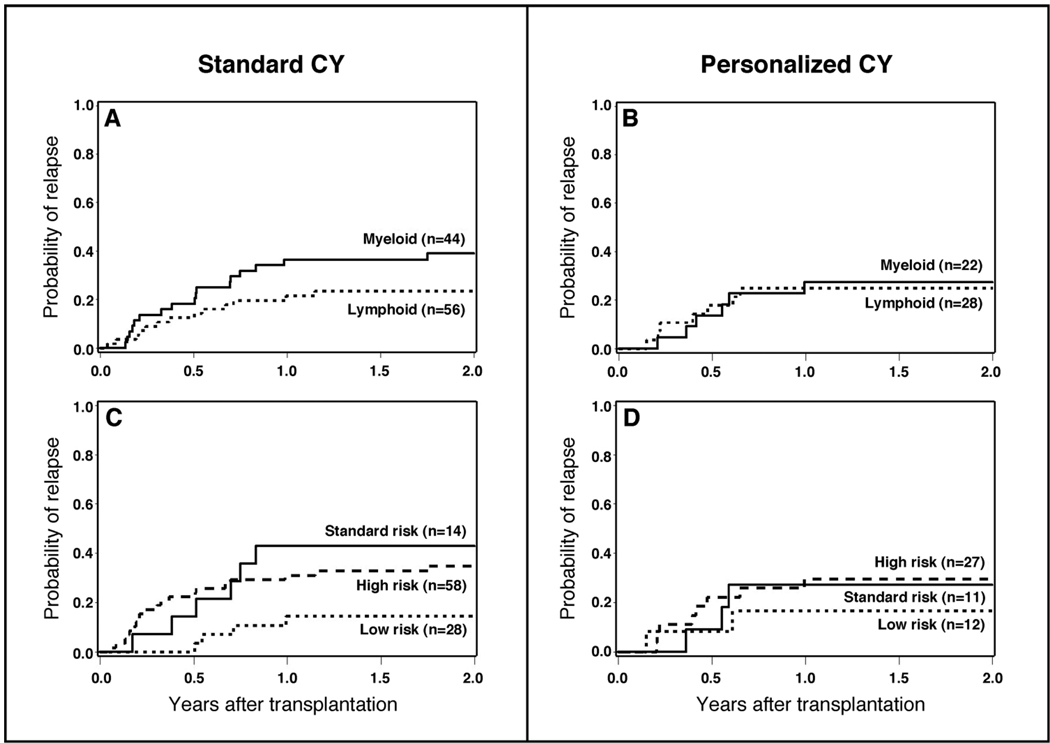
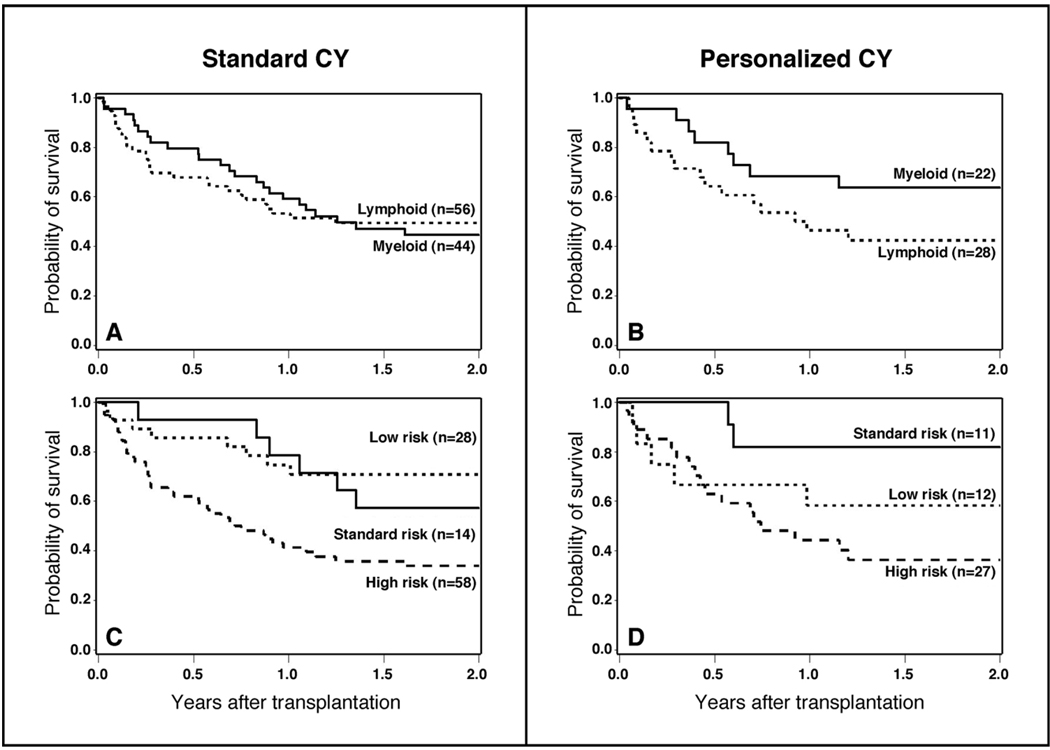
After analyzing outcome measures for the study cohort as a whole, we subsequently conducted subgroup analyses to determine if personalized CY dosing benefited a subgroup of the overall population. The purpose of this analysis was for hypothesis generation only. The distribution of patients with lymphoid and myeloid malignancies by risk of relapse and method of CY dosing is displayed in Table 1. Personalized CY dosing was not associated with worse outcomes (relapse, death) in either lymphoid or myeloid malignancies when these subgroups are considered separately (Table 2, Figure 3A and 3B, Figure 4A and 4B). There was, however, a 40% reduction in the hazard of death in patients with myeloid malignancies that were conditioned with TBI and personalized CY dosing that was not statistically significant (95% CI, 0.27 – 1.34) (Table 2, Figure 3A and 3B). Similarly, personalized CY dosing was not associated with worse outcomes (compared to standard CY dosing) when patients were analyzed by their risk of recurrent malignancy (Table 2, Figure 3C and 3D, Figure 4C and 4D).
Figure 3.
Relapse of malignancy among patients receiving TBI 12 Gy plus standard CY dosing (120 mg/kg) vs. personalized CY dosing by type of hematologic malignancy (A and B) and by risk of relapse of malignancy after transplant (C and D).
Figure 4.
Overall survival among patients receiving TBI 12 Gy plus standard CY dosing (120 mg/kg) vs. personalized CY dosing by type of hematologic malignancy (A and B) and by risk of relapse of malignancy after transplant (C and D).
DISCUSSION
This phase II trial of a conditioning regimen that combines total body irradiation 12 Gy with personalized dosing of CY demonstrates that 1) it is feasible to accurately target AUC of CY metabolite levels in individual patients using a Bayesian pharmacokinetic method; 2) the CY dose range to achieve the same metabolic endpoint varies over 3-fold (45–145 mg/kg total dose) and; 3) there was less liver and kidney injury with personalized CY dosing, when compared to the frequency of injury among a cohort of concomitant, well-matched patients receiving TBI and standard CY dosing at 120 mg/kg.
