Abstract
Aims
Tobacco dependence treatments achieve abstinence rates of 25–30% at 1 year. Low rates may reflect failure to conceptualize tobacco dependence as a chronic disorder. The aims of the present study were to determine the efficacy of extended cognitive behavioral and pharmacological interventions in smokers ≥ 50 years of age, and to determine if gender differences in efficacy existed.
Design
Open randomized clinical trial.
Setting
A free-standing, smoking treatment research clinic.
Participants
A total of 402 smokers of ≥ 10 cigarettes per day, all 50 years of age or older.
Intervention
Participants completed a 12-week treatment that included group counseling, nicotine replacement therapy (NRT) and bupropion. Participants, independent of smoking status, were then assigned randomly to follow-up conditions: (i) standard treatment (ST; no further treatment); (ii) extended NRT (E-NRT; 40 weeks of nicotine gum availability); (iii) extended cognitive behavioral therapy (E-CBT; 11 cognitive behavioral sessions over a 40-week period); or (iv) E-CBT plus E-NRT (E-combined; 11 cognitive behavioral sessions plus 40 weeks nicotine gum availability).
Measurements
Primary outcome variable was 7-day point prevalence cigarette abstinence verified biochemically at weeks 24, 52, 64 and 104.
Findings
The most clinically important findings were significant main effects for treatment condition, time and the treatment × time interaction. The E-CBT condition produced high cigarette abstinence rates that were maintained throughout the 2-year study period [(week 24 (58%), 52 (55%), 64 (55%) and 104 (55%)], and was significantly more effective than E-NRT and ST across that period. No other treatment condition was significantly different to ST. No effects for gender were found.
Conclusions
Extended cognitive behavioral treatments can produce high and stable cigarette abstinence rates for both men and women. NRT does not add to the efficacy of extended CBT, and may hamper its efficacy. Research is needed to determine if these results can be replicated in a sample with a greater range of ages, and improved upon with the addition of medications other than NRT.
Keywords: Bupropion, chronic disorders, cigarette smoking, NRT, older adults, tobacco dependence
INTRODUCTION
The goal of this study was to evaluate an extended cigarette smoking treatment program for one group of chronic, heavy smokers: adults 50 years of age and older. The study was guided by the concept of tobacco dependence as an addiction with a chronic, relapsing course [1,2]. Most smokers try to quit many times. Generally, these quit attempts fail and relapse is the norm [3,4]. For most addictions, the recognition that addictive disorders are chronic and relapsing has led to services that include treatment of extended duration, follow-up support and encouragement of treatment re-entry. This does not describe the usual provision of services to cigarette smokers. For the most part, intervention models have been either inexpensive or time-limited, and have used brief courses of treatment. There are three corollaries to a chronic disorder model: first, pharmacological treatments should be used if possible, at least when physical symptoms predominate. Secondly, smoking comes to serve diverse functions for the smoker—for example, weight and mood regulation—and modalities to overcome the multiple deficits presented by loss of these functions need to be provided. Thirdly, independent of the content of the intervention, the model of tobacco dependence as a chronic disorder suggests that long-term treatment, perhaps even treatment re-occurring over a life-time, may be necessary.
Few interventions take into account the implications of this model. This failure may explain the low long-term cigarette abstinence rates we have come to expect for tobacco dependence treatment—usually 25–30% at 1 year, even with combined pharmacotherapy behavioral therapy [2,5].
There are studies of extended treatments in the literature. An early study assessed the effects of extended combined nicotine replacement therapy (NRT) versus single modality NRT. Kornitzer et al. [6] compared active patch plus active nicotine gum, active patch plus placebo gum and placebo patch plus placebo gum over 6 months, and found significant differences favoring active patch plus active gum. More recently, four studies addressed the efficacy of extended bupropion administration, with mixed results. Hays et al. treated participants for 7 weeks with open-label bupropion, then assigned randomly the cigarette-abstinent participants only (59% of the initial sample) to active or placebo bupropion for 45 weeks. Cigarette abstinence was significantly higher in the bupropion group than in the placebo group after 1 year of drug therapy but the conditions did not differ at 2 years [7]. In a second study by this group, smokers were treated with nicotine patches calibrated to their level of cigarette intake. Cigarette-abstinent participants were assigned randomly to either active or placebo bupropion for 6 months. Abstinence rates did not differ between conditions [8]. Cox et al. [9] randomized abstinent smokers who had been treated with bupropion for 7 weeks to either continued bupropion for the remainder of 1 year or to placebo. Bupropion produced higher cigarette abstinence at the end of medication treatment when compared to placebo, but no differences at 1 year follow-up. Killen