Abstract
Background
Vascular endothelial growth factor (VEGF) plays an important role in the growth and metastatic progression of melanoma. Exposure of melanoma cells to chemotherapy induces VEGF overproduction, which, in turn, may allow melanoma cells to evade cell death and become chemotherapy resistant. Therefore, in patients with metastatic melanoma, the combination of chemotherapy with an agent that specifically targets VEGF might be able to control tumor growth and progression more effectively than chemotherapy alone.
Methods
A two-stage phase II clinical trial was conducted in patients with unresectable stage IV (metastatic) melanoma to assess anti-tumor activity and toxicity profile of the combination of carboplatin (AUC 6 IV on day 1 of a 28-day cycle), paclitaxel (80 mg/m2 IV on days 1, 8 and 15), and bevacizumab (10 mg/kg IV on days 1 and 15). Treatment was continued until progression or intolerable toxicity.
Results
Fifty-three patients (62.3% male) were enrolled. Nine patients (17%) achieved partial remission and another 30 (57%) stable disease for at least 8 weeks. Median PFS and median overall survival were 6 months and 12 months, respectively. One patient died after 8 treatment cycles from intracranial hemorrhage into undiagnosed brain metastases. The most common severe (≥ grade 3) toxicities were neutropenia (53%), thrombocytopenia (11%), hypertension (9%), and anemia (8%).
Conclusion
This combination of carboplatin, paclitaxel, and bevacizumab appears to be moderately well tolerated and clinically beneficial in patients with metastatic melanoma. Further study of this combination is warranted.
Keywords: Metastatic melanoma, angiogenesis, chemotherapy, phase II trials
INTRODUCTION
The growth of solid tumors depends to a large extent on angiogenesis (1). After a tumor grows beyond 100 µm to 200 µm in size, diffusion alone is insufficient to maintain tumor oxygenation and the development of new blood vessels becomes necessary for continued tumor growth. Angiogenesis involves the recruitment of sprouting vessels from existing blood vessels and incorporation of endothelial progenitors into the growing vascular bed.
Vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, is the most potent, specific and well-defined soluble mediator of angiogenesis (2). VEGF appears to play a crucial role in the pathogenesis, growth and metastatic progression of melanoma (3–8). Anti-VEGF therapy has been shown to inhibit growth in human melanoma xenografts (9). In addition, exposure of melanoma cells with a non-aggressive phenotype to dacarbazine (DTIC) results in the acquisition of a much more tumorigenic and metastatic phenotype (10) through, at least in part, overproduction of VEGF, which may render both endothelial and cancer cells resistant to chemotherapy through a variety of mechanisms: (a) enhancement of tumor growth through induction of angiogenesis; (b) impairment of delivery of chemotherapy to the tumor through increase in interstitial fluid pressure (11); (c) protection of tumor-associated endothelial cells against cytotoxicity (12); and (d) initiation of autocrine survival signals in cancer cells (13,14).
Bevacizumab is a monoclonal antibody that binds VEGF-A (the most common VEGF isoform) and blocks binding to its receptors (15). In comparison with conventional chemotherapy, the antiangiogenic effects of bevacizumab are indirect (through inhibition of VEGF) and not necessarily lethal, and this is probably why as a single agent, bevacizumab is not very active in patients with metastatic melanoma (and other malignancies) (16). However, the addition of bevacizumab to conventional chemotherapy has been shown to control tumor growth and progression more effectively than chemotherapy alone in patients with metastatic non-small cell lung cancer (NSCLC) (17) and colon cancer (18,19). This is probably explained by bevacizumab’s ability to dampen (or even block) the effects of VEGF up-regulation induced by chemotherapy.
Because of the role that VEGF appears to play in the resistance of malignant melanoma to chemotherapy and the proven synergy between chemotherapy and bevacizumab in other malignancies, we decided to explore the safety and efficacy of a combination of carboplatin and paclitaxel with bevacizumab in patients with metastatic melanoma. Though the combination of paclitaxel and carboplatin is not commonplace in the treatment of metastatic melanoma, we selected this chemotherapy regimen because its use in combination with bevacizumab had already been shown to be clinically beneficial in patients with metastatic NSCLC (17), and data from prospective studies (20–23) as well as our own experience with this combination (24) suggested that its efficacy in patients with metastatic melanoma was at least comparable to that of DTIC or temozolomide.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients were required to have histologically confirmed unresectable metastatic melanoma. Additional eligibility criteria included bi-dimensionally measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, life expectancy ≥ 4 months, and adequate hematologic, hepatic and renal function (including urinary excretion of < 1g of protein per day if urine protein to creatinine [UPC] ratio > 0.5). Exclusion criteria included: prior chemotherapy with carboplatin, paclitaxel or agents known to disrupt VEGF activity; known central nervous system metastases; radiographic evidence of tumor invasion of major blood vessels or a hollow viscus; grade 2 peripheral neuropathy; ongoing need for full dose oral or parenteral anticoagulation; active bleeding or bleeding diathesis; serious, non-healing wound, poorly controlled high blood pressure despite appropriate treatment; major surgical procedure, significant traumatic injury or history of abdominal fistula, gastrointestinal perforation or intraabdominal abscess ≤ 4 weeks prior to registration. All patients provided written informed consent in accordance with institutional review board requirements. The study was approved by the institutional review boards of all participating centers and conducted in accordance with the US Food and Drug Administration’s (FDA) Good Clinical Practice requirements.
