Abstract
In this paper, the estabished and possible roles of CCK1 and CCK2 receptors in gastrointestinal (GI) and metabolic diseases are reviewed and available results from human agonist/antagonist studies are discussed. While there is evidence for the involvement of CCK1R in numerous diseases including pancreatic disorders, motility disorders, tumor growth, regulation of satiety and a number of CCK-deficient states, the role of CCK1R in these conditions is not clearly defined. There are encouraging data from several clinical studies of CCK1R antagonists in some of these conditions, but their role as therapeutic agents remains unclear. The role of CCK2R in physiological (atrophic gastritis, pernicious anemia) and pathological (Zollinger-Ellison syndrome) hypergastrinemic states, its effects on the gastric mucosa (ECL cell hyperplasia, carcinoids, parietal cell mass) and its role in acid-peptic disorders are clearly defined. Furthermore, recent studies point to a possible role for CCK2R in a number of GI malignancies. Current data from human studies of CCK2R antagonists are presented and their potential role in the treatment of these conditions reviewed. Furthermore, the role of CCK2 receptors as targets for medical imaging is discussed.
Even though cholecystokinin (CCK) and gastrin were among the first gastrointestinal hormones discovered [1,2], both their physiological roles as well as their roles in clinically relevant gastrointestinal diseases remain unclear and even controversial in many cases [3–6]. The structural characterization of CCK and gastrin [7,8], pharmacological identification [9–13] and cloning [14,15] of CCK and gastrin receptors (CCK1R, CCK2R), characterization of receptor location, peptide and receptor genes, development of receptor antagonists and receptor/agonist knockout animals [16–21] have led to important advancements in our understanding of the physiological and pathophysiological role of CCK and gastrin signaling [3]. Most of these topics are dealt with in other papers in this volume. The present review will focus on the role of CCK and gastrin and their receptors (CCK1R and CCK2R) in gastrointestinal and metabolic diseases with special emphasis on human studies and the assessments and potential for their use for treatments for human diseases
1.INTRODUCTION
Multiple gastrointestinal tissues express CCK1R, CCK2R or both. Importantly, there is a relevant inter-species variation of the tissue distribution of CCK1R and CCK2R [4,22], so that data from animal studies cannot always be extrapolated to humans. The human CCK1R is expressed at the protein level in the mucosa of the stomach [23,24], the exocrine pancreas [25] and in smooth muscle cells of the gallbladder [26], stomach [24] and intestine [27,28]. Moreover, human CCK1R mRNA has been reported in vagal afferent fibers [29], the adrenal gland [30], the kidney [22] and mononuclear blood cells [23]. In contrast to most animals, very low or non-detectable levels of CCK1R mRNA are expressed in human pancreatic acini and these cells do not respond to CCK1R agonists [4,31]. CCK2R protein has been demonstrated in the human exocrine [32] and endocrine [33] pancreas, the stomach mucosa [24] and muscularis [24]. Moreover, CCK2R receptor mRNA expression has been shown in human blood mononuclear cells [23], adrenal gland [30] and vagal afferent fibers [29].
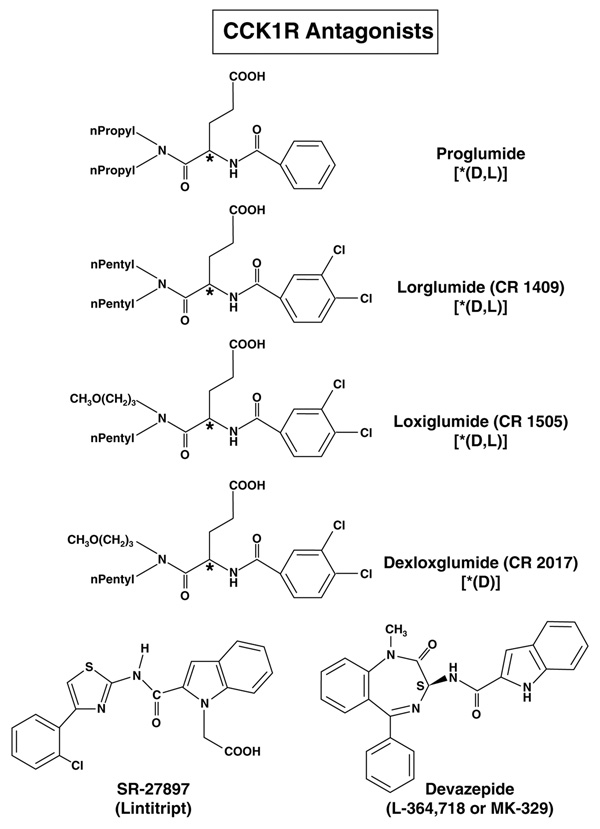
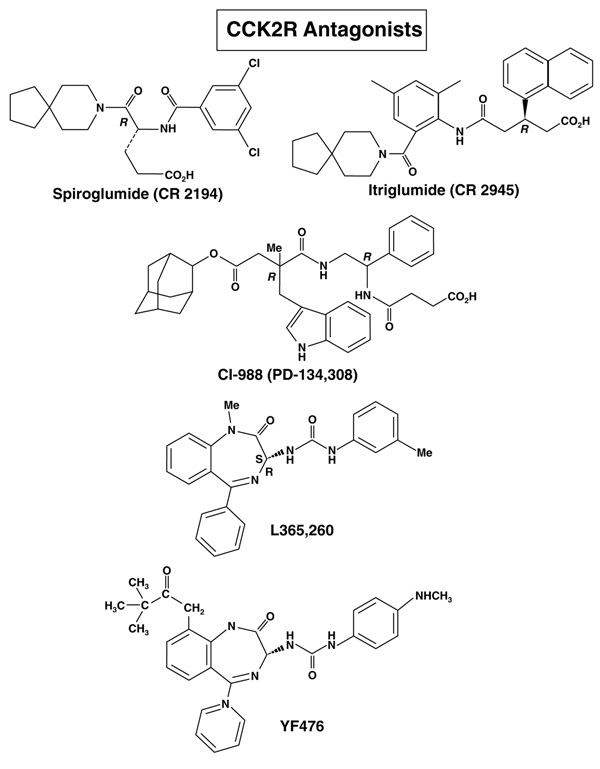
The CCK1R has a high affinity (Kd in the nanomolar range) for CCK and sulfated CCK analogues but a low affinity (Kd in the micromolar range) for gastrin, which is a poor activator of CCK1Rs at physiological concentrations [34–36]. The CCK1R has been shown to exist in a high- and low-affinity state, which are coupled to different intracellular signaling mechanisms [3,17,37–39].The CCK2R has almost equal affinity for gastrin and CCK as well as for desulfated CCK analogues [3,17,37,38]. As postprandial serum gastrin values are 5- to 10-fold higher than those of CCK, gastrin is probably the physiological ligand of most of the peripheral (i.e. non-CNS) CCK2R receptors [3]. For both receptors, numerous specific agonists and antagonists have been developed (for reviews, see [6,21,40]). The CCK1R and CCK2R antagonists that have been assessed in humans (physiologically or in diseases) are shown in Fig. (1) and Fig. (2), respectively.
Figure 1.
Structure of CCK1 receptor antagonists used in human studies. CCK1R and CCK2R affinities, chemical structures and references are listed in Table 3.
Figure 2.
Structure of CCK2 receptor antagonists used in human studies. CCK1R and CCK2R CCK1R and CCK2R affinities, chemical structures and references are listed in Table 3.
Numerous selective CCK1R agonists and antagonists have been developed [3,6,21,41–43]. CCK1R selective agonists include peptides (sulfated CCK analogues as will as CCK tetra-peptide analogues [A-71378, A-71623, AR-R 15849]), benzodiazepine derivatives (GSK compound GI 18177, GW 7178, GW 5823) and thiazole derivatives (SR 146131, SR 146131) [21,41,44–46]. CCK1R agonist have primarily been investigated in appetite control and will not be discussed here because this is covered in other papers in this volume. CCK1R selective antagonists include glutaramic acid derivatives (lorglumide, loxiglumide, dexloxiglumide, A-65186), 1,4-benzodiazepine derivatives (L-364,718 [MK-329, devazepide], pranazepide [FK-480], tarazepide), various conformationally constrained dipeptoid analogues, various 1,3-dioxoperhydropyrido[1,2-c]pyrimidine analogues, 1,3,5-substituted pyrrolidinones analogues (SC-50,998), 1,3,3-substituted indol-2-one derivatives (T-0632) as well as others identified by randon screening (SR-27,897[lintitript], TP-680) [6,21,45,47]. In the present review only CCK1R antagonists that have been used in humans will be discussed [Fig. (1), Table 3].
Table 3.
CCK1R and CCK2R Antagonist used in human studies1
| IC50 (µM) | ||||
|---|---|---|---|---|
| CCK1R | CCK2R | Fold CCK1R preferring |
||
| CCK1R preferring | ||||
| A. | Glutaramic acid analogues | |||
| Proglumide2 | 6,000 | 11,000 | 1.8 | |
| Lorglumide (CR 1409)3 | 0.13 | 300 | 2,300 | |
| Loxiglumide (CR 1505)4 | 0.33 | 9.1 | 27 | |
| Dexloxiglumide (CR 2017)5 | 0.12 | 22 | 170 | |
| B. | 1,4 Benzodiazepines | |||
| L-364,718 (MK-329, Devazepide)6 | 0.08 | 270 | 3,400 | |
| C. | Other | |||
| Lintript (SI-27897)7 | 0.58 | 489 | 843 | |
| Fold CCK2R preferring |
||||
| CCK2R preferring | ||||
| A. | Glutaramic acid analogues | |||
| Spiroglumide (CR 2194)8 | 13,500 | 1,400 | 9.6 | |
| Itriglumide (CR 2945)9 | 20,700 | 2.3 | 9,000 | |
| B. | 1.4 Benzodiazepines | |||
| L-365,36010 | 280 | 2 | 140 | |
| YF47611 | 502 | 0.11 | 5,020 | |
| C. | Dipeptoids | |||
| CI-988 (PD-134,308)12 | 4,300 | 1.1 | 2,501 | |
Data are from [6,40,47,334–338,373–375]
D,L-4-benzamido-N,N-dipropyl-glutaramic acid
[D, L-4-(3,4-dichlorobenzoylamino)-5-(di-N-pentylamino)-5-oxopentanoic oxid]
[D, L-4+(3,4 dichlorobenzamido)-N-(3-methoxypropyl)-N-pentylglutaramic acid]
[(R)-4-(3,4-dichlorobenzoylamino)-5-[N-(3-methoxylpropyl)-N-pentylamino]-5-oxopentanoic acid]
[3S(−)-N(2,3-Dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-yl)-1H-indole-2- carboxamide]
1-([2-(4-(2-chlorophenyl)thiazole-2-yl)aminocarbonyl]indolyl) acetic acid
(R)-8-Azaspiro[4,5]decaane-8-pentanoic acid
(R)-1-naphtalene propionic acid
3-R(+)-(N-2,3-Dihydro-1methyl-2-oxo-5-phenyl-1 H-1,4 benzodiazepin-3-yl)-N’-(3- methylphenyl)urea
((R)-1-[2.3 dihydro-2-oxo-1-pivaloylmethyl-5-(2’pyridyl)-1H-1,4-benzodiazepin-3-yl]-3- (methylamino-phenyl)urea
4-[[2-[[3-(1H-indol-3-yl)-2-methyl-1-oxo-2[[(tricyclo[3.3[12.17]dec-2-yloxy)- carbonyl]amino]-propyl]amino]-1-phenyethyl]amino]-4-oxo-[R-(R*,R*)]-butanoate N-methyl- D-glucamine
II. CCK AND CCK1R
II.A. Physiological functions mediated by CCK1Rs (Table 1)
Table 1.
CCK and CCK1R in the gastrointestinal tract: physiological functions and possible disorders
| Physiological functions | ||
| 1. | Contraction of gallbladder/relaxation sphincter of Oddi | |
| 2. | Stimulation of pancreatic secretion | |
| 3. | Inhibit colonic motility | |
| 4. | Inhibit gastric emptying | |
| 5. | Decrease lower sphincter pressure /increase in sphincter relaxation | |
| 6. | Inhibition of acid secretion | |
| Possible disease involvement. | ||
| 1. | CCK-deficient states (celiac sprue, bulimia, diabetes mellitus, autoimmune polyglandular syndrome-type 1) |
|
| 2. | Pancreatic disorders (acute/chronic pancreatitis) | |
| 3. | GI motility disorders [gallbladder disease (cholesterol stores, acalculous cholecystitis), irritable bowel syndrome, functional dyspepsia, chronic constipation] |
|
| 4. | Tumor growth | |
| 5. | Satiety disorders | |
In humans strong evidence suggests CCK1R activation is involved in the regulation of numerous physiological processes, including gallbladder contraction and sphincter of Oddi relaxation, stimulation of pancreatic secretion, inhibition of gastric emptying and acid secretion, lower esophageal sphincter relaxation, slowing of colonic motility and regulation of satiety [4,16,19,20,48–62].
II.B. Gastrointestinal (GI) diseases likely involving CCK or CCK1Rs (Table 1)
Although CCK1Rs have been reported to be relevant in various gastrointestinal diseases, the role of CCK1Rs in these conditions has not been firmly established [4,16,48]. Several studies reviewed below report CCK deficiency states that could have clinical relevance. Others suggest that CCK1Rs could be involved in various pancreatic disorders (acute, chronic pancreatitis); GI motility disorders including gallbladder disease, irritable bowel syndrome, functional dyspepsia, chronic constipation, gastroesophageal reflux disease; appetite/satiety regulation, modulation of pain and regulation of tumor growth. The latter two subjects will be treated in other papers in this journal and thus will not be dealt with further in the following review.
II.B.1. CCK deficiency states
Reduced serum levels of CCK, possibly contributing to impaired gallbladder contractility and cholelithiasis, have been reported in patients with celiac disease [63–68], the short bowel syndrome [69], in diabetics [70], in newborns with infantile colic [71] and in patients receiving total parenteral nutrition [30,72]. In this later group of patients, it is controversial whether CCK administration can prevent parenteral nutrition-induced cholestasis or sludge/stone formation [73–77]. It has been proposed that diarrhea and malabsorption in patients with autoimmune polyglandular syndrome type 1 are due to CCK deficiency induced by loss of CCK-producing enteroendocrine cells in the proximal small intestine [78], however, this proposal has been questioned by others [79].
II.B.2. Role of CCK1R in pancreatic disorders
II.B.2.a. General
The relevance of CCK1R signaling in clinical pancreatic disorders is not clearly defined. This has occurred because important inter-species variation of pancreatic CCK1R and CCK2R expression complicates the development of suitable animal models. While in rat and mice, the two animals used in most experimental models of human pancreatic disease, pancreatic acini express exclusively CCK1R, human pancreatic acini express almost 2exclusively CCK2R receptors [4,22,80–82] and CCK causes no alteration in human acinar cell function [31]. Other reports indicate that CCK-induced pancreatic enzyme secretion in humans is mediated by a cholinergic mechanism [83,84]. Therefore, several authors conclude that it is unlikely that CCK1Rs on human acini mediate important cellular functions, such as enzyme secretion or proliferation [31,83], as reported in animal models.
II.B.2.b. CCK1R in acute pancreatitis
II.B.2.b.1 CCK1R in acute pancreatitis.General
Several lines of evidence in animal studies suggest that CCK1Rs may mediate induction and development of acute pancreatitis in experimental models [55,85–87]. First, in rats and mice, parenteral administration of supraphysiological doses of CCK agonists can cause acute pancreatitis [88,89]. Second, in the CDE model of acute pancreatitis in mice (choline-deficient ethionine-supplemented diet), CCK worsens the pancreatitis [89]. Third, OLETF rats lacking CCK1R expression develop less severe pancreatitis in several experimental models [90,91]. Forth, administration of CCK1R antagonists reduced the severity of pancreatitis in most [87,89,92–99] but not all [100–102] animal studies of experimental pancreatitis. One study [102] even showed that the specific CCK1R antagonist L-364,718 [Fig. (1), Table 3] worsened the course of bile-pancreatic-duct obstruction (PDO) pancreatitis in rats, but it has been criticized by others for methodological problems [103]. Another report by the same group suggested that CCK1R blockade by, L-364,718 could increase intra-acinar free-radical generation, thereby worsening PDO pancreatitis [104]. Fifth, ethanol, one of the common causes of pancreatitis in men, has been shown to sensitize pancreatic acinar cells to CCK in in vitro [92,105] and in vivo [106] rat studies. Moreover, recent studies reporting an up-regulation of CCK1Rs during pancreatic regeneration after taurocholate-induced pancreatitis in rats [107] and a delayed pancreatic regeneration in CCK1R deficient OLETF rats after ethionine-induced pancreatitis [91] suggest a possible role for CCK1R in pancreas regeneration. In animal studies, intracellular activation of zymogens appears to be one of the early events in the initiation of acute pancreatitis [93,108–111].
At present, little data is available on the pathogenesis of the most common forms of pancreatitis in man, i.e. pancreatitis induced by biliary tract disorders, alcohol, drugs and metabolic abnormalities. There is only indirect evidence for a potential role of CCK1Rs in clinical pancreatitis. First, patients with biliary pancreatitis have been reported to have higher serum CCK levels than control patients or patients with non-biliary acute pancreatitis [112]. Second, studies in hereditary pancreatitis support the role of premature zymogen activation in clinical pancreatitis, because different mutations of the cationic trypsinogen [113–115] and the serine protease inhibitor Kazal type 1 [116–118], two molecules regulating zymogen activation, have been found to correlate with different forms of hereditary pancreatitis. This analogy to animal models of pancreatitis, including CCK-induced pancreatitis, might provide indirect evidence for the role of CCK in certain forms of clinical pancreatitis, but does not clearly establish the importance of CCK1R signaling in this disease.
II.B.2.b.2 CCK1R antagonists in acute pancreatitis
Currently, data are available on only one human clinical study of a CCK1R antagonist in acute pancreatitis [119]. In this double-blind study, 189 patients from 104 Japanese centers were treated with three different doses of the selective CCK1R antagonist loxiglumide [Fig. (1), Table 3], administered intravenously twice a day. Pain disappearance, changes in clinical symptoms (nausea, vomiting), changes in physical findings and changes in serum amylase were similar in all three groups. Serum lipase levels returned to normal more quickly in the high-dose group, suggesting that loxiglumide could be useful to treat acute pancreatitis, especially as the reported side effects were rare and usually mild. However, this study lacks a placebo group and therefore is inconclusive. Loxiglumide has entered a phase III trial for acute pancreatitis, however no data are currently available [42]. So far, no study shows unequivocally a clinical benefit of CCK1R antagonists in acute pancreatitis in humans.
