Abstract
In the budding yeast, Saccharomyces cerevisiae, actively transcribed tRNA genes can negatively regulate adjacent RNA polymerase II (pol II)-transcribed promoters. This tRNA gene-mediated silencing is independent of the orientation of the tRNA gene and does not require direct, steric interference with the binding of either upstream pol II factors or the pol II holoenzyme. A mutant was isolated in which this form of silencing is suppressed. The responsible point mutation affects expression of the Cbf5 protein, a small nucleolar ribonucleoprotein protein required for correct processing of rRNA. Because some early steps in the S. cerevisiae pre-tRNA biosynthetic pathway are nucleolar, we examined whether the CBF5 mutation might affect this localization. Nucleoli were slightly fragmented, and the pre-tRNAs went from their normal, mostly nucleolar location to being dispersed in the nucleoplasm. A possible mechanism for tRNA gene-mediated silencing is suggested in which subnuclear localization of tRNA genes antagonizes transcription of nearby genes by pol II.
Keywords: nucleolus, RNA polymerase III
It has previously been shown that tRNA-class RNA polymerase III (pol III) promoters can exert negative transcriptional regulation on neighboring DNA in the budding yeast, Saccharomyces cerevisiae (1). The degree to which this tRNA gene-mediated silencing (tgm silencing) affects nearby RNA polymerase II-transcribed genes varies among different pol II promoters. Although complete inhibition of some nearby pol II promoters has been achieved in selected artificial juxtapositions, promoters that are found bordering the 274 tRNA genes in their normal chromosomal locations must somehow be exempt, or at least conditionally resistant to this form of negative regulation.
There is a particularly close association of tRNA genes with naturally occurring copies of most classes of the Ty retrotransposons (2). One possible selective pressure for maintaining an association between tRNA genes and neighboring pol II promoters is that the proximity serves some regulatory function that is beneficial for maintenance of the retrotransposon at that position. Although the presence of a Ty3 sigma element does not strongly affect expression of a neighboring tRNA gene (3), temperature-dependent silencing of the chromosomal Ty3 and sigma elements was shown to be dependent on RNA polymerase III, suggesting possible involvement of the neighboring tRNA transcription units (1). It is interesting to note that the one class of Ty retrotransposon that is not found adjacent to tRNA genes, the Ty5 class, is found instead at other silenced locations, namely telomeres and the silent mating type loci (4, 5).
At this time, it is not clear what relationship the mechanism of tgm silencing might have to silencing at silent mating type loci, telomeres, ribosomal RNA genes, or other forms of negative regulation, but several types of interference between the transcription units appear to be ruled out. It seems unlikely that either readthrough by pol III or positive supercoils propagated in front of the transcribing pol III are disrupting neighboring pol II upstream activator sequences (UAS) or promoter complexes. These exclusions are inferred from the fact that the tRNA genes repress in both orientations with respect to the pol II promoter. Direct steric interference with binding of pol II transcription factors to the UAS elements and promoters is also improbable for several reasons. Not only do the tRNA genes repress at considerable distance from the pol II UAS elements, but direct examination of the chromatin showed that the pol II promoter UAS is occupied by its cognate transcription factor, even when pol II transcription is repressed by the tRNA gene (1). The ability of the tRNA genes to repress in both orientations also argues against direct occlusion of the UAS, because different ends of the tRNA complex would have to be involved in the two cases. Modification of local chromatin structure in the region of a tRNA gene has not been ruled out. Although detailed examination of chromatin nucleoprotein structure in regions of some tRNA genes did not show drastic rearrangements on inactivation of the tRNA gene promoter (6), there are reported instances of nucleosome rearrangements because of active transcription of tRNA-class promoters in yeast (7, 8).
To investigate the mechanism of tgm silencing, we have selected for chromosomal mutations that were defective in silencing of an artificial pol II promoter by a neighboring tRNA gene. One of the mutations that alleviated tgm silencing affected expression of the Cbf5 protein, a probable pseudouridine synthetase associated with small nucleolar RNAs (snoRNAs) and implicated in ribosomal RNA maturation. Because early pre-tRNA biogenesis has been localized primarily to nucleoli in yeast (9), we examined effects of the CBF5 mutation on pre-tRNA localization. The observed complete dissociation of the pre-tRNAs from the nucleolus in the mutant strain suggests a model in which tgm silencing is caused by subnuclear localization of the tRNA genes.