Study Design and Treatment
Patients were treated with bevacizumab 10 mg/kg IV on days 1 and 15 of a 28-day cycle, paclitaxel 80 mg/m2 IV on days 1, 8 and 15, and carboplatin AUC 6 IV on day 1. Paclitaxel was administered over 1 hour after completion of bevacizumab infusion. Carboplatin was administered over 15 to 30 minutes after completion of the paclitaxel infusion. On day 1 of the cycle, dose reductions were permitted based on interval toxicity, including febrile neutropenia, absolute neutrophil count < 1,000/mm3, platelet count < 75,000/mm3, grade 3/4 nausea or vomiting not controlled by antiemetics, and grade 2 motor or sensory neuropathy. Paclitaxel and carboplatin were held on day 1 if ANC < 1500/mm3, platelet count < 100,000/mm3 or there were any grade 3 or 4 toxicities until counts recovered or toxicity returned to baseline or ≤ grade 1. If ANC <1000/mm3 or platelet count < 50,000/mm3 on days 8 or 15, paclitaxel was omitted. If platelet count was between 50,000 and 75,000/mm3 on days 8 or 15, the dose of paclitaxel was reduced. A maximum of 2 dose reductions were allowed for both carboplatin and paclitaxel. The first dose reduction resulted in a target AUC of 5 for carboplatin and a dose of 65 mg/m2 for paclitaxel; the second dose reduction resulted in a target AUC of 4 for carboplatin and a dose of 50 mg/m2 for paclitaxel. Bevacizumab was administered by intravenous infusion over 90 minutes. If the first infusion was well tolerated, subsequent infusions were shortened to a minimum of 30 minutes. The dose of bevacizumab was not modified during this study. All patients received standard supportive care, including antiemetics, antibiotics and blood and platelet transfusions, as appropriate. Granulocyte colony-stimulating and recombinant erythropoietin were administered at the discretion of the treating physician.
Study Parameters
Within 14 days of registration, patients underwent a complete physical exam, assessment of ECOG PS, complete blood cell count (CBC), comprehensive metabolic panel including lactic dehydrogenase (LDH), measurement of UPC ratio and baseline radiographic tumor assessment. All patients underwent a brain imaging to rule out brain metastases. Blood was also drawn to measure baseline VEGF levels. After initiation of treatment, physical exams, toxicity evaluations and serum chemistries were repeated every 4 weeks. CBC was measured weekly during treatment. UPC ratio was measured ≤ 48 hours prior to each bevacizumab infusion. Additional blood was drawn for correlative studies (VEGF levels) on day 1 of cycles 2, 3, 4 and every even cycle thereafter during treatment and at discontinuation of treatment. Tumor status was assessed every 8 weeks during treatment using RECIST criteria.
Quantitation of plasma VEGFA
Blood samples for analysis of plasma VEGFA levels were collected before initiation of therapy and prior to every subsequent cycle of therapy (every 4 weeks). Platelet-poor plasma was collected from heparinized vacutainer tubes and immediately frozen for storage at −80°C until analysis. To minimize inter-assay variability, all samples were analyzed in the same assay using a commercial human VEGFA ELISA kit, following manufacturer’s instructions (R&D Systems Inc. Minneapolis, MN).
Statistical Considerations
The primary endpoint of this trial was the 8-week event-free survival (EFS) rate, which is defined as the percentage of eligible patients who remain progression-free and on study treatment for at least 56 days post-registration divided by the number of eligible patients who began treatment. A Simon two-stage phase II clinical trial design enrolling a minimum of 20 or a maximum of 47 eligible patients was chosen to test whether the true 8-week event-free rate was at most 35% against the alternative hypothesis that the true 8-week event-free rate was at least 55% (25). If at most 7 (20) of the first 20 (47) eligible patients remained progression-free and on treatment for at least 8 weeks, the regimen would not be recommended for further testing in this patient population. If at least 21 of the 47 eligible patients remained progression-free and on treatment for at least 8 weeks without excessive toxicity, the regimen would be recommended for further investigation in this patient population. With the significance level set at 0.10, the power to detect that the 8-week event-free rate was greater than 35% when it was at least 55% was 90%.