II.B.2.c. CCK1R in chronic pancreatitis
II.B.2.c.1. CCK1R in chronic pancreatitis. General
Animal studies in rats, chicken and pigs suggest that exocrine pancreatic secretion is regulated by a negative feedback mechanism [112,120]. A CCK-releasing peptide stimulates secretion of CCK, which triggers pancreatic enzyme secretion. Pancreatic enzymes inactivate the CCK-releasing peptide in the duodenum, thereby reducing their own secretion in a negative feedback loop. Several findings have led to the proposal that the inhibition of this feedback mechanism in chronic pancreatitis could cause elevated CCK levels inducing excessive stimulation of pancreatic secretion with elevated intraductal pressure, causing abdominal pain. First, some patients with chronic pancreatitis are reported to have higher basal CCK levels than healthy controls [121–123]. Second, some studies of pancreatic enzyme preparations in chronic pancreatitis showed pain relief [121,124], especially in mild to moderate disease and minimal/no changes in ERCP [125]. Some authors propose that this pain-relieving effect of the pancreatic enzymes is mediated by feedback inhibition of CCK [125], probably by denaturation of CCK-releasing peptide by the pancreatic enzymes. The role of CCK in the pathogenesis of pain in chronic pancreatitis is however controversial [120,126], as three studies of pancreatic enzyme preparations in chronic pancreatitis showed no decrease in abdominal pain [120,127,128].
II.B.2.c.2. CCK1R antagonists in chronic pancreatitis
Data on one double-blinded, randomized, placebo-controlled study of three doses (300, 600, 1200 mg/day) of oral loxiglumide in chronic pancreatitis in 207 Japanese patients are available [129]. Physical signs, clinical symptoms and serum pancreatic enzyme levels were evaluated. In the 600 mg group, back/abdominal pain, serum amylase and trypsin levels decreased significantly while in all three groups, the abdominal tenderness/resistance improved. Adverse side effects were rare and mostly mild to moderate. The authors concluded that 600 mg/day loxeglumide may be useful in the treatment of chronic pancreatitis. Further adequately designed placebo-controlled studies with appropriate end points are needed to confirm this conclusion. A phase III trial of loxeglumide in chronic pancreatitis has been started, but no data are available [42].
II.B.3. Role of CCK1R in motility disorders
II.B.3.a. Role of CCK1R in gallbladder disorders
II.B.3.a.1.General
Numerous studies evidence that gallbladders from cholesterol stone patients have a CCK contractile defect [130,131], due to altered membrane fluidity, which results in dysfunction of G-protein coupled transmembrane receptors, such as the CCK1R [130–132]. Moreover, recent studies in a knockout mouse model [133] provide evidence that disruption of CCK1R signaling leads to enhanced gallstone formation. Furthermore, some clinical studies show reduced CCK1R expression in cholesterol stone patients with non-contractile gallbladders [134] and in diabetic patients with gallstones [135].
The role of CCK in acalculous gallbladder disease and the clinical entity itself as a cause of chronic abdominal pain are controversial [136–139]. Multiple approaches to study gallbladder emptying after CCK or a fatty meal, triggering release of endogenous CCK, to identify symptomatic patients with acalculous cholecystitis who might benefit from a cholecystectomy have led to contradictory results [136,137,140,141]. While some recent studies on CCK-cholescintigraphy, the most widely used of these tests in the United States, have found a good reproducibility [142] and a good prediction of the presence of acalculous chronic/acute cholecystitis [143], others have found that cholescintigraphy may only be of limited use [144]. Therefore, further investigations with standardized test procedures are needed to define the optimal methodology for studying gallbladder emptying.
II.B.3.a.2. CCK1R antagonists in gallbladder disease
Pain relief after oral administration of the CCK1R inhibitor loxiglumide has been first reported by Beglinger et al. in patients with biliary colic refractory to conventional therapy after extracorporeal shock-wave lithotripsy of gallbladder stones [145]. In a pilot study of 14 patients with biliary colic, Malesci et al. report significantly greater and faster pain reduction after 50 mg of intravenous loxiglumide than after standard intravenous anticholinergic treatment [146]. Further randomized double-blind trials with sufficient patient numbers are needed to confirm this finding.
II.B.3.b. Role of CCK1R in irritable bowel syndrome (IBS)
II.B.3.b.1. Role of CCK1R in IBS: general
Numerous abnormalities of bowel motility, impaired sensitivity to gastric acid and visceral sensitivity have been described in IBS [4,147–150]. Some studies suggest that exaggerated release of CCK or altered responses to CCK could contribute to the symptoms of IBS [149,151–154]. A recent study provides evidence that the presence of intraduodenal lipids increases visceral sensitivity in constipation-and diarrhea-predominant IBS [155] and that CCK is a possible mediator of this effect.
II.B.3.b.2. Role of CCK1R in IBS: CCK1R antagonists in IBS
In a study involving eight healthy volunteers and eight patients with IBS, loxiglumide failed to inhibit the gastrocolic response in both patient groups [156]. In a pilot double-blind placebo-controlled multicenter study of 72 patients with IBS, 200 or 400 mg t.i.d. oral loxiglumide were administered for eight weeks. 400 mg were reported to cause a significant clinical improvement when compared to the placebo group or 200 mg group, especially in constipation-predominant IBS [157,158]. A recent study of the CCK1 selective antagonist dexloxiglumide [Fig. (1), Table 3] (200 mg t.i.d) in 36 women with constipation-predominant IBS found accelerated gastric emptying and slower ascending colon emptying, but no significant effect on overall colon transit or relief of IBS symptoms [159]. In a multicenter, randomized, placebo-controlled, double-blind phase II study of 405 patients of oral dexloxiglumide (200 mg t.i.d.), female constipation-predominant IBS patients responded significantly better to dexloxiglumide than to placebo and dexloxiglumide significantly improved clinical symptoms (pain, bloating, stool consistency) in constipation-dominant IBS patients. Furthermore, dexloxiglumide was generally well tolerated [160–162]. Two phase III studies involving 1400 women with constipation-predominant IBS found a trend towards a benefit of oral dexloxiglumide vs. placebo, but it did not reach statistical difference [163]. Results from another phase III trials of 1800 patients with constipation-predominant IBS are still pending [43]. To date, the usefulness of CCK1R inhibitors in the treatment of IBS remains unclear.
II.B.3.c. Role of CCK1R functional dyspepsia
II.B.3.c.1. Role of CCK1R functional dyspepsia.General
Dyspepsia is defined as a discomfort or pain in the upper abdomen and is a very common problem in patients in clinical practice [164]. Patients with functional dyspepsia often present with a hypersensitivity to distension of the stomach [165]. Chua et al. showed that infusion of CCK can reproduce specific symptoms in patients with functional dyspepsia [166]. Consumption of fat, which is known to release CCK, is often associated with dyspeptic symptoms and the indcution of symptoms is accompanied by a rise in plasma CCK levels (62,65). In healthy subjects, CCK1R antagonist loxiglumide has been found to accelerate liquid and solid gastric emptying [42], whereas a CCK1R agonist, GI181771X, has been found to delay gastric emptying of solids [167].
II.B.3.c.2. CCK1R antagonists in functional dyspepsia
In a randomized, double-blind, placebo-controlled study of 28 patients with functional dyspepsia, the authors found that oral loxiglumide relieved dyspeptic symptoms significantly better than placebo [166,168]. In another double-blind study of 12 patients with functional dyspepsia [165], intravenous dexloxiglumide relieved dyspeptic symptoms induced by gastric distension and duodenal lipid infusions significantly better than placebo. Larger studies are needed to confirm these encouraging findings.
II.B.3.d. Role of CCK1R in chronic constipation
II.B.3.d.1. Chronic constipation and CCK1R.General
A number of studies have demonstrated that CCK affects the activity of colonic muscle [169–171]. In human and animals a number of studies provide evidence that CCK increases colonic transit time [5,172,173], however, other studies found that physiological concentrations of CCK do not effect transit in healthy subjects [156,174]. In healthy volunteers CCK1R antagonists have accelerated colonic transit [48,175,176], however, no increase in colonic transit occurred after treatment with CCK1R antagonists in patients with irritable bowel syndrome [159,177].
II.B.3.d.2. Chronic constipation and CCK1R antagonists
A randomized, double-blind, placebo-controlled trial of 21 chronically constipated geriatric patients found a significant benefit (acceleration of transit time, increase in stool frequency, diminution of number of enemas) after three weeks of oral loxiglumide (800 mg 3 times daily) [178]. In another study in 8 younger men, dexloxiglumide 200 b.i.d. over 7 days partly reversed increased colonic transit time induced by fiber-supplemented liquid formula diet, suggesting it might be useful as pro-kinetic in patients with chronic obstipation [175]. However, larger studies are needed to confirm these findings.
II.B.3.d. Role of CCK1R in gastroesophageal reflux disease (GERD)
II.B.3.d.1. GERD and CCK1R.General
Multiple studies have shown in animal models and man that CCK can contribute to GERD by increasing transient lower esophageal sphincter relaxations indirectly by causing gastric fundal distension as well as through a direct interaction with esophageal CCK1Rs result in lower basal lower esophageal sphincter pressure [179–186]. The CCK1R antagonists loxiglumide and lintript [Fig. (1), Table 3) can inhibit the effect of CCK on the human lower esophageal sphincter (LOS)[187] and loxiglumide can significantly decrease transient relaxation of the LOS caused by either CCK infusions or mechanical distension [5]. A number of studies in humans demonstrated that the CCK1R antagonist, loxiglumide could decrease transient relaxations of the LOS induced by various means (air or mechanical distension, CCK infusion, fat meal) [5,60,185,188].
II.B.3.d.1. Use of CCK1R antagonists in GERD
In a study of 10 healthy volunteers and 9 GERD patients, Trudgill et al. found that loxiglumide inhibits postprandial LOS relaxation significantly better than placebo in GERD patients and controls, however, there was only a modest effect on acid exposure [186]. In a study of 12 patients with morbid obesity, Hirsch et al. found that loxeglumide reduced postprandial lower esophageal sphincter relaxation but did not reduce significantly episodes of transient lower esophageal sphincter relaxations [189]. Further well-designed trials with sufficient patient numbers are needed to define the role of CCK1R inhibitors in GERD.
II.B.4.CCK1R gene mutations
A number of recent studies provide evidence that reduced CCK1R expression or expression of a non-functional receptor could predispose a subset of patients to cholecystolithiasis. In an obese patient with gallstones, Miller et al. described a 262 bp deletion resulting in a non-functional receptor [85]. Miyasaka et al. [190] reported that CCK1R expression was decreased in gallbladders with stones when compared to gallbladders without gallstones and described a polymorphism in the CCK1R promoter in gallstone patients. However, this polymorphism did not influence promoter activity. The authors further show that CCK1R knockout mice had increased gallstone formation [190]. Another study reported that CCK1R knockout mice showed increased sludge and gallstone formation at 12 and 24 months of life when compared to wild-type mice [191]. Recently, decreased CCK1R expression in the gallbladder was reported in patients with gallstones and a non-contracting gallbladder [134] and in patients with gallstones and diabetes mellitus [135]. Some recent studies suggest that CCK1R polymorphisms might be related to obesity. Funakoshi et al. [192] report that a polymorphism in the CCK1R promoter [G to T (n-128) and A to G (n-81)] is correlated with higher percent body fat and increased serum levels for insulin and leptin [193]. However, the mechanism of this association remains unclear and polymorphic promoters did not affect CCK1R promoter activity when transiently expressed in STC-1 endocrine tumor cells [192]. In another study, the same group reported that this CCK1R promoter polymorphism is associated with midlife weight gain in men only when combined with a β3-adrenergic receptor polymorphism [194], although the mechanism underlying this association remains unclear. Marchal-Victorion et al. reported a V365I mutation in the CCK1R of obese diabetic patients [195]. This mutated receptor demonstrated a decreased expression and a low efficacy for activating phospholipase C when transfected into COS-7 cells. However, it remains unclear whether this mutation contributes to diabetes mellitus or obesity in these patients.
III. GASTRIN AND CCK2R
III.A. General
Gastrins well-established physiological effects are mediating acid secretion and stimulation of gastric mucosal growth, especially the enterochromaffin-like cells (ECL) [4,18] (Table 4). Recent studies on CCK2R knockout mice demonstrate that inhibition of gastric emptying is likely also a physiological effect of gastrin[196](Table 4). In contrast to the CCK1R and CCK, the role of the CCK2R or gastrin has been defined in a number of gastrointestinal disorders (Table 4). Gastrin causes gastric mucosal growth/hyperplasia of gastric enterochromaffin-like cells (ECL cells), which can progress to the formation of carcinoid tumors [197–201]; stimulates gastric mucosal growth with increased parietal cells, and is a mediator of gastric acid secretion in acid-peptic disorders and in various gastric acid hypersecretory studies including Zollinger-Ellison syndrome (ZES) [202–208]. Studies suggest gastrin-related peptides also may have important growth effects on a number of GI malignancies, because they frequently overexpress or ectopically express CCK2R [209–213]. Furthermore, gastrin itself or gastrin-related peptides, interacting with either the CCK2R or an unknown receptor, may have growth effects in some tumors, especially colon cancer [209–217]. The effect of gastrin, CCK and CCK-R’s in cancer is dealt with in a separate section in this volume, so it will not be dealt with further, except in the use of radiolabeled CCK/gastrin analogues for localization of various tissues/tumors over-expressing these receptors.
Table 4.
Gastrin and CCK2R in the gastrointestinal tract: physiological functions and possible disorders
| Physiological functions | |||
| 1. | Stimulation of gastric acid secretion | ||
| 2. | Stimulation of gastric mucosa growth (esp ECL cells) | ||
| 3. | Inhibit of gastric empyting | ||
| Possible disease involvement | |||
| I. | Proven: | ||
| Hypergastrinemic states [physiological (atrophic gastritis, pernicious anemia), and pathological (Zollinger-Ellison syndrome)] | |||
| Abnormalities due to gastric mucosal effects of hypergastrinemia (ECL cell hyperplasia, carcinoids, ↑ parietal cell mass) | |||
| Acid-peptic disorders | |||
| II. | Possible: | ||
| a. Tumor growth (colon, gastric, pancreatic, liver) | |||
III.B. Gastrin or CCK2R alterations in clinical GI disease
CCK2R and /or gastrin is important in human hypergastrinemic states (Table 2,4), in acid-peptic disorders, in imaging disease processes over-expressing CCK2R’s, and CCK2R mutations have recently been described which may mediate important abnormal growth effects. In a number of these conditions the possible utility of CCK2R antagonists has been proposed and in some cases studied in humans. Furthermore, there is a long established use of gastrin analogues in provocative testing to examine for medullary thyroid cancer (MTC) or to assess gastric maximal acid output (MAO) to aid in the diagnosis or management of various disorders of gastric acid secretion [218–221]. Each of these will be briefly dealt with in the following sections.
Table 2.
Causes of chronic hypergastrinemia
| a. | Associated with gastric acid hyposecretion-achlorhydria | ||
| 1. | Pernicious anemia-atrophic gastritis | ||
| 2. | Treatment with potent acid antisecretory agents (especially with H+-K+-ATPase inhibitors) |
||
| 3. | Chronic renal failure (common) | ||
| 4. | H. pylori infection | ||
| 5. | Post-gastric acid-reducing surgery | ||
| B. | Associated with gastric acid hypersecretion | ||
| H. pylori infection | |||
| Gastric outlet obstruction | |||
| Antral G-cell hyperfunction-hyperplasia | |||
| Chronic renal failure (rare) | |||
| Retained gastric antrum syndrome | |||
| Short-bowel syndrome | |||
| Gastrinoma (Zollinger-Ellison syndrome) | |||
III.B.1. Gastrin analogues for provocative testing
A number of analogues of gastrin, primarily derivatives of the active C-terminal gastrin tetrapeptide sequence (Tyr-Met-Asp.Phe-NH2) were used in humans, principally to assess maximal acid output (MAO) [221]. Currently, only pentagastrin (N-t-butyl oxycarbonyl-β-Ala.Tyr.Met.Asp.Phe-NH2) is generally used for this purpose or as a provocative agent for medullary thyroid cancer [218–221]. Assessment of MAO was used much more frequently in the past than at present [220,221]. In the past the MAO was assessed for possible prediction of acid- peptic recurrences, for the diagnosis of Zollinger-Ellison syndrome and to determine the presence of pernicious anemia and other hypochlorhydria/achlorhydric states [220–223]. Today, although underutilized, it remains important in the differential diagnosis of hypergastrinemic states, especially in the differentiation between physiologically hypergastrinemia due to hypo-/achlorhydria and pathological hypergastrinemia due to disorders such as Zollinger-Ellison syndrome (Table 4)[220,222–224]. Medullary thyroid cancers (MTC) are derived form parafollicular C-cells of the thyroid and release calcitonin which is used to assess for the possible presence of MTC as well as the ability of pentagastrin to stimulate calcitonin release from these cells [218,219,225]. MTC ectopically express CCK2R in 92% of cases whereas the other cells of the thyroid do not which is the basis of this widely used provocative test [226].
III.B.2. Gastrin and CCK2R in hypergastrinemic states
III.B.2.A. Gastrin and CCK2R in hypergastrinemic states-diseases
Human hypergastrinemic states result from two principal causes: a physiological response due to acid hypo-/achlorhydria occurring in such disorders as pernicious anemia/atrophic gastritis (Table 4), and in disorders causing hypergastrinemia with acid hypersecretion [203] (Table 4). Long-lasting hypergastrinemia in both categories can have clinical significance, because it can result in gastric ECL cell hyperplasia and the development of gastric carcinoid tumors [197,200,227–230]. This consequence of hypergastrinemia is receiving increased attention, because a proportion of gastric carcinoid tumors can be malignant [59,231,232]. Hypergastrinemia with acid hypersecretion is significant because the acid hypersecretion can result in aggressive peptic ulcer disease. Hypergastrinemia with acid hypersecretion can be caused by a number of abnormalities (Table 4). The most frequent cause is H. pylori infections, because in a proportion of infected patients hypergastrinemia with hyperchlorhydria develops[233,234]. The most aggressive hypersecretory disease occurs in the Zollinger-Ellison syndrome (ZES), which is due to the presence of a neuroendocrine tumor ectopically secreting gastrin (i.e., a gastrinoma) [203,224,235].
Various forms of gastrin peptides as well as gastrin mRNA has been reported in bronchogenic carcinoma, acoustic neuromas, pheochromocytomas, ovarian carcinomas, colorectal carcinomas, and other pancreatic endocrine tumor syndromes than those causing the Zollinger-Ellison syndrome [18,236–238]. Except for ovarian carcinoma and a single case of small cell lung cancer, these tumors do not cause ZES, because they do not secrete biologically active amidated gastrins, which are the only forms that interact with high affinity with the CCK2R and stimulate potently acid secretion[18,236,238–242].
Gastrinomas producing the ZES until recently were thought to be entirely intra-abdominal in location (primary location: duodenum >pancreas >lymph nodes >liver >other abdominal sites) [224,235,243,244]. However, recent studies show gastrinomas can arise in the heart [245,246] and ZES can also occur from gastrin secretion by a non-small cell lung cancer [239]. Because, patients with ZES are cured surgically in <50% of cases, the majority have life-long hypergastrinemia [59,244,247–250]. Furthermore, because their acid hypersecretion can now be controlled in almost every patient medically (i.e. with proton pump inhibitors, histamine H2 antagonists) [59,251,251–253],today ZES patients rarely die of refractory peptic disease as they did in the past [254,255], and have extended survival; hence they are an excellent natural model to study the long-term consequences of hypergastrinemia in man.
