Abstract
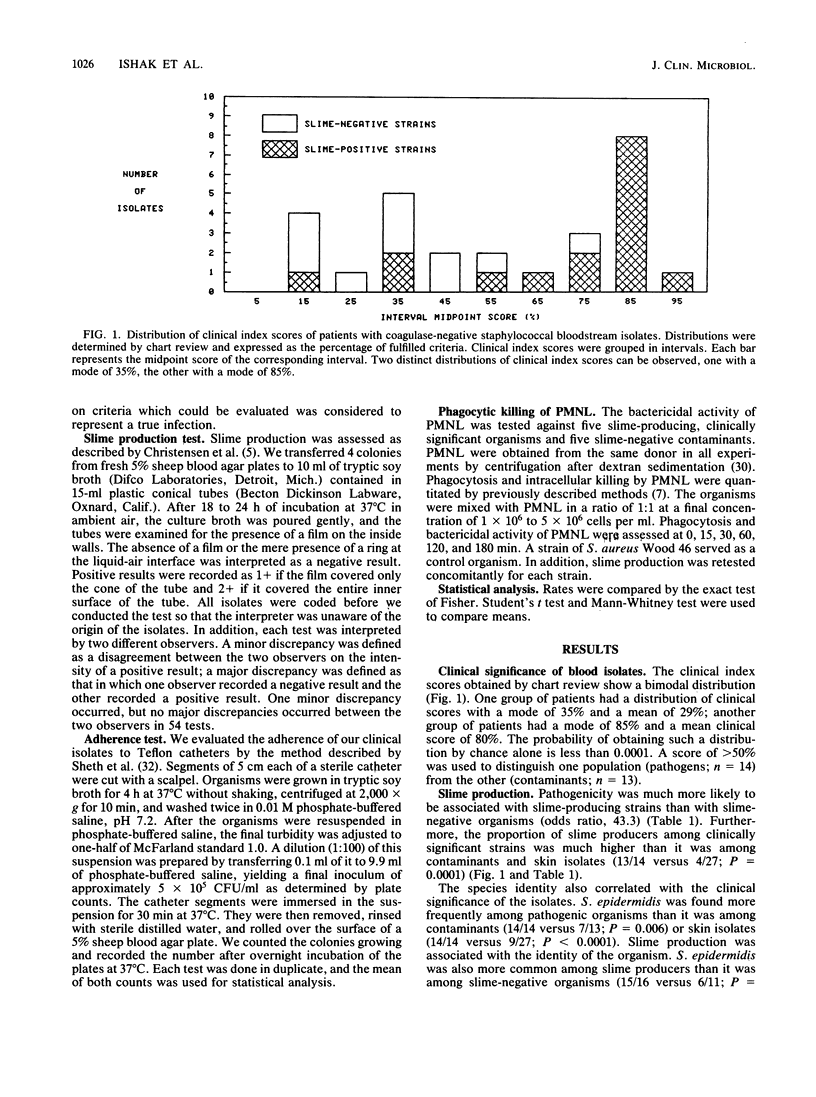
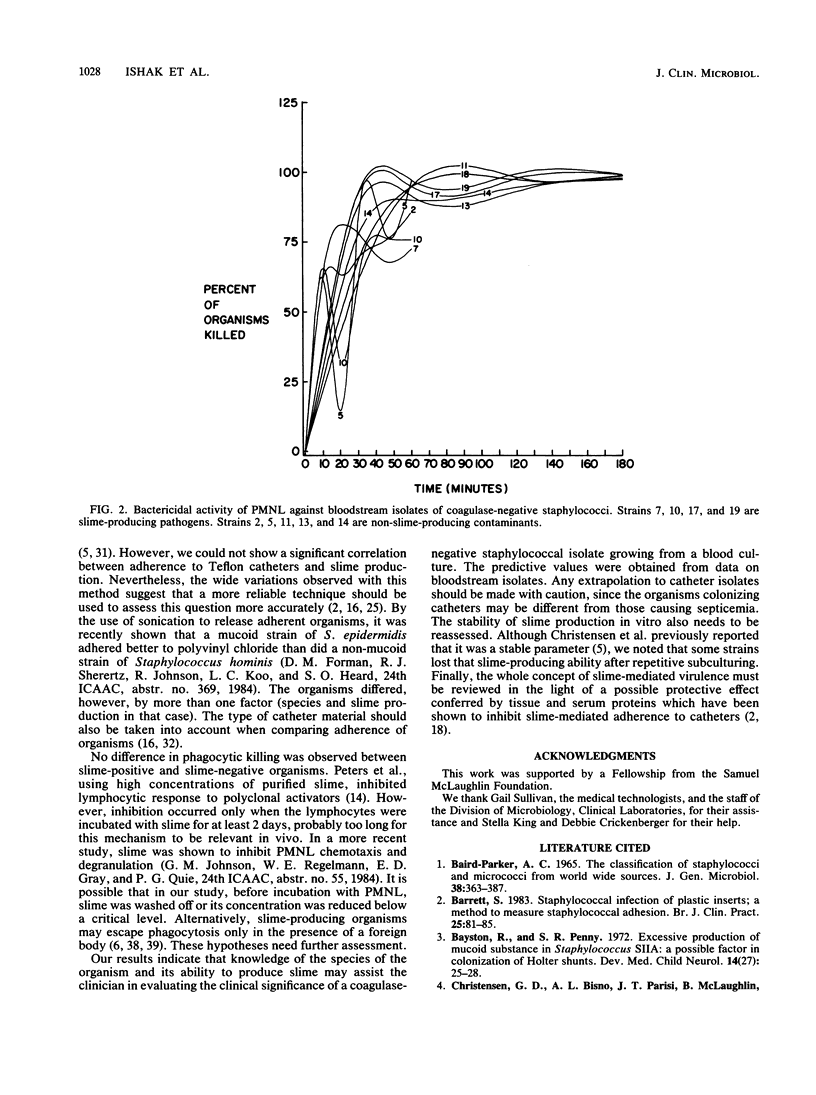
To assess the role of slime in the pathogenesis of nosocomial bloodstream infections caused by coagulase-negative staphylococci, we compared the characteristics of 27 nosocomial bloodstream isolates with those of 27 skin isolates from non-hospital personnel. Of 27 bloodstream isolates, 14 were judged to be significant by a clinical index, and 13 were contaminants. Slime production was observed in 13 of 14 significant isolates but in only 3 of 13 contaminants (P = 0.0003) and 4 of 27 skin isolates (P = 0.0001). The 14 pathogens were identified as Staphylococcus epidermidis. Only 7 of 13 contaminants and 9 of 27 skin isolates belonged to the same species (P less than 0.006). Slime-producing strains of S. epidermidis represented 13 of 14 pathogens but only 2 of 13 contaminants (P less than 0.0003). Neither adherence to Teflon catheters nor phagocytosis and killing of coagulase-negative staphylococci by polymorphonuclear leukocytes was significantly influenced by slime production. Nevertheless, the identity of the organism and the slime production test predicted the clinical significance of blood isolates of coagulase-negative staphylococci with an overall accuracy of 89%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller A., Schleifer K. H. Modified oxidase and benzidine tests for separation of staphylococci from micrococci. J Clin Microbiol. 1981 Jun;13(6):1031–1035. doi: 10.1128/jcm.13.6.1031-1035.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forse R. A., Dixon C., Bernard K., Martinez L., McLean A. P., Meakins J. L. Staphylococcus epidermidis: an important pathogen. Surgery. 1979 Sep;86(3):507–514. [PubMed] [Google Scholar]
- Fowler J. E., Jr, Stamey T. A. Studies of introital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J Urol. 1977 Apr;117(4):472–476. doi: 10.1016/s0022-5347(17)58501-8. [DOI] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Grieble H. G., Krause S. L., Pappas S. A., DiCostanzo M. B. The prevalence of high-level methicillin resistance in multiply resistant hospital staphylococci. Medicine (Baltimore) 1981 Jan;60(1):62–69. doi: 10.1097/00005792-198101000-00006. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., de Vries J. A., Feijen J. Adhesion of coagulase-negative staphylococci to biomaterials. J Gen Microbiol. 1983 Sep;129(9):2959–2968. doi: 10.1099/00221287-129-9-2959. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Katz S., Izhar M., Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection. Ann Surg. 1981 Jul;194(1):35–41. doi: 10.1097/00000658-198107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Wolfshohl J. F. Identification of Staphylococcus species with the API STAPH-IDENT system. J Clin Microbiol. 