Materials and Methods
Yeast Strains and Genetic Manipulations.
All strains used are wild type at GAL4 and GAL80. The mutation that suppressed tgm silencing [originally designated art1-1 (art = alleviation of repression by tRNA genes)] was isolated in YM2062 (MATα ura3-52 ade2-101 his3-200 lys2-801 tyr1-501 GAL4 GAL80 leu2∷GAL1-lacZ). Dominance was tested by mating with YM705 (MATa ura3-52 ade2-101 his3-200 lys2-801 trp1-901 met− GAL4 GAL80). Transformations were done by using the lithium acetate method (10). For tetrad analysis, mutant and parental wild-type strains were mated to the W3031A (MATa ura3-1 ade2-1 his3-11,15 trp1-1 can1-100 GAL4 GAL80). Mating type tests, sporulation and tetrad dissection were done by standard methods (11). Expression of the SUP4 ochre suppressor tRNA gene was tested by growth of ade2-1 or ade2-101 strains on media lacking adenine.
Construction of Plasmids.
The construction of pSUP4o was described previously (1) and is described in Fig. 1 and in Results. The wild-type and mutated CBF5 genes were inserted at the PshAI site of pSUP4o to give pSUP4oCBF5-AUG and pSUP4oCBF5-AUU by gap repair. Briefly, pSUP4o was cut at the PshAI site and was transformed into W3031A along with a PCR product of the CBF5 gene, which had ends homologous to the vector on both sides of the PshA1 site. The chromosomal CBF5 gene from 10 nucleotides upstream of the initiating AUG to just past the termination codon was subsequently deleted in W3031A containing the CBF5 plasmids by replacement with a bacterial kanamycin gene.
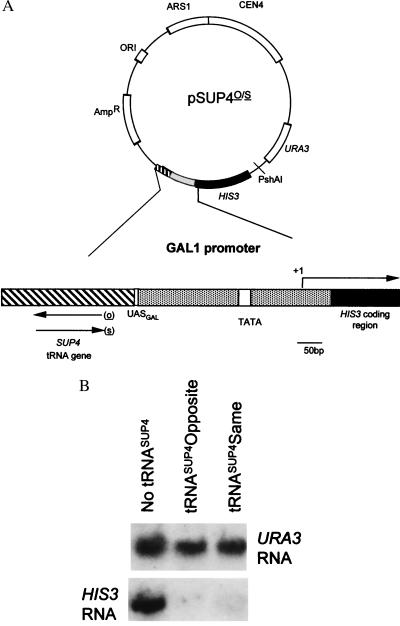
Figure 1.
(A) Plasmid used to monitor tgm silencing. In this plasmid, the tRNASUP4 gene is placed in the same (s) or opposite (o) orientation as transcription of the HIS3 coding region. HIS3 expression is controlled by a consensus UASGAL and the GAL1 promoter as described previously (1). (B) A Northern blot analysis of HIS3 mRNA and URA3 mRNA, expressed from the reporter plasmid (pSUP4o/s). Both orientations of the neighboring tRNA gene drastically reduce HIS3 mRNA levels.
Isolation, Identification and Cloning of a Silencing Suppressor.
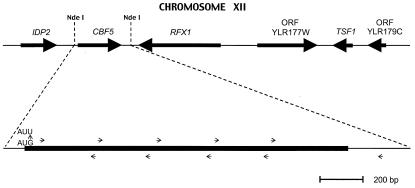
Spontaneous mutants were isolated from YM2062 containing pSUP4o by plating on media lacking histidine and uracil and by using a mixture of galactose and raffinose as a carbon source. His+ suppressors that were still His− on glucose were mated with YM705 to determine whether the mutation was dominant or recessive. Dominant mutations were most often the result of plasmid rearrangement whereby the tRNA gene was deleted or transcriptionally inactive. Recessive mutations, including the one characterized here, were routinely slow growing and sporulated poorly. Tetrad analysis revealed that the His+ (suppressor of tgm silencing) phenotype cosegregated with a slow-growing phenotype. Complementation of the slow-growing phenotype with plasmids from a library in Yep24 (gift of N. Woychik, University of Medicine and Dentistry of New Jersey) was used to identify multiple, overlapping clones in one region of the yeast genome. This region contained five open reading frames, three of which were independently subcloned into a CEN plasmid, pRS316 (10). The complementary CBF5 ORF (Fig. 2) was cloned from the NdeI site (end-filled) 99 bp upstream of the ORF to the NdeI (end filled) site 282 bp downstream of the coding region, inserted into pRS316 at the SpeI end-filled site.