et al. [10] treated smokers for 12 weeks with open-label bupropion, nicotine patch and weekly relapse prevention training. All participants independent of smoking status were then offered four relapse prevention sessions, and continued on either active or placebo bupropion for an additional 14 weeks. There were no differences in abstinence rates between conditions at 1 year. Using nortriptyline, we completed a study in which smokers were assigned to one of four treatment conditions in a 2 × 2 (nortriptyline versus placebo by brief treatment versus extended treatment) design. Participants in extended treatment continued taking drug or placebo and received monthly individual counseling sessions to week 52, with telephone calls between sessions. At week 52 we found a 50% cigarette abstinence rate for smokers given extended nortriptyline plus counseling over a 1 year period, and a 42% cigarette abstinence rate at 1 year for those receiving counseling plus placebo [11], both of which exceeded the effects of short-term treatment. Studies assessing the effects of extended varenicline administration appear to indicate that this drug may enhance abstinence. Tonstad et al. [12] randomized abstinent smokers who had been treated with 12 weeks of varenicline to either continued varenicline treatment or to placebo for an additional 12 weeks. Continuous cigarette abstinence rates were higher for the varenicline group than the placebo group for weeks 13–24 and 13–52. Williams et al. [13] administered either varenicline or placebo over a 1-year period, and found that varenicline was superior to placebo at both 12 and 52 weeks. One study assessed the effects of cognitive behavioral therapy (CBT) in promoting long-term abstinence [14]. Participants received bupropion, nicotine patch and CBT for 8 weeks, and were then assigned randomly to receive either 12 weeks of CBT plus voice-mail monitoring and telephone counseling, or telephone-based general support. These investigators reported significant differences at 20 weeks in favor of the CBT condition, but differences at 52 weeks were not significant. A predicted gender × treatment interaction was not found although history of depression was a treatment moderator, with individuals with a positive history showing a better response when assigned to the less intensive condition.
In summary, the existing data present a mixed picture with respect to the efficacy of most extended treatments. There are few follow-ups after the end of treatment, and in those that have been completed there was only modest evidence of maintenance of treatment effects. It is difficult to interpret cigarette abstinence rates in most studies, because only cigarette-abstinent smokers continued into the extended treatment portion of the study.
In the present study, all participants continued into the extended treatment phase of the study, thus allowing comparisons to the cigarette abstinence rates reported in most of the literature. The intervention was directed specifically at components of relapse and a follow-up was conducted 1 year after the end of extended treatment. The CBT-based intervention addressed the five areas that the 2000 Practice Guidelines indicated were important in relapse [2].
We selected smokers 50 years of age and older as participants for five reasons. First, surveys [15] and descriptive data from randomized controlled trials conducted in the mid-1990s suggested that older smokers are long-term, heavy smokers who are dependent on nicotine and who are motivated to quit [15–19]. Secondly, older Americans are the fastest-growing population segment, and this growth will increase the sheer number of older smokers [20]. Thirdly, smoking is a risk factor for seven of the 14 major causes of death in older people [21]. Fourthly, even though both smokers and their physicians seem to assume that quitting smoking when older will have limited benefit, for this age group, as for other age groups, risks for heart disease, stroke and even lung cancer decline after quitting [22,23]. Fifthly, there are few recent treatment studies of older smokers. There has not been a randomized control trial of tobacco dependence interventions for older smokers reported in the literature in the past 14 years. Age 50 was selected because it was the definition of ‘older smokers’ used by the 2000 Practice Guidelines [2].
There were four experimental conditions: standard treatment (ST); extended NRT (E-NRT; extended cognitive–behavioral treatment alone (E-CBT); and extended cognitive behavioral treatment plus extended NRT combined (E-combined). The following hypotheses were proposed: (i) over weeks 24, 52, 64 and 104, the E-CBT condition, the E-combined condition and the E-NRT condition will produce higher point prevalence cigarette abstinence rates than the ST condition; (ii) over weeks 24, 52, 64 and 104, E-combined will have higher point prevalence cigarette abstinence rates than the remaining three conditions; (iii) while both men and women will have the highest point prevalent cigarette abstinence rates in the E-combined condition, the difference between this condition and the other three conditions will be greater for women than for men. This hypothesis, formulated before the publication of Killen et al.’s 2008 paper [14], was based on the frequently voiced supposition that women are helped more by social support than are men while quitting smoking [1].