The results of the phase II trial reported here confirm previous observations about the extreme variability in CY metabolism.10,12 Among patients receiving personalized CY dosing, there was a 3-fold CY dosing range that was needed to achieve the same target metabolic endpoint. Normalizing exposure to the toxic metabolites of CY resulted in statistically significantly lower peak bilirubin levels and a lower frequency of kidney injury than in a comparison cohort of patients who received TBI followed by standard CY dosing of 120 mg/kg over two days. Although this was not a randomized trial, the comparison cohort was remarkably similar to the cohort receiving personalized CY dosing. Both cohorts were transplanted in approximately the same time frame (see Methods, Patient Selection), when transplant practices and supportive care, including drugs used for prophylaxis against infection, graft-versus-host disease (GVHD), and cholestatic liver injury, were identical. The results of this study confirm the hypothesis that CY is a proximate cause of liver injury when given in conjunction with TBI 12 Gy.10,11
The expected result was that reducing exposure to the toxic metabolites of CY in patients whose metabolism was destined to create high levels of toxin exposure would prevent liver and kidney injury, which was indeed true. Competing concerns, however, were that patients who received doses of CY lower than the standard 120 mg/kg would have reduced anti-tumor efficacy and a greater rate of relapse of the underlying malignancy, and conversely, that those who received higher CY doses would have more toxicity. The data show that personalized CY dosing in this regimen had no impact on the frequency of either relapse of malignancy or overall survival, compared to the concurrent control cohort. This finding is in agreement with our prior study that indicated no relationship exists between relapse of hematologic malignancies and exposure to CY and its metabolites when receiving TBI/CY conditioning.10
This somewhat discordant result—less organ toxicity but nearly identical outcome measures—may have resulted from the fixed 12 Gy dose of TBI that both cases and controls received. Our previous analysis demonstrated that aberrant CY metabolism and the total dose of TBI were independent factors in the pathogenesis of sinusoidal liver injury and non-relapse mortality.10 That is, patients at highest risk for SOS and mortality were those whose metabolism of CY yielded a greater quantity of toxins and who received the highest doses of TBI. Limiting the dose of TBI to 12 Gy appears to have reduced the frequency of liver injury even among patients who received standard CY dosing (our control cohort), compared to our historical patients whose doses of TBI varied.10 It is noteworthy that transplant centers that have used TBI doses of 10–12 Gy following standard doses of CY have consistently reported a lower frequency of fatal SOS14 than our center, where doses of TBI up to 14.4 Gy have been used along with CY 120 mg/kg.4 The lack of an effect on non-relapse mortality after personalized CY dosing, despite less liver and kidney injury, may also be related to the fact that acute GVHD and infection during periods of intense immune suppression are important causes of non-relapse mortality. We had no expectation that personalized CY dosing would affect the frequency of severe acute GVHD or fatal infection.
The average total dose of CY in our cohort who received personalized CY dosing (111 mg/kg) was ~9% less than the standard dose; 14% of patients received less than 80 mg/kg total CY dose. There appeared to be no obvious penalty for a lower total dose of CY, as the rates of relapse of malignancy and survival were not different in the cohort who received personalized CY dosing, even when examined in subgroups sorted by risk of relapse after transplant and by type of malignancy. Given the small number of patients in each disease category, however, a modest impact on relapse rates could have been missed. We note that 15 of 50 patients received total CY doses in excess of 120 mg/kg to achieve the target metabolic endpoint; there was no evidence for either greater organ toxicity or improved anti-tumor outcomes in such patients.
There are some practical implications of this study. While the standard dose of CY (120 mg/kg) was chosen on an empiric basis over 30 years ago, the extreme variability in CY metabolism means that most patients who receive this dose are being exposed to increased liver toxicity, particularly when CY is combined with a second modality like TBI that is also toxic to sinusoidal endothelial cells.15 If a fixed dose of CY is to be used along with TBI, our data suggest that it should be 110 mg/kg. While we have demonstrated that it is feasible to personalize doses of CY, the methods for doing so are complicated and resource-intense, as they require bedside processing of blood specimens, refrigerated transport, and just-in-time, highly-specialized pharmacokinetic analysis that allows calculation of the second CY dose.12,13 If TBI doses greater than 12 Gy are to be used in a TBI/CY regimen, personalized CY dosing may be necessary to avoid fatal regimen-related toxicity. There also may be individuals at increased risk of liver damage from conditioning therapy because of prior liver injury for whom the use of personalized CY dosing might be justified.16 Finally, one could argue that if personalized CY dosing were available, there could be a dose-escalation of TBI to achieve better outcomes related to relapse of malignancy, without jeopardizing survival because of regimen-related toxicity.
