Abstract
Runx2 and androgen receptor (AR) are master transcription factors with pivotal roles in bone metabolism and prostate cancer (PCa). We dissected AR-mediated repression of Runx2 in dihydrotestosterone (DHT)-treated osteoblastic and PCa cells using reporter assays and endogenous Runx2 target genes. Repression required DHT, but not AR’s transactivation function, and was associated with nuclear colocalization of the two proteins. Runx2 and AR coimmunoprecipitated and interacted directly in glutathione-S-transferase pull-down assays. Interaction was ionic in nature. Intact AR DNA-binding domain (DBD) was necessary and sufficient for both interaction with Runx2 and its repression. Runx2 sequences required for interaction were the C-terminal 132 amino acid residues together with the Runt DBD. Runx2 DNA binding was abrogated by endogenous AR in chromatin immunoprecipitation assays and by recombinant AR-DBD in gel shift assays. Furthermore, AR caused increased nuclear mobility of Runx2 as indicated by faster fluorescence recovery after photobleaching. Thus, AR binds Runx2 and abrogates its binding to DNA and possibly to other nuclear components. Clinical relevance of our results was suggested by an inverse correlation between expression of AR-responsive prostate-specific antigen and osteocalcin genes in PCa biopsies. Given the tumor suppressor properties of Runx2, its repression by AR may constitute a mechanism of hormone carcinogenesis. Attenuation of Runx2 by AR in osteoblasts may play a role in skeletal metabolism: the bone-sparing effect of androgens is attributable, in part, to keeping Runx2 activity in check and preventing high-turnover bone disease such as seen after castration and in transgenic mice overexpressing Runx2 in osteoblasts.
AR directly interacts with Runx2 and inhibits its DNA-binding and transcriptional activity, potentially contributing to the bone-sparing and prostate carcinogenic properties of androgens.
Runx2 is one of the three mammalian transcription factors sharing the highly conserved DNA-binding Runt domain. It is best known for its mandatory role in the differentiation of osteoblasts and chondrocytes from mesenchymal precursors (1,2,3,4). In addition to promoting embryonic bone formation, Runx2 plays a role in postnatal bone metabolism and the control of bone mass (5,6,7,8,9). Runx2 is also expressed in breast and prostate cancer cells, possibly contributing to their tumorogenicity and metastatic potential, in part by stimulating the expression of matrix metalloproteases (10,11). In other contexts, the Runx proteins, including Runx2, function as tumor suppressors (12,13).
The most well-established target gene for Runx2 is osteocalcin (OC). The osteoblast specific element 2 (OSE2) at the OC promoter served as a crucial molecular tool by which Runx2 was discovered as an osteoblast master transcription factor (5,14). However, OC does not mediate the role of Runx2 in osteoblast differentiation and bone metabolism (15,16). Many other Runx2 target genes in osteoblasts have also been identified (17,18,19,20), but most of them have not been tested yet for their roles in bone cell function. Even less is known about target genes that mediate the tumor suppressor function of Runx2. Notable candidates are the cyclin-dependent kinase inhibitor p21 (21) and the proapoptotic protein Bax (22).
In addition to the Runt domain, Runx2 has an N-terminal glutamine-alanine domain (QA) and a C-terminal proline-serine-threonine (PST) domain. Within the PST domain are Runx2’s nuclear localization and the nuclear matrix targeting signals (NMTS) (23). A number of coregulatory proteins interact with, and orchestrate Runx2 activities. Binding of Cbfβ to the Runt domain enhances the affinity of Runx2 to OSE2-like response elements and is required for proper control of skeletogenesis (24). The PST domain recruits coactivators such as p300, MOZ, and MORF (25,26), as well as corepressors such as TLE/Groucho, HDAC6, and YAP, to activate or repress target genes in a context-dependent manner (21,27). Of particular interest, the positive role of osteogenic bone morphogenetic proteins and the negative role of TGFβ in skeletal development are mediated, in part, by the interaction of their signal transducers [Sma- and Mad-related protein (SMAD) 1, 5, and 8 and SMAD 2 and 3, respectively] with Runx2 (28,29). Runx2 expression and activity are also controlled at the levels of transcription (30), translation (31), posttranslational modifications (32), nuclear and subnuclear localization (33), and access to chromatin-embedded targets (34). Interestingly, Runx2’s SMAD interaction domain (SMID) overlaps with its NMTS (28,35). There is also evidence that growth factors influence the function of osteoblasts and other cells in part by MAPK-mediated phosphorylation of residues in Runx2’s PST domain (36,37,38).