METHODS OF PROCEDURE
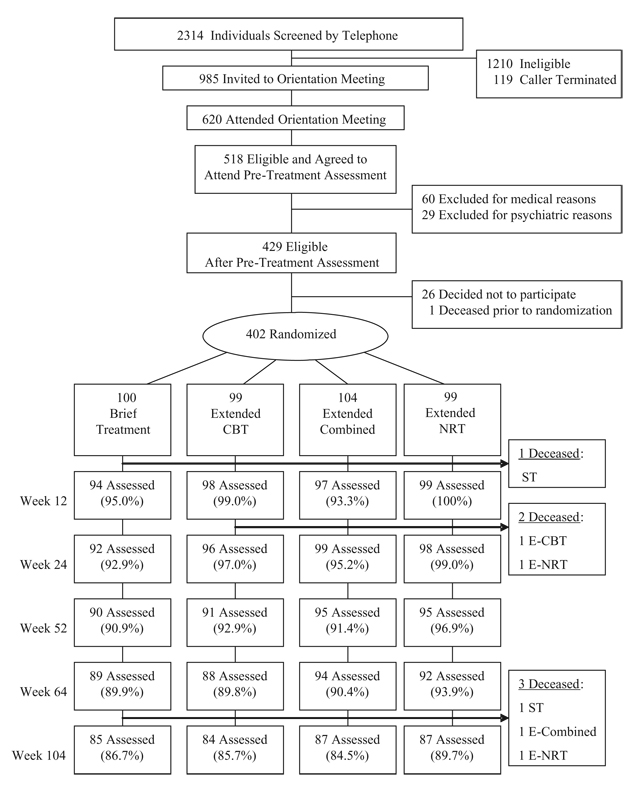
Participants
The Consolidated Standards of Reporting Trials (CONSORT) [24] chart in Fig. 1 shows participant recruitment and attrition from the first contact with the program to the week 104 assessment. Study treatments were approved by the University of California, San Francisco (UCSF) Institutional Review Board before recruiting was initiated. All participants signed written informed consents before entering treatment. Participants were recruited by advertising, public service announcements and flyers. After a telephone screening, participants were invited to an orientation meeting, where they completed informed consent and were invited to a baseline assessment including a physical examination, electrocardiographic (EKG) and blood samples for basic blood chemistry analyses. Each participant then completed the depression, alcohol and nicotine sections of the Computerized Diagnostic Interview Schedule for DSM-IV (CDIS) [25] administered by research staff. Participants were ≥ 50 years of age and smoked ≥ 10 cigarettes per day. Exclusionary criteria included cardiovascular disease, history of seizure, severe allergies, life-threatening disease, life-time bipolar disorder, current major depressive disorder (MDD), current use of any psychiatric medication, suicidal or psychotic symptoms, treatment for drugor alcohol use within 6 months, psychiatric hospitalization within 1 year and pregnancy or lactation.
Figure 1.
Recruitment and follow-up chart. ST: standard treatment; CBT: cognitive–behavioral therapy; NRT: nicotine replacement therapy
Four hundred and three participants completed baseline tests and screenings, and consented to participate. One participant died at week 5, before randomization, reducing the sample available for randomization to 402. Six more deaths occurred during the course of the study. All deaths were unrelated to study procedures. The mean age was 56.7 years [standard deviation (SD) = 5.87]. The mean years of regular smoking was 37.8 (SD = 8.23).The mean number of cigarettes smoked per day was 20.5 (SD = 8.72). The mean score on the Fagerström Test of Nicotine Dependence (FTND)was 4.8 (SD = 2.1).Women comprised 40% of the sample, and Caucasians comprised 76.9%. They were divided about equally between being married or living with a partner (42.5%) and separated/divorced/widowed (39.7). Only 12.1% were high school graduates or less; 35.5% had some college, 30.5% were college graduates and 21.9% had a graduate degree. In terms of diagnosis, 21.1% had a history of MDD, 63% had a diagnosis of life-time nicotine dependence, 38.6% had a diagnosis of life-time nicotine withdrawal and 18.1% had a diagnosis of life-time alcohol dependence. Only one variable, marital status (married/living with partner versus not), was significantly different among conditions. However, marital status did not correlate with cigarette abstinence at any assessment.
At week 8, all 402 participants were stratified on gender, history of MDD (positive history of MDD versus not) and current cigarette abstinence status, and assigned randomly to one of four experimental conditions using a computerized allocation list by the project statistician (Ms Robbins), who had no contact with participants. The assignment of individual participants by subject number was then transmitted electronically to clinical staff.
Conditions did not differ on the percentage of participants from whom we were able to collect smoking data at weeks 12, 24, 52, 64 or 104. Determination of predictors of attrition from assessments was not useful due to the low attrition rates; rates were sufficiently low that attrition bias was not a concern (week 12 = 3.2%; week 24 = 4.0%, week 52 = 7.0%; week 64 = 9.0%; week 104 = 13.4%).
Assessments
Data were collected at baseline and weeks 12, 24, 52, 64 and 104. All participants were contacted for all assessments, independent of whether or not they continued in treatment. Participants were paid $25.00 for completing each assessment at weeks 12, 24, 52, 64 and 104.