METHODS
Patient selection
From 2005–2007, patients over 18 years of age who had a malignant hematological disease unlikely to respond to conventional treatment and a suitable donor from an HLA-identical family member or unrelated allogeneic donor were considered for participation in a protocol where CY dosing was determined by therapeutic drug monitoring. High-resolution typing was performed for unrelated donors; donors who were an allele match or a one allele mismatch for HLA A, B, C, DRB1 or DQB1 were selected for favorable prognosis patients.17 Written consent was obtained using forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. This study was registered with Clinical Trials.gov as trial identifier # NCT00317785. In order to select control patients whose treatment differed only in the method of CY dosing, we reviewed the medical records of consecutive patients over 18 years of age who were treated for hematologic malignancy with allogeneic HCT following conditioning with TBI 12 Gy and CY 120 mg/kg during the years 2003–2007, under the aegis of a protocol approved by our Institutional Review Board. There were 102 such patients, two of whom had not given permission for review of records for research purposes, leaving 100 evaluable patients as controls.
Technique of hematopoietic cell transplantation
Starting six days before infusion of donor hematopoietic cells, hyperfractionated total body irradiation (total dose 12 Gy) was delivered via a linear accelerator as 200 cGy fractions twice daily over 3 days, followed by intravenous CY administration on each of two consecutive days. Male patients with a diagnosis of ALL, biphenotypic leukemia, NHL, and CML in blast crisis remission also received testicular irradiation of 400 cGy during conditioning therapy. For patients whose CY dose was to be personalized by therapeutic drug monitoring, CY was infused through a central venous access catheter over one to two hours on the first day at a dose of 45 mg/kg based on adjusted ideal body weight.12 On the following day, a second infusion of CY was given to a maximum of 100 mg/kg (maximal total CY dose 145 mg/kg), with dosing as described below. For patients whose CY dose was fixed at 120 mg/kg (standard CY dosing), CY was infused at a dose of 60 mg/kg on each of two consecutive days. During CY infusion days, patients received MESNA (2-mercaptoethane sulfonate) for uroepithelial prophylaxis, at a milligram dose equal to that of CY. A day of rest followed, with donor hematopoietic cell infusion occurring on day 0. For patients in both groups, a calcineurin inhibitor and methotrexate were given as prophylaxis against GVHD, ursodiol against cholestatic liver disease, and fluconazole, acyclovir and trimethoprim-sulfamethoxazole as infection prophylaxis.18,19
Therapeutic drug monitoring and dose adjustment of cyclophosphamide
For patients receiving personalized CY dosing, blood samples were drawn from a central venous access catheter at the end of the first CY infusion and at 2, 4, 8, 16, 20, and 24 hours after the start of infusion. At each of these times, blood was collected in two tubes, one containing EDTA for CEPM and CY quantitation, and the other containing phenylhydrazine HCl to stabilize 4-hydroxycyclophosphamide (HCY).12 All samples were stored at the bedside at 4°C prior to sample transport. Following collection of the 16-hour blood sample, all samples were taken to the Pharmacology Laboratory for quantitation of the plasma concentrations of CEPM and HCY using liquid chromatography and mass spectroscopy methods, as described previously.12 Each patient’s CY dose was adjusted based on pharmacokinetics to achieve a target plasma AUC over 0–148 hours for CEPM and HCY. The target AUCCEPM was set at 325±25 µM•h, a value derived from clinical endpoints and their relationship to CY pharmacokinetics from our prospective study of 147 patients.10 Briefly, 325 µM•h was the highest value in the lowest quartile of CEPM exposure; patients whose AUCCEPM was within this quartile had the lowest frequency of organ toxicity, the lowest non-relapse mortality, and the best survival.10 Because of concern that lower CY doses needed to achieve the target AUCCEPM would lead to insufficient AUCHCY, a minimum AUCHCY was set at 50 µM•h, based on prior data showing that this level of HCY exposure was consistent with engraftment.10 Personalized CY dosing was accomplished using Maximum A posteriori Probability (MAP) Bayesian estimation of the pharmacokinetic parameters, incorporating a blend of individualized pharmacokinetic data and a population parameter prior.13 This method was previously shown to more accurately predict 48-hour AUCs than a method combining noncompartmental pharmacokinetic analysis of individual patient concentration-time data for CY metabolites and a regression equation relating the AUC0–16 hr to AUC0–48 hr.12,13 The MAP Bayesian method used individual patient 0–16 hour HCY and CEPM concentration-time data, together with a pharmacokinetic model and mean parameter values and their variance (derived from the prior study of 147 patients), to obtain the most likely estimates of the pharmacokinetic parameter values for that individual.20 This integrated model simultaneously describes the pharmacokinetics of CY, HCY and CEPM and includes autoinduction of CY to HCY, thus allowing for a prediction of exposure conditional on any potential dosing scenario. The population prior parameters were not changed during this study.