Like Runx2, the androgen receptor (AR) is also a transcription factor that plays important roles in bone and cancer biology. It is crucial for the survival and growth of both androgen-dependent and ablation-resistant prostate cancer (PCa) cells (39,40,41), and its target gene prostate-specific antigen (PSA) is frequently used as a surrogate for AR activity in clinical settings and to study its mechanism of action (42). Similar to other steroid hormone receptors, AR contains an N-terminal domain (NTD) that contains activation function-1 (AF1), a conserved zinc-finger DNA-binding domain (DBD), and a ligand-binding domain (LBD) that contains activation function-2 (AF2). Testosterone or its more potent derivative, dihyrotestosterone (DHT), binds to the LBD to induce a conformational change that triggers AR nuclear translocation and transcription of target genes (43), which mediate the hormone’s roles in diverse physiological and disease processes.
The proskeletal properties of androgens include promotion of bone formation and attenuation of bone resorption. This is achieved both via aromatization to estrogens, which then activate estrogen receptor-α (ERα), and by aromatization-independent mechanisms (44,45,46,47). Lack of androgens or AR results in progressive loss of bone mass and increased risk of fracture (46,48,49,50). Although AR is clearly implicated in the regulation of bone cell function, the underlying molecular mechanisms remain unclear.
We recently demonstrated that ERα interacts with and modulates Runx2 activity in osteoblasts and breast cancer epithelial cells (51). Attenuation of Runx2 by ERα likely contributes to the beneficial effect of estrogens in the adult skeleton because unrestrained Runx2 in osteoblasts leads to increased osteoclast activation and loss of bone mass (7,52). In breast cancer cells, inhibition of Runx2 activity by estrogens may promote carcinogenesis given the tumor suppressor properties of Runx proteins (12,13). Runx2 is also inhibited by the AR (53). In the present study, we dissected the regulation of Runx2 activity by AR in osteoblastic and PCa cell lines. We report that, like ERα, AR interacts with Runx2 directly, but the two receptors bind different regions of Runx2. Of particular importance, whereas both receptors require the PST domain of Runx2 for interaction, only the AR requires, in addition, the Runt domain, interaction with which diminishes the DNA-binding function of Runx2.
Results
AR represses Runx2 independently of its own transactivation property
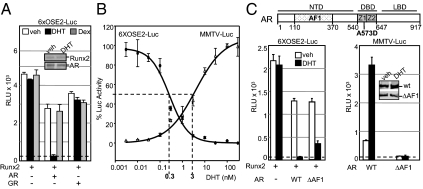
Initially, we examined the effect of AR on the transcriptional potential of Runx2 using transient transfection reporter assays. COS7 cells, which express neither transcription factor, were transfected with plasmids encoding Runx2 and AR, along with the 6XOSE2-Luc reporter, in which luciferase is controlled by six copies of the OSE2 Runx2-binding site (54). The reporter had very low basal activity and was strongly activated upon Runx2 expression (Fig. 1A). In the absence of AR, its ligand dihydrotestosterone (DHT) did not affect Runx2 activity. However, DHT treatment in the presence of AR repressed Runx2 activity to basal levels (Fig. 1A). The repression was not due to reduced Runx2 protein level as shown by Western blotting (Fig. 1A, inset). DHT did not alter Runx2 activity in the presence of the glucocorticoid receptor (GR); neither did dexamethasone (DEX) in the presence of GR or AR (Fig. 1A). Thus, repression of Runx2 by DHT is both receptor and ligand specific.
Figure 1.