Recent studies of ZES patients has provided important insights into the effects of chronic hypergastrinemia in man, especially in regards to its gastric effects [4,59,249]. These studies show hypergastrinemia can cause marked gastric acid hypersecretion which can result in severe refractory peptic ulcer disease, malabsorption and gastro-esophageal reflux disease [223,249,254,256,257]; can cause increased gastric mucosal thickness and increased parietal cell mass 4- to 6-fold with no increase in peptic cells [258–261]; and result in a mean 2-fold increase in mucosal argyrophil cells [262–267]. Of the seven types of gastric endocrine cells the increase in gastric argyrophil cells with hypergastrinemia in man is due to an increase only in the gastric ECL cells [267], which is similar to results in animal studies [268–271]. No other clinical effects of chronic hypergastrinemia have been clearly demonstrated from studies of patients with Zollinger-Ellison syndrome, especially in regard to increased tumor growth or frequency of tumors in other sites [4,59,272,273]. These points will be discussed in more detail in the following sections.
A number of animal studies show that chronic hypergastrinemia (antisecretory drug treatment, gastrin infusions, surgical procedures) can cause gastric ECL proliferation and in some cases (rat, mouse, mastomys), the development of gastric carcinoids [198,270,271,274–278], which can occasionally be malignant [279]. It is proposed the gastric carcinoids arise from the ECL cell through a progression of ECL cell proliferative changes from increasing hyperplasia (linear, micronodular, adenomatoid) to dysplasia and carcinoid formation [200,270,280,281]. It is thought a similar sequence of events occurs in humans because various human conditions with chronic hypergastrinemia [especially atrophic gastritis/pernicious anemia and ZES (Table 4)] as well as in patients with chronic acid suppressive treatment, ECL cell proliferative changes can occur [200,230,263,266,270,280,282–287]. In addition, in patients with atrophic gastritis [231,284,287,288] or with ZES with multiple endocrine neoplasia-type 1 (MEN1) [231] and rarely without MEN1 [231,249,289,290], gastric carcinoid tumors develop.
Recently, increasing attention is being given to the effect of chronic hypergastrinemia on gastric ECL cell proliferation primarily for two reasons [59]. First, increasing numbers of patients with idiopathic gastro-esophageal reflux disease/peptic ulcer disease (PUD) are treated long-term with potent acid suppressants such as PPIs and in 80–100% of these patients [283,291] hypergastrinemia develops. In 20–30% of patients the fasting gastrin levels can reach levels frequently seen in ZES [249,283,291–293]. Second, in a subset (4–30%) of patients with hypergastrinemic states who develop gastric carcinoids, the tumors are malignant [231,232,294].
A recent study [230] in 106 ZES patients provided a number of insights into the long-term effects of hypergastrinemia on gastric ECL cells in man. Because these patients infrequently have gastritis or gastric atrophy, as occurs in atrophic gastritis and which can effect ECL cell behavior, they are a good model to study the effects of chronic hypergastrinemia alone in man [230]. Furthermore, at least one-half of the ZES patients have fasting gastrin levels in the range seen with chronic PPI treatment [230,249,282,291,293]. In this study [230] only 1% of the patients with active disease had a normal ECL cell pattern and 52% having at least linear hyperplasia or a more advanced ECL change [230] including 7% with dysplasia and 0% with carcinoid tumors [230]. Fasting gastrin levels correlated closely with the magnitude of the ECL change (p <0.0001) and no threshold effect of gastrin on ECL cell hyperplasia was found, as proposed by others [200,295,296]. In contrast to animal studies, gender [274,297,298], age, or vagal tone [198,299,300] did not affect the degree of ECL change. These results show the risk of developing gastric carcinoids with chronic hypergastrinemia alone in man is low; however, even mild chronic hypergastrinemia in man can cause ECL hyperplasic changes without a threshold effect.
III.B.2.B. Use of CCK2R antagonists in human hypergastrinemic states
Whereas it appears logical to consider treating human hypergastrinemic states with CCK2R antagonists and numerous papers have proposed such a use for them [6,40,301,302] there is very little data from human studies. Particularly appealing would be the ability to prevent the ECL hyperplasia that occurs in these states as well as the ECL changes seen after the long-term use of potent acid suppressant drugs such as the PPI’s and therefore reduces the potential development of gastric carcinoids with long-term hypergastrinemia [59]. In animal studies a number of CCK2R antagonists have been shown to cause prompt inhibition of gastrin stimulated ECL-cell histamine and pancreastatin secretion and synthesis as well as gastrin-stimulated acid secretion [303]. A few studies have examined the effect of CCK2R antagonists on gastric acid secretion in humans, which will be discussed in the next section, but almost no data exist on their potential effective in hypergastrinemic states. Whether this potential clinical indication for a CCK2R antagonist would actually be clinically helpful in many of these patients is unclear at present. Whereas it is clear chronic treatment with PPI’s increases ECL hyperplasia in many patients it is not established in what proportion, if any, its long-term use in man leads to the development of carcinoids [59,304–306]. Furthermore, except for ZES patients with MEN1 who develop gastric carcinoids in 13–43%, the proportion of patients with atrophic gastritis (2–8%) or with idiopathic ZES (<1%) who develop gastric carcinoids over many years is relatively low [4,59,230,307]. Lastly, other forms of treatment currently exist for hypergastrinemic patients with carcinoids with the demonstration that parental somatostatin analogues can reverse the hypergastrinemia and even the carcinoids presence in these patients [308,309]. Proglumide (DL-4-benzamido-N,N-di-n-propylglutaramic acid) [Fig. (2), (Table 3], a low affinity CCK1R and CCK2R antagonist (Kd, 3=11 uM) [6,310,311] was given intravenously to 3 patients with ZES [312]. Proglumide was given as a bolus injection (50 mg/kg) and as a bolus followed by a continuous intravenous infusion (50 mg/kg/hr). It did not alter serum gastrin levels but inhibited acid secretion by 13–62%, which was less than the 83–86% inhibition caused by an infusion of the histamine H2 receptor antagonist cimetidine (2 mg/kg/hr). It was concluded that proglumide is a weak inhibitor of acid secretion in these patients [312].
III.B.3. Gastrin and CCK2 receptors in peptic ulcer disease
III.B.3.A. Gastrin and CCK2 receptors in peptic ulcer disease/acid secretion
One of the most important physiological effects of gastrin is its ability to stimulate gastric acid secretion [18].Even though studies in various species show the parietal cell possesses CCK2R, most evidence suggests the principal pathway of stimulation of acid secretion by gastrin is by stimulating release of histamine from ECL cells [18,313,314]. That gastrin is the major hormonal mediator of the gastric phase of acid secretion [18] is supported by studies utilizing immuno-neutralization of circulating gastrin, which completely inhibits acid secretion stimulated by peptone or glucose-induced gastric distension [315]. Gastrin also plays a variable role in the cephalic and intestinal phases of acid secretion in different species [18]. CCK2R antagonists in rats inhibit gastrin-stimulated acid secretion and histamine release from ECL cells [303,313,316]. In humans spiroglumide, a selective CCK2R antagonist [Fig. (2), Table 3], inhibited acid secretion stimulated by a meal as well as sham feeding stimulated secretion [317]. These results support an important physiological role for gastrin in regulating acid secretion in humans, as well as other species. The central role of the CCK2R in mediating the action of gastrin on acid secretion is also supported by the results of CCK2R and gastrin knockout studies in mice [19,53,318]. In both cases the mice have an elevated gastric pH and do not secrete acid in response to gastrin [53,318].
Gastrin also has a trophic effect on parietal cells and chronic hypergastrinemia results in an increased parietal cell mass [224,319,320]. This is important clinically because the parietal cell mass correlates with the maximal acid output [221]. In various chronic hypergastrinemic states in man, such as ZES, both increased parietal cell mass and an increased maximal acid output are frequently found and can contributed to the marked gastric acid hypersecretion that occurs[223,224,258–261].
Numerous studies demonstrate that Helicobacter pylori (H. pylori) infections are the principal cause of duodenal ulcer disease; however, the exact mechanisms by which H. pylori causes duodenal ulcer are still unclear [321–324]. Furthermore, the exact role of gastrin or abnormalities in gastric secretion in the development of H. pylori-mediated duodenal ulcer disease remain unclear [321–324]. A proportion of patients with duodenal ulcers have an increased parietal cell mass resulting in an increased MAO, an exaggerated acid and gastrin release with meals or gastrin-releasing peptide administration, an altered sensitivity to gastrin, impairment in inhibiting responses mediating secretion of acid and gastrin, in addition to an increased basal acid output in 30% of these patients [322].
Patients with duodenal ulcer disease caused by H. pylori characteristically have an antrum-dominant, body-sparing, non-atrophic gastritis [323,324], which results in increased acid secretion and gastrin release. The increased gastrin release is mediated primarily by an impairment of the acid-mediated inhibitory control of gastrin release, which is regulated by somatostatin release from antral D cells [323–325]. Lower levels of somatostatin-IR are found in the antral mucosa of H. pylori infected subjects and the levels increase post-eradication of the H. pylori [323–325]. Functional studies suggest that alterations in the ability of CCK functioning through CCK1R may be involved in the impairment of acid-mediated inhibitory control of gastrin release in H. pylori infected subjects [326,327]. CCK acting via CCK1R on antral D cells stimulates somatostatin release, which has an inhibitory effect on gastrin secretion from antral G cells [50,323,328]. The CCK1R antagonist, loxiglumide (Table 3), increased meal-stimulated acid output in healthy controls, but not in duodenal ulcer patients [327]. Eradicating H. pylori results in correction of the abnormal response to CCK1R blockage in duodenal ulcer patients [326,327]. The mechanism by which H. pylori infection or accompanying gastritis alters the acid-inhibitory control of gastrin release and somatostatin levels is not completely clear [323,324]. Possible mechanisms include secondary to increased cytokine production, and alterations induced by ammonia production by H. pylori [317,323,329].
Some studies [324], but not others [321], propose that alterations in gastrin regulation can explain most of the acid secretory abnormalities seen in patients with H. pylori infection. This includes the proposal that 1) the increased acid output is, in large part, due to the altered gastrin release, and this results in increased duodenal acid load which progressively damages the duodenal mucosa leading to gastric metaplasia and eventually to duodenal ulcers; and 2) the increased gastrin release results in an increased BAO and the trophic effects of gastrin cause the increased MAO [324].
III.B.3.B. Use of CCK2R antagonists in peptic ulcer disease/acid secretion
A number of CCK2R antagonists have been studied in man for their affect on acid secretion or in peptic ulcer disease.
Glutaramic acid derivatives
Proglumide, a non selective CCK1R and CCK2R low affinity antagonist (Table 3) was marketed by Rotta Laboratories (Italy) for treatment for peptic ulcers prior to the widespread use of histamine H2 antagonists and PPIs and some studies reported increased healing rates [330–332]. Subsequent structure function studies of glutaramic acid analogues of proglumide yielded the CCK1R antagonists; lorglumide, loxiglumide, dexloxiglumide [Table 3, Fig. (2)] which are discussed in the previous section on CCK1R disease states, as well as the CCK2R preferring antagonists: spiroglumide and itriglumide [(Table 3), Fig. (2)]. Spiroglumide given intravenously in 3 doses (1,2.5,7.5 mg/kg/hr dose dependently inhibited gastrin, sham and meal stimulated acid secretion in normal volunteers [317] and in another study the inhibition of gastrin stimulated secretion was competitive in nature [333]. This drug was not developed further even though it had excellent oral bioavailability, because of it relatively low anti-gastrin activity in vitro and its poor selectivity for CCK2R compared to CCK1R[334]. Further structure-function studies of spiroglumide yielded itraglumide (CR 2945) which had a 9000 fold higher affinity for CCK2R than CCK1R (Table 3)[334,335] This compound is reported to now be in Phase 1 trials as an anti-ulcer and anxiolytic agent [6].
Benzodiazepine derivatives
Structure function studies of asperlicin, the first potent nonpeptide CCK1R antagonist [336] identified the highly selective CCAR antagonist, L-364,718 [337] and the CCK2R antagonist, L-365,260 [338](Table 3). L-365,260 was active after oral administration, had a extended duration of action and inhibited stimulated acid secretion in a number of animals [339]. In a double-blind study in eight normal human volunteers, L365,260 inhibited gastrin-stimulated acid secretion, however the duration was not prolonged [340]. L-365,260 subsequently underwent a number of human studies assessing its possible anxiolytic effects, however it generally gave disappointing results, which were attributed to its limited oral bioavailability [6,341]. Subsequently, additional 1,4 substituted benzodiazepine derivatives (L-368,730, L369,466, L-736,380, L-740,093, YM022) as well as 1,5 substituted analogues (GV1500013X, GV191869X, Z-360) were developed [6,40,334,342] with enhanced potency and bioavailability, however no human studies assessing their effect on acid secretion/secretory disorders are reported with these compounds. YF476 (Table 3) was identified by structure-function studies of YM022 and shown to have a 5000 higher affinity for CCK2R than CCK1R, to have good bioavailability and to inhibit acid secretion in animals [302,343]. A single dose of YF474 caused dose-dependent inhibition of gastric acid secretion in human volunteers and the antisecretory effect was longer lasting than seen with ranitidine [344]. In later trials in humans when YF476 was administered twice per day for 7 or 14 days there was initially a substantial reduction in acid secretion, however this decreased with repeated doses and after 7 and 14 days in the two trials the gastric acidity was not different from placebo [345,346]. The mechanism of this loss of efficacy of YF476 with continued treatment was not clear. However, this is the first report of tachyphylaxis with prolonged CCK2R antagonist use in humans.
Peptoids
Using the C-terminal tetrapeptide sequence of CCK/gastrin (Trp-Met-Asp-Phe-NH2) Parke Davis researchers developed a series of CCK2R antagonists [6,347]. One of the most potent and selective was CI-988 (PD-134,308) (Table 3) which potently inhibited pentagastrin-stimulated acid secretion in animal studies [348,349].CI-988 has been examined in a number of human studies to investigate its ability to prevent panic attacks [350–353],however there are no studies on its effect on acid secretion in humans.
Other CCK2R antagonists in human acid secretory studies
A number of other potent CCK2R antagonists have been developed, but not assessed in human acid secretory disorders, including ureido-acetamide derivatives (RP 69758, RP 72540, RP 73870, DA-3934, D51-9927), quinazolinone derivatives LY-202769), benzazepine derivatives (CP 310,713) [6,40,334], dibenzobicyclo [2.2.2]octane and bicycloheteroaromatic scaffold-based analogues (compounds 83,86,89,91, JB93182) [6,354,355].
What the role for CCK2R antagonists in the treatment of human peptic disease or gastro-esophageal reflux disease at present is unclear [334,342,356]. There is a number of classes of drugs such as histamine H2 receptor antagonists (cimetidine, ranitidine, famotidine) and proton pump inhibitors (omeprazole, lansoprazole, esomeprazole, rabeprazole, pantoprazole) that are generally very effective at inhibiting acid secretion and treating these diseases. PPI’s have a long duration of action so that there is in general not a need for an inhibitor that might even have longer duration of activity. Furthermore, each of the above compounds has an excellent safety record with prolonged use in many patients. Therefore an additional class of acid-suppressant agents such as the CCK2R antagonists is not need in most patients. Furthermore, if the tachyphylaxis seen with repeated use of YF476 [345,346] is a characteristic acid secretory response in humans to repeated dosing with a potent CCK2R antagonist, this will greatly limit the therapeutic potential of these compounds for acid secretory disorders. The main role for CCK2R antagonists in these common diseases might be to possibly prevent the results of the hypergastrinemia that occurs in almost all patients with prolonged treatment with potent acid anti-suppressants such as PPI’s in gastroesophageal reflux patients [59].At present the risk of this hypergastrinemia short term (<5 years) is very low, although the long term risk is unclear [59,357,358]. The definition of this risk will be important in assessing the possible need for prevention of this effect with concomitant treatment with CCK2R antagonists [59].
III.B.4. CCK2R abnormalities in diseases
CCK2R mutations occur in a number of cancers including those of the pancreas [359], colon [113,114], and stomach [114]. A misspliced form of the CCK2R in which intron 4 is retained is reported in pancreatic [359] and colon cancer [113]. The occurrence of the misspliced receptor form was associated with decreased amounts of the U2 small nuclear ribonucleoprotein particle auxiliary splicing donor in pancreatic cancer [359] (U2HF35) [359]. The abnormal spliced receptor showed constitutive activation and had trophic activity in cells expressing it [113], suggesting its presence might confer t growth-promoting effects. In 43 gastrointestinal tumors with a high microsatellite instability, frameshift mutations were found in the CCK2R in 19% [114]. Frameshift mutations in the CCK2R occurred in 23% of gastric cancers, 13% of sporadic colorectal cancers and 20% of hereditary colorectal carriers, and all tumors also had frameshift mutations in other genes [114]. The LoVo colorectal cancer cell line responds to gastrin also showed a similar frameshift mutation in the CCK2R [114]. The above results [114] led the authors to propose that the human CCK2R gene is a new candidate target gene possibly playing a role in the tumorigenesis of a fraction of MSI tumors.
In obese, diabetic patients, 2 of 18 families with type-2 diabetes mellitus were found to have a V125I mutation in the CCK2R [195]. This mutated receptor, when expressed in COS-7 cells, had an increased affinity for CCK and enhanced potency for activating phospholipase C [195]. Co-segregation studies showed the mutation was not associated with diabetes or early age at diagnosis of the disease. At present, the role of this CCK2R mutation in the pathogenesis of either the obesity or diabetes mellitus in the families remains unclear.
III.B.5. Gastrin, gastrin-related peptides on normal and tumor growth (non-ECL cell growth)
Numerous studies demonstrate that gastrin related peptides can have important growth effects on a number of tumors [4,201,209–211,215,217,319,360]. This is dealt with in another paper in this volume so it will not be dealt with further here.
I.C. CCK2R imaging for localization of disease
Recent studies especially with radiolabeled somatostatin analogues demonstrate that overexpression of G protein-coupled receptors by tumors or other disease processes can be used for localization, clinical assessment and for receptor directed delivery of therapeutic agents [361–364]. CCK2R receptors are overexpressed in a number of human tumors particularly medullary thyroid cancer, (92%), but also some gastroenteropancreatic tumors [226,365]. In addition, over-expression of CCK2R occurs in a significant number of small cell lung cancers, ovarian cancers and astrocytomas [365]. This has lead a number of groups to develop specific radiolabeled analogues of gastrin that could be used to assess CCK2R expression using imaging and provide information on its localization and over-expression in vivo in different disease processes [366–372]. [111In-DTPA]-[D-Asp26, Nle28, 31]-CCK (26–33[366] and [111In-DTPA] minigastrin analogues [368,372] are reported to image CCK2R bearing medullary thyroid cancers in patients as well as to image the gastric mucosa which contains a high density of CCK2R cells. Whether this approach will be clinically useful in localizing these tumors or in allowing peptide receptor targeting of cytotoxic agents, as has been done with somatostatin analogues in a number of tumors over-expressing somatostatin [361–364], is at present unclear.
Acknowledgments
This publication is partially supported by the Intramural Research Program of the NIDDK, NIH.