The trial was designed with a stopping rule for excessive toxicity which required that enrollment be suspended, adverse event data examined, and a trial recommendation formulated for consideration by the DSMC and CTEP. Excessive toxicity was considered to be when 3 or more of the first 10 study patients or 30% or more of study patients at any point after 11 patients have been enrolled develop a grade 3+ non-hematologic toxicity or grade 4+ hematologic toxicity considered to be possibly, probably or definitely related to treatment.
A 90% confidence interval for the 8-week event-free rate was constructed using the Duffy-Santer approach (26). Tumor response rate was defined as the number of patients whose disease status met the RECIST criteria for complete or partial response on two consecutive evaluations 8 weeks apart (as determined by patient’s institution) divided by the number of eligible patients who started study treatment. Progression-free survival time was defined as the time elapsed from registration to progression of disease or death without prior documentation of progressive disease. Survival time was defined as the time elapsed from registration to death due to any cause. Time to event distributions was estimated using the Kaplan- Meier method (27). Toxicities were graded and attribution assigned by treating physicians using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. A Spearman rank correlation coefficient was used to examine the association between tumor burden and pre-treatment VEGF levels.
RESULTS
Patient Characteristics
Fifty-three patients (62.3% male) were enrolled onto the study between December 30, 2005 and November 3, 2006. None of the patients were found to be ineligible or canceled participation prior to the start of treatment after signing the consent form. The characteristics of these patients are presented in Table 1. The median age at enrollment was 55 years (range 30–84). Prior therapies included immunotherapy (52.8%), radiation therapy (26.4%), chemotherapy (24.5%) or vaccines (13.2%). AJCC M classification was M1 for 7 patients (13%), M2 for 3 patients (6%), M3 for 42 patients, and unknown for 1 patient with soft tissue disease but unknown LDH level.
Table 1.
Selected Demographic and Baseline Characteristics
| Patient Characteristics | n=53 |
|---|---|
| Median Age (range) | 55 (range 30–84) |
| Male | 62.3% |
| M Stage | |
| M1: distant skin, subcutaneous, or lymph node metastases with LDH in normal range | 13% |
| M2: lung metastases with LDH in normal range | 6% |
| M2: all other visceral or any distant metastases with LDH in normal range or any metastatic disease with elevated LDH | 79% |
| unknown | 2% |
| Prior Systemic Therapies | |
| None | 28.3% |
| Immunotherapy | 52.8% |
| Adjuvant setting | |
| Interferon | 30.0% |
| GM-CSF | 11.3% |
| Metastatic setting | |
| Interferon or IL-2 | 9.4% |
| Other | 11.3% |
| Radiation therapy | 26.4% |
| Chemotherapy | 24.5% |
| Vaccine therapy | 13.2% |
| ECOG Performance Status | |
| 0 | 81.1% |
| 1 | 17.0% |
| 2 | 1.9% |
| Pre-existing Signs and Symptoms | |
| Grade 3 hypertension | 1.9% |
| Grade 2 fatigue | 7.6% |
| Grade 2 anorexia | 5.6% |
| Grade 2 nausea | 1.9% |
| Grade 2 musculoskeletal pain | 1.9% |
| Serum LDH pre-treatment | |
| < 240 U/I | 73.5% |
| ≥ 240 U/I | 20.8% |
| Not Done | 5.7% |
Treatment Status
At the time of this report, all patients have discontinued active treatment. A total of 330 cycles of treatment were administered. The median number of cycles administered was 5 (range 1–17). Dose reductions were required for 33 patients (62.2%). Nineteen patients (36%) received G-CSF support. The most common reason for dose reduction/omission was severe hematology toxicity. The most common severe (≥ grade 3) toxicities (possibly, probably or definitely related to treatment) were neutropenia (53%), leukopenia (38%), thrombocytopenia (11%), anemia (8%), hypertension (9%), nausea (6%), hypersensitivity (6%) and fatigue (6%) (Table 2). There were 40 instances of hemorrhaging reported among 31 patients. Four of these events were more severe than a grade 1, namely, two grade 2 bronchopulmonary hemorrhages, one grade 1 intra-abdominal hemorrhage and one case of grade 5 central nervous system hemorrhage.
