Abstract
Objective
To examine the association between number of born children and neuropathology of Alzheimer’s disease (AD).
Methods
The brains of 86 subjects with data on the number of biological children born, were studied postmortem. Primary analyses included 73 subjects (average age at death=80; 42 women) devoid of cerebrovascular disease associated lesions (i.e, infarcts) or of non-AD related neuropathology. Women were significantly older at death than men (85.6 vs. 73.4; p<.0005) but did not differ significantly from men in number of children or dementia severity. Secondary analyses included 13 additional subjects who had concomitant cerebrovascular disease. Density of neuritic plaques (NPs) and neurofibrillary tangles (NFTs) in the hippocampus, entorhinal cortex, amygdala, and multiple regions of the cerebral cortex, as well as composites of these indices reflecting overall neuropathology, were analyzed. For men and women separately, partial correlations, controlling for age at death and dementia severity, were used to assess the associations of number of children with these neuropathological variables.
Results
Among women, all the partial correlations were positive, with statistical significance for overall neuropathology (r=.37;p=.02), overall NPs (r=.36;p=.02), and for NPs in the amygdala (r=0.47; p=.002). Among men, none of the partial correlations were statistically significant. Results of the secondary analyses were similar.
Conclusions
Since the associations between number of children and neuropathology of AD were found for women only, they might reflect sex-specific mechanisms (such as variations in estrogen or luteinizing hormone levels) rather than social, economic, biological or other mechanisms common to both men and women.
Keywords: Alzheimer’s disease, neuropathology, number of children, estrogen, neuritic plaques, neurofibrillary tangles
1. Introduction
Alzheimer’s disease (AD) is more prevalent in women than in men and this differential prevalence remains after adjusting for age and level of education(2;27). Therefore, identifying factors that make women more susceptible to this disease may help to reveal underlying causal mechanisms. Several studies have found associations of measures of fertility with risk and age of onset of AD, as well as with cognitive decline. Having had one or more children, rather than none, was significantly associated with risk of AD in women(12) and in women but not in men(44), and with AD age at onset in women without the APOE4 allele but not in those with it(13). Having children was also associated with cognitive decline based on the Mini Mental State Exam(36). Number of pregnancies was associated with risk of AD (9) and lower age of onset(12;52). However, in other studies, number of children was not associated with risk of AD(44) or age of onset(13). To our knowledge, no previous study has examined the association between number of children and the hallmark neuropathological lesions of AD - neuritic plaques (NPs) and neurofibrillary tangles (NFTs)(7).
The current study examined the associations of number of born children with the severity of NPs and NFTs. These associations were examined in specific brain regions (hippocampus, entorhinal cortex, amygdala, and multiple cerebral cortical regions) as well as in composite measures of the severity of NP and NFT neuropathology, reflecting overall neuropathology across the examined brain areas. This study included samples of both men and women. Dissimilar associations between men and women would suggest the possibility of an underlying biological mechanism on which men and women differ, for example, lifetime hormonal histories. Alternatively, similar associations would suggest the possibility of common socio-demographic, biological, or environmental influences.
2. Methods
2.1 Subjects
Analyses were based on the study of 150 brain donations from the Jewish Home and Hospital (JHH), a nursing home that collaborates with the Mount Sinai School of Medicine for many years in studies of aging and early dementia. All cases had valid data on the number of biological children born to each donor, over a period of 17 years. Forty-seven cases had a primary neuropathological diagnosis of cerebrovascular disease or neuropathology other than AD, and 17 other cases had missing values in at least one of the variables in the analyses. In addition, 13 had a secondary neuropathological diagnosis of cerebrovascular disease (i.e., both AD neuropathology and infarcts). Of the remaining 73 cases (31 men and 42 women), with either a normal brain or a primary neuropathological diagnosis of AD, 6 were categorized by the CERAD neuropathological battery(37) as normal brain, 60 as definite AD, 2 as probable AD, and 5 as possible AD. These subjects did not differ significantly from the excluded subjects in age at death or dementia severity.