References
- 1.Ivy AC, Oldberg E. A hormone mechanism for gallbladder contraction and evacuation. Am. J. Physiol. 1928;65:599–613. [Google Scholar]
- 2.Edkins JS. On the chemical mechanism of gastric secretion. Proc. R. Soc. Lond. [Biol] 1905;76:376. [Google Scholar]
- 3.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 4.Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol. Toxicol. 2002;91:333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- 5.Peter SA, D'Amato M, Beglinger C. CCK1 antagonists: are they ready for clinical use? Dig. Dis. 2006;24:70–82. doi: 10.1159/000090310. [DOI] [PubMed] [Google Scholar]
- 6.Herranz R. Cholecystokinin antagonists: pharmacological and therapeutic potential. Med. Res. Rev. 2003;23:559–605. doi: 10.1002/med.10042. [DOI] [PubMed] [Google Scholar]
- 7.Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur. J. Biochem. 1968;6:156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 8.Gregory RA, Tracy HJ. The constitution and properties of two gastrins extracted from hog antral mucosa. Gut. 1964;5:103–117. [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen RT, Lemp GF, Gardner JD. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc. Natl. Acad. Sci. (USA) 1980;77:2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robberecht P, Deschodt-Lanckman M, Morgat JL, Christophe J. The interaction of caerulein with rat pancreas. 3. Structural requirements for in vitro binding of caerulein-like peptides and its relationship to increased calcium outflux, adenylate cyclase activation and secretion. Eur. J. Biochem. 1978;91:39–48. doi: 10.1111/j.1432-1033.1978.tb20934.x. [DOI] [PubMed] [Google Scholar]
- 11.Sankaran H, Goldfine ID, Deveney CW, Wong KY, Williams JA. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J. Biol. Chem. 1980;255:1849–1853. [PubMed] [Google Scholar]
- 12.Innis RB, Snyder SH. Distinct cholecystokinin receptors in brain and pancreas. Proc. Natl. Acad. Sci. USA. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito A, Sankaran H, Goldfine ID, Williams JA. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980;208:1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- 14.Wank SA, Harkins R, Jensen RT, Shapira H, DeWeerth DE, Slattery TS. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc. Natl. Acad. Sci. (USA) 1992;89(7):3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LF, Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. USA. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddle RA. Cholecystokinin. In: Walsh JH, Dockray GJ, editors. Gut Peptides. New York: Raven Press; 1994. pp. 175–216. [Google Scholar]
- 17.Jensen RT. Receptors on pancreatic acinar cells. In: Johnson LR 3 ed, Jacobson ED, Christensen J, Alpers DH, Walsh JH, editors. Physiology of the Gastrointestinal Tract, Third edition. 3 ed. New York: Raven Press; 1994. pp. 1377–1446. [Google Scholar]
- 18.Walsh JH. Gastrin. In: Walsh JH, Dockray GJ, editors. Gut Peptides. New York: Raven Press; 1994. pp. 75–121. [Google Scholar]
- 19.Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology. 1997;112:280–286. doi: 10.1016/s0016-5085(97)90000-7. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka K, Shinozaki H, Suzuki S, Sato Y, Kanai S, Masuda M, Jimi A, Nagata A, Matsui T, Noda T, Kono A, Funakoshi A. Disruption of cholecystokinin (CCK)-B receptor gene did not modify bile or pancreatic secretion or pancreatic growth: a study in CCK-B receptor gene knockout mice. Pancreas. 1999;19:114–118. [PubMed] [Google Scholar]
- 21.deTullio P, Delarge J, Pirotte B. Therapeutic and chemical developments of cholecystokinin receptor ligands. Exp. Opin. Invest. Drugs. 2000;9:129–136. doi: 10.1517/13543784.9.1.129. [DOI] [PubMed] [Google Scholar]
- 22.Monstein HJ, Nylander AG, Salehi A, Chen D, Lundquist I, Hakanson R. Cholecystokinin-A and cholecystokinin-B/gastrin receptor mRNA expression in the gatrointestinal tract and pancreas of the rat and man. A polymerase chain reaction study. Scand. J. Gastroent. 1996;31:383–390. doi: 10.3109/00365529609006415. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz F, Goke MN, Otte JM, Schrader H, Reimann B, Kruse ML, Siegel EG, Peters J, Herzig KH, cFolsch UR, Schmidt WE. Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul. Pept. 2001;102:101–110. doi: 10.1016/s0167-0115(01)00307-x. [DOI] [PubMed] [Google Scholar]
- 24.Reubi JC, Waser B, Laderach U, Stettler C, Friess H, Halter F, Schmassmann A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997;112:1197–1205. doi: 10.1016/s0016-5085(97)70131-8. [DOI] [PubMed] [Google Scholar]
- 25.Morisset J, Julien S, Laine J. Localization of cholecystokinin receptor subtypes in the endocine pancreas. J Histochem. Cytochem. 2003;51:1501–1513. doi: 10.1177/002215540305101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokunaga Y, Cox KL, Coleman R, Concepcion W, Nakazato P, Esquivel CO. Characterization of cholecystokinin receptors on the human gallbladder. Surgery. 1993;113:155–162. [PubMed] [Google Scholar]
- 27.Rettenbacher M, Reubi JC. Localization and characterization of neuropeptide receptors in human colon. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364:291–304. doi: 10.1007/s002100100454. [DOI] [PubMed] [Google Scholar]
- 28.Reubi JC, Waser B, Schmassmann A, Laissue JA. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastro-intestinal adenocarcinoma samples: where are they really located? Int. J Cancer. 1999;81:376–386. doi: 10.1002/(sici)1097-0215(19990505)81:3<376::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Moriarty P, Dimaline R, Thompson DG, Dockray GJ. Characterization of cholecystokininA and cholecystokininB receptors expressed by vagal afferent neurons. Neuroscience. 1997;79:905–913. doi: 10.1016/s0306-4522(96)00675-6. [DOI] [PubMed] [Google Scholar]
- 30.Mazzocchi G, Malendowicz LK, Aragona F, Spinazzi R, Nussdorfer GG. Cholecystokinin (CCK) stimulates aldosterone secretion from human adrenocortical cells via CCK2 receptors coupled to the adenylate cyclase/protein kinase A signaling cascade. J Clin Endocrinol. Metab. 2004;89:1277–1284. doi: 10.1210/jc.2003-030953. [DOI] [PubMed] [Google Scholar]
- 31.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- 32.Tang C, Biemond I, Lamers CB. Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study. Gastroenterology. 1996;111:1621–1626. doi: 10.1016/s0016-5085(96)70025-2. [DOI] [PubMed] [Google Scholar]
- 33.Reubi JC, Waser B, Gugger M, Friess H, Kleeff J, Kayed H, Buchler MW, Laissue JA. Distribution of CCK1 and CCK2 receptors in normal and diseased human pancreatic tissue. Gastroenterology. 2003;125:98–106. doi: 10.1016/s0016-5085(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 34.Yu DH, Huang SC, Wank SA, Mantey S, Gardner JD, Jensen RT. Pancreatic receptors for-cholecystokinin: Evidence for three receptor classes. Am. J. Physiol. 1990;258:G86–G95. doi: 10.1152/ajpgi.1990.258.1.G86. [DOI] [PubMed] [Google Scholar]
- 35.Yu DH, Noguchi M, Zhou ZC, Villanueva ML, Gardner JD, Jensen RT. Characterization of gastrin receptors on guinea pig pancreatic acini. Am. J. Physiol. 1987;253:G793–G801. doi: 10.1152/ajpgi.1987.253.6.G793. [DOI] [PubMed] [Google Scholar]
- 36.Huang SC, Yu DH, Wank SA, Mantey S, Gardner JD, Jensen RT. Importance of sulfation of gastrin or cholecystokinin (CCK) on affinity for gast CCK receptors. Peptides. 1989;10(4):785–789. doi: 10.1016/0196-9781(89)90114-9. [DOI] [PubMed] [Google Scholar]
- 37.Rivard N, Rydzewska G, Lods JS, Martinez J, Morisset J. Pancreas growth, tyrosine kinase, PtdIns 3-kinase, and PLD involve high-affinity CCK-receptor occupation. Am. J. Physiol. 1994;266:G62–G70. doi: 10.1152/ajpgi.1994.266.1.G62. [DOI] [PubMed] [Google Scholar]
- 38.Lankisch TO, Nozu F, Owyang C, Tsunoda Y. High-affinity cholecystokinin type A receptor/cytosolic phospholipase A2 pathways mediate Ca2+ oscillations via a positive feedback regulation by calmodulin kinase in pancreatic acini. Eur. J. Cell Biol. 1999;78:632–641. doi: 10.1016/S0171-9335(99)80048-X. [DOI] [PubMed] [Google Scholar]
- 39.Tapia JA, Ferris HA, Jensen RT, Marin LJ. Cholecystokinin activates PYK2/CAKβ, by a phospholipase C-dependent mechanism, and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J. Biol. Chem. 1999;274:31261–31271. doi: 10.1074/jbc.274.44.31261. [DOI] [PubMed] [Google Scholar]
- 40.Jensen RT. CCKB-gastrin receptor antagonists. Recent advances and potential uses in gastric secretory disorders. Yale J. Biol. Med. 1996;69:245–259. [PMC free article] [PubMed] [Google Scholar]
- 41.Szewczyk JR, Laudeman C. CCK1R agonists: a promising target for the pharmacological treatment of obesity. Curr. Top. Med. Chem. 2003;3:837–854. doi: 10.2174/1568026033452258. [DOI] [PubMed] [Google Scholar]
- 42.Katschinski M. Loxiglumide Rotta research. IDrugs. 2002;5:469–474. [PubMed] [Google Scholar]
- 43.Varga G. Dexloxiglumide Rotta Research Lab. Curr. Opin. Investig. Drugs. 2002;3:621–626. [PubMed] [Google Scholar]
- 44.Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RS. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 45.Satoh Y, Matsuo T, Sogabe H, Itoh H, Tada T, Kinoshita T, Yoshida K, Takaya T. Studies on a novel, potent and orally effective cholecystokinin A antagonist, FK-480. Synthesis and structure-activity relationships of FK-480 and related compounds. Chem. Pharm. Bull. (Tokyo) 1994;42:2071–2083. doi: 10.1248/cpb.42.2071. [DOI] [PubMed] [Google Scholar]
- 46.Bignon E, Bachy A, Boigegrain R, Brodin R, Cottineau M, Gully D, Herbert JM, Keane P, Labie C, Molimard JC, Olliero D, Oury-Donat F, Petereau C, Prabonnaud V, Rockstroh MP, Schaeffer P, Servant O, Thurneyssen O, Soubrie P, Pascal M, Maffrand JP, Le FG. SR146131: a new potent, orally active, and selective nonpeptide cholecystokinin subtype 1 receptor agonist. I. In vitro studies. J Pharmacol Exp. Ther. 1999;289:742–751. [PubMed] [Google Scholar]
- 47.Gully D, Frëhel D, Marcy C, Spinazzë A, Lespy L, Neliat G, Maffrand JP, Le Fur G. Peripheral biological activity of SR 27897: a new potent non- peptide antagonist of CCKA receptors. Eur. J. Pharmacol. 1993;232:13–19. doi: 10.1016/0014-2999(93)90722-t. [DOI] [PubMed] [Google Scholar]
- 48.Meyer BM, Werth BA, Beglinger C, Hildebrand P, Jansen JB, Zach D, Rovati LC, Stalder GA. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet. 1989;2:12–15. doi: 10.1016/s0140-6736(89)90255-9. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt WE, Creutzfeldt W, Schleser A, Choudhury AR, Nustede R, Hocker M, Nitsche R, Sostmann H, Rovati LC, Folsch UR. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am. J. Physiol. 1991;260:G197–G206. doi: 10.1152/ajpgi.1991.260.2.G197. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt WE, Schenk S, Nustede R, Holst JJ, Folsch UR, Creutzfeldt W. Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology. 1994;107:1610–1620. doi: 10.1016/0016-5085(94)90799-4. [DOI] [PubMed] [Google Scholar]
- 51.Schwizer W, Borovicka J, Kunz P, Fraser R, Kreiss C, D'Amato M, Crelier G, Boesiger P, Friend M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: studies with the CCK antagonist loxiglumide. Gut. 1997;41:500–504. doi: 10.1136/gut.41.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoji E, Okumura T, Onodera S, Takahashi N, Harada K, Kohgo Y. Gastric emptying in OLETF rats not expressing CCK-A receptor gene. Dig. Dis. Sci. 1997;42:915–919. doi: 10.1023/a:1018860313674. [DOI] [PubMed] [Google Scholar]
- 53.Hinkle KL, Samuelson LC. Lessons from genetically engineered animal models. III. Lessons learned from gastrin gene deletion in mice. Am. J. Physiol. 1999;277:G500–G505. doi: 10.1152/ajpgi.1999.277.3.G500. [DOI] [PubMed] [Google Scholar]
- 54.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am. J. Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- 55.Beglinger C. Potential role of cholecystokinin in the development of acute pancreatitis. Digestion. 1999;60:61–63. doi: 10.1159/000051456. [DOI] [PubMed] [Google Scholar]
- 56.Degen L, Matzinger D, Drewe J, Beglinger C. The effect of cholecytokinin in controlling appetite and food intake in humans. Peptides. 2001;22:1265–1269. doi: 10.1016/s0196-9781(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki S, Takiguchi S, Sato N, Kanai S, Kawanami T, Yoshida Y, Miyasaka K, Takata Y, Funakoshi A, Noda T. Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: a study in CCK-A receptor knockout mice. Jpn J. Physiol. 2001;51:585–590. doi: 10.2170/jjphysiol.51.585. [DOI] [PubMed] [Google Scholar]
- 58.Takiguchi S, Suzuki S, Sato Y, Kanai S, Miyasaka K, Jimi A, Shinozaki H, Takata Y, Funakoshi A, Kono A, Minowa O, Kobayashi T, Noda T. Role of CCK-A receptor for pancreatic function in mice: a study in CCK-A receptor knockout mice. Pancreas. 2002;24:276–283. doi: 10.1097/00006676-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic Clin. Pharmacol. Toxicol. 2006;98:4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 60.Varga G, Balint A, Burghardt B, D'Amato M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br. J Pharmacol. 2004;141:1275–1284. doi: 10.1038/sj.bjp.0705769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes. Rev. 2005;6:297–306. doi: 10.1111/j.1467-789X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 62.Arora S. Anubhuti Role of neuropeptides in appetite regulation and obesity - A review. Neuropeptides. 2006 doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Low-Beer TS, Harvey RF, Davies ER, Read AF. Abnormalities of serum cholecystokinin and gallbladder emptying in celiac disease. N. Engl. J. Med. 1975;292:961–963. doi: 10.1056/NEJM197505012921807. [DOI] [PubMed] [Google Scholar]
- 64.Calam J, Ellis A, Dockray GJ. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J. Clin. Invest. 1982;69:218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masclee AA, Jansen JB, Driessen WM, Geuskens LM, Lamers CB. Gallbladder sensitivity to cholecystokinin in celiac disease. Correlation of gallbladder contraction with plasma cholecystokinin-like immunoreactivity during infusion of cerulein. Scand. J. Gastroent. 1991;26:1279–1284. doi: 10.3109/00365529108998625. [DOI] [PubMed] [Google Scholar]
- 66.Deprez P, Sempoux C, Van Beers BE, Jouret A, Robert A, Rahier J, Geubel A, Pauwels S, Mainguet P. Persistent decreased plasma cholecystokinin levels in celiac patients under gluten-free diet: respective roles of histological changes and nutrient hydrolysis. Regul. Pept. 2002;110:55–63. doi: 10.1016/s0167-0115(02)00162-3. [DOI] [PubMed] [Google Scholar]
- 67.Deprez PH, Sempoux C, De SC, Rahier J, Mainguet P, Pauwels S, Geubel A. Expression of cholecystokinin in the duodenum of patients with coeliac disease: respective role of atrophy and lymphocytic infiltration. Clin Sci (Lond) 2002;103:171–177. doi: 10.1042/cs1030171. [DOI] [PubMed] [Google Scholar]
- 68.Nousia-Arvanitakis S, Fotoulaki M, Tendzidou K, Vassilaki C, Agguridaki C, Karamouzis M. Subclinical exocrine pancreatic dysfunction resulting from decreased cholecystokinin secretion in the presence of intestinal villous atrophy. J Pediatr. Gastroenterol. Nutr. 2006;43:307–312. doi: 10.1097/01.mpg.0000228098.66583.85. [DOI] [PubMed] [Google Scholar]
- 69.Ling PR, Sheikh M, Boyce P, Keane-Ellison M, Thibault A. Cholecystokinin (CCK) secretion in patients with severe short bowel syndrome. Dig. Dis. Sci. 2001;46:859–864. doi: 10.1023/a:1010772922341. [DOI] [PubMed] [Google Scholar]
- 70.Bucceri AM, Calogero AE, Brogna A. Gallbladder and gastric emptying: relationship to cholecystokininemia in diabetics. Elsevier Sci. 2002;13:123–128. doi: 10.1016/s0953-6205(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 71.Huhtala V, Lehtonen L, Uvnas-Moberg K, Korvenranta H. Low plasma cholecystokinin levels in colicky infants. J Pediatr. Gastroenterol. Nutr. 2003;37:42–46. doi: 10.1097/00005176-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Mashako MN, Bernard C, Cezard JP, Chayvialle JA, Navarro J. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr. Gastroenterol. Nutr. 1987;6:948–952. doi: 10.1097/00005176-198711000-00022. [DOI] [PubMed] [Google Scholar]
- 73.Sitzmann JV, Pitt HA, Steinborn PA, Pasha ZR, Sanders RC. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg. Gynecol. Obstet. 1990;170:25–31. [PubMed] [Google Scholar]
- 74.Curran TJ, Uzoaru I, Das JB, Ansari G, Raffensperger JG. The effect of cholecystokinin-octapeptide on the hepatobiliary dysfunction caused by total parenteral nutrition. J Pediatr. Surg. 1995;30:242–246. doi: 10.1016/0022-3468(95)90568-5. [DOI] [PubMed] [Google Scholar]
- 75.Dawes LG, Muldoon JP, Greiner MA, Bertolotti M. Cholecystokinin increases bile acid synthesis with total parenteral nutrition but does not prevent stone formation. J Surg. Res. 1997;67:84–89. doi: 10.1006/jsre.1996.4953. [DOI] [PubMed] [Google Scholar]
- 76.Teitelbaum DH, Tracy TF, Jr, Aouthmany MM, Llanos A, Brown MB, Yu S, Brown MR, Shulman RJ, Hirschl RB, Derusso PA, Cox J, Dahlgren J, Groner JI, Strouse PJ. Use of cholecystokinin-octapeptide for the prevention of parenteral nutrition-associated cholestasis. Pediatrics. 2005;115:1332–1340. doi: 10.1542/peds.2004-1014. [DOI] [PubMed] [Google Scholar]