Table 2.
Hemorrhaging, Infections, and the Most Common Severe toxicities reported as possibly, probably or definitely related to treatment among the 53 patients enrolled
| Toxicity | Any Grade | ≥ grade 3 |
|---|---|---|
| Most Common Severe Toxicities Reported | ||
| Neutropenia | 75% | 53% |
| Leukopenia | 79% | 38% |
| Anemia | 92% | 8% |
| Thrombocytopenia | 64% | 11% |
| Fatigue | 89% | 6% |
| Nausea | 53% | 6% |
| Hypertension | 30% | 9% |
| Hypersensitivity | 9% | 6% |
| Infection | ||
| Cather-related infection | 4% | 4% |
| Bladder infection | 4% | 2% |
| Febrile Neutropenia | 2% | 2% |
| Other infections* including nail bed, pharynx sinus, upper airway, urinary tract, NOS | 15% | 0% |
| Hemorrhaging | ||
| CNS hemorrhage | 4% | 2% |
| Epistaxis | 19% | 0% |
| Broncho-pulmonary hemorrhage | 34% | 0% |
| Intra-abdominal hemorrhage | 8% | 0% |
| Nasal, rectal, urogenial, vaginal or NOS hemorrhaging | 9% | 0% |
at most 2 patients had a particular kind of the infection listed
The reason for treatment discontinuation included: tumor progression (39 patients.), refusal (5 patients.), adverse events including hypersensitivity reaction to carboplatin (1 patient), sensory neuropathy (1 patient), motor neuropathy (1 patient), hypertension and proteinuria (1 patient) and catheter-related infection (1 patient), desire for alternative treatment (3 patients), and death due to hemorrhaging into a previously undiagnosed brain metastases (1 patient).