The complexity of the CY pharmacokinetic model and the necessity of rapid calculations led us to develop a customized software tool to perform MAP estimation of pharmacokinetic parameters.13 We developed the dose adjustment software in the statistical program R (R Development Core Team, Vienna, Austria), and named it BaRD, or “Bayesian R Dose” adjustment. With BaRD, we could read individual patient pharmacokinetic data of HCY and CEPM directly from an Excel® spreadsheet (Microsoft Corp, Redmond WA), estimate the individual CY pharmacokinetic model parameters, and then report the predicted AUC0–48 hr for both HCY and CEPM over a wide range of potential CY second doses. The summary spreadsheet (BaRD input) included the first CY dose, the patient’s age, and the HCY and CEPM concentration-time from the five samples obtained over 0–16 hours after the first CY dose. The output consisted of potential second CY doses in 5 mg/kg increments, with the option to obtain the output in 1 mg/kg increments. At each potential second CY dose, the expected AUC0–48 hr for both HCY and CEPM along with the 95% confidence intervals of achieving the combined target AUCs was obtained. The recommended second CY dose was subject to review and approval by one of us (JSM) such that the personalized CY dosing was the result of the BaRD proposed dose and review by a human expert. The second CY dose was communicated to the treating physician approximately 6 hours after the 16 hour blood sample had been obtained. Blood samples were also obtained after the personalized CY dose, at the end of infusion and at 2, 4, 8, 16, 20, and 24 hours after the start of infusion.
Statistical analyses
The outcomes of interest were liver disease, acute kidney injury, non-relapse mortality, relapse, and overall survival. Maximum total serum bilirubin levels in the first 20 days (reflecting liver injury caused by the conditioning regimen) and from day 21 to 100 (reflecting liver injury from other causes) were used as measures of liver disease for this analysis. Acute kidney injury (AKI) was defined as a doubling of baseline serum creatinine at any time during the first 100 days post-transplant.7 Cumulative incidence curves were used to estimate the probabilities of time-to-event outcomes. The statistical significance of differences in event rates was evaluated with the proportional hazards regression model. Death was treated as a competing risk in the analysis of AKI and relapse. Relapse of the underlying malignancy was treated as a competing risk in the analysis of non-relapse mortality. The maximum total serum bilirubin values were log-transformed to approximate normality, and compared by group via linear regression models. The association of AKI and the incidence of a maximum bilirubin value in days 0 – 20 higher than the median value for all patients were estimated with the logistic regression model. Factors considered as potential confounders of the relationships between the method of CY dosing and the outcomes included age, race, donor-recipient sex match, transplant year, donor type, receipt of testicular irradiation as part of the conditioning regimen, and risk of relapse as defined by disease type and stage.21 Such factors were retained in the model if their presence influenced the coefficient of interest (use of personalized CY dosing) by 10% or more. Reported p-values are two-sided, and based on the Wald statistic. No adjustments are made for multiple comparisons. The target accrual for this phase II study was 50 participants, which provided 88% power to observe a 16% decrease in day 200 non-relapse mortality, based on our historical experience with conditioning with TBI and standard doses of CY (120 mg/kg for 2 days).