Postmortem donations were received by the Mount Sinai School of Medicine Department of Psychiatry Brain Bank from the next of kin of deceased residents of the Jewish Home and Hospital (JHH) in Manhattan, NY and Bronx, NY. Patients with severe psychiatric disorders (e.g. schizophrenia) were excluded from this cohort. Before death, subjects participated in a family study of AD in which at least one relative of each subject was interviewed and also the subject if cognitively intact. All children (i.e. all live births) of the probands were identified and tabulated. As part of the family study, all assessments were approved by the institutional review boards of both the JHH and the Mount Sinai School of Medicine. Autopsies were performed after receiving consent from the legal next of kin. Research staff reviewed detailed medical records, which were available on all subjects, and whenever possible conducted in depth interviews with staff and family caregivers to obtain information about the donor’s antemortem functional and cognitive status.
The Clinical Dementia Rating (CDR) assesses cognitive and functional impairments associated with dementia and provides specific severity criteria for classifying subjects as non-demented (CDR= 0), questionably demented (CDR= 0.5), or increasing levels of severity of dementia from CDR=1 to CDR=5(18;38). A previously described(23;24) multi-step approach was applied to the assignment of postmortem CDR scale scores based on cognitive and functional status during the last 6 months of life. Assessments of CDR were made without prior knowledge of neuropathology findings or parity.
2.2 Neuropathological Assessment
The neuropathological assessment procedures employed have been extensively described previously(23;24) and were performed without knowledge of the donor’s medical, cognitive, parity or functional status. Representative blocks obtained from standardized sites in the superior and midfrontal gyrus, orbital cortex, basal ganglia with basal forebrain, amygdala, hippocampus (rostral and caudal levels with adjacent parahippocampal and inferior temporal cortex), superior temporal gyrus, parietal cortex (angular gyrus), calcarine cortex, hypothalamus with mammillary bodies, thalamus, midbrain, pons, medulla, cerebellar vermis, and lateral cerebellar hemisphere were examined using hematoxylin and eosin, modified Bielschowsky, modified thioflavin S, anti-β amyloid and anti-tau. Any case showing evidence of Lewy body formation in the substantia nigra or locus ceruleus underwent anti-ubiquitin and anti-α-synuclein staining of representative cerebral cortical and subcortical sections for the identification of cortical Lewy bodies(25;48).
Every case was evaluated for the extent of neuropathologic lesions using the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) neuropathologic battery(37). Sections from each of the tissue blocks described above were rated for the extent of NPs and NFTs using the CERAD four-point scale of 0=none, 1=sparse, 3=moderate, or 5=severe, as described previously(23). NP and NFT scores derived from examination of the neocortical blocks were aggregated by addition into a single summary variable, and the entorhinal cortex, hippocampus, and amygdala were used in the primary analyses reported here since previous studies had shown the relevance of NP and NFT densities in these regions with respect to dementia severity(23;24).
Neuropathological and final diagnosis was based on a consensus diagnosis (VH, DPP, HG and clinical neuropsychologists) and was derived after review of all medical, clinical, neuropathological, and other research records according to procedures described in detail previously(23;24).
2.3 Statistical Analysis
The primary analyses included the 73 subjects that had normal brain or only AD-associated neuropathology. Since concomitant cerebrovascular disease is relatively common in the elderly(26), we also performed the same analyses after including the 13 subjects with secondary neuropathological diagnosis of cerebrovascular disease. In addition to analyses for each of the neuropathological dependent variables separately, we created summarizing variables in two ways. We calculated separate sums of the NP ratings and of the NFT ratings in the four areas of interest (hippocampus, entorhinal cortex, amygdala and the cerebral cortex). In addition, a factor analysis of all eight NP and NFT ratings was performed including both men and women. The first principal component accounted for over half of the variation and was used as an overall neuropathology summary variable. Each of the variables was strongly positively associated with the principal component. Partial correlations controlling for age at death and CDR at death were performed to assess the associations between the number of children and each of these eleven measures of neuropathology of AD. The possibility of a non-linear relationship was tested by a stepwise linear regression to add a quadratic association, with the same covariates. A p-value less than or equal to .05 was considered significant.