- 77.Teitelbaum DH, Han-Markey T, Drongowski RA, Coran AG, Bayar B, Geiger JD, Uitvlugt N, Schork MA. Use of cholecystokinin to prevent the development of parenteral nutrition-associated cholestasis. JPEN J Parenter. Enteral Nutr. 1997;21:100–103. doi: 10.1177/0148607197021002100. [DOI] [PubMed] [Google Scholar]
- 78.Hogenauer C, Meyer RL, Netto GJ, Bell D, Little KH, Ferries L, Santa Ana CA, Porter JL, Fordtran JS. Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N. Engl. J. Med. 2001;344:270–274. doi: 10.1056/NEJM200101253440405. [DOI] [PubMed] [Google Scholar]
- 79.Creutzfeldt W. Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N. Engl. J. Med. 2001;345:65. [PubMed] [Google Scholar]
- 80.Wank SA, Pisegna JR, DeWeerth A. Cholecystokinin receptor family. Molecular cloning, structure, and functional expression in rat, guinea pig, and human. Ann. N. Y. Acad. Sci. 1994;713:49–66. doi: 10.1111/j.1749-6632.1994.tb44052.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhou W, Povoski SP, Bell RH., Jr Characterization of cholecystokinin receptors and messenger RNA expression in rat pancreas: evidence for expression of cholecystokinin-A receptors but not cholecystokinin-B (gastrin) receptors. J. Surg. Res. 1995;58:281–289. doi: 10.1006/jsre.1995.1044. [DOI] [PubMed] [Google Scholar]
- 82.Morisset J, Wong H, Walsh JH, Laine J, Bourassa J. Pancreatic CCK(B) receptors: their potential roles in somatostatin release and delta-cell proliferation. Am. J. Physiol. (Gastrointest. Liver Physiol. 1) 2000;279:G148–G156. doi: 10.1152/ajpgi.2000.279.1.G148. [DOI] [PubMed] [Google Scholar]
- 83.Adler G, Beglinger C, Braun U, Reinshagen M, Koop I, Schafmayer A, Rovati L, Arnold R. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology. 1991;100:537–543. doi: 10.1016/0016-5085(91)90227-c. [DOI] [PubMed] [Google Scholar]
- 84.Soudah HC, Lu YA, Hasler WL, Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am. J. Physiol. 1992;263:G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- 85.Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J. Clin. Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saluja AK, Saluja M, Printz H, Zavertnik A, Sengupta A, Steer ML. Experimental pancreatitis is mediated by low-affinity cholecystokinin receptors that inhibit digestive enzyme secretion. PNAS (USA) 1989;86:8968–8971. doi: 10.1073/pnas.86.22.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niederau C, Grendell JH. Role of cholecystokinin in the development and progression of acute pancreatitis and the potential of therapeutic application of cholecystokinin receptor antagonists. Digestion. 1999;60:69–74. doi: 10.1159/000051458. [DOI] [PubMed] [Google Scholar]
- 88.Adler G, Hupp T, Kearn HF. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol. Anat. Histol. 1979;382:31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- 89.Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88:1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- 90.Tachibana I, Shirohara H, Czako L, Akiyama T, Nakano S, Watanabe N, Hirohata Y, Otsuki M. Role of endogenous cholecystokinin and cholecystokinin-A receptors in the development of acute pancreatitis in rats. Pancreas. 1997;14:113–121. doi: 10.1097/00006676-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Sato T, Niikawa J, Usui I, Imamura T, Yoshida H, Tanaka S, Mitamura K. Pancreatic regeneration after ethionine-induced acute pancreatitis in rats lacking pancreatic CCK-A receptor gene expression. J Gastroenterol. 2003;38:672–680. doi: 10.1007/s00535-003-1120-0. [DOI] [PubMed] [Google Scholar]
- 92.Katz M, Carangelo R, Miller LJ, Gorelick F. Effect of ethanol on cholecystokinin-stimulated zymogen conversion in pancreatic acinar cells. Am. J. Physiol. 1996;270:G171–G175. doi: 10.1152/ajpgi.1996.270.1.G171. [DOI] [PubMed] [Google Scholar]
- 93.Gorelick FS, Otani T. Mechanisms of intracellular zymogen activation. Bailliere's Best Practice-Research. Clin. Gastroenterol. 1999;13:227–240. doi: 10.1053/bega.1999.0021. [DOI] [PubMed] [Google Scholar]
- 94.Taniguchi H, Yomota E, Kume E, Shikano T, Endo T, Nagasaki M. Effect of T-0632, a cholecystokininA receptor antagonist, on experimental acute pancreatitis. Jpn. J Pharmacol. 1997;73:105–112. doi: 10.1254/jjp.73.105. [DOI] [PubMed] [Google Scholar]
- 95.Yoshinaga K, Washizuka M, Segawa Y. Cholecystokinin acts as an essential factor in the exacerbation of pancreatic bile duct ligation-induced rat pancreatitis model under non-fasting condition. Jpn. J Pharmacol. 2000;84:44–50. doi: 10.1254/jjp.84.44. [DOI] [PubMed] [Google Scholar]
- 96.Niederau C, Borchard F, Luthen R, Niederau M. Early development of experimental biliary pancreatitis and its amelioration by CCK-receptor blockade. Hepatogastroenterology. 1996;43:1442–1453. [PubMed] [Google Scholar]
- 97.Murayama KM, Drew JB, Nahrwold DL, Joehl RJ. Cholecystokinin antagonist prevents hyperamylasemia and improves pancreatic exocrine function in cerulein-induced acute pancreatitis. Pancreas. 1990;5:439–444. doi: 10.1097/00006676-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Montero AC, Manso MA, Rodriguez AI, De Dios I. Therapeutic and protective effect of subcutaneous injections of L-364,718 on caerulein-induced acute pancreatitis. Pancreas. 1994;9:309–315. doi: 10.1097/00006676-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Makovec F, Bani M, Cereda R, Chiste R, Revel L, Rovati LC, Setnikar I, Rovati LA. Protective effect of CR 1409 (cholecystokinin antagonist) on experimental pancreatitis in rats and mice. Peptides. 1986;7:1159–1164. doi: 10.1016/0196-9781(86)90147-6. [DOI] [PubMed] [Google Scholar]
- 100.Ohshio G, Saluja A, Leli U, Sengupta A, Steer ML. Failure of a potent cholecystokinin antagonist to protect against diet-induced pancreatitis in mice. Pancreas. 1989;4:739–743. doi: 10.1097/00006676-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Czako L, Takacs T, Varga IS, Hai DQ, Tiszlavicz L, Hegyi P, Mandi Y, Matkovics B, Lonovics J. The pathogenesis of L-arginine-induced acute necrotizing pancreatitis: inflammatory mediators and endogenous cholecystokinin. J Physiol Paris. 2000;94:43–50. doi: 10.1016/s0928-4257(99)00104-7. [DOI] [PubMed] [Google Scholar]
- 102.De Dios I, Urunuela A, Orfao A, Manso MA. Cholecystokinin antagonist L364,718 induces alterations in acinar cells that prevent improvement of acute pancreatitis induced by obstruction. Dig. Dis. Sci. 2002;47:1800–1809. doi: 10.1023/a:1016400829744. [DOI] [PubMed] [Google Scholar]
- 103.Niederau C. Do cholecystokinin antagonists increase cytosolic calcium in pancreatic acinar cells and thereby promote pancreatitis? Dig. Dis. Sci. 2004;49:266–269. doi: 10.1023/b:ddas.0000017449.51877.fc. [DOI] [PubMed] [Google Scholar]
- 104.Urunuela A, Manso MA, de la Mano AM, De Dios I. Cholecystokinin blockade triggers earlier and enhanced intra-acinar oxygen free radical generation in acute pancreatitis induced by pancreatic duct obstruction in rats. Clin Sci (Lond) 2003;105:203–212. doi: 10.1042/CS20030072. [DOI] [PubMed] [Google Scholar]
- 105.Satoh A, Gukovskaya AS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-{kappa}B activation in pancreatic acinar cells through effects on protein kinase C-{epsilon} Am. J Physiol Gastrointest Liver Physiol. 2006;291:G432–G438. doi: 10.1152/ajpgi.00579.2005. [DOI] [PubMed] [Google Scholar]
- 106.Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 107.Yoshikawa H, Nakamura H, Tashiro M, Yamaguchi T, Taguchi M, Fukumitsu K, Otsuki M. Cholecystokinin-1 receptor protein up-regulation during pancreatic regeneration after acute haemorrhagic pancreatitis in rats. Eur. J Clin Invest. 2004;34:498–507. doi: 10.1111/j.1365-2362.2004.01363.x. [DOI] [PubMed] [Google Scholar]
- 108.Bialek R, Willemer S, Arnold R, Adler G. Evidence of intracellular activation of serine proteases in acute cerulein-induced pancreatitis in rats. Scand. J Gastroenterol. 1991;26:190–196. doi: 10.3109/00365529109025030. [DOI] [PubMed] [Google Scholar]
- 109.Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991;87:362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steer ML. Early events in acute pancreatitis. Baillieres Best Pract. Res. Clin. Gastroenterol. 1999;13:213–225. doi: 10.1053/bega.1999.0020. [DOI] [PubMed] [Google Scholar]
- 111.Nagar AB, Gorelick FS. Acute pancreatitis. Curr. Opin. Gastroenterol. 2004;20:439–443. doi: 10.1097/00001574-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Otsuki M. Pathophysiological role of cholecystokinin in humans. J. Gastroenterol. Hepatol. 2000;15:D71–D83. doi: 10.1046/j.1440-1746.2000.02178.x. [DOI] [PubMed] [Google Scholar]
- 113.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, Liddle RA, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat. Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 114.Whitcomb DC, Ulrich CD. 2. Hereditary pancreatitis: new insights, new directions. Baillieres Best Pract. Res. Clin. Gastroenterol. 1999;13:253–263. doi: 10.1053/bega.1999.0023. [DOI] [PubMed] [Google Scholar]
- 115.Teich N, Ockenga J, Hoffmeister A, Manns M, Mossner J, Keim V. Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology. 2000;119:461–465. doi: 10.1053/gast.2000.9312. [DOI] [PubMed] [Google Scholar]
- 116.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat. Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 117.Pfutzer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 118.Chen JM, Mercier B, Audrezet MP, Ferec C. Mutational analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene in hereditary and sporadic chronic pancreatitis. J Med. Genet. 2000;37:67–69. doi: 10.1136/jmg.37.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ochi K, Harada H, Satake K. Clinical evaluation of cholecystokinin-A- receptor antagonist (loxiglumide) for the treatment of acute pancreatitis. A preliminary clinical trial. Study Group of Loxiglumide in Japan. Digestion. 1999;60:81–85. doi: 10.1159/000051460. [DOI] [PubMed] [Google Scholar]
- 120.Mossner J, Secknus R, Meyer J, Niederau C, Adler G. Treatment of pain with pancreatic extracts in chronic pancreatitis: results of a prospective placebo-controlled multicenter trial. Digestion. 1992;53:54–66. doi: 10.1159/000200971. [DOI] [PubMed] [Google Scholar]
- 121.Slaff J, Jacobson D, Tillman CR, Curington C, Toskes P. Protease-specific suppression of pancreatic exocrine secretion. Gastroenterology. 1984;87:44–52. [PubMed] [Google Scholar]
- 122.Schafmayer A, Becker HD, Werner M, Folsch UR, Creutzfeldt W. Plasma cholecystokinin levels in patients with chronic pancreatitis. Digestion. 1985;32:136–139. doi: 10.1159/000199231. [DOI] [PubMed] [Google Scholar]
- 123.Gomez CJ, Codoceo R, Fernandez CP, Molina F, Tenias JM, Vazquez JJ. Basal and postprandial cholecystokinin values in chronic pancreatitis with and without abdominal pain. Digestion. 1991;48:134–140. doi: 10.1159/000200685. [DOI] [PubMed] [Google Scholar]
- 124.Isaksson G, Ihse I. Pain reduction by an oral pancreatic enzyme preparation in chronic pancreatitis. Dig. Dis. Sci. 1983;28:97–102. doi: 10.1007/BF01315137. [DOI] [PubMed] [Google Scholar]
- 125.Toskes PP. Feedback control of pancreatic exocrine secretion. Trans. Am. Clin. Climatological Assoc. 2001;112:61–67. [PMC free article] [PubMed] [Google Scholar]
- 126.Jin HO, Song CW, Chang TM, Chey WY. Roles of gut hormones in negative-feedback regulation of pancreatic exocrine secretion in humans. Gastroenterology. 1994;107:1828–1834. doi: 10.1016/0016-5085(94)90827-3. [DOI] [PubMed] [Google Scholar]
- 127.Halgreen H, Pederssen NT, Worning H. Symptomatic effect of pancreatic enzyme therapy in patients with chronic pancreatitis. Scand. J. Gastroenterol. 1986;21:104–108. doi: 10.3109/00365528609034631. [DOI] [PubMed] [Google Scholar]
- 128.Malesci A, Gaia E, Fioretta A, Bocchia P, Ciravegna G, Cantor P, Vantini I. No effect of long-term treatment with pancreatic extract on recurrent abdominal pain in patients with chronic pancreatitis. Scand. J. Gastroenterol. 1995;30:392–398. doi: 10.3109/00365529509093296. [DOI] [PubMed] [Google Scholar]
- 129.Shiratori K, Takeuchi T, Satake K, Matsuno S. Clinical evaluation of oral administration of a cholecystokinin-A receptor antagonist (loxiglumide) to patients with acute, painful attacks of chronic pancreatitis: a multicenter dose-response study in Japan. Pancreas. 2002;25:e1–e5. doi: 10.1097/00006676-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 130.Behar J, Lee KY, Thompson WR, Biancani P. Gallbladder contraction in patients with pigment and cholesterol stones. Gastroenterology. 1989;97:1479–1484. doi: 10.1016/0016-5085(89)90392-2. [DOI] [PubMed] [Google Scholar]
- 131.Amaral J, Xiao ZL, Chen Q, Yu P, Biancani P, Behar J. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506–511. doi: 10.1053/gast.2001.21190. [DOI] [PubMed] [Google Scholar]
- 132.Xiao ZL, Chen Q, Amaral J, Biancani P, Behar J. Defect of receptor-G protein coupling in human gallbladder with cholesterol stones. Am. J. Physiol. (Gastrointest. Liver Physiol.) 2000;278:G251–G258. doi: 10.1152/ajpgi.2000.278.2.G251. [DOI] [PubMed] [Google Scholar]
- 133.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521–528. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol. 2005;11:1685–1689. doi: 10.3748/wjg.v11.i11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ding X, Lu CY, Mei Y, Liu CA, Shi YJ. Correlation between gene expression of CCK-A receptor and emptying dysfunction of the gallbladder in patients with gallstones and diabetes mellitus. Hepatobiliary Pancreat. Dis. Int. 2005;4:295–298. [PubMed] [Google Scholar]
- 136.Strasberg SM. Acute acalculus cholecystitis. Gastroenterology. 1995:2665–2673. [Google Scholar]
- 137.Ott DJ. Acalculous gallbladder disease: a controversial entity and imaging dilemma revisited. Am. J. Gastroenterol. 1998;93:1181–1183. doi: 10.1111/j.1572-0241.1998.01181.x. [DOI] [PubMed] [Google Scholar]
- 138.Lillemoe KD. Chronic acalculous cholecystitis: are we diagnosing a disease or a myth? Radiology. 1997;204:13–14. doi: 10.1148/radiology.204.1.9205215. [DOI] [PubMed] [Google Scholar]
- 139.Reitter D, Aaning HL. Chronic acalculous cholecystitis: reproduction of pain with cholecystokinin and relief of symptoms with cholecystectomy. S. D. J. Med. 1999;52:197–200. [PubMed] [Google Scholar]
- 140.Barr RG, Agnesi JN, Schaub CR. Acalculous gallbladder disease: US evaluation after slow-infusion cholecystokinin stimulation in symptomatic and asymptomatic adults. Radiology. 1997;204:105–111. doi: 10.1148/radiology.204.1.9205230. [DOI] [PubMed] [Google Scholar]
- 141.Middleton GW, Williams JH. Diagnostic accuracy of 99Tcm-HIDA with cholecystokinin and gallbladder ejection fraction in acalculous gallbladder disease. Nucl. Med. Commun. 2001;22:657–661. doi: 10.1097/00006231-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 142.Krishnamurthy GT, Krishnamurthy S, Brown PH. Constancy and variability of gallbladder ejection fraction: impact on diagnosis and therapy. J Nucl. Med. 2004;45:1872–1877. [PubMed] [Google Scholar]
- 143.Gunna BR, Yannam GR, Kavita N, Pathak S, Alla BR. Acalculous biliary tract disorders: the value of fatty meal-cholescintigraphyq. Surgeon. 2003;1:293–295. doi: 10.1016/s1479-666x(03)80049-5. [DOI] [PubMed] [Google Scholar]
- 144.Ozden N, DiBaise JK. Gallbladder ejection fraction and symptom outcome in patients with acalculous biliary-like pain. Dig. Dis. Sci. 2003;48:890–897. doi: 10.1023/a:1023039310574. [DOI] [PubMed] [Google Scholar]
- 145.Beglinger C, Dill S, Meyer B, Werth B, Adler G. Treatment of biliary colic with loxiglumide. Lancet. 1989;2:167. doi: 10.1016/s0140-6736(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 146.Malesci A, Pezzilli R, D'Amato M, Rovati L. CCK-1 receptor blockade for treatment of biliary colic: a pilot study. Aliment. Pharmacol Ther. 2003;18:333–337. doi: 10.1046/j.1365-2036.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- 147.Townsend CM, Jr, Singh P, Thompson JC. Effects of gastrointestinal peptides on gastrointestinal cancer growth. Gastroenterol. Clin. North Am. 1989;18:777–791. [PubMed] [Google Scholar]
- 148.Abrahamsson H. Gastrointestinal motility in patients with the irritable bowel syndrome. Scand. J. Gastroenterol. 1987;130:21–26. doi: 10.3109/00365528709090996. [DOI] [PubMed] [Google Scholar]
- 149.Kellow JE, Phillips SF, Miller LJ, Zinsmeister AR. Dysmotility of the small intestine in irritable bowel syndrome. Gut. 1988;29:1236–1243. doi: 10.1136/gut.29.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am. J. Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 151.Harvey RF, Read AE. Effect of cholecystokinin on colonic motility and symptoms in patients with the irritable-bowel syndrome. Lancet. 1973;1:1–3. doi: 10.1016/s0140-6736(73)91219-1. [DOI] [PubMed] [Google Scholar]
- 152.Snape WJ, Jr, Carlson GM, Matarazzo SA, Cohen S. Evidence that abnormal myoelectrical activity produces colonic motor dysfunction in the irritable bowel syndrome. Gastroenterology. 1977;72:383–387. [PubMed] [Google Scholar]
- 153.Sjolund K, Ekman R, Lindgren S, Rehfeld JF. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand. J. Gastroenterol. 1996;31:1110–1114. doi: 10.3109/00365529609036895. [DOI] [PubMed] [Google Scholar]