Figure 2.
The mutation leading to suppression of tgm silencing in the CBF5 gene. The gene corresponding to the art1-1 mutation was cloned, and the entire coding region of the parental wild-type and mutant CBF5 genes was sequenced. The mutant contained a single AUG to AUU change in the translation initiation codon.
Disruption of CBF5 Gene.
The CBF5 gene was disrupted by one-step gene replacement (12). The kanr gene was generated by PCR of pkanMX2 with 50 nucleotides of homology on each side of the CBF5 gene. This gene includes 60 nucleotides upstream of the start site AUG and 80 nucleotides downstream of the UGA. Transformation of this PCR product resulted in colonies that grew on media with Geneticin (G418, GIBCO/BRL).
Assays for Expression of HIS3.
Expression of the galactose-driven HIS3 gene was monitored in two ways. In all cases, strains were tested for their ability to grow on galactose-containing media lacking histidine. Growth on this media is the simplest test for suppression of silencing. To directly look at the level of transcription of the HIS3 coding region, Northern blots were performed, using an oligodeoxynucleotide complementary to the 5′ end of the HIS3 mRNA. (5′-GGGCTTTCTGCTCTGTCATCTTTGCCC). As a control, an oligo complementary to the 5′ end of the URA3 mRNA was used to probe the same RNA blot (5′TGTAGCTTTCGACATG).
Fluorescent in Situ Hybridizations.
Yeast cells in logarithmic growth phase in liquid culture were fixed, probed with fluorescently labeled oligodeoxynucleotides, stained with 4′,6-diamidino-2-phenylindole (DAPI), and imaged as described previously (9). Probes for the introns of pre-tRNATrp and pre-tRNALeu3 were labeled with a 5′ fluorescein and had the sequences 5′GATTGCAATCTTATTCCGTGGAATTTCCAAGATTTAATTG and 5′TGAGTATTCCCACAGTTAACTGCGGTCAAGATATTTCTTG. The Cy3-derivatized antisense probe for U14 snoRNA was as described (9).
Results
Selection for Mutations Affecting tgm Silencing.
To probe for the mechanism of tgm silencing, spontaneous mutants were isolated that abolished silencing of a Gal4p-regulated promoter by an upstream tRNASUP4 gene. The selection construct, pSUP4o was located on a low copy number plasmid and is shown in Fig. 1A. In the absence of a functional upstream tRNA gene, HIS3 expression can be induced with galactose in the growth media (1). In the presence of the tRNA gene with a functional intragenic promoter, the wild-type strain does not express enough HIS3 mRNA to grow without added histidine. Fig. 1B shows an RNA blot probed for either HIS3 mRNA or URA3 mRNA as an internal control. The results confirm that histidine auxotrophy coincides with a severe reduction in HIS3 mRNA levels in the presence of a neighboring tRNA gene in either transcriptional orientation.
Spontaneous mutants were sought that could grow without histidine in the presence of galactose, but in which HIS3 expression was still repressed by glucose in the medium. Most His+ phenotypes resulted from mutations on the plasmid, as judged by loss of the phenotype where the plasmid in the isolated mutant was removed and replaced by the original plasmid (data not shown). A very small number of chromosomal mutations (1 in 108-109cells) were identified that allowed galactose-dependent growth in the absence of histidine. These mutations were generally difficult to characterize because most grew slowly and did not mate and sporulate well in this strain. To date, extensive characterization has been accomplished for only one mutant, originally termed art1-1, for alleviation of repression by tRNA genes. This mutant was found to have a recessive mutation in which the tgm silencing suppression (His+) cosegregated with a slow growth phenotype in tetrad analysis (not shown).
To identify the gene corresponding to the mutation, we sought a clone of S. cerevisiae genomic DNA that both allowed normal growth rates and caused reversion to a His− phenotype. Complementation of the slow growth phenotype with a genomic DNA library of S. cerevisiae in a high-copy number plasmid identified multiple clones that had a common region, shown at the top of Fig. 2. This region contains five full predicted ORFs, as well as one partial ORF at each end. The ORFs were individually cloned into a low-copy centromere plasmid. The CBF5 gene alone was shown to both complement the slow growth phenotype and eliminate the suppression of tgm silencing by the art1-1 mutation.