All analyses were performed separately for men and women. It was not feasible to perform analyses of covariance (ANCOVA) with sex as an additional independent variable since there were significant interactions of sex with age at death or CDR (i.e. regression coefficients significantly different between men and women). Even for dependent variables for which the assumptions of ANCOVA were not violated, the significant difference between ages (see Table 1), argued against a direct comparison of men and women.
Table 1.
Characteristics of the sample (mean ± SD; range)
| Characteristics | Men (n=31) | Women (n=42) | t (df=71), p |
|---|---|---|---|
| Number of children | 2.2 (1.8); 0–8 | 1.8 (1.5); 0–7 | 1.1, .29 |
| Age of death | 73.4 (9.3); 55–94 | 85.6 (10.6); 59–102 | 5.1, <.0005 |
| CDR | 3.7 (1.4); 0–5 | 3.2 (1.5); 0–5 | 1.3, .19 |
| NFTs hippocampus1 | 3.7 (1.7); 0–5 | 3.8 (1.8); 0–5 | .16, .87 |
| NFTs entorhinal cortex1 | 4.2 (1.3); 0–5 | 4.4 (1.1); 1–5 | .43, .67 |
| NFTs amygdala1 | 3.7 (1.7); 0–5 | 2.5 (2.0); 0–5 | 2.7, .01 |
| NFTs cerebral cortex2 | 10.4 (5.1); 0–18 | 7.1 (6.6); 0–20 | 2.3, .03 |
| NPs hippocampus1 | 2.6 (1.7); 0–5 | 1.8 (1.4); 0–5 | 2.0, .045 |
| NPs entorhinal cortex1 | 3.6 (1.7); 0–5 | 2.8 (1.5); 0–5 | 2.1, .04 |
| NPs amygdala1 | 3.6 (1.8); 0–5 | 3.1 (1.9); 0–5 | 1.2, .23 |
| NPs cerebral cortex2 | 15.3 (6.6); 0–20 | 13.2 (5.9); 0–20 | 1.4, .16 |
0=none, 1=sparse, 3=moderate, 5=severe
Summary of four cerebral cortex areas (midfrontal gyrus, superior middle temporal gyrus, inferior parietal gyrus, and occipital primary visual cortex) by addition of scores on the same scale as in 1.
3. Results
Table 1 summarizes the age and neuropathological features of the 42 women and 31 men in the sample. With the exception of NFTs in the entorhinal cortex, for which women had a minimum of mild neuropathology, all neuropathological ratings had the full range from no to severe pathology. Dementia severity ranged from no dementia to severe dementia in both women and men. Women were significantly older at death than men but did not differ significantly from men in number of children or dementia severity.
Table 2 presents the results of the partial correlations. Among women, all the partial correlations were positive, with significance for overall neuropathology (r=.37; p=.02), overall NPs (r=.36; p=.02), and NPs in the amygdala (r=0.47; p=.002; see Figure 1). Results approached significance for NPs in the entorhinal cortex (r=.29; p=.06). Among men, none of the partial correlations were statistically significant. None of these analyses for men or women included a significant quadratic association.
Table 2.
Partial correlation§ of number of children and Alzheimer’s disease neuropathology-df=38 for women and 27 for men(r; p-value).
| AD Neuropathology | Women | Men |
|---|---|---|
| Neuritic Plaques (NPs) | ||
| Hippocampus | .24 (.14) | −.08 (.66) |
| Entorhinal Cortex | .29 (.06) | −.10 (.59) |
| Amygdala | .47 (.002) | .11 (.59) |
| Cerebral Cortex | .22 (.18) | −.14 (.48) |
| Total NPs | .36 (.02) | −.10 (.59) |
| Neurofibrillary Tangles (NFTs) | ||
| Hippocampus | .18 (.26) | .07 (.73) |
| Entorhinal Cortex | .15 (.37) | .24 (.22) |
| Amygdala | .22 (.16) | .03 (.87) |
| Cerebral Cortex | .22 (.18) | .09 (.64) |
| Total NFTs | .26 (.10) | .12 (.52) |
| General neuropathology score$ | .37 (.02) | .02 (.93) |
Controlling for age at death and CDR
first principal component of a factor analysis including NPs and NFTs in the hippocampus, entorhinal cortex, amygdala, and cerebral cortex.