- 154.Roberts-Thomson IC, Fettman MJ, Jonsson JR, Frewin DB. Responses to cholecystokinin octapeptide in patients with functional abdominal pain syndromes. J. Gastroenterol. Hepatol. 1992;7:293–297. doi: 10.1111/j.1440-1746.1992.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 155.Caldarella MP, Milano A, Laterza F, Sacco F, Balatsinou C, Lapenna D, Pierdomenico SD, Cuccurullo F, Neri M. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am. J Gastroenterol. 2005;100:383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 156.Niederau C, Faber S, Karaus M. Cholecystokinin's role in regulation of colonic motility in health and in irritable bowel syndrome. Gastroenterology. 1992;102:1889–1898. doi: 10.1016/0016-5085(92)90310-u. [DOI] [PubMed] [Google Scholar]
- 157.Cann PA, Rovati LC, Smart HL, Spiller RC, Whorwell PJ. Loxiglumide, a CCK-A antagonist, in irritable bowel syndrome. A pilot multicenter clinical studyq. Ann. N. Y. Acad. Sci. 1994;713:449–450. doi: 10.1111/j.1749-6632.1994.tb44123.x. [DOI] [PubMed] [Google Scholar]
- 158.Cann PA, Rovati LC, Smart H, Spiller RC, Whorwell PJ. Loxiglumide, a CCK-A antagonist in irritable bowel syndrome: a pilot multicentre clinical study. Gastroenterology. 1993;104:A486. doi: 10.1111/j.1749-6632.1994.tb44123.x. [DOI] [PubMed] [Google Scholar]
- 159.Cremonini F, Camilleri M, McKinzie S, Carlson P, Camilleri CE, Burton D, Thomforde G, Urrutia R, Zinsmeister AR. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am. J Gastroenterol. 2005;100:652–663. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 160.D'Amato M, Whorwell PJ, Thompson DG, Spiller RC, Giacovelli G, Rovati LC. The CCK-A recptor-antagonist dexloxiglumide in the tratment of IBS. Gastroenterology. 1999;116:A981. [Google Scholar]
- 161.D'Amato M, Whorwell PJ, Thompson DG, Spiller RC, Giacovelli G, Rovati LC. The efficacy and safety of the CCK-A receptor antagonist dexloxiglumide in the treatment of IBS. Gut. 1999;45:5. [Google Scholar]
- 162.D'Amato M, Whorwell PJ, Thompson DG, Spiller RC, Giacovelli G, Griffin G. The CCK-1 receptor antagonist dexloxiglumide is effective and safe in female patients with constipation predominant irritable bowel syndrome. Am. J Gastroenterol. 2001;96:S31. [Google Scholar]
- 163.Camilleri M. Treating irritable bowel syndrome: overview, perspective and future therapies. Br. J Pharmacol. 2004;141:1237–1248. doi: 10.1038/sj.bjp.0705741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cremonini F, gado-Aros S, Talley NJ. Functional dyspepsia: drugs for new (and old) therapeutic targets. Best Pract. Res. Clin Gastroenterol. 2004;18:717–733. doi: 10.1016/j.bpg.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 165.Feinle C, Meier O, Otto B, D'Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347–355. doi: 10.1136/gut.48.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chua AS, Dinan TG, Rovati LC, Keeling PW. Cholecystokinin hyperresponsiveness in dysmotility-type nonulcer dyspepsia. Ann. N. Y. Acad. Sci. 1994;713:298–299. doi: 10.1111/j.1749-6632.1994.tb44077.x. [DOI] [PubMed] [Google Scholar]
- 167.Castillo EJ, gado-Aros S, Camilleri M, Burton D, Stephens D, O'Connor-Semmes R, Walker A, Shachoy-Clark A, Zinsmeister AR. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am. J Physiol Gastrointest Liver Physiol. 2004;287:G363–G369. doi: 10.1152/ajpgi.00074.2004. [DOI] [PubMed] [Google Scholar]
- 168.Chua A, Bekkering M, Rovati LC, Keeling PWN. Clinical efficacy and prokinetic effect of the CCK-A antagonist loxiglumide in non-ulcer dyspepsia. Gastroenterology. 1993;104:A491. doi: 10.1111/j.1749-6632.1994.tb44124.x. [DOI] [PubMed] [Google Scholar]
- 169.D'Amato M, Stamford IF, Bennett A. The effects of cholecystokinin octapeptide on human isolated alimentary muscle. Br. J Pharmacol. 1990;100:126–130. doi: 10.1111/j.1476-5381.1990.tb12063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.D'Amato M, Stamford IF, Bennett A. Studies of three non-peptide cholecystokinin antagonists (devazepide, lorglumide and loxiglumide) in human isolated alimentary muscle and guinea-pig ileum. Br. J Pharmacol. 1991;102:391–395. doi: 10.1111/j.1476-5381.1991.tb12184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morton MF, Welsh NJ, Tavares IA, Shankley NP. Pharmacological characterization of cholecystokinin receptors mediating contraction of human gallbladder and ascending colon. Regul. Pept. 2002;105:59–64. doi: 10.1016/s0167-0115(01)00383-4. [DOI] [PubMed] [Google Scholar]
- 172.D'Amato M, Rovati LC. Cholecystokinin-A receptor antagonists: therapies for gastrointestinal disorders. Expert Opin. Investig. Drugs. 1997;6:819–836. doi: 10.1517/13543784.6.7.819. [DOI] [PubMed] [Google Scholar]
- 173.Scarpignato C, Pelosini I. Management of irritable bowel syndrome: novel approaches to the pharmacology of gut motility. Can. J Gastroenterol. 1999;13 Suppl A:50A–65A. doi: 10.1155/1999/183697. [DOI] [PubMed] [Google Scholar]
- 174.O'Brien MD, Camilleri M, Thomforde GM, Wiste JA, Hanson RB, Zinsmeister AR. Effect of cholecystokinin octapeptide and atropine on human colonic motility, tone, and transit. Dig. Dis. Sci. 1997;42:26–33. doi: 10.1023/a:1018868601475. [DOI] [PubMed] [Google Scholar]
- 175.Meier R, D'Amato M, Pullwott A, Schneider H, Rovati LC, Beglinger C. Effect of a CCK A receptor antagonist in an exerimental model of delyaed colonic transit in man. Gastroenterology. 1994;106:A538. [Google Scholar]
- 176.Meier R, Thumshirn M, Meyer B, Rovati LC, Gyr K, Beglinger C. Treatment of chronic constipation in geriatic patients with loxiglumide (LOX), a cholecystokinin anatgonist. Gastroenterology. 1990;98:A374. doi: 10.1016/0016-5085(90)90484-i. [DOI] [PubMed] [Google Scholar]
- 177.Barrow L, Blackshaw PE, Wilson CG, Rovati L, Beglinger C. Selective slowing of proximal colon transit in irritable bowel syndrome by the cholecystokinin-receptor anatagonist, loxiglumide. Eur. J Gastroenterol. Hepatol. 1994;6:381–387. [Google Scholar]
- 178.Meier R, Beglinger C, Thumshirn M, Meyer B, Rovati LC, Giacovelli G, D'Amato M, Gyr K. Therapeutic effects of loxiglumide, a cholecystokinin antagonist, on chronic constipation in elderly patients: a prospective, randomized, double-blind, controlled trial. J. Gastrointest. Mot. 1993;5:129–135. [Google Scholar]
- 179.Fisher RS, DiMarino AJ, Cohen S. Mechanism of cholecystokinin inhibition of lower esophageal sphincter pressure. Am. J Physiol. 1975;228:1469–1473. doi: 10.1152/ajplegacy.1975.228.5.1469. [DOI] [PubMed] [Google Scholar]
- 180.Behar J, Biancani P. Effect of cholecystokinin-octapeptide on lower esophageal sphincter. Gastroenterology. 1977;73:57–61. [PubMed] [Google Scholar]
- 181.Dent J, Dodds WJ, Hogan WJ, Arndorfer RC, Teeter BC. Effect of cholecystokinin-octapeptide on opossum lower esophageal sphincter. Am. J Physiol. 1980;239:G230–G235. doi: 10.1152/ajpgi.1980.239.3.G230. [DOI] [PubMed] [Google Scholar]
- 182.Ledeboer M, Masclee AA, Batstra MR, Jansen JB, Lamers CB. Effect of cholecystokinin on lower oesophageal sphincter pressure and transient lower oesophageal sphincter relaxations in humans. Gut. 1995;36:39–44. doi: 10.1136/gut.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boulant J, Mathieu S, D'Amato M, Abergel A, Dapoigny M, Bommelaer G. Cholecystokinin in transient lower oesophageal sphincter relaxation due to gastric distension in humans. Gut. 1997;40:575–581. doi: 10.1136/gut.40.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Clave P, Gonzalez A, Moreno A, Lopez R, Farre A, Cusso X, D'Amato M, Azpiroz F, Lluis F. Endogenous cholecystokinin enhances postprandial gastroesophageal reflux in humans through extrasphincteric receptors. Gastroenterology. 1998;115:597–604. doi: 10.1016/s0016-5085(98)70139-8. [DOI] [PubMed] [Google Scholar]
- 185.Zerbib F, Bruley DV, Scarpignato C, Leray V, D'Amato M, Roze C, Galmiche JP. Endogenous cholecystokinin in postprandial lower esophageal sphincter function and fundic tone in humans. Am. J Physiol. 1998;275:G1266–G1273. doi: 10.1152/ajpgi.1998.275.6.G1266. [DOI] [PubMed] [Google Scholar]
- 186.Trudgill NJ, Hussain FN, Moustafa M, Ajjan R, D'Amato M, Riley SA. The effect of cholecystokinin antagonism on postprandial lower oesophageal sphincter function in asymptomatic volunteers and patients with reflux disease. Aliment. Pharmacol Ther. 2001;15:1357–1364. doi: 10.1046/j.1365-2036.2001.01045.x. [DOI] [PubMed] [Google Scholar]
- 187.Gonzalez AA, Farre R, Mones J, Capella G, Clave P. Pharmacological and molecular characterization of muscular cholecystokinin receptors in the human lower oesophageal sphincter. Neurogastroenterol. Motil. 2000;12:539–546. doi: 10.1046/j.1365-2982.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 188.Boeckxstaens GE, Hirsch DP, Fakhry N, Holloway RH, D'Amato M, Tytgat GN. Involvement of cholecystokininA receptors in transient lower esophageal sphincter relaxations triggered by gastric distension. Am. J Gastroenterol. 1998;93:1823–1828. doi: 10.1111/j.1572-0241.1998.00527.x. [DOI] [PubMed] [Google Scholar]
- 189.Hirsch DP, Mathus-Vliegen EM, Holloway RH, Fakhry N, D'Amato M, Boeckxstaens GE. Role of CCK(A) receptors in postprandial lower esophageal sphincter function in morbidly obese subjects. Dig. Dis. Sci. 2002;47:2531–2537. doi: 10.1023/a:1020520311938. [DOI] [PubMed] [Google Scholar]
- 190.Miyasaka K, Takata Y, Funakoshi A. Association of cholecystokinin A receptor gene polymorphism with cholelithiasis and the molecular mechanisms of this polymorphism. J Gastroenterol. 2002;37 Suppl 14:102–106. doi: 10.1007/BF03326426. [DOI] [PubMed] [Google Scholar]
- 191.Sato N, Miyasaka K, Suzuki S, Kanai S, Ohta M, Kawanami T, Yoshida Y, Takiguchi S, Noda T, Takata Y, Funakoshi A. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig. Dis. Sci. 2003;48:1944–1947. doi: 10.1023/a:1026110002713. [DOI] [PubMed] [Google Scholar]
- 192.Takata Y, Takeda S, Kawanami T, Takiguchi S, Yoshida Y, Miyasaka K, Funakoshi A. Promoter analysis of human cholecystokinin type-A receptor gene. J Gastroenterol. 2002;37:815–820. doi: 10.1007/s005350200135. [DOI] [PubMed] [Google Scholar]
- 193.Funakoshi A, Miyasaka K, Matsumoto H, Yamamori S, Takiguchi S, Kataoka K, Takata Y, Matsusue K, Kono A, Shimokata H. Gene structure of human cholecystokinin (CCK) type-A receptor: body fat content is related to CCK type-A receptor gene promoter polymorphism. FEBS Lett. 2000;466:264–266. doi: 10.1016/s0014-5793(00)01080-2. [DOI] [PubMed] [Google Scholar]
- 194.Koda M, Ando F, Niino N, Shimokata H, Miyasaka K, Funakoshi A. Association of cholecystokinin 1 receptor and beta3-adrenergic receptor polymorphisms with midlife weight gain. Obes. Res. 2004;12:1212–1216. doi: 10.1038/oby.2004.152. [DOI] [PubMed] [Google Scholar]
- 195.Marchal-Victorion S, Vionnet N, Escrieut C, Dematos F, Dina C, Dufresne M, Vaysse N, Pradayrol L, Froguel P, Fourmy D. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics. 2002;12:23–30. doi: 10.1097/00008571-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Miyasaka K, Ohta M, Kanai S, Yoshida Y, Sato N, Nagata A, Matsui T, Noda T, Jimi A, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Enhanced gastric emptying of a liquid gastric load in mice lacking cholecystokinin-B receptor: a study of CCK-A,B, and AB receptor gene knockout mice. J Gastroenterol. 2004;39:319–323. doi: 10.1007/s00535-003-1297-2. [DOI] [PubMed] [Google Scholar]
- 197.Solcia E, Rindi G, Silini E, Villani L. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Bailliere's Clin. Gastroenterol. 1993;7:149–165. doi: 10.1016/0950-3528(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 198.Hakanson R, Bottcher G, Sundler F, Vallgren S. Activation and hyperplasia of gastrin and enterochromaffin-like cells in the stomach. Digestion. 1986;1:23–41. doi: 10.1159/000199380. [DOI] [PubMed] [Google Scholar]
- 199.Bordi C, D'Adda T, Azzoni C, Ferraro G. Pathogenesis of ECL cell tumors in humans. Yale J. Biol. Med. 1999;71:273–284. [PMC free article] [PubMed] [Google Scholar]
- 200.Bordi C, D'Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am. J. Surg. Pathol. 1995;19:S8–S19. [PubMed] [Google Scholar]
- 201.Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc. Natl. Acad. Sci. (USA) 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Arnold A. Molecular mechanisms of parathyroid neoplasia. Endocrinol. Metab. Clin North Am. 1994;23:93–107. [PubMed] [Google Scholar]
- 203.Jensen RT. Gastrinoma. Bailliere's Clin. Gastroenterol. 1996;10:555–766. doi: 10.1016/s0950-3528(96)90016-0. [DOI] [PubMed] [Google Scholar]
- 204.Szabo I, Rumi G, Bodis B, Nemeth P, Mozsik G. Gastrin and pentagastrin enhance the tumour proliferation of human stable cultured gastric adenocarcinoma cells. J. Physiol. Paris. 2000;94:71–74. doi: 10.1016/s0928-4257(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 205.Ohlsson B, Fredang N, Axelson J. The effect of bombesin, cholecystokinin, gastrin, and their antagonists on proliferation of pancreatic cancer cell lines. Scand. J. Gastroenterol. 1999;34:1224–1229. doi: 10.1080/003655299750024742. [DOI] [PubMed] [Google Scholar]
- 206.Goetze JP, Nielsen FC, Burcharth F, Rehfeld JF. Closing the gastrin loop in pancreatic carcinoma: coexpression of gastrin and its receptor in solid human pancreatic adenocarcinoma. Cancer. 2000;88:2487–2494. doi: 10.1002/1097-0142(20000601)88:11<2487::aid-cncr9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 207.DeWeerth A, vonSchrenck T, Lohr M, Mirau S, Greten H, Kalthoff H. Human pancreatic cancer cell lines express the CCKB receptor. Hepatogastroenterology. 1999;46:472–478. [PubMed] [Google Scholar]
- 208.Smith JP, Fantaskey AP, Liu G, Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am. J. Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 209.Baldwin GS. The role of gastrin and cholecystokinin in normal and neoplastic gastrointestinal growth. J. Gastroenterol. Hepatol. 1995;10:215–232. doi: 10.1111/j.1440-1746.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 210.Rozengurt E, Walsh JH, Gastrin CCK. signaling, and cancer. Annu. Rev. Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 211.Guo YS, Townsend CM., Jr Roles of gastrointestinal hormones in pancreatic cancer. J. Hepatobiliary Pancreat. Surg. 2000;7:276–285. doi: 10.1007/s005340070049. [DOI] [PubMed] [Google Scholar]
- 212.Rehfeld JF. Gastrin and colorectal cancer: A never-ending dispute? (Editorial) Gastroenterology. 1995;108:1307–1310. doi: 10.1016/0016-5085(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 213.Smith AM, Watson SA. Review article: gastrin and colorectal cancer. Aliment. Pharmacol. Ther. 2000;14:1231–1247. doi: 10.1046/j.1365-2036.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- 214.Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47:820–824. doi: 10.1136/gut.47.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Baldwin GS, Shulkes A. Gastrin as an autocrine growth factor in colorectal carcinoma: implications for therapy. World J. Gastroenterol. 1998;4:461–463. doi: 10.3748/wjg.v4.i6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.McWilliams DF, Watson SA, Crosbee DM, Michaeli D, Seth R. Coexpression of gastrin and gastrin receptors (CCK-B and delta CCK-B) in gastrointestinal tumour cell lines. Gut. 1998;42:795–798. doi: 10.1136/gut.42.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996;56:4111–4115. [PubMed] [Google Scholar]
- 218.Karges W, Dralle H, Raue F, Mann K, Reiners C, Grussendorf M, Hufner M, Niederle B, Brabant G. Calcitonin measurement to detect medullary thyroid carcinoma in nodular goiter: German evidence-based consensus recommendation. Exp. Clin Endocrinol. Diabetes. 2004;112:52–58. doi: 10.1055/s-2004-815727. [DOI] [PubMed] [Google Scholar]
- 219.Massoll N, Mazzaferri EL. Diagnosis and management of medullary thyroid carcinoma. Clin Lab Med. 2004;24:49–83. doi: 10.1016/j.cll.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 220.Metz DC, Starr JA. A retrospective study of the usefulness of acid secretory testing. Aliment. Pharmacol Ther. 2000;14:103–111. doi: 10.1046/j.1365-2036.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 221.Baron JH. Clinical tests of gastric secretion--history, methodology and interpretation. New York: Oxford University Press; 1972. [Google Scholar]
- 222.Jensen RT, Doppman JL, Gardner JD. Gastrinoma. In: Go VLW, Brooks FA, DiMagno EP, Gardner JD, Lebenthal E, Scheele GA, editors. The Exocrine Pancreas: Biology, Pathobiology and Disease. 1 ed. New York: Raven Press; 1986. pp. 727–744. [Google Scholar]
- 223.Roy P, Venzon DJ, Feigenbaum KM, Koviack PD, Bashir S, Ojeaburu JV, Gibril F, Jensen RT. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore) 2001;80:189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 224.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathobiology and Disease. 2 ed. New York: Raven Press Publishing Co; 1993. pp. 931–978. [Google Scholar]