AR represses Runx2 independently of its transcriptional activation property. A, COS7 cells were transiently transfected in 96-well plates with the Runx2 reporter 6XOSE2-Luc (firefly luciferase) along with expression vectors for human Runx2, AR, and GR as indicated. Cells were treated for 24 h with DHT, DEX, or vehicle and subjected to luciferase assay. The dotted line indicates the basal level of luciferase activity in the absence of Runx2, which was not affected by any of the receptors or ligands when present individually. Inset, Western blot analysis of Runx2 and AR in corresponding cultures treated with DHT or vehicle. B, Two sets of COS7 cell cultures were transfected in parallel with either the AR reporter MMTV-Luc or the Runx2 reporter 6XOSE-Luc, along with 1 ng each of AR and Runx2 expression plasmids. Cells were treated with the indicated DHT concentrations for 24 h, followed by luciferase assay. DHT concentrations required for 50% Runx2 repression and 50% AR activation were intrapolated from the curves as shown by the dotted lines. C, Block diagram at the top depicts the AR-NTD, -DBD, and -LBD, as well as the AF1 domain within the NTD and the position of the A573D mutation within the DBD. The two zinc-finger motifs constituting the AR-DBD are indicated by Z1 and Z2. Bar graphs represent luciferase assays, in which the reporters 6XOSE2-Luc (left panel) and MMTV-Luc (right panel), were transfected along with expression plasmids for the WT AR (WT) or a mutant lacking the AF1 domain (ΔAF1). Inset in the right panel represents side-by-side immunoblot analysis of AR and ARΔAF1 using anti-AR antibody. Results in all panels were corrected for expression of the internal control CMV-Renilla luciferase and presented as mean relative light units (RLU) ± sem, with n = 4 dish replicates of a representative experiment, repeated at least three times. Veh, Vehicle.
Because AR is a potent transcription factor, we investigated whether its transactivation function was required for repressing Runx2. First, we took advantage of the fact that ligand was required for both the specific AR-mediated transactivation function and its Runx2 repression property and derived DHT dose-response curves for each function (Fig. 1B). Mouse mammary tumor virus (MMTV)-Luc and 6XOSE2-Luc were used as reporters for monitoring the transactivation and Runx2 repression functions of AR, respectively, with equal amounts of each of AR and Runx2 expression plasmids cotransfected in both assays. As shown in Fig. 1B, the DHT concentration required to achieve 50% Runx2 repression was 10-fold lower than that required for 50% AR transactivation. Thus, AR-mediated Runx2 repression occurs more readily than AR-mediated transactivation, suggesting that Runx2 repression does not require stimulation of direct AR target gene(s).
To further clarify the dissociation between AR’s transactivation and Runx2 repression functions, we tested an AR mutant that lacks the AF1 domain (ARΔAF1) (Fig. 1C, block diagram). DHT-bound ARΔAF1 repressed Runx2 similar to the wild-type (WT) AR (Fig. 1C, left panel) even though it was transcriptionally inactive (Fig. 1C, right panel). These results show that the transactivation function of AR is not required for Runx2 repression.