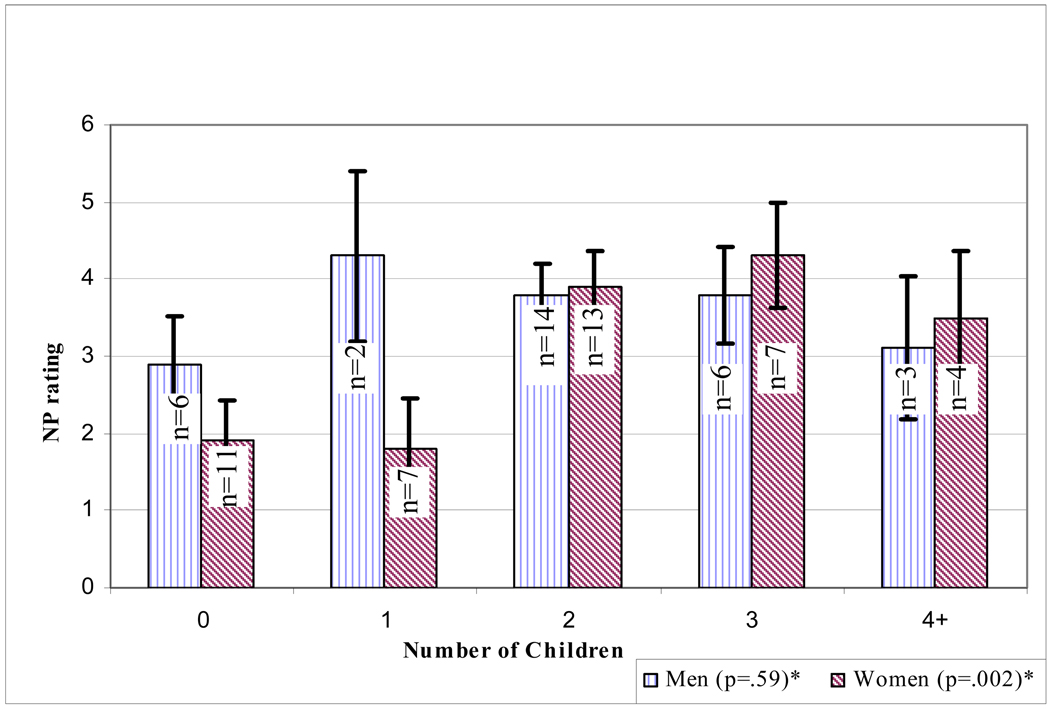
Figure 1. Adjusted means of neuritic plaques (NP) according to number of children by sex.
*In women, NP rating in the amygdala increased with the number of children (p=.002 for linear trend controlling for CDR and age at death). In men, NP ratings in the amygdala were not associated with number of children (p=.59).
Supplementary analyses included subjects who, in addition to a primary neuropathological diagnosis limited to AD, evidenced cerebrovascular disease (CERAD neuropathologic diagnosis category 7). Correlations of NP and NFT densities in the examined brain regions with number of children born for women were smaller, with the exception of NPs in the entorhinal cortex (r=.29, df=49, p=.04); none of the other correlations changed status of statistical significance. Among men, all the partial correlations remained statistically non significant.
Since nulliparity has been suggested to be protective against AD(12;44), we performed similar partial correlations on the dichotomy between nulliparous subjects (11 women and 6 men) and those who had at least one child. Results were in the same direction as in the partial correlations reported above but were not statistically significant for any of the neuropathological variables assessed except for NPs in the amygdale (r=.36; p=.02).