Characterization of the CBF5 Mutation.
CBF5 is an essential gene that was first identified as a loosely associated centromere binding factor affecting chromosomal segregation in meiosis/mitosis (13). Subsequently, it was shown to encode a nucleolar antigen (14) with strong homology to NAP57, a rat cell nucleolar protein. Very recently Cbf5p has been shown to be a possible pseudouridine synthetase for rRNA and to be required for efficient processing of rRNA precursors (15, 16). Although it was not immediately obvious why a defect in an apparent nucleolar pre-rRNA-processing enzyme might affect tgm silencing, we identified the mutation in CBF5 responsible for this phenotype.
The CBF5 gene region from the YM2062 parent strain and the mutant strain were amplified by PCR. The amplified fragment population was sequenced directly (without cloning) to avoid mutational artifacts that can occur in single PCR-generated clones. The series of internal primers used for detecting sequence are indicated by small arrows in Fig. 2. Only a single point mutation was identified, a change from AUG to AUU at the translation initiation codon. Because deletion of CBF5 is lethal, we assume the mutation allows some level of expression of the Cbf5 protein. This interpretation would be consistent with the observation that an AUU codon can be used for translation with low efficiency in the context of the HIS4 gene (17). Because we were unable to unambiguously determine the levels of Cbf5p in the wild-type and mutant strains with available antibodies, we opted to prove that the initiator codon mutation caused the observed phenotypes by recreating that point mutation in another wild-type strain.
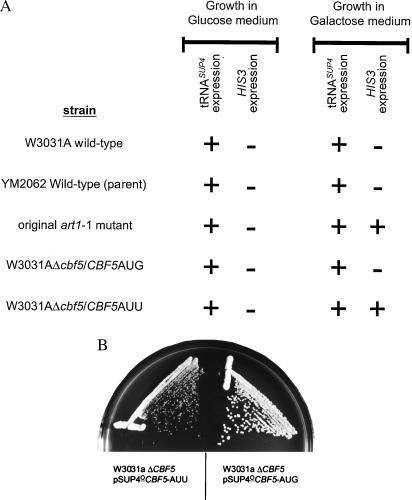
To demonstrate conclusively that the AUU mutation in CBF5 is responsible for the loss of tgm silencing phenotype, the wild-type (AUG) and the mutant (AUU) CBF5 gene were expressed in an unrelated strain, W3031A. The wild-type or mutant CBF5 genes, including their endogenous expression signals, were inserted into the pSUP4o plasmid at the PshAI site, downstream from the HIS3 gene. After transformation of the W3031A haploid strain with these two plasmids, the chromosomal CBF5 locus was deleted in each strain. Only the strain receiving the mutant CBF5 gene, CBF5-AUU, demonstrated the galactose-dependent His+ phenotype (Fig. 3A). This strain also grew slowly (Fig. 3B), confirming that the CBF5-AUU mutation is responsible for both phenotypes seen in the original art1-1 mutant strain.
Figure 3.
Loss of silencing and slow growth recreated by an AUG to AUU change in the CBF5 initiation codon. (A) The expression of the tRNASUP4 gene and the HIS3 gene on media containing glucose (represses pol II promoter) or galactose (induces pol II promoter). The tRNASUP4 gene is always expressed when present, tested by the suppression of a chromosomal ade2-101 mutation. In the original mutant (art1-1, which is derived from strain YM2062), expression of HIS3 from the GAL1 promoter requires galactose induction. To show that this galactose-inducible His+ phenotype is due solely to the CBF5 AUG to AUU mutation, either the mutated or wild-type CBF5 gene was introduced into an unrelated strain (W3031A) on a plasmid, and the chromosomal CBF5 was deleted. The AUU mutation conferred galactose-inducible HIS3 expression. (B) Recreation of the AUU translational initiation codon in CBF5 (W3031aΔcbf5/pSUP4oCBF5-AUU) also recreates the slow growth phenotype of the original mutant compared with the same construct with the wild-type AUG in the plasmid-borne CBF5 gene.
Mislocalization of pre-tRNA Biosynthesis in the CBF5-AUU Mutant.