Biochemically verified, 7-day point prevalent abstinence from cigarettes, indicated by self-report of cigarette abstinence (‘no smoking, not even a puff’), expired air carbon monoxide (CO) levels ≤ 10 parts per million (p.p.m.) and anatabine/anabasine levels of ≤ 2 mg/ml was the primary dependent variable. Anatabine and anabasine are two alkaloids present in tobacco. They are not present in nicotine-containing medications, however, and measuring concentrations of these alkaloids is useful for detecting tobacco use in people who are being treated with NRT [26]. Cotinine verification could be used, as it is a by-product of the nicotine in both tobacco and NRT. Anatabine/anabasine were assayed only if the participant’s self-report and CO levels were consistent with cigarette abstinence, and participants were coded as abstinent only if the anatabine/anabasine assay value also fell below the cut-off point [27].
We also administered a questionnaire that assessed gender, education, ethnicity and marital status, the Profile of Mood States (POMS) [28], measures of in-treatment and partner support for quitting adapted from Mermelstein (1983) [29], the FTND [30], measures of drug and alcohol use developed by us; the Thoughts about Abstinence Questionnaire [31], the Medical Outcomes Scale, Short-Form (SF-36) [32], the Geriatric Depression Scale [33] and the Perceived Stress Scale [34].
Treatment conditions
Standard treatment (ST)
Participants were provided with 12 weeks of sustained release bupropion and 10 weeks of 2 mg and 4 mg nicotine gum, and received counseling based on Clear Horizons [35]. This manual, designed originally as a self-help aid for smokers ≥ 50 years of age, was used by the counselor to guide smokers through the steps of quitting, and was given to each participant to use as a guide. To take the possibility of age-related, slower drug clearance into account, we modified the standard bupropion dosing protocol. Dose was 150 mg/day for the first week. If no adverse effects were noted the dose was increased to 300 mg for the second week, where it remained for the rest of the 12-week period, barring adverse events during that period. All participants were provided with 10 weeks of nicotine gum, beginning at the quit date during week 3. Participants who smoked ≥ 25 cigarettes per day received 6 weeks of 4 mg gum, followed by 4 weeks of 2 mg gum. Participants who smoked ≤25 cigarettes per day received 10 weeks of 2 mg gum; if they used more than 12 pieces a day or reported withdrawal symptoms they were given 4 mg gum. At the week 8 meeting, participants were instructed to begin tapering from the gum if they had not already done so, with the taper to be completed by week 12. All participants received five group counseling sessions held at weeks 1, 3 (two sessions, one immediately after the quit date), 5 and 8. Participants assigned to ST received no further treatment after week 12.
Extended NRT (E-NRT)
This condition provided evidence about the efficacy and cost-effectiveness of long-term NRT when used as a relapse prevention strategy. Bupropion, NRT and counseling were provided during the first 12 weeks as they were provided in ST, except that participants did not taper from NRT. NRT was available through week 52. Participants received instructions at week 8, both orally from the counselor and in writing, on the use of nicotine gum during the extended treatment period but no counseling about its use was provided during the remainder of the study. Specifically, participants were instructed to keep a ‘shelf ’ of nicotine gum with them, and to use it if the urge to smoke occurred. We used extended NRT rather than extended bupropion, because there was some concern about the accumulation of bupropion in older individuals [36] and no long-term clinical trials with older patients had been published when this study was initiated.
E-CBT alone
Bupropion, NRT and group counseling were provided during the first 12 weeks of treatment as in the ST condition. The E-CBT intervention was based on a cognitive–behavioral model.
The content areas, taken from the 2000 Practice Guidelines’ recommendation for relapse prevention, were motivation, social support, dysphoria, dependence/withdrawal and weight gain [2]. This version of the intervention was tailored to older smokers, and was designed to be self-directed with coaching and instruction from the therapist.
(i) Motivation
The motivational component of the intervention identified cues to elicit motivation, and used a decisional balance chart to emphasize the benefits of quitting and costs of smoking. Participants were to make a repeated commitment to cigarette abstinence to themselves and to significant others.
(ii) Mood management
Participants received a self-administered mood management guide. Materials for charting the number of cigarettes smoked, number of pleasant activities and mood level were distributed [37,38]. Participants were instructed to increase pleasant activities and to note the correspondence of activities with mood, and mood with the number of cigarettes smoked. Ideas for increasing pleasant activities were provided.
(iii) Weight control
Physical activity may both control weight and decrease depression [39,40]. The goal of the activity program was for participants to have completed 30 minutes of moderate exercise most days of the week. Participants received a pedometer, and a form was provided to record time spent in activity not assessed by the pedometer for study counselors to convert into step estimates. The goal was 10 000 steps per day, which corresponds to the public health activity guideline of >30 minutes of moderate physical activity per day [41]. Subsequent sessions were devoted to assisting participants to gradually increase their activity in order to reach that goal, using computerized graphs, feedback and counselor encouragement.
(iv) Social support
The social support intervention included managing the current support network and building a larger non-smoking network. We determined the smoking status of network members and identified those supporting cessation and smoking. Using this information, the participant and the counselor practised methods for eliciting positive support and handling negative support. We also provided participants with lists of groups and activities developed from community sources and the internet.
