Abstract
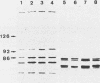
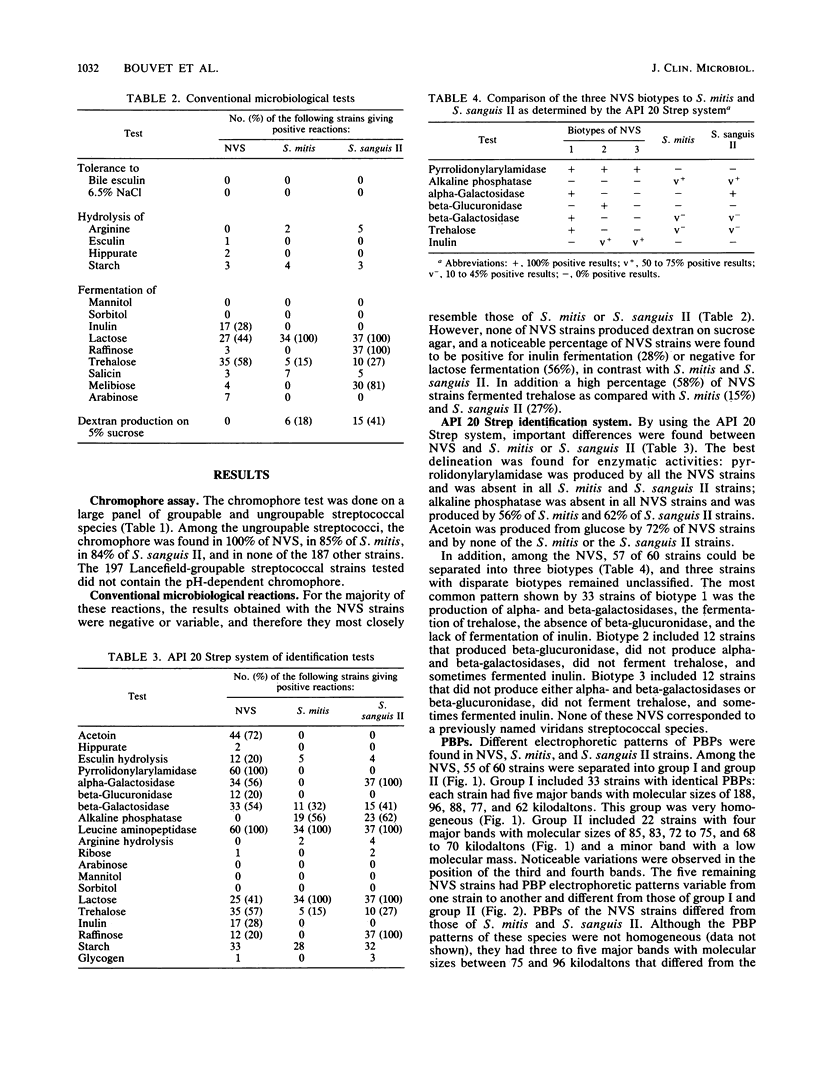
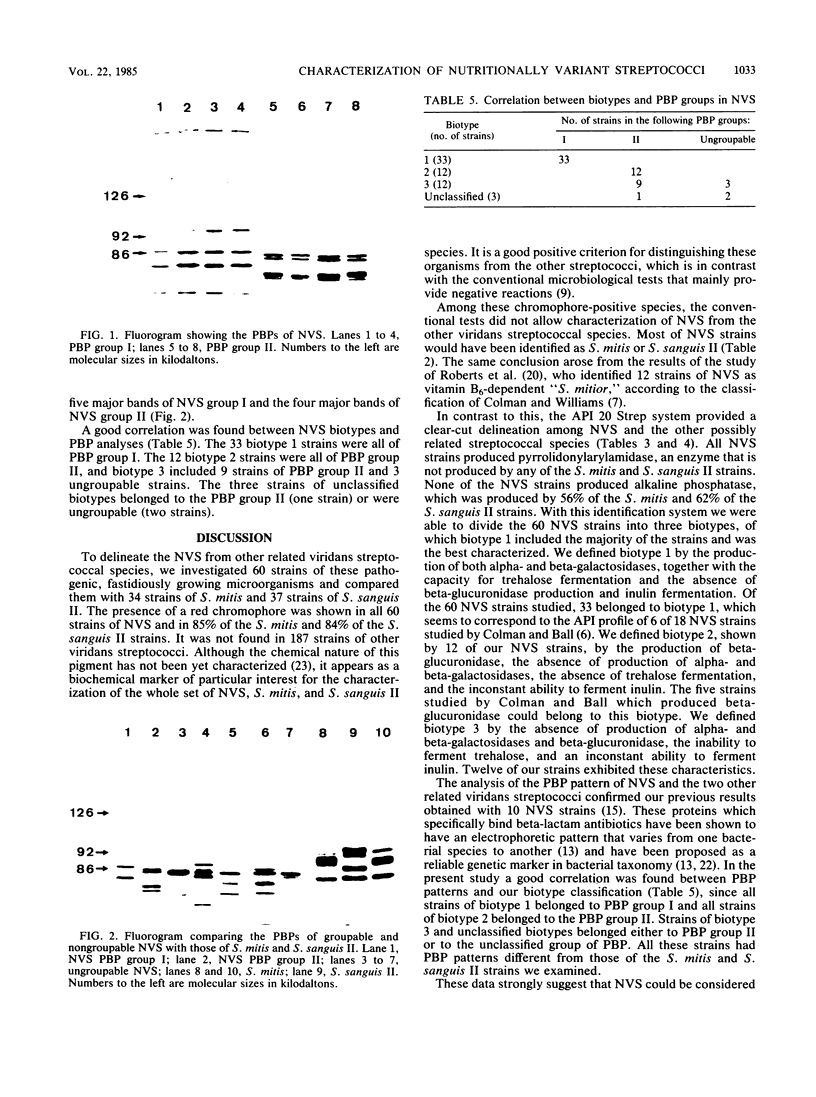
A comparative study of 60 strains of nutritionally variant streptococci (NVS) with 34 strains of Streptococcus mitis and 37 strains of Streptococcus sanguis II showed the presence of a red chromophore which was absent in the other streptococcal species. By using the conventional microbiological tests, only small differences were found between the NVS and the two other related species. In contrast a clear-cut delineation was found by the API 20 Strep system of identification. All NVS contained pyrrolidonylarylamidase, an enzyme which was absent in S. mitis and S. sanguis II strains, and lacked the alkaline phosphatase enzyme which was present in 56% of S. mitis strains and 62% of S. sanguis II strains. According to the additional enzymatic and biochemical tests of the API 20 Strep system, there were three biotypes among NVS. The major biotype included 33 of 60 strains which were characterized by the presence of both alpha- and beta-galactosidases and the capacity to hydrolyze trehalose. This biotype also showed a specific pattern of penicillin-binding proteins. These results show that NVS are recognized as a separate variety distinct from S. mitis and S. sanguis II species, despite some common biochemical properties. Moreover, the delineation of 33 strains with a specific biotype and a specific penicillin-binding protein pattern strongly suggests that a large part of NVS strains belong to an individual species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvet A., Ryter A., Fréhel C., Acar J. F. Nutritionally deficient streptococci: electron microscopic study of 14 strains isolated in bacterial endocarditis. Ann Microbiol (Paris) 1980 Sep-Oct;131B(2):101–120. [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. B., Gross K. C., Roberts R. B. Vitamin B6-dependent Streptococcus mitior (mitis) isolated from patients with systemic infections. J Infect Dis. 1975 Jun;131(6):722–726. doi: 10.1093/infdis/131.6.722. [DOI] [PubMed] [Google Scholar]
- Cayeux P., Acar J. F., Chabbert Y. A. Bacterial persistence in streptococcal endocarditis due to thiol-requiring mutants. J Infect Dis. 1971 Sep;124(3):247–254. doi: 10.1093/infdis/124.3.247. [DOI] [PubMed] [Google Scholar]