After study accrual was complete, we sought to confirm our prior observation12 that MAP Bayesian estimation provided a more accurate method for determination of the second CY dose than the noncompartmental analysis method. Noncompartmental analysis was used to estimate each individual patient’s AUCCEPM values for the time from 0 to 16 hours. The estimate of the expected AUCCEPM for time 0 to 48 hours was then calculated using a regression equation relating the AUCCEPM from 0 to 16 hours and the AUCCEPM from 0 to 48 hours12: AUCCEPM from 0–48 hours equals 8.47(AUCCEPM for 0–16 hours)0.813. The dose recommendation based on this noncompartmental analysis was calculated. In this manuscript, this method is referred to as the “regression method.” In addition, the predicted AUCCEPM was calculated using the regression equation with the actual administered second CY dose. An assessment of predictive performance was based on the predicted AUCCEPM from 0 to 48 hours from either the regression method or the MAP Bayesian pharmacokinetics method, using the AUCCEPM as the actual measurement value in the calculation of precision. Precision was calculated by use of absolute percent error as follows: [(Observed-Predicted/Observed) * 100].22
ACKNOWLEDGEMENTS
Our research was supported by grants from the National Institutes of Health, National Cancer Institute (CA 18029, CA 15704). We are grateful to the study participants and the many nurses at the Seattle Cancer Care Alliance and University of Washington Medical Center who supported this protocol.
Footnotes
Conflict of Interest
None of the authors declared a conflict of interest.
REFERENCES
- 1.Santos GW, et al. Marrow transplantation in man following cyclophosphamide. Transplant Proc. 1971;3:400–404. [PubMed] [Google Scholar]
- 2.Thomas ED, et al. Aplastic anaemia treated by marrow transplantation. Lancet. 1972;1:284–289. doi: 10.1016/s0140-6736(72)90292-9. [DOI] [PubMed] [Google Scholar]
- 3.Thomas ED, et al. Cure of leukemia by marrow transplantation. Leukemia Research. 1977;1:67–70. [Google Scholar]
- 4.McDonald GB, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 5.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 6.Fink JC, Cooper MA, Burkhart KM, McDonald GB, Zager RA. Marked enzymuria after bone marrow transplantation: a correlate of veno-occlusive disease-induced “hepatorenal syndrome”. J Am Soc Nephrol. 1995;6:1655–1660. doi: 10.1681/ASN.V661655. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int. 2005;67:272–277. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345–352. doi: 10.1002/hep.20529. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge ME, Huitema AD, Beijnen JH, Rodenhuis S. High exposures to bioactivated cyclophosphamide are related to the occurrence of veno-occlusive disease of the liver following high-dose chemotherapy. Br J Cancer. 2006;94:1226–1230. doi: 10.1038/sj.bjc.6603097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald GB, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003 Mar 1;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 11.DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837. doi: 10.1002/hep.510240414. [DOI] [PubMed] [Google Scholar]
- 12.McDonald GB, et al. Metabolism-based cyclophosphamide dosing for hematopoietic cell transplant. Clin Pharmacol Ther. 2005;78:298–308. doi: 10.1016/j.clpt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Salinger DH, et al. Real-time dose adjustment of cyclophosphamide in a preparative regimen for hematopoietic cell transplant: a Bayesian pharmacokinetic approach. Clin Cancer Res. 2006;12:4888–4898. doi: 10.1158/1078-0432.CCR-05-2079. [DOI] [PubMed] [Google Scholar]
- 14.Ringden O, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- 15.Geraci JP, Mariano MS, Jackson KL. Radiation hepatology of the rat: microvascular fibrosis and enhancement of liver dysfunction by diet and drugs. Radiat Res. 1992;129:322–332. [PubMed] [Google Scholar]
- 16.McDonald GB. Review article: management of hepatic disease following haematopoietic cell transplant. Alimentary pharmacology & therapeutics. 2006;24:441–452. doi: 10.1111/j.1365-2036.2006.03001.x. [DOI] [PubMed] [Google Scholar]
- 17.Petersdorf EW, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 18.Ruutu T, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 19.Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplant. 4th edn. Malden, MA: Blackwell Publishing; 2009. [Google Scholar]
- 20.Qiu R, et al. Diminishing the risk of nonrelapse mortality in hematopoietic stem cell transplantation: Prediction of exposure to the cyclophosphamide metabolite carboxyethylphosphoramide mustard. Clin Pharmacol Ther. 2004;76:270–280. doi: 10.1016/j.clpt.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Kahl C, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]