Clinical Efficacy
Patients were followed for a minimum of 8.5 months or until death. Among the 53 patients enrolled, 9 (17%) achieved a partial remission (PR) and an additional 30 (57%) completed 2 cycles of treatment with stable disease. No complete remissions (CR) were observed. Thus, the 8-week event-free rate among these 53 patients was 74% (90% CI: 62–83%). Of the 13 patients who had received chemotherapy in the adjuvant or metastatic setting, 3 achieved a partial remission and an additional 7 patients completed 2 cycles of treatment with stable disease.
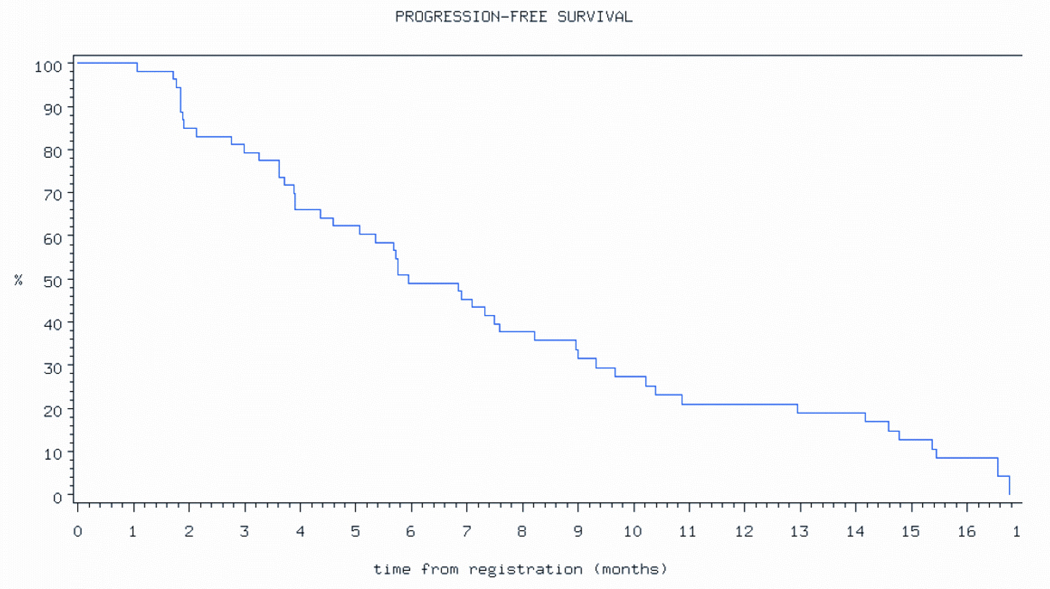
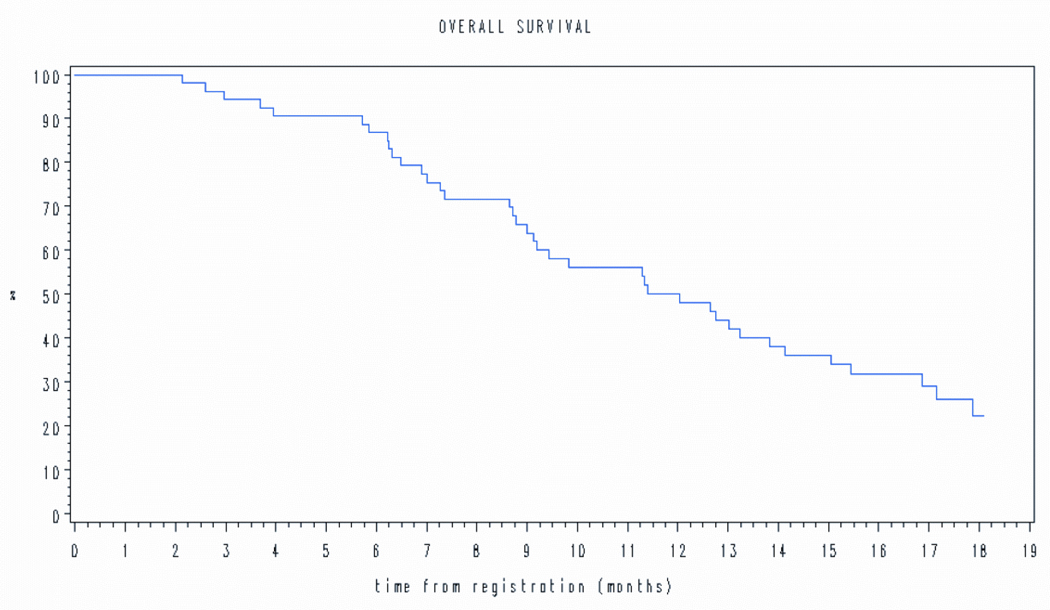
At last follow-up, 4 patients are alive without disease progression, 9 are alive with disease progression, and 40 have died of disease. The estimated median PFS was181 days (6 months) (Figure 1a) and median overall survival (OS) was 366 days (12 months) (Figure 1b).
Figure 1.
Progression free survival.
Tumor Response by M stage
Tumor response among the 10 patients with M1 or M2 disease was such that 1 patient had a partial remission and 4 completed 2 cycles of treatment with stable disease. Tumor response among the 42 patients with M3 disease (29 with LDH > ULN) was such that 8 patients (2 with LDH > ULN) had a partial remission and 26 (14 with LDH > ULN) completed 2 cycles of treatment with stable disease.