4. Discussion
This study shows that the number of children born is associated with the extent of overall neuropathologic lesions of AD in women but not in men. This association was more robust for NPs, especially in the amygdala. Since these associations were not found for men, they might reflect sex-specific mechanisms, rather than social, economic, biological, or other mechanisms common to both men and women. However, the difference in age mitigates direct comparison of these samples of men and women.
The observed associations between the number of children and neuropathology of AD are consistent with and may explain findings of a relationship between the number of children and AD or cognitive decline assessed clinically(12;44;52);(36). Nonetheless, in the current sample, the association between number of children and CDR was weak and non-significant and almost entirely accounted for by the association of each with neuropathology (data not shown).
The associations found in this study might be explained by biological mechanisms and/or by social and behavioral factors associated with women’s child rearing and family life. Pregnancy and childbirth are accompanied by wide-ranging changes in endocrine regulation and activity resulting in profound metabolic changes. Not only do these changes alter blood lipoprotein levels(17;28), increase insulin concentrations(29), and enhance generation of reactive oxygen species(54), but they also provoke structural and functional alterations of the cardiovascular and other systems(10;46). Consistent with this, of the several studies that have considered the association between parity and coronary heart disease risk later in life among women, most have found increasing disease and mortality with increasing number of children(22;32;40). Having more children has been associated with obesity and central adiposity(30), BMI(31), high triglyceride levels(17), low HDL(33), diabetes(41) and stroke(45). It has also been associated with directly measured carotid intima-media thickness and carotid artery atherosclerosis. In addition, number of children has recently been linearly associated with the risk of metabolic syndrome in the Third National Health and Nutrition Examination Survey. After controlling for age, race, income, education, and other sociodemographic, reproductive, and behavioral risk factors, the odds of metabolic syndrome increased by 13% (95% CI, 6%–20%) with each additional child. Since cardiovascular risk factors and diseases have been associated with AD(5;35), increased cardiovascular disease risk might provide an additional explanation for the associations found in this study.
Lifetime hormonal patterns are a major biological difference between men and women. In particular, estrogen has been suggested as an explanation for the differential risk for AD between men and women(4;11). Estrogen has been shown to have neuroprotective effects(39) against β-amyloid induced neurotoxicity(16;35;58) in animal(9) and in vitro(19) models, by enhancing β-amyloid scavengers(53), increasing survival of cholinergic synapses(51), and through anti-apoptotic(43) and antioxidant mechanisms(55). Estrogen deprivation has been implicated as a risk factor for Alzheimer’s disease (AD)(57). There is evidence that parity “resets” ovarian function in the non-gravid state(6), resulting in lower estrogen concentrations in parous compared to nulliparous women (but the duration of lower levels is not well established). Furthermore, estrogen concentrations are negatively associated with the number of children(21) i.e., over the life span, women who have more children have lower circulating estrogen than women with few or no children. Thus, having more children could be associated with AD neuropathology through a reduction in estrogen.
Although less consistently than estrogen, progesterone, which increases dramatically during pregnancy and drops quickly thereafter, has also been associated with neuroprotection (for a review, see(50)). It reduces neuronal vulnerability to glutamate and Aβ toxicity(20), and neuronal loss following cortical contusion(3) and global ischemia(8). Nonetheless, the interactions between the brain and the reproductive endocrine system are very complex, and suggesting that estrogen and progesterone are the primary hormonal explanation for the current results would be simplistic (Webber 2007). The hormones of the hypothalamic-pituitary-gonadal (HPG) axis include gonadortorpin-releasing hormone (GRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estrogen, progesterone, testosterone, activin, inhibin, and follistatin. These hormones are involved in regulating reproductive function at multiple levels including participation in a complex feedback loop that is initiated by the hypothalamic secretion of GRH (Genazzani, 1992), that stimulates the anterior pituitary to secrete the gonadotropins, LH and FSH. These gonadotropins then bind to receptors on the gonads and stimulate the production of the sex steroids. The latter complete the negative feedback loop by decreasing gonadotropin secretion from the hypothalamus and pituitary gland. There is growing evidence supporting a role for gonadotropins, particularly LH, in the pathogenesis of AD (Webber,2005a, 2005b, 2007). A 2-fold increase in circulating gonadotropins in AD patients compared with age matched controls was found in two studies (Bowen 2000; Short 2001). LH was significantly elevated in vulnerable neuronal populations in AD patients (Bowen 2002). Furthermore, while LH did not alter amyloid protein precursor (APP) expression, it did alter APP processing toward the amyloidogenic pathway (Bowen 2004a 2004b). Notably, hCG (the pregnancy hormone), which has very similar primary and secondary structures to LH, binds the same receptors, and has similar biological functions (Rao and Lei 2007).