1982 Sep;16(3):509–516. doi: 10.1128/jcm.16.3.509-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laharrague P. F., Corberand J. X., Fillola G., Gleizes B. J., Fontanilles A. M., Gyrard E. In vitro effect of the slime of Pseudomonas aeruginosa on the function of human polymorphonuclear neutrophils. Infect Immun. 1984 Jun;44(3):760–762. doi: 10.1128/iai.44.3.760-762.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liekweg W. G., Jr, Greenfield L. J. Vascular prosthetic infections: collected experience and results of treatment. Surgery. 1977 Mar;81(3):335–342. [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. I. Microtopographical characteristics of intravenous catheters as detected by scanning electron microscopy. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):285–292. [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. III. Adhesion of staphylococci to lumina of intravenous catheters perfused with bacterial suspensions. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):300–307. [PubMed] [Google Scholar]
- Lowder J. N., Lazarus H. M., Herzig R. H. Bacteremias and fungemias in oncologic patients with central venous catheters: changing spectrum of infection. Arch Intern Med. 1982 Aug;142(8):1456–1459. [PubMed] [Google Scholar]
- Malmvall B. E., Alestig K., Dottori O., Seeberg S. Septicaemia in patients with central vein catheters. An evaluation of a method including weekly exchange of catheter. Acta Chir Scand. 1980;146(3):155–159. [PubMed] [Google Scholar]
- McNeish A. S., Turner P., Fleming J., Evans N. Mucosal adherence of human enteropathogenic Escherichia coli. Lancet. 1975 Nov 15;2(7942):946–948. doi: 10.1016/s0140-6736(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Sheth N. K., Rose H. D., Franson T. R., Buckmire F. L., Sohnle P. G. In vitro quantitative adherence of bacteria to intravascular catheters. J Surg Res. 1983 Mar;34(3):213–218. doi: 10.1016/0022-4804(83)90062-8. [DOI] [PubMed] [Google Scholar]
- Shurtleff D. B., Foltz E. L., Weeks R. D., Loeser J. Therapy of staphylococcus epidermidis: infections associated with cerebrospinal fluid shunts. Pediatrics. 1974 Jan;53(1):55–62. [PubMed] [Google Scholar]
- Sitges-Serra A., Puig P., Jaurrieta E., Garau J., Alastrue A., Sitges-Creus A. Catheter sepsis due to Staphylococcus epidermidis during parenteral nutrition. Surg Gynecol Obstet. 1980 Oct;151(4):481–483. [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Dudnick D. V., Chapin M., Ho W. G., Gale R. P., Martin W. J. Coagulase-negative staphylococcal bacteremia in patients receiving immunosuppressive therapy. Arch Intern Med. 1983 Jan;143(1):32–36. [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]