Physical interaction between AR and Runx2: AR DBD is necessary and sufficient for Runx2 repression
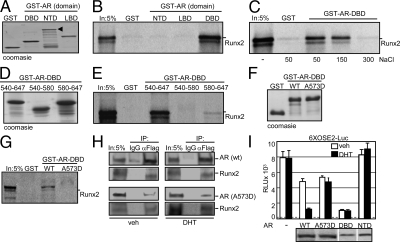
Repression of Runx2 by AR could be mediated by direct interaction between the two proteins as previously observed in glutathione-S-transferase (GST) pull-down assays (55). To determine which part of AR participates in the interaction with Runx2, we performed GST pull-down assays using the three major domains of AR (Fig. 1C, block diagram) as baits. The prey was in vitro transcribed and translated full-length Runx2. Of the three baits (Fig. 2A), Runx2 bound only the AR-DBD (Fig. 2B). Increasing concentrations of NaCl inhibited the AR-DBD/Runx2 binding (Fig. 2C), suggesting that the interaction was of ionic nature. Next we examined the contribution of the two zinc fingers constituting the AR-DBD to the interaction. GST-tagged fragments containing the individual zinc fingers, amino acid (aa) residues 540-580 and 580-647 (Fig. 2D), were tested for binding to Runx2. Unlike the intact AR-DBD (aa 540-647), none of these fragments significantly bound Runx2 (Fig. 2E), indicating that structural integrity of the whole AR-DBD is crucial for the interaction. We further substantiated specificity of our results by using an AR-DBD mutant, in which a highly conserved alanine residue within the first zinc finger (position 573) is replaced by aspartic acid (AR-DBDA573D). This mutation disrupts the AR DNA-binding function and causes Reifenstein Syndrome. In contrast to its WT counterpart, GST-AR-DBDA573D (Fig. 2F) failed to pull down Runx2 (Fig. 2G), corroborating our conclusion that an intact AR-DBD is mandatory for binding Runx2. Furthermore, coimmunoprecipitation (co-IP) of ARA573D with Runx2 was much less efficient than that of WT AR (Fig. 2H). Finally, given the extreme specificity of the AR-DBD/Runx2 interaction, we investigated whether the A573D mutation abrogated Runx2 repression in transient transfection assays. As shown in Fig. 2I, ARA573D did not mediate the repression of Runx2 as observed with the WT receptor. These results implicate the physical interaction between AR-DBD and Runx2 (Fig. 2B) in AR-mediated Runx2 repression (Fig. 1A).
Figure 2.
DBD of AR mediates its interaction with Runx2. A, GST and the indicated AR-derived GST-fusion proteins were expressed and purified from E. coli, and 10 μg of each was subjected to SDS-PAGE and Coomassie blue staining. B, Baits shown in panel A were used to pull-down 35S-labled murine Runx2 produced using reticulocyte lysates. SDS-PAGE and autoradiography show the exclusive interaction between GST-AR-DBD and Runx2. C, 35S-labeled Runx2 was pulled down using GST and GST-AR-DBD as baits in the presence of the indicated millimolar concentrations of NaCl. D, Coomassie blue-stained SDS-PAGE of intact or the indicated fragments of AR-DBD fused to GST. E, The baits shown in D were used to pull-down 35S-labeled Runx2. SDS-PAGE and autoradiography show that only the intact AR-DBD bound Runx2. F, Coomassie blue-stained SDS-PAGE showing WT and mutant AR-DBD used as GST-fusion baits to pull-down 35S-labeled Runx2. G, GST pull-down assay using the baits shown in F and 35S-labeled Runx2. AR-DBDA573D did not bind Runx2. In each pull-down assay, 5% of the input (In) prey is shown for reference. H, Whole-cell extracts from COS7 cells coexpressing Flag-Runx2 with either AR or ARA573D were subjected to co-IP assays using anti-Flag or nonspecific (IgG) antibodies followed by Western blot analysis with anti-AR and anti-Flag antibodies. I, Luciferase assay of COS7 cells transiently transfected in 96-well plates with the 6XOSE-Luc reporter and expression vectors for human Runx2 alone or with those encoding WT AR, ARA573D, Flag-AR-DBD, or Flag-AR-NTD as indicated. Below the bar diagram are side-by-side Western blots showing comparable expression of ARA573D and WT AR (N-20 antibody) and of AR-DBD and AR-NTD (α-Flag antibody). The dotted line indicates the background luciferase activity in the absence of Runx2 (mean ± sem, with n = 4 dish replicates of a representative experiment, repeated at least three times). Veh, Vehicle.
Although AR-DBD is sufficient for binding Runx2, it may not be sufficient for executing the repression function. We therefore tested the ability of the AR-DBD to repress Runx2. Remarkably, transiently expressed AR-DBD repressed Runx2 as effectively as the full-length receptor (Fig. 2I). As expected, the repression was DHT independent. As control, AR-NTD, which did not bind Runx2 (Fig. 2B), did not influence Runx2 activity (Fig. 2I). Thus, the structurally intact AR-DBD is necessary and sufficient for both binding to and repressing Runx2.