- 225.The French Calcitonin Tumours Study Group (GETC) Niccoli-Sire P, Murat A, Baudin E, Henry JF, Proye C, Bigorgne JC, Bstandig B, Modigliani E, Morange S, Schlumberger M, Conte-Devolx B. Early or prophylactic thyroidectomy in MEN 2/FMTC gene carriers: results in 71 thyroidectomized patients. Eur. J Endocrinol. 1999;141:468–474. doi: 10.1530/eje.0.1410468. [DOI] [PubMed] [Google Scholar]
- 226.Reubi JC, Waser B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J Cancer. 1996;67:644–647. doi: 10.1002/(SICI)1097-0215(19960904)67:5<644::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 227.Bordi C, Pilato F, Carfagna G, Ferrarari C, D'Adda T, Sivelli R, Bertele A, Missale G. Argyrophil cell hyperplasia of fundic mucosa in patients with chronic atrophic gastritis. Digestion. 1986;35:130–143. doi: 10.1159/000199389. [DOI] [PubMed] [Google Scholar]
- 228.Bordi C, Cocconi G, Togni R, Vezzadini P, Missale G. Gastric endocrine cell proliferation. Association with Zollinger-Ellison syndrome. Arch. Pathol. 1974;98:274–278. [PubMed] [Google Scholar]
- 229.Hakanson R, Chen D, Sundler F. The ECL cells. In: Johnson LR, editor. In Physiology of the Gastrointestinal Tract. 3rd ed. New York: Raven Press; 1994. pp. 1171–1184. [Google Scholar]
- 230.Peghini PL, Annibale B, Azzoni C, Milione M, Corleto VD, Gibril F, Venzon DJ, Delle Fave G, Bordi C, Jensen RT. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- 231.Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J. Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 232.Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, Stolte M, Capella C, Bordi C, Solcia E. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542. doi: 10.1016/s0016-5085(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 233.Metz DC, Weber HC, Orbuch M, Strader DB, Lubensky IA, Jensen RT. Helicobacter pylori infection: a reversible cause of hypergastrinemia and hyperchlorhydria which can mimic Zollinger-Ellison syndrome. Dig. Dis. Sci. 1995;40:153–159. doi: 10.1007/BF02063959. [DOI] [PubMed] [Google Scholar]
- 234.el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 235.Jensen RT. Gastrin-producing tumors. Cancer Treat. Res. 1997;89:293–334. doi: 10.1007/978-1-4615-6355-6_14. [DOI] [PubMed] [Google Scholar]
- 236.Rehfeld JF, van Solinge WW. The tumor biology of gastrin and cholecystokinin. Adv. Cancer Res. 1994;63:295–347. doi: 10.1016/s0065-230x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- 237.Kochman ML, DelValle J, Dickinson CJ, Boland CR. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem. Biophys. Res. Commun. 1992;189:1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- 238.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu. Rev. Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 239.Abou-Saif A, Lei J, McDonald TJ, Chakrabarti S, Waxman I, Shojamanesh H, Schrump DS, Kleiner DE, Gibril F, Jensen RT. A new cause of Zollinger-Ellison syndrome: non-small cell lung cancer. Gastroenterology. 2001;120:1271–1278. doi: 10.1053/gast.2001.23242. [DOI] [PubMed] [Google Scholar]
- 240.Maton PN, Mackem SM, Norton JA, Gardner JD, O'Dorisio TM, Jensen RT. Ovarian carcinoma as a cause of Zollinger-Ellison syndrome. Natural history, secretory products and response to provocative tests. Gastroenterology. 1989;97:468–471. doi: 10.1016/0016-5085(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 241.Levi S, Beardshall K, Swift I, Foulkes W, Playford R, Ghosh P, Calam J. Antral Helicobacter pylori, hypergastrinaemia, and duodenal ulcers: effect of eradicating the organism. BMJ. 1989;299:1504–1505. doi: 10.1136/bmj.299.6714.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.van Solinge WW, Odum L, Rehfeld JF. Ovarian cancers express and process progastrin. Cancer Res. 1993;53:1823–1828. [PubMed] [Google Scholar]
- 243.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B, editors. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 291–344. [Google Scholar]
- 244.Gibril F, Jensen RT. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr. Gastroenterol. Rep. 2005;7:114–121. doi: 10.1007/s11894-005-0049-2. [DOI] [PubMed] [Google Scholar]
- 245.Rossel M, Pasini A, Chappuis S, Geneste O, Fournier L, Schuffenecker I, Takahashi M, van Grunsven LA, Urdiales JL, Rudkin BB, Lenoir GM, Billaud M. Distinct biological properties of two RET isoforms activated by MEN 2A and MEN 2B mutations. Oncogene. 1997;14:265–275. doi: 10.1038/sj.onc.1200831. [DOI] [PubMed] [Google Scholar]
- 246.Noda S, Norton JA, Jensen RT, Gay WA., Jr Surgical resection of intracardiac gastrinoma. Ann. Thorac. Surg. 1999;67:532–533. doi: 10.1016/s0003-4975(98)01205-3. [DOI] [PubMed] [Google Scholar]
- 247.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann. Surg. 2004;240:757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini P, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N. Engl. J. Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 249.Jensen RT. Gastrinoma as a model for prolonged hypergastrinemia in man. In: Walsh JH, editor. Gastrin. New York, NY: Raven Press Publishing Co; 1993. pp. 373–393. [Google Scholar]
- 250.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann. Surg. 2004;239:617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr. Gastroenterol. Rep. 2004;6:454–463. doi: 10.1007/s11894-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 252.Jensen RT. Use of omeprazole and other proton pump inhibitors in the Zollinger-Ellison syndrome. In: Olbe L, editor. Milestones in Drug Therapy. Basel, Switzerland: Birkhauser Verlag AG Publish. Co; 1999. pp. 205–221. [Google Scholar]
- 253.Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of omeprazole in Zollinger-Ellison: A prospective nine-year study of efficacy and safety. Aliment. Pharmacol. Ther. 1993;7:597–610. doi: 10.1111/j.1365-2036.1993.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 254.Ellison EH, Wilson SD. The Zollinger-Ellison syndrome: Re-appraisal and evaluation of 260 registered cases. Ann. Surg. 1964;160:512–530. doi: 10.1097/00000658-196409000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J. Clin. Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 256.Roy P, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 257.Mignon M, Jais P, Cadiot G, Yedder D, Vatier J. Clinical features and advances in biological diagnostic criteria for Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, editors. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995. pp. 223–239. [Google Scholar]
- 258.Neuburger P, Lewin M, Recherche Cd, Bonfils S. Parietal and chief cell population in four cases of the Zollinger-Ellison syndrome. Gastroenterology. 1972;63:937–942. [PubMed] [Google Scholar]
- 259.Polacek MA, Ellison EH. Parietal cell mass and gastric acid secretion in the Zollinger-Ellison syndrome. Surgery. 1966;60:606–614. [PubMed] [Google Scholar]
- 260.Sum P, Perey BJ. Parietal-cell mass (PCM) in a man with Zollinger-Ellison syndrome. Can. J. Surg. 1969;12:285–288. [PubMed] [Google Scholar]
- 261.Rosenlund ML. The Zollinger-Ellison syndrome in children. A review. Am. J. Med. Sci. 1967;254:884–892. doi: 10.1097/00000441-196712000-00018. [DOI] [PubMed] [Google Scholar]
- 262.Solcia E, Capella C, Sessa F, Rindi S, Cornaggia M, Riva C, Villani L. Gastric carcinoids and related endocrine growths. Digestion. 1986;35 Suppl 1:3–22. doi: 10.1159/000199378. [DOI] [PubMed] [Google Scholar]
- 263.Lehy T, Mignon M, Cadiot G, Elouaer-Blanc L, Ruszniewski P, Lewin MJ, Bonfils S. Gastric endocrine cell behavior in Zollinger-Ellison patients upon long-term potent antisecretory treatment. Gastroenterology. 1989;96:1029–1040. doi: 10.1016/0016-5085(89)91620-x. [DOI] [PubMed] [Google Scholar]
- 264.Bardram L, Thomsen P, Stadil F. Gastric endocrine cells in omeprazole treated and untreated patients with Zollinger-Ellison syndrome. Digestion. 1986;35 Suppl 1:116–122. doi: 10.1159/000199387. [DOI] [PubMed] [Google Scholar]
- 265.Helander HF. Oxyntic mucosa histology in omeprazole treated patients suffering from duodenal ulcer or Zollinger-Ellison syndrome. Digestion. 1986;35 Suppl 1:123–129. doi: 10.1159/000199388. [DOI] [PubMed] [Google Scholar]
- 266.Maton PN, Lack EE, Collen MJ, Cornelius MJ, David E, Gardner JD, Jensen RT. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990;99:943–950. doi: 10.1016/0016-5085(90)90611-4. [DOI] [PubMed] [Google Scholar]
- 267.D'Adda T, Corleto V, Pilato FP, Baggi MT, Robutti F, Delle Fave G, Bordi C. Quantitative ultrastructure of endocrine cells of oxyntic mucosa in Zollinger-Ellison syndrome. Correspondence with light microscopic findings. Gastroenterology. 1990;99:17–26. doi: 10.1016/0016-5085(90)91224-t. [DOI] [PubMed] [Google Scholar]
- 268.Hakanson R, Sundler F. Mechanisms for the development of gastric carcinoids. Digestion. 1986;35 Suppl 1:1–151. [Google Scholar]
- 269.Larsson H, Carlsson E, Mattsson H, Lundell L, Sundler F, Sundell G, Wallmark B, Watanabe T, Hakanson R. Plasma gastrin and gastric enterochromaffin-like cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986;90:391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- 270.Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39:61–79. doi: 10.1159/000199609. [DOI] [PubMed] [Google Scholar]
- 271.Havu N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion. 1986;35 Suppl 1:42–55. doi: 10.1159/000199381. [DOI] [PubMed] [Google Scholar]
- 272.Orbuch M, Venzon DJ, Lubensky IA, Weber HC, Gibril F, Jensen RT. Prolonged hypergastrinemia does not increase the frequency of colonic neoplasia in patients with Zollinger-Ellison syndrome. Dig. Dis. Sci. 1996;41:604–613. doi: 10.1007/BF02282349. [DOI] [PubMed] [Google Scholar]
- 273.Jensen RT. Recent insights from studies of gastrinomas. In: Merchant JL, Buchan AMJ, Wang TC, editors. Gastrin in the New Millenium (2004) Los Angeles, CA: The CURE Foundation; 2004. pp. 339–352. [Google Scholar]
- 274.Poynter D, Pick CR, Harcourt RA, Selway SA, Ainge G, Harman IW, Spurling NW, Fluck PA, Cook JL. Association of long lasting unsurmountable histamine H2 blockade and gastric carcinoid tumours in the rat. Gut. 1985;26:1284–1295. doi: 10.1136/gut.26.12.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ryberg B, Axelson J, Hakanson R, Sundler F, Mattsson H. Trophic effects of continuous infusion of [Leu15]-gastrin-17 in the rat. Gastroenterology. 1990;98:33–38. doi: 10.1016/0016-5085(90)91287-g. [DOI] [PubMed] [Google Scholar]
- 276.Poynter D, Selway SA, Papworth SA, Riches SR. Changes in the gastric mucosa of the mouse associated with long lasting unsurmountable histamine H2 blockade. Gut. 1986;27:1338–1346. doi: 10.1136/gut.27.11.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mattsson H, Havu N, Brautigam J, Carlsson K, Lundell L, Carlsson E. Partial gastric corpectomy results in hypergastrinemia and development of gastric enterochromaffinlike-cell carcinoids in the rat. Gastroenterology. 1991;100:311–319. doi: 10.1016/0016-5085(91)90197-s. [DOI] [PubMed] [Google Scholar]
- 278.Sundler F, Hakanson R, Carlsson E, Larsson H, Mattsson H. Hypergastrinemia after blockade of acid secretion in the rat: trophic effects. Digestion. 1986;35:56–69. doi: 10.1159/000199382. [DOI] [PubMed] [Google Scholar]
- 279.Gasslander T, Permert J, Feng W, Adrian TE, Larsson J. Trophic effects by epidermal growth factor on duodenal mucosa and exocrine pancreas in rats. Eur. Surg. Res. 1997;29:142–149. doi: 10.1159/000129518. [DOI] [PubMed] [Google Scholar]
- 280.Solcia E, Bordi C, Creutzfeldt W, Dayal Y, Dayan AD, Falkmer S, Grimelius L, Havu N. Histopathological classification of nonantral gastric endocrine growths in man. Digestion. 1988;41:185–200. doi: 10.1159/000199786. [DOI] [PubMed] [Google Scholar]
- 281.Solcia E, Rindi G, LaRosa S, Capella C. Morphological, molecular, and prognostic aspects of gastric endocrine tumors. Microsc. Res. Tech. 2000;48:339–348. doi: 10.1002/(SICI)1097-0029(20000315)48:6<339::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 282.Lamberts R, Creutzfeldt W, Stockmann F, Jacubaschke U, Maas S, Brunner G. Long term omeprazole treatment in man: effects on gastric endocrine cell populations. Digestion. 1988;39:126–135. doi: 10.1159/000199615. [DOI] [PubMed] [Google Scholar]
- 283.Lamberts R, Creutzfeldt W, Struber HG, Brunner G, Solcia E. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Gastroenterology. 1993;104:1356–1370. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- 284.Delle Fave G, Marignani M, Moretti A, D'Ambra G, Martino G, Annibale B. Hypergastrinemia and enterochromaffin-like cell hyperplasia. Yale J. Biol. Med. 1998;71:291–301. [PMC free article] [PubMed] [Google Scholar]
- 285.Lehy T, Cadiot G, Mignon M, Ruszniewski P, Bonfils S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut. 1992;33:1275–1279. doi: 10.1136/gut.33.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cadiot G, Lehy T, Ruszniewski P, Bonfils S, Mignon M. Gastric endocrine cell evolution in patients with Zollinger-Ellison syndrome. Influence of gastrinoma growth and long-term omeprazole treatment. Dig. Dis. Sci. 1993;38:1307–1317. doi: 10.1007/BF01296083. [DOI] [PubMed] [Google Scholar]
- 287.Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology. 1985;88:638–648. doi: 10.1016/0016-5085(85)90131-3. [DOI] [PubMed] [Google Scholar]
- 288.Solcia E, Fiocca R, Villani L, Gianatti A, Cornaggia M, Chiaravalli A, Curzio M, Capella C. Morphology and pathogenesis of endocrine hyperplasias, precarcinoid lesions, and carcinoids arising in chronic atrophic gastritis. Scand. J. Gastroenterol. 1991;26:146–159. doi: 10.3109/00365529109093193. [DOI] [PubMed] [Google Scholar]
- 289.Feurle GE. Argyrophil cell hyperplasia and a carcinoid tumour in the stomach of a patient with sporadic Zollinger-Ellison syndrome. Gut. 1994;35:275–277. doi: 10.1136/gut.35.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cadiot G, Vissuzaine C, Potet F, Mignon M. Fundic argyrophil carcinoid tumor in a patient with sporadic-type Zollinger-Ellison syndrome. Dig. Dis. Sci. 1995;40:1275–1278. doi: 10.1007/BF02065537. [DOI] [PubMed] [Google Scholar]
- 291.Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, De Bruijne JW, Festen HP, Snel P, Luckers AE, Biemond I, Lamers CB. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990;99:621–628. doi: 10.1016/0016-5085(90)90946-x. [DOI] [PubMed] [Google Scholar]
- 292.Ligumsky M, Lysy J, Siguencia G, Friedlander Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J. Clin. Gastroenterol. 2001;33:32–35. doi: 10.1097/00004836-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 293.Berna MJ, Hoffmann KM, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;10:12–15. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Norton JA, Melcher ML, Gibril F, Jensen RT. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgery. Surgery. 2004;136:1267–1274. doi: 10.1016/j.surg.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 295.Modlin IM, Tang LH. The gastric enterochromaffin-like cell: an enigmatic cellular link. Gastroenterology. 1996;111:783–810. doi: 10.1053/gast.1996.v111.agast961110783. [DOI] [PubMed] [Google Scholar]
- 296.Coupe M, Rees H, Springer CJ, Bishop AE, Morris JA, Polak JM, Calam J. Gastric enterochromaffin-like (ECL) cells in hypergastrinaemic duodenal ulcer disease. Gut. 1990;31:144–147. doi: 10.1136/gut.31.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ekman L, Hansson E, Havu N, Carlsson E, Lundberg C. Toxicological studies on omeprazole. Scand. J. Gastroenterol. 1985;20 Suppl 108:53–69. [PubMed] [Google Scholar]
- 298.Cui G, Qvigstad G, Falkmer S, Sandvik AK, Kawase S, Waldum HL. Spontaneous ECLomas in cotton rats (Sigmodon hispidus): tumours occurring in hypoacidic/hypergastrinaemic animals with normal parietal cells. Carcinogenesis. 2000;21:23–27. doi: 10.1093/carcin/21.1.23. [DOI] [PubMed] [Google Scholar]
- 299.Axelson J, Ekelund M, Hakanson R, Sundler F. Gastrin and the vagus interact in the trophic control of the rat oxyntic mucosa. Regul. Pept. 1988;22:237–243. doi: 10.1016/0167-0115(88)90036-5. [DOI] [PubMed] [Google Scholar]
- 300.Cao G, Zhu L, Liao D. The influence of vagotomy on stomach endocrine cells in rat. Hua Xi Yi Ke Da Xue Xue Bao. 1991;22:282–286. [PubMed] [Google Scholar]
- 301.Scarpignato C, Pelosini I, Di MF. Acid suppression therapy: where do we go from here? Dig. Dis. 2006;24:11–46. doi: 10.1159/000091298. [DOI] [PubMed] [Google Scholar]
- 302.Parsons ME, Keeling DJ. Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin. Investig. Drugs. 2005;14:411–421. doi: 10.1517/13543784.14.4.411. [DOI] [PubMed] [Google Scholar]
- 303.Hakanson R, Ding XQ, Norlén P, Lindstrom E. CCK2 receptor antagonists: pharmacological tools to study the gastrin-ECL cell-parietal cell axis. Regul. Pept. 1999;80:1–12. doi: 10.1016/s0167-0115(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 304.Waldum HL, Brenna E, Sandvik AK. Long-term safety of proton pump inhibitors: risks of gastric neoplasia and infections. Expert Opin. Drug Saf. 2002;1:29–38. doi: 10.1517/14740338.1.1.29. [DOI] [PubMed] [Google Scholar]
- 305.Norton JA, Alexander HA, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann. Surg. 2003;237:650–659. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Creutzfeldt W. Consequences of gastric acid inhibition in man. In: Olbe L, editor. Proton Pump Inhibitors. Basel, Switzerland: Birkhauser Verlag; 1999. pp. 91–115. [Google Scholar]