(v) Withdrawal/dependence
Counselors were alerted to reports of symptoms such as craving and weight gain. They worked with participants to develop strategies to deal with emerging symptoms. They also reminded participants that using the strategies taught in the intervention would be helpful in preventing the emergence of symptoms.
Eleven individual extended treatment sessions were provided after the five group sessions included in the ST protocol, from weeks 10 to 52. Time between sessions was more frequent early in the extended treatment period, and increased gradually throughout the study (2 weeks apart during weeks 10–16; 4 weeks apart during weeks 20–36 and 8 weeks apart during weeks 44 and 52). Sessions lasted 20–40 minutes. Copies of the treatment manual are available from the senior author.
Extended CBT plus extended NRT (e-combined)
Bupropion, NRT and group counseling were provided during the first 12 weeks of treatment as in the ST condition. The relapse prevention intervention was identical to that provided in the E-CBT condition. The use of NRT paralleled that in the E-NRT condition, but NRT use was reinforced by the counselor.
Counselor training and monitoring
Counselors were trained by Drs Humfleet and Muñoz, both clinical psychologists. Initial training consisted of reading study manuals and observing one of these three individuals treating one cohort of participants. Counselors were then observed for a cohort by either Dr Humfleet, Dr Muñoz or the project coordinator. The counselors met weekly with Drs Humfleet and Muñoz, who reviewed the previous week’s sessions with them. Reviews of progress, clinical difficulties and how to handle therapeutic issues most effectively within the theoretical confines of the study were addressed at these meetings.
Statistical methods
The planned sample size was based on the primary hypothesis testing differences in 7-day point prevalence cigarette abstinence rates across weeks 24–104. The estimated effect size was taken from Hall et al. [42] and factored in the anticipated attrition rate. Generalized estimating equations (GEE) were used to test the hypothesis about point prevalence cigarette abstinence across the follow-up period. Power analyses were based on this method of analysis. Power was set at 80% with a Type I error rate of 0.05 using the method of Rochon [43]. SAS version 9.1 was used for all analyses [44].
To ensure that randomization had not been compromised, we compared the four experimental conditions on the baseline variables in Table 1 using analysis of variance (ANOVA) for continuous variables and Pearson’s χ2 tests for categorical variables. To identify potential covariates, we correlated these baseline variables with point prevalence cigarette abstinence at weeks 12, 24, 52, 64 and 104. We included in preliminary hypothesis testing models those variables that had significant correlations with cigarette abstinence. These variables were eliminated if they did not contribute significantly to the final model.
Table 1.
Number and percentage abstinent from cigarettes by treatment condition × treatment condition at weeks 12, 24, 52, 64 and 104.
| Week 12 | Week 24 | Week 52 | Week 64 | Week 104 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST | 59 | 63% | 50 | 54% | 30 | 33% | 30 | 34% | 31 | 36% |
| E-CBT | 63 | 64% | 56 | 58% | 50 | 55% | 48 | 55% | 46 | 55% |
| E-combined | 64 | 66% | 58 | 59% | 46 | 48% | 48 | 51% | 39 | 45% |
| E-NRT | 62 | 63% | 55 | 56% | 39 | 41% | 42 | 46% | 35 | 40% |
ST: standard threatment; E-CBT: extended cognitive behavioral treatment; E-NRT: extended nicotine replacement therapy; E-combined: extended cognitive behavioral treatment plus extended NRT combined.
Differences in the number of days of study NRT use were tested by a one-way analysis of variance. Differences among the four conditions in the use of non-study prescription medications and the use of non-study NRT at each assessment were determined by a Pearson’s χ2 analysis for categorical variables. All tests were two-tailed.