AR colocalizes with and alters the mobility of Runx2 in living cells
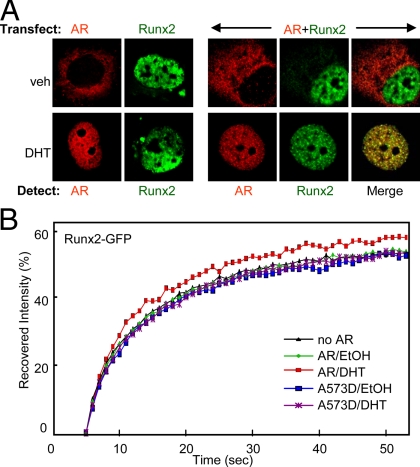
The interaction and behavior of AR and Runx2 in living cells were investigated by confocal microscopy. First, using indirect immunofluorescence, we visualized the intracellular localization of AR and Runx2 that were transiently expressed in COS7 cells in the presence of DHT or vehicle. Whether expressed alone or together, AR was primarily cytoplasmic and became nuclear upon DHT treatment, whereas Runx2 was nuclear irrespective of treatment (Fig. 3A). Merged confocal images showed that the two transcription factors colocalize in the nucleus only after DHT treatment (Fig. 3A), offering a potential explanation for hormone requirement in the functional inhibition assays (Fig. 1A) despite the hormone-independent interaction in cell homogenates (Fig. 2H). The DHT-mediated colocalization was associated with alterations in the patterns of AR and Runx2 subnuclear distribution. Similar to the recent observations of Kawate et al. (53), the AR and Runx2 colocalized in subnuclear structures, which were only observed when the two transcription factors were coexpressed and the cells were treated with DHT (Fig. 3A).
Figure 3.
Colocalization and evidence for interaction between AR and Runx2 in living cells. A, COS7 cells were transfected with the indicated plasmid/s (top), immunostained and analyzed by confocal microscopy to visualize the AR (red) and/or Runx2 (green). B, Runx2-GFP fusion protein was expressed in COS7 cells alone or together with WT AR or ARA573D. The cells were treated with DHT or vehicle and subjected to FRAP analysis. Curves represent fluorescence intensity relative to the respective prephotobleaching levels. veh, Vehicle.
Interaction of transcription factors with nuclear components is often linked to their intranuclear mobility (56,57). We employed fluorescence recovery after photobleaching (FRAP) to examine the mobility of Runx2 in response to AR activation. COS7 cells expressing Runx2-green fluorescent protein (GFP), alone or together with AR, were treated with either DHT or vehicle, and a small nuclear section was briefly photobleached, followed by measurements of the fluorescence recovery over time. As shown in Fig. 3B, the Runx2-GFP repopulated the photobleached section faster in the presence of DHT-bound AR as compared with Runx2-GFP alone or in the presence of unliganded AR. Furthermore, the ARA573D mutant, whose interaction with Runx2 is impaired (Fig. 2, G and H), did not significantly influence the mobility of Runx2-GFP (Fig. 3B). These results provide further evidence for in vivo interaction between AR and Runx2 and suggest that AR competes out another, relatively immobile nuclear component that otherwise engages Runx2 (see Discussion).