- 307.Annibale B, Azzoni C, Corleto VD, diGiulio E, Caruana P, D'Ambra G, Bordi C, Delle Fave G. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur. J. Gastroenterol. Hepatol. 2001;13:1449–1456. doi: 10.1097/00042737-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 308.Annibale B, Delle Fave G, Azzoni C, Corleto V, Camboni G, D'Ambra G, Pilato FP, Bordi C. Three months of octreotide treatment decreases gastric acid secretion and argyrophil cell density in patients with Zollinger-Ellison syndrome and antral G-cell hyperfunction. Aliment. Pharmacol. Ther. 1994;8:95–104. doi: 10.1111/j.1365-2036.1994.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 309.Tomassetti P, Migliori M, Caletti GC, Fusaroli P, Corinaldesi R, Gullo L. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N. Engl. J. Med. 2000;343:551–554. doi: 10.1056/NEJM200008243430805. [DOI] [PubMed] [Google Scholar]
- 310.Hahne WF, Jensen RT, Lemp GF, Gardner JD. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc. Natl. Acad. Sci. USA. 1981;78:6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Makovec F, Bani M, Chistë R, Revel L, Rovati LC, Rovati LA. Differentiation of central and peripheral cholecystokinin receptors by new glutaramic acid derivatives with cholecystokinin- antagonistic activity. Arzneimittelforschung. 1986;36:98–102. [PubMed] [Google Scholar]
- 312.Lamers CBHW, Jansen JBMJ. The effect of a gastrin receptor antagonist on gastric acid secretion and serum gastrin the Zollinger-Ellison syndrome. J. Clin. Gastroenterol. 1983;5:21–23. doi: 10.1097/00004836-198302000-00005. [DOI] [PubMed] [Google Scholar]
- 313.Chen D, Zhao CM, Norlén P, Bjorkqvist M, Ding XQ, Kitano N, Hakanson R. Effect of cholecystokinin-2 receptor blockade on rat stomach ECL cells. A histochemical, electron-microscopic and chemical study. Cell Tissue Res. 2000;299:81–95. doi: 10.1007/s004419900136. [DOI] [PubMed] [Google Scholar]
- 314.Lindstrom E, Chen D, Norlén P, Andersson K, Hakanson R. Control of gastric acid secretion: the gastrin-ECL cell-parietal cell axis. Med. Imaging and Graphics. 2001;128:505–514. doi: 10.1016/s1095-6433(00)00331-7. [DOI] [PubMed] [Google Scholar]
- 315.Kovacs TO, Walsh JH, Maxwell V, Wong HC, Azuma T, Katt E. Gastrin is a major mediator of the gastric phase of acid secretion in dogs: proof by monoclonal antibody neutralization. Gastroenterology. 1989;97:1406–1413. doi: 10.1016/0016-5085(89)90383-1. [DOI] [PubMed] [Google Scholar]
- 316.Bjorkqvist M, Norlén P, Kitano N, Chen D, Zhao CM, delaCour CD, Gagnemo-Persson C, Hakanson R. Effects of CCK2 receptor blockade on growth parameters in gastrointestinal tract and pancreas in rats. Pharmacol. Toxicol. 2001;89:208–213. doi: 10.1111/j.0901-9928.2001.890411.x. [DOI] [PubMed] [Google Scholar]
- 317.Beltinger J, Hildebrand P, Drewe J, Christ A, Hlobil K, Ritzel U, D'Amato M, Rovati L, Beglinger C. Effects of spiroglumide, a gastrin receptor antagonist, on acid secretion in humans. Eur. J. Clin. Invest. 1999;29:153–159. doi: 10.1046/j.1365-2362.1999.00424.x. [DOI] [PubMed] [Google Scholar]
- 318.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am. J. Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 319.Koh TJ, Chen D. Gastrin as a growth factor in the gastrointestinal tract. Regul. Pept. 2000;25:37–44. doi: 10.1016/s0167-0115(00)00176-2. [DOI] [PubMed] [Google Scholar]
- 320.Eissele R, Patberg H, Koop H, Krack W, Lorenz W, McKnight AT, Arnold R. Effect of gastrin receptor blockade on endocrine cells in rats during achlorhydria. Gastroenterology. 1992;103:1596–1601. doi: 10.1016/0016-5085(92)91183-5. [DOI] [PubMed] [Google Scholar]
- 321.Dore MP, Graham DY. Pathogenesis of duodenal ulcer disease: the rest of the story. Baillieres Best Pract. Res. Clin. Gastroenterol. 2000;14:97–107. doi: 10.1053/bega.1999.0061. [DOI] [PubMed] [Google Scholar]
- 322.Soll AH. Peptic ulcer and its complications. In: Feldman M, Sleisenger MH, Scharschmidt BF, editors. Gastrointestinal and Liver Disease. 6th ed. Philadelphia,PA: W.B. Saunders Co; 1998. pp. 620–678. [Google Scholar]
- 323.Calam J. Helicobacter pylori modulation of gastric acid. Yale J. Biol. Med. 1999;72:195–202. [PMC free article] [PubMed] [Google Scholar]
- 324.McColl KE, Gillen D, El-Omar E. The role of gastrin in ulcer pathogenesis. Baillieres Best Pract. Res. Clin. Gastroenterol. 2000;14:13–26. doi: 10.1053/bega.1999.0056. [DOI] [PubMed] [Google Scholar]
- 325.Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- 326.Konturek JW, Gillessen A, Konturek SJ, Domschke W. Eradication of Helicobacter pylori restores the inhibitory effect of cholecystokinin on postprandial gastrin release in duodenal ulcer patients. Gut. 1995;37:482–487. doi: 10.1136/gut.37.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Konturek JW, Konturek SJ, Domschke W. Cholecystokinin in the control of gastric acid secretion and gastrin release in response to a meal at low and high pH in healthy subjects and duodenal ulcer patients. Scand. J. Gastroenterol. 1995;30:738–744. doi: 10.3109/00365529509096321. [DOI] [PubMed] [Google Scholar]
- 328.Eissele R, Koop I, Schaar M, Koop H, Arnold R. Role of cholecystokinin in the control of gastric somatostatin in the rat: in vivo and in vitro studies. Regul. Pept. 1991;32:333–340. doi: 10.1016/0167-0115(91)90026-d. [DOI] [PubMed] [Google Scholar]
- 329.Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989;1:1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- 330.Geoffroy H, el-Haddad A, Coudoux P. [Proglumide (milide) in gastroduodenal therapeutics] Sem. Hop. Ther. 1974;50:207–216. [PubMed] [Google Scholar]
- 331.Galeone M, Moise G, Ferrante F, Cacioli D, Casula PL, Bignamini AA. Double-blind clinical comparison between a gastrin-receptor antagonist, proglumide, and a histamine H2-blocker, cimetidine. Curr. Med. Res. Opin. 1978;5:376–382. doi: 10.1185/03007997809111901. [DOI] [PubMed] [Google Scholar]
- 332.Bergemann W, Consentius K, Braun HE, Hirschmann H, Marowski B, Munck A, Rehs HU, Stopik D, Wilke G. [Duodenal ulcer - multicenter double-blind study with proglumide] Med. Klin. 1981;76:226–229. [PubMed] [Google Scholar]
- 333.Beltinger J, Hildebrand P, Howald A, D'Amato M, Beglinger C. Effect of CR 2194, a novel CCKB/gastrin receptor antagonist, on gastrin-stimulated acid secretion in man. Gastroenterology. 1994;106:A50. [Google Scholar]
- 334.Makovec F, D'Amato M. CCKB/gastrin receptor anatgonists as potential drugs for peptic ulcer therapy. Drug Discovery Today. 1997;2:283–293. [Google Scholar]
- 335.Makovec F, Revel L, Letari O, Mennuni L, Impicciatore M. Characterization of antisecretory and antiulcer activity of CR 2945, a new potent and selective gastrin/CCK(B) receptor antagonist. Eur. J Pharmacol. 1999;369:81–90. doi: 10.1016/s0014-2999(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 336.Chang RS, Lotti VJ, Monaghan RL, Birnbaum J, Stapley EO, Goetz MA, Albers-Schonberg G, Patchett AA, Liesch JM, Hensens OD, Springer JP. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from. Aspergillus Allicaceus. Science. 1985;230:177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- 337.Chang RS, Lotti VJ. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokonin antagonist. Proc. Natl. Acad. Sci. USA. 1986;83:4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Bock MG, DiGardo RM, Evans BE, Rittle KE, Whitter WE, Veber DE, Anderson PS, Freidinger RM. Benzodiazepine gastrin and cholecystokinin receptor ligands: L- 365 260. J. Med. Chem. 1989;32:13–16. doi: 10.1021/jm00121a004. [DOI] [PubMed] [Google Scholar]
- 339.Lotti VJ, Chang RS. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur. J. Pharmacol. 1989;162:273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- 340.Murphy MG, Sytnik B, Kovacks TOG, Mertz H, Ewanik D, Shingo S, Lin JH, Gertz BJ, Walsh JH. The gastrin-receptor antagonist L-365,260 inhibits stimulated acid secretion in humans. Clin. Pharmacol. Ther. 1993;54:533–539. doi: 10.1038/clpt.1993.185. [DOI] [PubMed] [Google Scholar]
- 341.Kramer MS, Cutler NR, Ballenger JC, Patterson WM, Mendels J, Chenault A, Shrivastava R, Matzura-Wolfe D, Lines C, Reines S. A placebo-controlled trial of L-365-260, a CCKB antagonist, in panic disorder. Biol. Psychiatry. 1995;37:462–466. doi: 10.1016/0006-3223(94)00190-E. [DOI] [PubMed] [Google Scholar]
- 342.Steel K. Gastrin and gastrin receptor ligands - a review of recent patent literature. IDrugs. 2002;5:689–695. [PubMed] [Google Scholar]
- 343.Semple G, Ryder H, Rooker DP, Batt AR, Kendrick DA, Szelke M, Ohta M, Satoh M, Nishida A, Akuzawa S, Miyata K. (3R)-N-(1-(tert-butylcarbonylmethyl)-2,3-dihydro-2-oxo-5-(2-pyridyl)-1H-1, 4- benzodiazepin-3-yl)-N'-(3-(methylamino)phenyl)urea (YF476): a potent and orally active gastrin/CCK-B antagonist. J Med. Chem. 1997;40:331–341. doi: 10.1021/jm960669+. [DOI] [PubMed] [Google Scholar]
- 344.Boyce M, Warrington S, Johnston A, Harris A. Effect on gastric pH of single doses of YF476, a new gastrin antagonist, compared with ranitidine and placebo. Br. J Clin Pharmacol. 2000;49:493P–494P. [Google Scholar]
- 345.Boyce M, Warrington S, Johnston A, Harris A. Effect on gastric pH of repeated doses of YF476, a new gastrin antagonist, compared with omeprazole and placebo. Br. J Clin Pharmacol. 2000;50:383P–384P. [Google Scholar]
- 346.Boyce M, Warrington S, Lewis Y, Nentwich H, Harris A. Adaptation to the antisecretory effect of YF474, a new gastrin antagonist, in healthy man. Br. J Clin Pharmacol. 2002;53:437P. [Google Scholar]
- 347.Hughes J, Boden P, Costall B, Domeney A, Kelly E, Horwell DC, Hunter JC, Pinnock RD, Woodruff GN. Development of a class of selective cholecystokinin type B receptor antagonists having potent anxiolytic activity. Proc. Natl. Acad. Sci. USA. 1990;87:6728–6732. doi: 10.1073/pnas.87.17.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Horwell DC, Hughes J, Hunter JC, Pritchard MC, Richardson RS, Roberts E, Woodruff GN. Rationally designed “dipeptoid”analogues of CCK. alpha-Methyltryptophan derivatives as highly selective and orally active gastrin and CCK-B antagonists with potent anxiolytic properties. J Med. Chem. 1991;34:404–414. doi: 10.1021/jm00105a062. [DOI] [PubMed] [Google Scholar]
- 349.Nakamura T, Ozawa T, Kitagawa M, Takehira Y, Yamada M, Yasumi K, Tamakoshi K, Kobayashi Y, Nakamura H. Endoscopic resection of gangliocytic paraganglioma of the minor duodenal papilla: case report and review. Gastrointest. Endosc. 2002;55:270–273. doi: 10.1067/mge.2002.120782. [DOI] [PubMed] [Google Scholar]
- 350.Adams JB, Pyke RE, Costa J, Cutler NR, Schweizer E, Wilcox CS, Wisselink PG, Greiner M, Pierce MW, Pande AC. A double-blind, placebo-controlled study of a CCK-B receptor antagonist, CI-988, in patients with generalized anxiety disorder. J Clin Psychopharmacol. 1995;15:428–434. doi: 10.1097/00004714-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 351.van Megen HJ, Westenberg HG, den Boer JA, Slaap B, van Es-Radhakishun F, Pande AC. The cholecystokinin-B receptor antagonist CI-988 failed to affect CCK-4 induced symptoms in panic disorder patients. Psychopharmacology (Berl) 1997;129:243–248. doi: 10.1007/s002130050186. [DOI] [PubMed] [Google Scholar]
- 352.Pande AC, Greiner M, Adams JB, Lydiard RB, Pierce MW. Placebo-controlled trial of the CCK-B antagonist, CI-988, in panic disorder. Biol. Psychiatry. 1999;46:860–862. doi: 10.1016/s0006-3223(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 353.Goddard AW, Woods SW, Money R, Pande AC, Charney DS, Goodman WK, Heninger GR, Price LH. Effects of the CCK(B) antagonist CI-988 on responses to mCPP in generalized anxiety disorder. Psychiatry Res. 1999;85:225–240. doi: 10.1016/s0165-1781(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 354.Buck IM, Black JW, Cooke T, Dunstone DJ, Gaffen JD, Griffin EP, Harper EA, Hull RA, Kalindjian SB, Lilley EJ, Linney ID, Low CM, McDonald IM, Pether MJ, Roberts SP, Shankley NP, Shaxted ME, Steel KI, Sykes DA, Tozer MJ, Watt GF, Walker MK, Wright L, Wright PT. Optimization of the in vitro and in vivo properties of a novel series of 2,4,5-trisubstituted imidazoles as potent cholecystokinin-2 (CCK2) antagonists. J Med. Chem. 2005;48:6803–6812. doi: 10.1021/jm0490686. [DOI] [PubMed] [Google Scholar]
- 355.Kalindjian SB, Buck IM, Davies JM, Dunstone DJ, Hudson ML, Low CM, McDonald IM, Pether MJ, Steel KI, Tozer MJ, Vinter JG. Non-peptide cholecystokinin-B/gastrin receptor antagonists based on bicyclic, heteroaromatic skeletons. J Med. Chem. 1996;39:1806–1815. doi: 10.1021/jm9508907. [DOI] [PubMed] [Google Scholar]
- 356.Lehmann F, Hildebrand P, Beglinger C. New molecular targets for treatment of peptic ulcer disease. Drugs. 2003;63:1785–1797. doi: 10.2165/00003495-200363170-00002. [DOI] [PubMed] [Google Scholar]
- 357.Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, Fox JG, Wang TC. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–1983. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 358.Waldum HL, Brenna E. Personal review: is profound acid inhibition safe? Aliment. Pharmacol. Ther. 2000;14:15–22. doi: 10.1046/j.1365-2036.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 359.Ding WQ, Kuntz SM, Miller LJ. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: role of reduced sellular U2AF35 and a suboptimal 3'-splicing site leading to retention of the fourth intron. Cancer Res. 2002;62:947–952. [PubMed] [Google Scholar]
- 360.Lamers CB, Jansen JB, Woutersen RA. Cholecystokinin and gastrointestinal cancer. J. Steroid Biochem. Mol. Biol. 1990;37:1069–1072. doi: 10.1016/0960-0760(90)90467-y. [DOI] [PubMed] [Google Scholar]
- 361.Reubi JC, Macke HR, Krenning EP. Candidates for peptide receptor radiotherapy today and in the future. J Nucl. Med. 2005;46 Suppl 1:67S–75S. [PubMed] [Google Scholar]
- 362.Jensen RT. Somatostatin receptor based scintigraphy and anti-tumor treatment. An expanding vista? J. Clin. Endocrinol. Metab. 2000;85:3507–3508. doi: 10.1210/jcem.85.10.6958. [DOI] [PubMed] [Google Scholar]
- 363.Kwekkeboom D, Krenning EP, deJong M. Peptide receptor imaging and therapy. J. Nucl. Med. 2000;41:1704–1713. [PubMed] [Google Scholar]
- 364.Weiner RE, Thakur ML. Radiolabeled peptides in oncology : role in diagnosis and treatment. BioDrugs. 2005;19:145–163. doi: 10.2165/00063030-200519030-00002. [DOI] [PubMed] [Google Scholar]
- 365.Reubi JC, Schaer JC, Waser B. Cholecystokinin (CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997;57:1377–1386. [PubMed] [Google Scholar]
- 366.Kwekkeboom DJ, Bakker WH, Kooij PP, Erion J, Srinivasan A, de JM, Reubi JC, Krenning EP. Cholecystokinin receptor imaging using an octapeptide DTPA-CCK analogue in patients with medullary thyroid carcinoma. Eur. J Nucl. Med. 2000;27:1312–1317. doi: 10.1007/s002590000296. [DOI] [PubMed] [Google Scholar]
- 367.Behr TM, Jenner N, Behe M, Angerstein C, Gratz S, Raue F, Becker W. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J Nucl. Med. 1999;40:1029–1044. [PubMed] [Google Scholar]
- 368.Behr TM, Behe M, Angerstein C, Gratz S, Mach R, Hagemann L, Jenner N, Stiehler M, Frank-Raue K, Raue F, Becker W. Cholecystokinin-B/gastrin receptor binding peptides: preclinical development and evaluation of their diagnostic and therapeutic potential. Clin Cancer Res. 1999;5:3124s–3138s. [PubMed] [Google Scholar]
- 369.de Jong M, Bakker WH, Bernard BF, Valkema R, Kwekkeboom DJ, Reubi JC, Srinivasan A, Schmidt M, Krenning EP. Preclinical and initial clinical evaluation of 111In-labeled nonsulfated CCK8 analog: a peptide for CCK-B receptor-targeted scintigraphy and radionuclide therapy. J Nucl. Med. 1999;40:2081–2087. [PubMed] [Google Scholar]
- 370.Aloj L, Caraco C, Panico M, Zannetti A, Del VS, Tesauro D, De LS, Arra C, Pedone C, Morelli G, Salvatore M. In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging. J Nucl. Med. 2004;45:485–494. [PubMed] [Google Scholar]
- 371.Nock BA, Maina T, Behe M, Nikolopoulou A, Gotthardt M, Schmitt JS, Behr TM, Macke HR. CCK-2/gastrin receptor-targeted tumor imaging with (99m)Tc-labeled minigastrin analogs. J Nucl. Med. 2005;46:1727–1736. [PubMed] [Google Scholar]
- 372.Behr TM, Behe MP. Cholecystokinin-B/gastrin receptor--Targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Sem. Nucl. Med. 2002;32:97–109. doi: 10.1053/snuc.2002.31028. [DOI] [PubMed] [Google Scholar]
- 373.Makovec F, Christe R, Bani M, Pacini MA, Setnikar I, Rovati LA. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung. 1985;35:1048–1051. [PubMed] [Google Scholar]
- 374.Revel L, Ferrari F, Makovec F, Rovati LC, Impicciatore M. Characterization of antigastrin activity in vivo of CR 2194, a new R-4-benzamido-5-oxo-pentanoic acid derivative. Eur. J Pharmacol. 1992;216:217–224. doi: 10.1016/0014-2999(92)90363-9. [DOI] [PubMed] [Google Scholar]
- 375.Huang SC, Zhang L, Chiang HC, Wank SA, Maton PN, Gardner JD, Jensen RT. Benzodiazepine analogues L-365,260 and L-364,718 as gastrin and pancreatic CCK receptor antagonists. Am. J. Physiol. 1989;257:G169–G174. doi: 10.1152/ajpgi.1989.257.1.G169. [DOI] [PubMed] [Google Scholar]