Recently, it has been shown that at least some early steps of tRNA processing take place in the nucleolus (9) alongside ribosomal biogenesis. Ribonuclease P, which cleaves the 5′ termini of pre-tRNAs early in the nuclear processing pathway, and several intron-containing and intronless pre-tRNAs were shown to be concentrated in nucleoli. In addition, the nuclear pool of a tRNA modification enzyme that makes an isopentenyl adenosine modification has been shown to be nucleolar (18). These findings suggest that biogenesis of at least some tRNAs either begins with transcription at the nucleolus (as with ribosomal genes), or that the nascent transcripts are transported to the nucleolus for processing. Whereas some pre-tRNAs and pre-tRNA-processing enzymes have also been found in the nucleoplasm and nuclear periphery (19–21), the extent to which the position of the pathway is enzyme specific or tRNA specific has yet to be explored in any depth.
In light of this information, the involvement by Cbf5p, a small nucleolar ribonucleoprotein (snoRNP) component, created the suspicion that tgm silencing is linked to the nucleolus. This suspicion is reinforced by previous genetic studies in yeast that suggest links between tRNA gene expression and the nucleolus (22). Nucleoli are specialized for rRNA and tRNA biogenesis, and are relatively poor in pol II transcription components. An interesting factor to tgm silencing effects might be that tRNA genes, or a subset of actively transcribed genes, are physically located at the nucleolus. If this localization contributes to the silencing of nearby pol II-transcribed genes, the CBF5 mutation might alleviate the silencing by disrupting nucleolar organization and indirectly causing a loss of gene localization.
The most direct way to test this hypothesis would be to determine the nuclear location of the tRNA genes, in particular a gene that has effectively silenced a neighboring pol II promoter. It would be necessary to visualize the tRNA gene sequences, rather than RNA polymerase III or transcription factors, because the nucleolar 5S rRNA genes use the same proteins. To date, it has not been possible to directly visualize the tRNA gene positions in yeast. As an alternative approach, we have asked whether the CBF5 mutation that alleviates tgm silencing also affects localization of early tRNA transcripts.
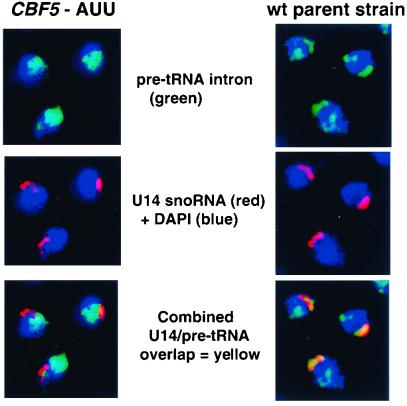
We examined both the nucleolar morphology and the localization of the nuclear pre-tRNAs in the CBF5-AUU mutant compared with the parental wild-type strain. In situ fluorescence of these two strains is shown in Fig. 4. The blue color in all panels is the DAPI stain of the nucleoplasm. Pre-tRNAs were visualized with fluorescein-labeled probes to two tRNA introns (green), and the position of the nucleolus was detected with a Cy3-labeled probe to U14, a snoRNA (red). The blue-green coloring in some panels results when a lower intensity intron signal (green) coincides with the blue DAPI staining. In the wild-type parent strain, the majority of the pre-tRNA signal is colocalized to the single, crescent-shaped nucleolus, coincident with U14 snoRNA. There are also punctate, secondary loci of pre-tRNA staining in the nucleoplasm. These secondary sites vary in prominence between strains (9) and might indicate the presence of both nucleolar and nonnucleolar foci of pre-tRNA biosynthesis. In the CBF5-AUU strain, the U14 snoRNA signal is slightly fragmented compared with the wild-type parent, although it still maintains the overall crescent shape at one end of the nucleus. The change in the pre-tRNA positions is more dramatic, with little overlap remaining between the green pre-tRNA signal and the red U14 signal. Instead, the pre-tRNA signal has become both diffuse and nucleoplasmic. This severe mislocalization of pre-tRNAs suggests that the spatial organization of early tRNA biosynthesis is linked to tgm silencing effects by tRNA genes.
Figure 4.
In situ fluorescence of the mutant strain CBF5-AUU and the parental wild-type strain. DAPI stains of the nucleoplasm are blue in all views. The fluorescein-labeled probe to the introns of pre-tRNAs are green, and the Cy3-labeled probe to U14 nucleolar RNA is red. In the wild-type strain, most of the pre-tRNA signal is colocalized to the nucleolus, along with the U14 snoRNA, although there are some secondary loci of pre-tRNA staining. In the CBF5-AUU strain, the U14 nucleolar signal is slightly fragmented, but the pre-tRNA signal is dispersed to the nucleoplasm.