- Colman G., Ball L. C. Identification of streptococci in a medical laboratory. J Appl Bacteriol. 1984 Aug;57(1):1–14. doi: 10.1111/j.1365-2672.1984.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cooksey R. C., Thompson F. S., Facklam R. R. Physiological characterization of nutritionally variant streptococci. J Clin Microbiol. 1979 Sep;10(3):326–330. doi: 10.1128/jcm.10.3.326-330.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL A., HIRSCH W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. 1961 Aug 12;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H. The isolation of symbiotic streptococci. J Med Microbiol. 1974 Feb;7(1):77–83. doi: 10.1099/00222615-7-1-77. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gephart J. F., Washington J. A., 2nd Antimicrobial susceptibilities of nutritionally variant streptococci. J Infect Dis. 1982 Oct;146(4):536–539. doi: 10.1093/infdis/146.4.536. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Tomasz A. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob Agents Chemother. 1981 May;19(5):872–880. doi: 10.1128/aac.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Delbos F. Les streptocoques non groupables dans les infections humaines: identification et sensibilité aux antibiotiques. Ann Microbiol (Paris) 1982 Sep-Oct;133(2):255–269. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarthy L. R., Bottone E. J. Bacteremia and endocarditis caused by satelliting streptococci. Am J Clin Pathol. 1974 May;61(5):585–591. doi: 10.1093/ajcp/61.5.585. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins in bacteria. Ann Intern Med. 1982 Apr;96(4):502–504. doi: 10.7326/0003-4819-96-4-502. [DOI] [PubMed] [Google Scholar]
- Verhaegen J., Vandepitte J. Endocarditis caused by nutritionally deficient streptococci. Acta Clin Belg. 1983;38(1):12–18. doi: 10.1080/22953337.1983.11718900. [DOI] [PubMed] [Google Scholar]
- Waitkins S. A., Anderson D. R., Todd F. K. An evaluation of the API-STREP identification system. Med Lab Sci. 1981 Jan;38(1):35–39. [PubMed] [Google Scholar]
- Williamson R., le Bouguénec C., Gutmann L., Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985 Aug;131(8):1933–1940. doi: 10.1099/00221287-131-8-1933. [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Bouvet A. Characterization of a pH-dependent chromophore from nutritionally variant streptococci. Infect Immun. 1984 Jan;43(1):28–31. doi: 10.1128/iai.43.1.28-31.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M. Immunochemical study of nutritionally variant streptococci. J Immunol. 1984 Oct;133(4):2220–2225. [PubMed] [Google Scholar]