RESULTS
Cigarette abstinence
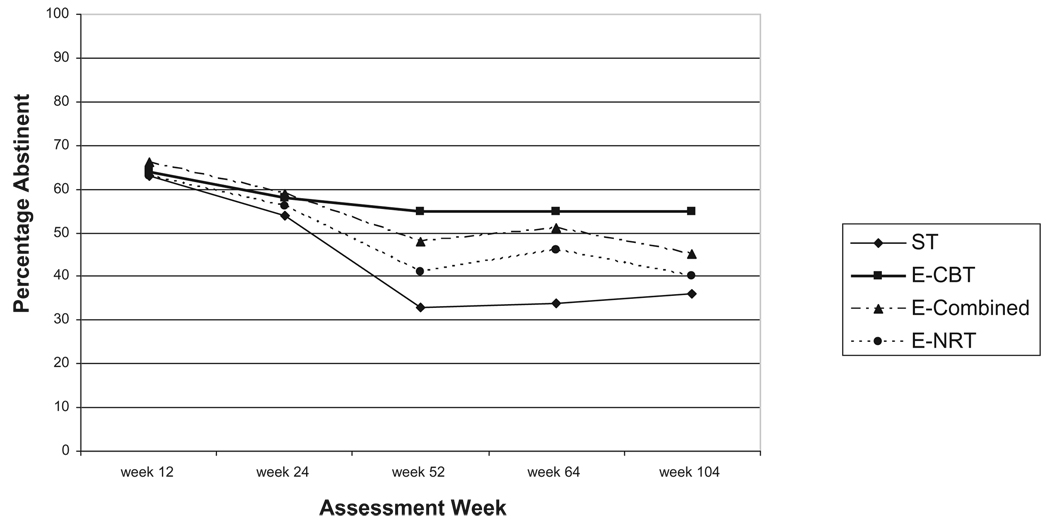
CO and anatabine/anabasine corrected cigarette abstinence rates for each treatment condition at each assessment are given in Table 1, and shown graphically in Fig. 2. Initial analyses of possible covariates revealed that FTND score, age, number of years of regular smoking, ethnicity social participation score and the POMS total score were the only baseline variables correlated significantly with outcome. Therefore, these baseline variables were entered as covariates in the full GEE model containing the variables of treatment condition, time and all possible two-way interactions with treatment condition. The interactions of treatment condition with the covariates age, number of years of regular smoking, ethnicity (non-Hispanic Caucasian versus not), social participation score and POMS total score did not contribute significantly, and were dropped from the final model. Significant main effects were found for treatment condition (χ2 (3, n=402) = 8.12, P = 0.0435), time (χ2 (1, n=402) = 48.78, P< 0.0001), baseline FTND (χ2 (1, n=402) = 19.45, P < 0.0001) and age (χ2 (1, n=402) = 3.91, P = 0.0481), along with significant interactions of treatment × time (χ2 (3, n=402) = 8.35, P = 0.0392) and treatment × FTND score (χ2 (3, n=402) = 8.17, P = 0.0427). With respect to main effects other than treatment, the younger the age, and the lower the FTND score, the greater the probability of cigarette abstinence. The interaction of FTND score and treatment reflected consistent negative correlations of FTND score with cigarette abstinence in the ST and E-combined conditions, and no relationship in the E-CBT and E-NRT conditions. Tests of the individual three hypotheses follow.
Figure 2.
Carbon monoxide and anatabine/anabasine corrected abstinence rates by treatment condition at weeks 12, 24, 52, 64 and 104
Hypothesis 1
Over the period of weeks 24, 52, 64 and 104, the E-CBT condition, the E-combined condition and the E-NRT condition will produce higher point prevalence cigarette abstinence rates than ST condition. This hypothesis was partially supported. The E-CBT condition had significantly higher cigarette abstinence rates than ST [odds ratio (OR) 1.27; 95% confidence interval (CI) 1.52, 1.05, P = 0.0122] and E-NRT (OR 1.22; 95% CI 1.45, 1.03, P = 0.0211) over time. Neither E-NRT (χ2 (1, 402) = 0.37, P = 0.7104) nor E-combined (χ2 (1, 402) = 0.73, P = 0.4683) differed significantly from ST.
Hypothesis 2
Over the period of weeks 24, 52, 64 and 104, E-combined will have higher point prevalence cigarette abstinence rates than the remaining three conditions. Contrary to prediction, E-CBT was more effective than E-combined (OR 1.18; 95% CI 1.40, 0.99, P = 0.0618), but the difference did not achieve traditional levels of significance. As noted above, E-combined was not significantly more effective than ST, nor was it more effective than E-NRT (χ2 (1, 402) = 0.38, P = 0.7031).
Hypothesis 3
While both men and women will have the highest point prevalent cigarette abstinence rates in the E-combined condition, the difference between this condition and the other three conditions will be greater for women than for men. There were no main or interaction effects for gender.
Other non-study medications and NRT use
There were no significant differences between treatment groups in use of non-study medications considered adjuncts to tobacco dependence treatment (i.e. bupropion, nortriptyline or varenicline) at any assessment, and overall rates of reported use were low (week 12 = 0.8%; week 24 = 3.1%; week 52 = 4.2%; week 64 = 5.2% week 104 = 8.4%).
The number of days of study provided NRT use in the E-NRT condition was significantly higher (x̄ = 85.31; SD = 103.62) than in the E-combined condition (x̄ = 48.23; SD = 78.74; F(1,201) = 8.21, P = 0.0046). The E-CBT condition reported a minimal number of days of non-study NRT use during weeks 12–52 (x̄ = 1.33, SD = 10.1), as did the ST condition (x̄ = 1.22, SD = 7.73).
There were also no differences observed in indicators of use of the behavioral techniques in the two extended treatment conditions. The two extended treatment conditions did not differ significantly in the number of counseling sessions attended (x̄RP = 5.5, x̄RP+NRT = 5.9 ), = nor in the use of the pedometer as part of the physical activity component (E-CBT = 42.2%, E-combined = 50%).