Discussion
Extensive investigations of transcriptional silencing at silent mating-type loci, telomeres, and ribosomal RNA genes in yeast have suggested that the nucleoprotein structure of affected chromatin regions is altered, but have also raised the possibility that some types of silencing involve subnuclear localization. rRNA genes have long been known to reside in a nucleolar structure that is specialized for ribosome biogenesis, and it was not surprising to find that pol II-transcribed genes placed in this environment are poorly transcribed (23, 24). Telomeres, also, have been found in interphase nucleoli, as well as in a limited number of other punctate nucleoplasmic loci (25, 26).
The experiments presented here demonstrate that a mutation in CBF5 that alleviates silencing near a tRNA gene also dissociates the early tRNA transcripts from the nucleolus. It is not clear why early pre-tRNA pathway localization should be affected by a snoRNP component, but the partial breakup of the nucleolar integrity in the CBF5-AUU mutant (Fig. 4) might provide a clue. It is possible, even likely, that the effects on the tRNA pathway and tgm silencing are indirect results of disrupting the ribosomal processing pathway. This inability to properly organize the nucleolus might result from inadequate supplies of the snoRNPs in some structural or transport role, or might be caused by loss of ribosomal RNA pseudouridylation, which is thought to be performed directly by the Cbf5 protein.
It is unlikely that tgm silencing can be comprehensively described by a simplistic model in which some interaction of the tRNA gene transcription complex carries the attached DNA to the nucleolus or some other subnuclear locus that is less accessible to pol II transcription complexes. Localization could, however, be one component in a balance between the silencing tendencies of the tRNA gene proximity and the activation mechanism for nearby pol II promoters. Unlike silencing at the silent mating type loci, ribosomal repeats, and telomeres, tgm silencing effects are relatively weak and variable among different pol II promoters. This variability would have evolved by necessity, because tRNA genes are dispersed throughout the closely packed yeast genome. Only in selected cases, such as the artificial Gal transcription elements used in the selection described here, are the pol II promoters seen to be strongly inhibited under otherwise inducing conditions. This selective silencing response suggests that promoters that normally function near tRNA genes, such as those in Ty elements, have activation mechanisms that are dominant over the influence of the tRNA gene under some conditions. Should it become possible to precisely identify the positions of individual DNA loci in yeast, it would be quite interesting to track the location of genes proximal to tRNA genes during activation of the neighboring pol II promoters. If the hypothesis is true that sequestered localization, whether nucleolar or elsewhere, contributes to tgm silencing, then it would be expected that the subnuclear position might change as a result of an event that allowed pol II transcription.
The possible difference between tRNA gene-mediated effects and other forms of silencing is underscored by the recent demonstration that a tRNA gene can serve as a “boundary” to an HMR silencer, preventing the propagation of the HMR silencing (27). At first glance this observation would seem to be at odds with our observed tgm silencing, but we interpret it to mean that whatever influence is being exerted by the tRNA gene is incompatible with the propagation of the silent mating locus silencing effects.
At this time, it is not known whether local silencing effects are limited to the tRNA class of pol-III-transcribed genes. The point is largely moot for 5S rRNA genes, which in yeast are clearly nucleolar because they are attached to the large ribosomal RNA gene repeat. In higher eukaryotes the 5S rRNA genes occur as clusters, rather than interspersed with pol II transcription units, and these clusters have been found localized to nucleoli in both animals and plants (28). An interesting group of highly repeated, short interspersed repetitive elements (SINEs) containing pol III promoters is widespread in eukaryotes and might be relevant to this discussion. These elements are derived from RNA-mediated duplication of different pol III transcription units, especially tRNA and 7SL RNA genes (29, 30). These repetitive elements can be found either dispersed as individual copies or as highly reiterated tandem copies, especially in heterochromatic regions. The elements are not generally transcribed into stable RNA commensurate with their copy number in vivo, although they can usually be transcribed in vitro, and there are numerous reports of widespread condition-specific or development-specific activation in vivo (31–40). Several hypotheses have been put forward regarding possible functions for these sequences. One particularly interesting suggestion in light of yeast tgm silencing is that these dispersed RNA polymerase III promoters might exert either a positive or negative influence on the transcriptional activity of overlapping or nearby RNA polymerase II promoters (37, 41–49). In yeast it was observed that active transcription of the tRNA gene was required for local tgm silencing to occur (1). If related types of regulation are applicable in other eukaryotes, one would expect that, under most circumstances, regulatory effects would occur only near the small minority of transcriptionally active pol III elements.