The majority of clinical trials investigating the neurocognitive effects of hormone replacement therapy (HRT)have found benefits associated with estrogen therapy (Gleason 2005, Morrison). Trials utilizing estradiol therapies were more likely to demonstrate enhanced cognition than those employing a conjugated equine estrogen (CEE)— which does not replicate pre-menopausal hormone profiles (Gleason 2005). Only the WHIMS trial suggested a possible harmful effect of opposed CEE plus progestin treatment on cognition(39;49). Since women who participated in the WHIMS trial started receiving HRT only at the age of 65 their HPG axis hormones were likely in disequilibrium for decades. It is possible that the administration of estrogen/progestin in these aged women was unable to restore the proper functioning of the HPG axis (Webber, 2007).
Since fetal stem cells penetrate and influence maternal blood(42), reactions to them might influence subsequent maternal hormonal patterns. This effect could be enhanced by multiple births, similarly to RH incompatibility, in which the sensitization induced by an incompatible fetus is only manifest at a subsequent pregnancy. This is consistent with increasing neuropathology as the number of children increases rather than an effect of parity per se.
Several studies have shown associations of non-biological factors with both number of children and the risk of AD, comparably in men and women(22). Number of children was positively associated with psychological stress(14;56), and negatively associated with socioeconomic status(1), and particularly with education(15) (47). Data on years of education were available for a 24 subjects (18 women) subset. In this subsample of women, there was no significant association between number of children and education (r= .07) and the association between education and overall neuropathology (r=.31) was positive and not significant. Thus, the significant association between overall neuropathology and number of children in this sub-sample (r=.40, p=.01) cannot be attributed to differences in education. Additionally, subjects of this study belonged to a relatively socio-economically homogeneous community (consistent with a narrow range of education—half of the subjects had 12 years of education and the rest had 14 or 16 years of education), so its influence on the results may have been limited.
A major limitation of this study was the lack of information on hormone replacement therapy, as well as the lack of serum samples of reproductive system hormones. Both are indicators of hormonal levels during life which could have mediated or changed the associations found in this study. Animal models and longitudinal studies that document gonadal hormone levels throughout child bearing and old age may be useful tools to study the inter-relationships of number of children, reproductive hormone levels, and AD neuropathology.
Acknowledgments
This study has been supported by NIA grants: K01 AG023515-01A2 (Dr. Beeri), AG02219 (Dr. Haroutunian) and AG05138 (Dr. Sano).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors reported no conflict of interest.