DISCUSSION
The most important finding of this study is the high and stable cigarette abstinence rates produced by the E-CBT condition. These rates are strikingly higher than those reported in the recent literature. It is particularly important that these cigarette abstinence rates were maintained for 1 year after the end of the extended treatment. A critical question is whether this was due to the content of the intervention, which was aimed at providing skills to maintain abstinence, or whether these results were due to continued long-term support. This question cannot be resolved without a control group that is equivalent in time to the E-CBT treatment, but has different content. However, that the results were maintained after the end of treatment suggests that the content, not just the therapeutic contact, may have played an important role in the outcome: it would not be expected that non-specific, supportive content would provide skills that would result in cigarette abstinence once it was withdrawn. These findings differ from those of Killen et al. [14], who found that 12 weeks of extended CBT did not facilitate abstinence at long-term follow-up. The present study and Killen’s differ in that the extended interventions differed in both length (40 weeks in the present study; 12 in Killen et al.’s study) and content, and in age of the subject sample. Additional work is needed to determine the variable that resulted in the difference between the two studies.
A second important finding is the failure of adding NRT to the E-CBT to increase cigarette abstinence rates. The reason for lack of an increase in cigarette abstinence is not clear. It is possible that availability of a pharmaceutical adjunct took participants’ attention away from the cognitive–behavioral intervention, and the skills provided were not so well learned. E-combined participants may have attributed their ability to quit to NRT rather than to themselves and newly acquired skills [45]. Also, use of NRT was modest in both conditions where it was available; it was used on significantly more days in the E-NRT condition than in the E-combined condition. It might be argued the low use rate of NRT in the E-combined condition renders comparisons between E-combined and E-CBT tentative, in that the E-combined condition may well have fared better had use of NRT been higher in that condition. However, when considered in terms of clinical utility, it must be remembered that the lower use rate reflects how participants chose to use NRT in the context of that condition. Therefore, the findings are an accurate reflection of the efficacy of the E-CBT and E-combined conditions as presented in this study. That NRT did not increase cigarette abstinence rates when provided over an extended period of time is consistent with the growing literature suggesting that NRT effects are not increased with increasing duration [46].
Strengths of the study include a large sample size, manualized treatment, a model-driven intervention and use of two biochemical verification assays. The limitations of this study include generalizability, as the population treated was relatively well-educated, willing to participate in research and to attend multiple treatment sessions. They were also predominantly Caucasian and able to read and speak in English.
As would be expected, lower FTND scores at baseline predicted cigarette abstinence. Younger age also predicted cigarette abstinence, suggesting that it may be the most difficult for the very oldest smokers to quit smoking. The interaction of FTND score with treatment condition is puzzling, and due most probably to chance.
So far as we could find, this is the only large-scale tobacco abstinence treatment study to use anatabine/anabasine assays as biochemical verification. In this study, these assays had only a modest effect on outcome. Of the 905 assays completed only 17 were changed as a function of anatabine/anabasine analyses, and these varied little by treatment condition (three in ST, three in E-NRT, six in E-CBT and five in E-combined). The present study suggests that the costs of obtaining urine for these assays should be weighed carefully against the increase in accuracy they may provide.
Older smokers are a rapidly increasing segment of the population that has generally been neglected in smoking treatment interventions. Despite setting 50 years of age as a lower-limit for recruiting, the mean age of smokers in this study was 56 years of age, younger than anticipated when the study was initiated. Nevertheless, the participants in this study are substantially older than individuals participating in our most recently published clinical trial, where the average age was 38.6 years [11]. The high cigarette abstinence rates obtained in the current study suggest that the provision of smoking treatment services to older adults is an efficient and valuable use of health-care resources. It also suggests that extended interventions should be studied in the general population of smokers, not simply those who are 50 years of age.
It is often argued that the ‘intensity’ of extended treatments precludes them from being adopted widely, due to constraints imposed by insurance companies’ restrictions and by providers’ time. However, when the cost of providing long-term treatment for cardiovascular disease, cancer or emphysema is considered, the cost of extended tobacco abstinence treatment is trivial indeed.
Acknowledgements
This publication was made possible by grant numbers R01 DA02538, K05 DA016752, K23 DA018691 and P50 DA 09253. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/NIDA. The authors wish to thank Dr Kevin Delucchi, University of California, San Francisco, for statistical consultation, Wynnie Wong for helping with data analysis, Jennifer Morris for manuscript preparation and Dr Ronald Pilato for his clinical contributions.
Footnotes
Preliminary versions of this paper were presented at the annual meetings of the Society for Research on Nicotine and Tobacco on 21–24 February 2007, in Austin, TX, and 27 February–1 March 2008, in Portland, OR, USA.
Clinical trial registration
Clinical trials.gov; NCT00086385; http://www.clinicaltrials.gov.
Declarations of interest
None.