Changes in plasma VEGF levels
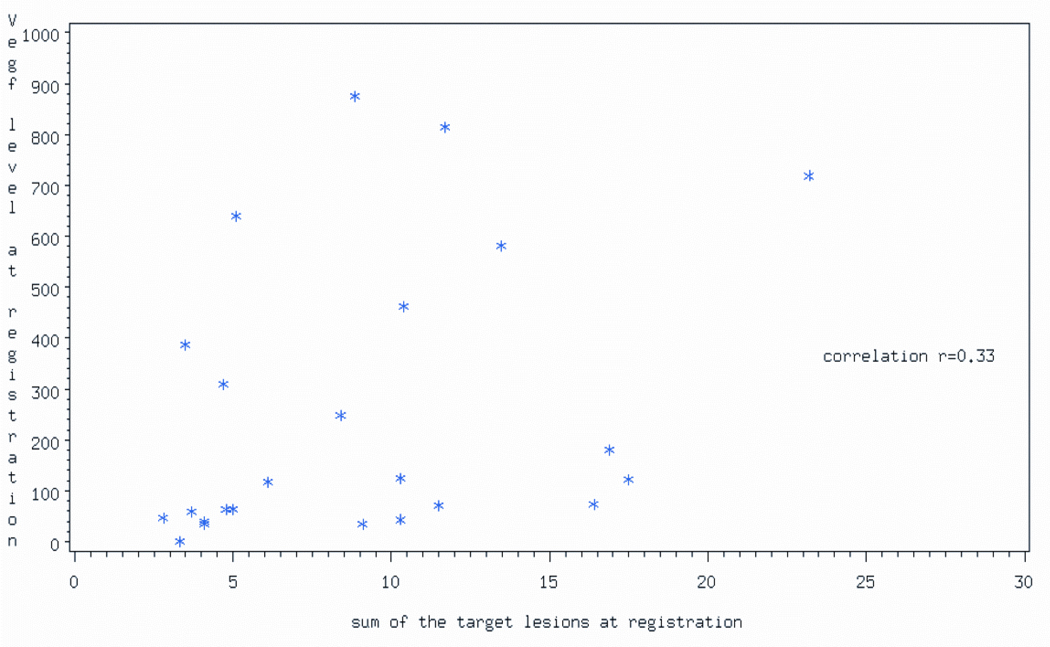
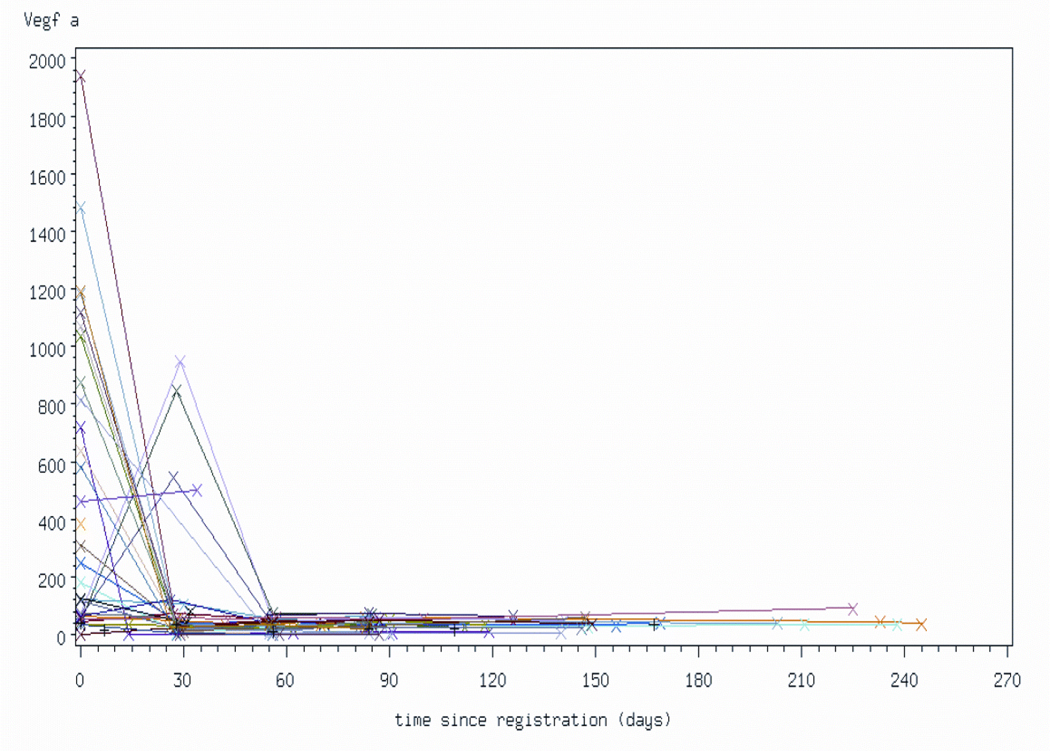
Thirty-one patients had pre-treatment VEGF-A levels measured. The median pre-treatment VEGF-A level was 181.1 pg/mL (range:1.3 –1938.6). There was weak positive correlation between tumor burden (estimated by the sum of the greatest diameters of the target lesions) and pre-treatment VEGF-A levels (rho = 0.33) (Figure 3). Of the 29 patients who had pre-treatment and week 4 VEGF-A measurements, 80% had VEGF-A levels below 85 pg/mL at week 4. (Figure 4). All of these 29 patients have had progression of disease. The percent change in VEGF-A levels after 4 weeks of treatment was not found to be associated with the amount of time the patient remained progression-free (Spearman rho=0.090)
Figure 3.
Correlation between serum VEGFA levels and sum of diameters (cm) of tumor target lesions at time of registration.
Figure 4.
Changes in plasma VEGFA levels as a function of protocol therapy. Patients underwent measurements of plasma VEGFA levels (pg/mL) prior to therapy and at monthly intervals while on treatment. All samples were analyzed in the same experiment. Last sample was analyzed at the time of discontinuation of protocol therapy. Each line represents data from an individual patient.