Reference List
- 1.Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. J Int Neuropsychol Soc. 2006;12(1):147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- 2.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A EURODEM Incidence Research Group. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology. 1999;53(9):1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 3.Asbury ET, Fritts ME, Horton JE, Isaac WL. Progesterone facilitates the acquisition of avoidance learning and protects against subcortical neuronal death following prefrontal cortex ablation in the rat. Behav Brain Res. 1998;97(1–2):99–106. doi: 10.1016/s0166-4328(98)00031-x. [DOI] [PubMed] [Google Scholar]
- 4.Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64(2):93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- 5.Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, Purohit DP, Perl DP, Siddiqui A, Lesser G, Rosendorff C, Haroutunian V. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006;66(9):1399–1404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74(4):741–745. [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996 doi: 10.1111/j.1600-0404.1996.tb05866.x. 1653-12. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33(1):6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 9.Cimarosti H, Siqueira IR, Zamin LL, Nassif M, Balk R, Frozza R, Dalmaz C, Netto CA, Salbego C. Neuroprotection and protein damage prevention by estradiol replacement in rat hippocampal slices exposed to oxygen-glucose deprivation. Neurochem Res. 2005;30(4):583–589. doi: 10.1007/s11064-005-2693-1. [DOI] [PubMed] [Google Scholar]
- 10.Clapp JF, III, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469–1473. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 11.Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55(2):169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 12.Colucci M, Cammarata S, Assini A, Croce R, Clerici F, Novello C, Mazzella L, Dagnino N, Mariani C, Tanganelli P. The number of pregnancies is a risk factor for Alzheimer's disease. Eur J Neurol. 2006;13(12):1374–1377. doi: 10.1111/j.1468-1331.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 13.Corbo RM, Gambina G, Ulizzi L, Monini P, Broggio E, Rosano A, Scacchi R. Combined Effect of Apolipoprotein E Genotype and Past Fertility on Age at Onset of Alzheimer's Disease in Women. Dement Geriatr Cogn Disord. 2007;24(2):82–85. doi: 10.1159/000103866. [DOI] [PubMed] [Google Scholar]
- 14.D'Elio MA, Ness RB, Matthews KA, Kuller LH. Are life stress and social support related to parity in women? Behav Med. 1997;23(2):87–94. doi: 10.1080/08964289709596732. [DOI] [PubMed] [Google Scholar]
- 15.Dodoo FN. Female education, age, parity, and reproduction cessation in Ghana. Soc Biol. 1992;39(1–2):102–108. doi: 10.1080/19485565.1992.9988807. [DOI] [PubMed] [Google Scholar]
- 16.Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol. 2004;183(3):605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- 17.Fahraeus L, Larsson-Cohn U, Wallentin L. Plasma lipoproteins including high density lipoprotein subfractions during normal pregnancy. Obstet Gynecol. 1985;66(4):468–472. [PubMed] [Google Scholar]
- 18.Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer's Disease. Aging (Milano) 1996;8(6):379–385. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 20.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66(5):1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson SE, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ, Longcope C, Speizer FE. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses' Health Study (United States) Cancer Causes Control. 1995;6(3):217–224. doi: 10.1007/BF00051793. [DOI] [PubMed] [Google Scholar]
- 22.Hardy R, Lawlor DA, Black S, Wadsworth ME, Kuh D. Number of children and coronary heart disease risk factors in men and women from a British birth cohort. BJOG. 2007;114(6):721–730. doi: 10.1111/j.1471-0528.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- 23.Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55(9):1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 24.Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56(6):713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 25.Haroutunian V, Serby M, Purohit DP, Perl DP, Marin D, Lantz M, Mohs RC, Davis KL. Contribution of Lewy body inclusions to dementia in patients with and without Alzheimer disease neuropathological conditions. Arch Neurol. 2000;57(8):1145–1150. doi: 10.1001/archneur.57.8.1145. [DOI] [PubMed] [Google Scholar]
- 26.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Centers Coordinating Center. Neurology. 2005;64(3):494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 27.Hy LX, Keller DM. Prevalence of AD among whites: a summary by levels of severity. Neurology. 2000;55(2):198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The relationship between multiparity and lipoprotein levels in older women. J Clin Epidemiol. 1992;45(7):761–767. doi: 10.1016/0895-4356(92)90053-p. [DOI] [PubMed] [Google Scholar]