AR interacts with and inhibits Runx2 in PCa cells
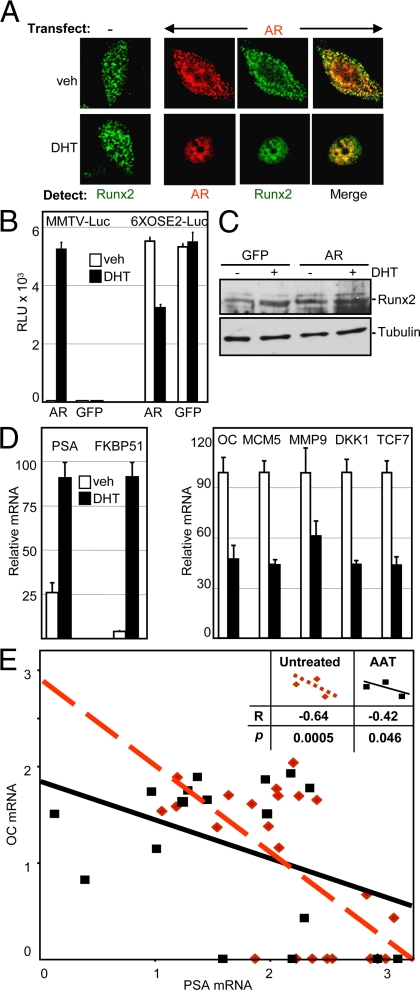
Runx2, which is implicated mostly in osteoblast differentiation, is also expressed in PCa cells (58). Because Runx proteins, including Runx2, possess tumor suppressor properties (12,13), inhibition of Runx2 by AR may play a role in prostate carcinogenesis. We chose to study the interaction between these two transcription factors in the PC3 PCa cell line, which exhibited the highest Runx2 activity among several PCa cell lines tested (data not shown). The AR, which is not expressed in these cells (59), was initially introduced by transient transfection and visualized along with the endogenous Runx2 using immunofluorescence confocal microscopy. In contrast to COS7 cells, where transiently expressed Runx2 was nuclear localized (Fig. 3A), the endogenous Runx2 in PC3 cells was nucleocytoplasmic (Fig. 4A). Without DHT, transiently expressed AR displayed a similar nucleocytoplasmic localization. Remarkably, treatment with DHT led to nuclear localization of not only AR, but also of Runx2 (Fig. 4A). Because DHT did not influence Runx2 localization in the absence of AR, it is likely that the physical interaction between the two proteins led to the DHT-induced nuclear localization of Runx2. Furthermore, merged images revealed a strong colocalization of AR and Runx2 in PC3 cells (Fig. 4A) and, similar to the co-IP results from COS7 cells, the two proteins were coimmunoprecipitated from PC3 cell homogenates (data not shown). These results suggest that AR interacts with and inhibits Runx2 activity in PCa cells.
Figure 4.
AR inhibits Runx2 in PCa cells. A, PC3 cells were transfected with AR-encoding or control vectors, fixed, immunostained, and analyzed by confocal microscopy to visualize the AR (red) and/or the endogenous Runx2 (green). B, PC3 cells that had been stably transduced with lentiviruses encoding AR or GFP as control were transiently transfected with the MMTV-Luc or 6XOSE2-Luc reporters, followed by DHT treatment and luciferase assay (mean ± sem, n = 4). C, Western analyses of cell extracts as in panel B showing the levels of Runx2 and tubulin (loading control). D, PC3-AR cells were treated with DHT or vehicle and subjected to RT-qPCR analyses of the indicated AR (left) and Runx2 target genes (right). Data were corrected for glyceraldehydes-3-phosphate dehydrogenase mRNA, which itself did not significantly change in response to DHT. The highest mean value for each gene was set at 100 (mean ± sem; n = 3). E, Primary prostate cancer tumors resected from 23 untreated patients (red diamonds) and 17 patients undergoing AAT (black squares) were previously subjected to comprehensive gene expression analysis (61). The negative correlation between OC and PSA mRNA levels in these tumors is demonstrated by best-fit linear regression of the respective values from the untreated patients (dashed line) and those undergoing AAT (solid line). The calculated r values and the levels of statistical significance assigned by the Wilcoxon signed-rank test are depicted in the inset. Veh, Vehicle.