DISCUSSION
The results of the present study suggest that the combination of carboplatin, weekly paclitaxel and biweekly bevacizumab has therapeutic activity in patients with metastatic melanoma. Treatment was moderately well tolerated with the incidence and severity of nausea/vomiting, neuropathy and renal toxicity at levels comparable to those observed with chemotherapy alone. The percentage of patients who developed severe (grade 3+) bleeding events with this regimen was 2%. This percentage is similar to the 3.7% (36/963) found by Scappaticci et al in a pooled analysis of 5 randomized trials of chemotherapy in combination with bevacizumab (28). The PFS and OS observed in our study compare favorably with those seen with single-agent temozolomide or dacarbazine (Table 3) as well as in other phase II studies conducted by the cooperative cancer groups in the United States (29). Obviously, without a well-designed randomized clinical trial of carboplatin and paclitaxel with or without bevacizumab in patients with metastatic melanoma it is impossible to know the relative value of adding bevacizumab to carboplatin and paclitaxel in this patient population. However, since the PFS and OS observed in our study also compare favorably to those seen in other prospective studies of the combination of carboplatin and bevacizumab (Table 3) and the addition of bevacizumab to carboplatin and paclitaxel has already been shown to increase the efficacy of this combination in patients with NSCLC, we believe that further study in the form of a randomized clinical trial is justified.
Table 3.
Results of prospective studies of the combination of carboplatin and paclitaxel in patients with metastatic melanoma
| Temozolomide (20) | Dacarbazine (20) | Current Study | Hodi (21) | Zimpfer-Rechner (22) | Agarwala (23) | |
|---|---|---|---|---|---|---|
| No. of patients | 156 | 149 | 53 | 15 | 19 | 135 |
| Median age in years | 58.5 | 58.8 | 55 | 54 | 57.6 | 56 |
| Prior chemotherapy (% patients) | 0 | 0 | 24.5% | 0 | 100% | 100% |
| Carboplatin/Paclitaxel Schedule | NA | NA | Hybrid (plus bevacizumab) | Every-3-weeks | Weekly | Every-3-weeks |
| CR | 3% | 3% | 0 | 0 | 0 | 0 |
| PR | 11% | 9% | 17% | 20% | 0 | 11% |
| Median PFS (weeks) | 7.6 | 6 | 26 | Not reported | 8 | 17.9 |
| Median OS (weeks) | 30.8 | 25.6 | 52 | 36 | 30 | 42 |
Siegal et al reported that the serum VEGF-A levels of patients with unresectable hepatocellular carcinoma receiving bevacizumab significantly decreased after 8 weeks of treatment and then increased to near pre-treatment levels at the time of progression (30). This study also found decreases in serum VEGF-A levels with 4 weeks of chemotherapy and bevacizumab but serum VEGF-A levels remained at these low levels at disease progression.
The results of phase II studies of different schedules of the combination of carboplatin and paclitaxel in patients with metastatic melanoma vary widely (Table 3). In the absence of data from randomized clinical trials, it is unclear which carboplatin/paclitaxel schedule is better or even best suited for combination with bevacizumab. In patients with metastatic NSCLC, carboplatin, paclitaxel and bevacizumab are given every three weeks (17). Clearly, paclitaxel/carboplatin administered every three weeks appear to have clinical activity in patients with metastatic melanoma (23). For our study, we chose to deliver paclitaxel on a weekly schedule in an attempt to increase the therapeutic index of the study regimen and enhance the intrinsic antiangiogenic properties of paclitaxel (31). To accomplish these goals, we considered delivering both carboplatin and placlitaxel on a weekly schedule. However, the poor results obtained by Zimpfer-Rechner et al. with weekly carboplatin/paclitaxel made this schedule less appealing. In addition, at the time of designing our study, data from a randomized phase II trial in patients with advanced NSCLC suggested that a “hybrid” schedule similar to the one used in our study had a better therapeutic index relative to weekly therapy (32).
In our study, we used the Taxol™ formulation of paclitaxel. However, it is not entirely clear that this is the best formulation of paclitaxel, particularly when used in combination with antiangiogenic agents. There has been concern about the possibility that clinically relevant concentrations of the formulation vehicle Cremophor-EL in Taxol™ could impair the antiangiogenic effects of paclitaxel (33). To explore this hypothesis, Ng et al. compared the antitumor effects of metronomic and maximum tolerated dose ABI-007, the albumin-bound, Cremofor-EL-free formulation of paclitaxel, and Taxol™ in two cancer xenograft models (34). Metronomic ABI-007, but not Taxol™, significantly suppressed tumor growth in their animal models. Moreover, the antitumor effect of minimally toxic metronomic ABI-007 approximated that of the maximum tolerated dose of Taxol™. Weekly ABI-007 has already been shown to be well tolerated and able to produce long-term disease control in patients with metastatic melanoma who have experienced rapid progression on other treatments (35). The clinical utility of the combination of ABI-007 and carboplatin in patients with metastatic melanoma is currently being evaluated by the North Central Cancer Treatment Group (protocol N057E).