- 29.Kritz-Silverstein D, Barrett-Connor E, Wingard DL, Friedlander NJ. Relation of pregnancy history to insulin levels in older, nondiabetic women. Am J Epidemiol. 1994;140(4):375–382. doi: 10.1093/oxfordjournals.aje.a117260. [DOI] [PubMed] [Google Scholar]
- 30.Lahmann PH, Lissner L, Gullberg B, Berglund G. Sociodemographic factors associated with long-term weight gain, current body fatness and central adiposity in Swedish women. Int J Obes Relat Metab Disord. 2000;24(6):685–694. doi: 10.1038/sj.ijo.0801219. [DOI] [PubMed] [Google Scholar]
- 31.Lao XQ, Thomas GN, Jiang CQ, Zhang WS, Yin P, Schooling M, Heys M, Leung GM, Adab P, Cheng KK, Lam TH. Parity and the metabolic syndrome in older Chinese women: the Guangzhou Biobank Cohort Study. Clin Endocrinol (Oxf) 2006;65(4):460–469. doi: 10.1111/j.1365-2265.2006.02615.x. [DOI] [PubMed] [Google Scholar]
- 32.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women's Heart and Health Study and the British Regional Heart Study. Circulation. 2003;107(9):1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 33.Lewis CE, Funkhouser E, Raczynski JM, Sidney S, Bild DE, Howard BV. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144(3):247–254. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 34.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6(4):261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 35.Marin R, Guerra B, Hernandez-Jimenez JG, Kang XL, Fraser JD, Lopez FJ, Alonso R. Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience. 2003;121(4):917–926. doi: 10.1016/s0306-4522(03)00464-0. [DOI] [PubMed] [Google Scholar]
- 36.McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. 2003;15(2):161–167. doi: 10.1176/jnp.15.2.161. [DOI] [PubMed] [Google Scholar]
- 37.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van BG, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 38.Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48(6):1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 39.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26(41):10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ness RB, Harris T, Cobb J, Flegal KM, Kelsey JL, Balanger A, Stunkard AJ, D'Agostino RB. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328(21):1528–1533. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2006;29(11):2349–2354. doi: 10.2337/dc06-0825. [DOI] [PubMed] [Google Scholar]
- 42.O'donoghue K, Choolani M, Chan J, de la FJ, Kumar S, Campagnoli C, Bennett PR, Roberts IA, Fisk NM. Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9(8):497–502. doi: 10.1093/molehr/gag063. [DOI] [PubMed] [Google Scholar]
- 43.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem. 1999;72(4):1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 44.Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer's disease. Arch Womens Ment Health. 2002;5(2):83–86. doi: 10.1007/s00737-002-0142-6. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi AI, Giles WH, Croft JB, Stern BJ. Number of pregnancies and risk for stroke and stroke subtypes. Arch Neurol. 1997;54(2):203–206. doi: 10.1001/archneur.1997.00550140073015. [DOI] [PubMed] [Google Scholar]
- 46.Sadaniantz A, Saint LL, Parisi AF. Long-term effects of multiple pregnancies on cardiac dimensions and systolic and diastolic function. Am J Obstet Gynecol. 1996;174(3):1061–1064. doi: 10.1016/s0002-9378(96)70351-4. [DOI] [PubMed] [Google Scholar]
- 47.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(3):308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serby M, Brickman AM, Haroutunian V, Purohit DP, Marin D, Lantz M, Mohs RC, Davis KL. Cognitive burden and excess Lewy-body pathology in the Lewy-body variant of Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(3):371–374. [PubMed] [Google Scholar]
- 49.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 50.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29(2):271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- 51.Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86(2):679–684. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- 52.Sobow T, Kloszewska I. Parity, number of pregnancies, and the age of onset of Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2004;16(1):120–121. doi: 10.1176/jnp.16.1.120-a. [DOI] [PubMed] [Google Scholar]
- 53.Tang YP, Haslam SZ, Conrad SE, Sisk CL. Estrogen increases brain expression of the mRNA encoding transthyretin, an amyloid beta scavenger protein. J Alzheimers Dis. 2004;6(4):413–420. doi: 10.3233/jad-2004-6409. [DOI] [PubMed] [Google Scholar]
- 54.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002;57(5):609–613. doi: 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]
- 55.Vural P, Akgul C, Canbaz M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res. 2006;54(4):298–302. doi: 10.1016/j.phrs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic Psychological Distress and Risk of Alzheimer's Disease in Old Age. Neuroepidemiology. 2006;27(3):143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 57.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005;102(52):19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J Neurosci. 2004;24(23):5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]