To study the influence of AR on Runx2 activity and on endogenous Runx2 target genes in PCa cells, AR was first stably introduced into PC3 cells by lentiviral infection. The transduced cells, designated PC3-AR, presumably resemble PCa cells that endogenously express both Runx2 and WT AR. These cells were transiently transfected with the MMTV-Luc or the 6XOSE2-Luc reporter plasmids. PC3 cells, transduced with a GFP-encoding lentiviral, were used as control (PC3-GFP). As shown in Fig. 4B, PC3-AR, but not PC3-GFP, cells showed robust MMTV-Luc activity in response to DHT, indicating expression of functional AR (60). The 6XOSE2-Luc Runx2 reporter was strongly expressed in both PC3-GFP and PC3-AR cells, indicating the presence of endogenous functional Runx2. As expected from our studies in COS7 cells, DHT treatment repressed the 6XOSE2-Luc reporter in PC3-AR (but not in PC3-GFP cells), which was not due to any decrease in Runx2 protein levels (Fig. 4, B and C). To test whether our plasmid-based reporter assays faithfully represented the activity of Runx2 and AR in the context of native chromatin, we analyzed the effect of DHT on the expression of their respective target genes in PC3-AR cells. RT-quantitative PCR (qPCR) analyses showed that DHT treatment strongly activated AR as assessed by the up-regulation of PSA and FKBP51 transcripts (Fig. 4D, left panel). As expected from our transient transfection experiments (Fig. 4B), DHT treatment of PC3-AR cells down-regulated the mRNAs encoding Runx2-regulated genes (Fig. 4D, right panel), including OC, MCM5, MMP9, DKK1, and TCF7 (17,18). Thus, AR inhibits the expression of plasmid-borne and endogenous Runx2 target genes in PCa cells.
We further investigated the relationship between AR activation and OC expression in a series of 40 PCa primary tumors, 23 from patients undergoing resection of the primary tumor without androgen ablation therapy (AAT), and 17 from patients who had initiated AAT 3 months before tumor resection. These tumors, which provide a wide range of AR activation levels, were previously subjected to comprehensive microarray-based analysis of gene expression (61). From the microarray data, we extracted the values of PSA and OC mRNAs as measures of AR and Runx2 activity, respectively. As shown in Fig. 4E, expression of OC negatively correlated with that of PSA whether or not the patients underwent AAT. A statistically significant negative correlation between PSA and OC expression (r = −0.53; P = 0.002) was also observed when the two groups were analyzed together. These results may be driven, at least in part, by the coordinated AR-mediated transcriptional stimulation and Runx2 repression functions, as demonstrated in the PC3-AR culture model (Fig. 4, A–D). Given the tumor suppressor property of Runx2 (12,13), our results are consistent with the hypothesis that AR-mediated inhibition of Runx2 in prostate epithelial cells contributes to the well-established role of AR in PCa initiation and progression.
Androgens inhibit Runx2 during late osteoblast differentiation
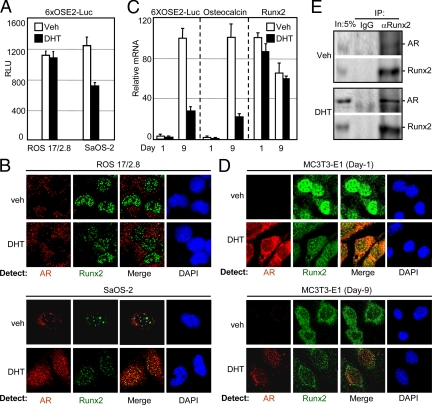
Manipulation of Runx2 in osteoblasts strongly influences bone turnover and bone mass; therefore modulation of Runx2 activity by AR in osteoblasts may explain some of the effects of androgens in bone. To address this, we initially transiently transfected SaOS-2 and ROS 17/2.8 osteosarcoma cells, which express endogenous Runx2 and AR, with the 6XOSE2-Luc reporter, followed by DHT treatment and luciferase assays. As shown in Fig. 5A, DHT inhibited luciferase activity in SaOS-2 but not in ROS 17/2.8 cells. This difference could be explained by the results of our confocal immunofluorescence microscopy studies (Fig. 5B): treatment of SaOS-2 cells with DHT resulted in nuclear translocation of AR and its colocalization with Runx2. In contrast, DHT treatment did not drive the AR to the nucleus in ROS 17/2.8 cells, avoiding colocalization with Runx2 (Fig. 5B). Conceivably, cytoplasmic retention of the AR preserved Runx2 activity in DHT-treated ROS 17/2.8 cells.
Figure 5.