In summary, the results of our study suggest that the combination of chemotherapy with an agent that specifically targets VEGF might be a valid therapeutic strategy for patients with metastatic melanoma. Further study is necessary to determine the relative value of bevacizumab in combination with carboplatin and paclitaxel, the best carboplatin/paclitaxel schedule and the best formulation of paclitaxel (Taxol™ versus ABI-007) in this patient population.
Figure 2.
Overall survival.
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-35267, CA-60276, CA-35101, CA-35090, CA-52352, CA-35195, CA-35269, CA-35431, CA-35103, CA-63849.
REFERENCES
- 1.Parikh AA, Ellis LM. The vascular endothelial growth factor family and its receptors. Hematol Oncol Clin North Am. 2004;18:951–971. doi: 10.1016/j.hoc.2004.06.004. vii. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A, Ralhan R, Kaur J. Angiogenesis in cutaneous melanoma: pathogenesis and clinical implications. Microsc Res Tech. 2003;60:208–224. doi: 10.1002/jemt.10259. [DOI] [PubMed] [Google Scholar]
- 4.Graells J, Vinyals A, Figueras A, et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol. 2004;123:1151–1161. doi: 10.1111/j.0022-202X.2004.23460.x. [DOI] [PubMed] [Google Scholar]
- 5.Streit M, Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003;22:3172–3179. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]
- 6.Demirkesen C, Buyukpinarbasili N, Ramazanoglu R, Oguz O, Mandel NM, Kaner G. The correlation of angiogenesis with metastasis in primary cutaneous melanoma: a comparative analysis of microvessel density, expression of vascular endothelial growth factor and basic fibroblastic growth factor. Pathology. 2006;38:132–137. doi: 10.1080/00313020600557565. [DOI] [PubMed] [Google Scholar]
- 7.Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer. 1997;76:930–934. doi: 10.1038/bjc.1997.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 9.Oku T, Tjuvajev JG, Miyagawa T, et al. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998;58:4185–4192. [PubMed] [Google Scholar]
- 10.Lev DC, Onn A, Melinkova VO, et al. Exposure of melanoma cells to dacarbazine results in enhanced tumor growth and metastasis in vivo. J Clin Oncol. 2004;22:2092–2100. doi: 10.1200/JCO.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 12.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- 14.Lacal PM, Failla CM, Pagani E, et al. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 16.Varker KA, Biber JE, Kefauver C, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–2376. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 17.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 18.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 19.Giantonio BJ, Catalano PJ, Meropol NJ, et al. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200. J Clin Oncol. 2005;23:1s. (Abstract) [Google Scholar]
- 20.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, Soiffer RJ, Clark J, Finkelstein DM, Haluska FG. Phase II study of paclitaxel and carboplatin for malignant melanoma. Am J Clin Oncol. 2002;25:283–286. doi: 10.1097/00000421-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Zimpfer-Rechner C, Hofmann U, Figl R, et al. Randomized phase II study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG) Melanoma Res. 2003;13:531–536. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Agarwala SS, Keilholz U, Hogg D, et al. Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma. J Clin Oncol. 2007;25(18S):8510. (Abstract) [Google Scholar]
- 24.Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006;106:375–382. doi: 10.1002/cncr.21611. [DOI] [PubMed] [Google Scholar]
- 25.Simon R. Optimal two-stage designs for Phase II clinical trial. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Duffy DE, Santner TJ. Confidence intervals for binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JASA. 1958;53:457–481. [Google Scholar]
- 28.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 29.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 30.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vacca A, Ribatti D, Iurlaro M, et al. Docetaxel versus paclitaxel for antiangiogenesis. J Hematother Stem Cell Res. 2002;11:103–118. doi: 10.1089/152581602753448577. [DOI] [PubMed] [Google Scholar]
- 32.Belani CP, Barstis J, Perry MC, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;21:2933–2939. doi: 10.1200/JCO.2003.02.563. [DOI] [PubMed] [Google Scholar]
- 33.Ng SS, Figg WD, Sparreboom A. Taxane-mediated antiangiogenesis in vitro: influence of formulation vehicles and binding proteins. Cancer Res. 2004;64:821–824. doi: 10.1158/0008-5472.can-03-3391. [DOI] [PubMed] [Google Scholar]
- 34.Ng SS, Sparreboom A, Shaked Y, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006;12:4331–4338. doi: 10.1158/1078-0432.CCR-05-2762. [DOI] [PubMed] [Google Scholar]
- 35.Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]