References
- 1.Surgeon General. Rockville, MD: US Government Printing Office; Women and Smoking: A Report of the Surgeon General. 2001
- 2.Fiore MC. Clinical practice guidelines for treating tobacco use and dependence: a US Public Health Service Report. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- 3.Garvey AJ, Bliss RE, Hitchcock JL, Heinhold JW, Rosner B. Predictors of smoking relapse among self-quitters: a report from the normative aging study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein E, Weiss SM, Hitchcock JL, Leveton LB, O’Connell KA, Prochaska JO. Patterns of smoking relapse. Health Psychol. 1986;5 suppl:29–40. [PubMed] [Google Scholar]
- 5.Fiore MC. Rockville, MD: US Department of Health and Human Services, Public Health Service Report; Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. 2008
- 6.Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med. 1995;24:41–47. doi: 10.1006/pmed.1995.1006. [DOI] [PubMed] [Google Scholar]
- 7.Hays JT, Hurt RD, Rigotti N, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: a randomized, controlled trial. Ann Intern Med. 2000;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 8.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21:914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 9.Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DP, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med. 2004;19:828–834. doi: 10.1111/j.1525-1497.2004.30423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 11.Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161:2100–2107. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 12.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation—a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Williams KE, Reeves KR, Billing CB, Jr, Gong AM, Pennington J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2007;23:793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- 14.Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103:1381–1390. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimer BK, Orleans T, Keintz M, Cristinzio S, Fleisher L. The older smoker: status challenges and opportunities. Chest. 1990;97:547–552. doi: 10.1378/chest.97.3.547. [DOI] [PubMed] [Google Scholar]
- 16.Rimer BK, Orleans TC. Tailoring smoking cessation for older adults. Cancer. 1994;74 suppl:2051–2054. doi: 10.1002/1097-0142(19941001)74:7+<2051::aid-cncr2820741711>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age Aging. 1990;19:164–168. doi: 10.1093/ageing/19.3.164. [DOI] [PubMed] [Google Scholar]
- 18.Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Prev Med. 1996;25:346–354. doi: 10.1006/pmed.1996.0065. [DOI] [PubMed] [Google Scholar]
- 19.Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6:188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husten CG, Shelton DM, Chrismon JH, Lin Y-CW, Mowery P, Powell F. Cigarette smoking and smoking cessation among older adults: United States, 1965–94. Tob Control. 1997;6:175–180. doi: 10.1136/tc.6.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaCroix AZ, Lang J, Scherr P, Wallace R, Cornoni-Huntley J, Berkman L, et al. Smoking and mortality among older men and women in three communities. N Engl J Med. 1991;324:1619–1625. doi: 10.1056/NEJM199106063242303. [DOI] [PubMed] [Google Scholar]
- 22.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case–control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gourlay SG, Benowitz NL. The benefits of stopping smoking and the role of nicotine replacement therapy in older patients. Drugs Aging. 1996;9:8–24. doi: 10.2165/00002512-199609010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 25.Robins LN, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV (DIS-IV) St Louis, MO: Department of Psychiatry, Washington University School of Medicine; 1995. [Google Scholar]
- 26.Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11:1668–1673. [PubMed] [Google Scholar]
- 27.Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 28.McNair DM, Lorr M, Droppleman LF. Manual: Profile of Mood States (POMS), revised. San Diego, CA: Educational and Instructional Testing Service; 1992. [Google Scholar]
- 29.Mermelstein R, Lichtenstein E, McIntyre K. Partner support and relapse in smoking-cessation programs. J Consult Clin Psychol. 1983;51:465–466. doi: 10.1037//0022-006x.51.3.465. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 33.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 35.Orleans C, Rimer B, Telepchak J, Fleisher L, Keintz M, Boyd N, et al. Clear Horizons: A Quit Smoking Guide for Smokers Aged 50 and Older. Philadelphia, PA: Fox Chase Cancer Center; 1997. [Google Scholar]
- 36.Sweet RA, Pollock BG, Kirshner M, Wright B, Altieri LP, DeVane CL. Pharmacokinetics of single- and multiple-dose bupropion in elderly patients with depression. J Clin Pharmacol. 1995;35:876–884. doi: 10.1002/j.1552-4604.1995.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 37.Hall SM, Munoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gumin smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- 38.Muñoz RF, Marín BV, Posner SF, Pérez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Commun Psychol. 1997;25:325–343. doi: 10.1023/a:1024676626955. [DOI] [PubMed] [Google Scholar]
- 39.Babyak MA, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch InternMed. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 41.Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S, et al. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 42.Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161:2100–2107. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 43.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6:188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeiss AM, Lewinsohn PM, Munoz RF. Nonspecific improvement effects in depression using interpersonal skills training, pleasant activity schedules, or cognitive training. J Consult Clin Psychol. 1979;47:427–439. doi: 10.1037//0022-006x.47.3.427. [DOI] [PubMed] [Google Scholar]
- 46.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]