Whether or not proximity to pol III transcription units is a widely used regulatory strategy in eukaryotes, it is clear that, in fungi, the distribution of pol II transcription units near tRNA genes is nonrandom and that this proximity affects the pol II units when it occurs. The mechanisms by which nucleolar components are involved in this form of silencing in yeast are under investigation.
Acknowledgments
We thank Nancy Woychik (Rutgers University) and the Roche Institute DNA sequencing facility for help in characterizing the CBF5 genomic DNA sequence. We also thank John Carbon for supplying us with antibodies to Cbf5p. This work was supported by National Institutes of Health Grants GM52907 and GM34869 (to D.R.E.) and National Institutes of Health Grants GM54887 and GM57071 (to R.H.S.). M.W.H. was a predoctoral fellow of the Molecular and Cellular Biology Training program, Grant T32GM07315.
Abbreviations
- pol II
polymerase II
- snoRNP
small nucleolar ribonucleoprotein
- tgm
tRNA gene-mediated
- UAS
upstream activator sequence
- DAPI
4′,6-diamidino-2-phenylindole
- art
alleviation of repression by tRNA genes
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240454997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240454997
References
- 1.Hull M, Erickson J, Johnston M, Engelke D R. Mol Cell Biol. 1994;14:3244–3252. doi: 10.1128/mcb.14.2.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boeke J D, Sandmeyer S B. In: Genome Dynamics, Protein Synthesis, and Energetics, The Molecular and Cellular Biology of the Yeast Saccharomyces. Broach J R, Pringle J, Jones E W, editors. Vol. 1. Plainview, NY: Cold Spring Harbor Lab. Press; 1991. pp. 193–261. [Google Scholar]
- 3.Kinsey P T, Sandmeyer S B. Nucleic Acids Res. 1991;19:1317–1324. doi: 10.1093/nar/19.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voytas D F, Boeke J D. Nature (London) 1992;358:717. doi: 10.1038/358717a0. (lett.). [DOI] [PubMed] [Google Scholar]
- 5.Zou S, Wright D A, Voytas D F. Proc Natl Acad Sci USA. 1995;92:920–924. doi: 10.1073/pnas.92.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huibregtse J M, Engelke D R. Mol Cell Biol. 1989;9:2195–2205. doi: 10.1128/mcb.9.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morse R H, Roth S Y, Simpson R T. Mol Cell Biol. 1992;12:4015–4025. doi: 10.1128/mcb.12.9.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsolier M C, Tanaka S, Livingston-Zatchej M, Grunstein M, Thoma F, Sentenac A. Genes Dev. 1995;9:410–422. doi: 10.1101/gad.9.4.410. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand E, Houser-Scott F, Kendall A, Singer R H, Engelke D R. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman F, Fink G R, Hicks J B. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 12.Wach A, Brachat A, Pohlmann R, Philippsen P. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Middleton K, Yoon H J, Fouquet C, Carbon J. Mol Cell Biol. 1993;13:4884–4893. doi: 10.1128/mcb.13.8.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadwell C, Yoon H, Zebarjadian Y, Carbon J. Mol Cell Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafontaine D, Bousquet-Antonelli C, Henry Y, Caizergues-Ferrer M, Tollervey D. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zebarjadian Y, King T, Fournier M J, Clarke L, Carbon J. Mol Cell Biol. 1999;19:7461–7472. doi: 10.1128/mcb.19.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinnebusch A G, Liebman S W. In: Genome Dynamics, Protein Synthesis, and Energetics, The Molecular and Cellular Biology of the Yeast Saccharomyces. Broach J R, Pringle J, Jones E W, editors. Vol. 1. Plainview, NY: Cold Spring Harbor Lab. Press; 1991. pp. 627–735. [Google Scholar]
- 18.Tolerico L H, Benko A L, Aris J P, Stanford D R, Martin N C, Hopper A K. Genetics. 1999;151:57–75. doi: 10.1093/genetics/151.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark M W, Abelson J. J Cell Biol. 1987;105:1515–1526. doi: 10.1083/jcb.105.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J-M, Hopper A K, Martin N C. J Cell Biol. 1989;109:1411–1419. doi: 10.1083/jcb.109.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar S, Hopper A K. Mol Biol Cell. 1998;9:3041–3055. doi: 10.1091/mbc.9.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre O, Ruth J, Sentenac A. J Biol Chem. 1994;269:23374–23381. [PubMed] [Google Scholar]
- 23.Smith J S, Boeke J D. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Bryk M, Banerjee M, Murphy M, Knudsen K E, Garfinkel D J, Curcio M J. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- 25.Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy B, Grunstein M, Gasser S. EMBO J. 1997;16:3243–3255. doi: 10.1093/emboj/16.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair D, Mills K, Guarente L. Science. 1997;277:1313–1316. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- 27.Donze D, Adams C R, Rine J, Kamakaka R T. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanswami S, Hamkalo B A. Cytometry. 1990;11:144–152. doi: 10.1002/cyto.990110117. [DOI] [PubMed] [Google Scholar]
- 29.Deininger P L. In: Mobile DNA. Berg D E, Howe M M, editors. Washington, DC: American Society for Microbiology; 1989. [Google Scholar]
- 30.Okada N. Curr Opin Genet Dev. 1991;1:498–504. doi: 10.1016/s0959-437x(05)80198-4. [DOI] [PubMed] [Google Scholar]
- 31.Carey M F, Singh K, Botchan M, Cozzarelli N R. Mol Cell Biol. 1986;6:3068–3076. doi: 10.1128/mcb.6.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang K L, Latchman D S. FEBS Lett. 1989;258:255–258. doi: 10.1016/0014-5793(89)81667-9. [DOI] [PubMed] [Google Scholar]
- 33.Lania L, Pannuti A, La Mantia G, Basilico C. FEBS Lett. 1987;219:400–404. doi: 10.1016/0014-5793(87)80260-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu W-M, Schmid C W. Nucleic Acids Res. 1993;21:1351–1359. doi: 10.1093/nar/21.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maraia R J, Driscoll C T, Bilyeu T, Hsu K, Darlington G J. Mol Cell Biol. 1993;13:4233–4241. doi: 10.1128/mcb.13.7.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matera G, Hellman U, Schmid C W. Mol Cell Biol. 1990;10:5424–5432. doi: 10.1128/mcb.10.10.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutcliffe J G, Milner R J, Gottesfeld J M, Lerner R A. Nature (London) 1984;305:237–241. doi: 10.1038/308237a0. [DOI] [PubMed] [Google Scholar]
- 38.Tiedge H, Fremeau R T, Weinstock P H, Arancio O, Brosius J. Proc Natl Acad Sci USA. 1991;88:2093–2097. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasseur M, Condamine H, Deprep P. EMBO J. 1985;4:1749–1753. doi: 10.1002/j.1460-2075.1985.tb03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson J B, Sutcliff J G. Mol Cell Biol. 1987;7:3324–3327. doi: 10.1128/mcb.7.9.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson D P, Ross J. Mol Cell Biol. 1986;6:3278–3282. doi: 10.1128/mcb.6.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung J, Sussman D J, Zeller R, Leder P. Cell. 1987;51:1001–1008. doi: 10.1016/0092-8674(87)90586-1. [DOI] [PubMed] [Google Scholar]
- 43.Huang W, Pruzan R, Flint S J. Proc Natl Acad Sci USA. 1994;91:1265–1269. doi: 10.1073/pnas.91.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saffer J D, Thurston S J. Mol Cell Biol. 1989;9:355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saksela K, Baltimore D. Mol Cell Biol. 1993;13:3698–3705. doi: 10.1128/mcb.13.6.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sussman D J, Chung J, Leder P. Nucleic Acids Res. 1991;19:5045–5052. doi: 10.1093/nar/19.18.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorey I S, Cecena G, Reynolds W, Oshima R G. Mol Cell Biol. 1993;13:6742–6751. doi: 10.1128/mcb.13.11.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomilin N V, Iguchi-Ariga S M M, Ariga H. FEBS Lett. 1990;263:69–72. doi: 10.1016/0014-5793(90)80707-p. [DOI] [PubMed] [Google Scholar]
- 49.Winoto A, Baltimore D. Cell. 1989;59:649–655. doi: 10.1016/0092-8674(89)90010-x. [DOI] [PubMed] [Google Scholar]