Differential intracellular localization of AR in osteoblastic cell lines correlates with the repression of Runx2. A, ROS 17/2.8 and SaOS-2 cells, which express both AR and Runx2, were transiently transfected with the 6XOSE2-Luc reporter and treated for 24 h with DHT or vehicle, followed by luciferase assay. B, Confocal micrographs of immunostained ROS 17/2.8 and SaOS-2 cells showing the intracellular distribution of AR (red) and Runx2 (green) after 24 h of treatment with DHT or vehicle. C, Differentiating MC3T3E-1 cells stably transfected with the 6XOSE2-Luc reporter were treated with DHT or vehicle commencing at confluence (d 0), and levels of the indicated mRNAs were measured on d 1 and d 9. Data were corrected for ribosomal protein L10A mRNA, which itself did not significantly change in response to DHT (mean ± sem; n = 3). D, d 1 and d 9 MC3T3E-1 cultures were treated for 24 h with DHT or vehicle, and subjected to confocal microscopy for visualization of AR (red) and Runx2 (green) after immunostaining with the respective antibodies. Staining with 4′,6-diamidino-2-phenylindole (DAPI) demarcates the cell nucleus. E, Co-IP assay of d 9 MC3T3-E1 cells that were treated for 24 h with DHT or vehicle. Immunoprecipitates were obtained using Runx2-specific or nonspecific IgG antibodies and were subjected, along with 5% of the input, to Western blot analysis for the detection of AR and Runx2. Data are representative of at least three independent experiments. IP, Immunoprecipitation; veh, vehicle.
To test whether AR inhibits Runx2 in nontumorigenic osteoblasts, we turned to the MC3T3-E1 preosteoblast-like cell line, which also expresses both Runx2 and AR. A subline that had been stably transfected with the 6XOSE2-Luc reporter (51) was employed to simultaneously test the influence of AR exclusively on Runx2 activity (luciferase) and on OC gene expression. The cells were treated with DHT for 24 h commencing at confluence (d 0) and subjected on d 1 and d 9 to RT-qPCR analysis of the mRNAs encoding luciferase and OC. As shown in Fig. 5C, expression of both 6XOSE2-Luc and OC was very low in the early cultures (d 1) and dramatically increased in late cultures (d 9). On d 9, expression of both 6XOSE2-Luc and OC was strongly inhibited by DHT, whether introduced chronically since confluence (Fig. 5C) or for only 24 h before harvest (data not shown). Thus, AR inhibits Runx2 in osteoblasts during late stages of their developmental program.
Immunofluorescence analysis of d 1 and d 9 MC3T3-E1 cultures provided interesting insights into the expression and localization of AR and Runx2 in response to DHT. First, similar to previous observations in osteoblasts (62,63), DHT strongly induced AR protein levels in both the early and the late cultures (Fig. 5D). Second, unlike the classical paradigm, AR remained primarily cytoplasmic in early cultures even after the DHT treatment. The more conventional AR behavior, i.e. nuclear localization in the presence of DHT, was observed only in the late cultures (Fig. 5D). The intracellular distribution of Runx2 also strongly depended on culture maturity. Without DHT treatment, most of the Runx2 on d 1 was nuclear and was found in numerous subnuclear foci. On d 9, Runx2 was more evenly distributed and occupied lesser subnuclear foci (Fig. 5D). Treatment of the early cultures with DHT resulted in redistribution of Runx2, a larger fraction of which became cytoplasmic and strongly colocalized with the AR (Fig. 5D). In the late cultures, Runx2 colocalized with AR within the nucleus (Fig. 5D).
Finally, we examined the interaction between AR and Runx2 in co-IP assays of d 9 MC3T3-E1 cultures. Runx2 was immunoprecipitated and the complexes were subjected to Western blot analysis. As shown in Fig. 5E, AR was only present in immunoprecipitates obtained using Runx2 antibodies but not in those obtained using nonspecific IgG. Similar to the results with COS7 (Fig. 2H) and PC3 cells (data not shown), the interaction did not require hormone treatment in the co-IP assay. Thus, DHT inhibits Runx2 during late stages of osteoblast differentiation most likely via nuclear translocation of the AR that leads to direct interaction with Runx2 (also see Fig. 2).
Runx2’s PST and Runt domains are necessary for interaction with AR
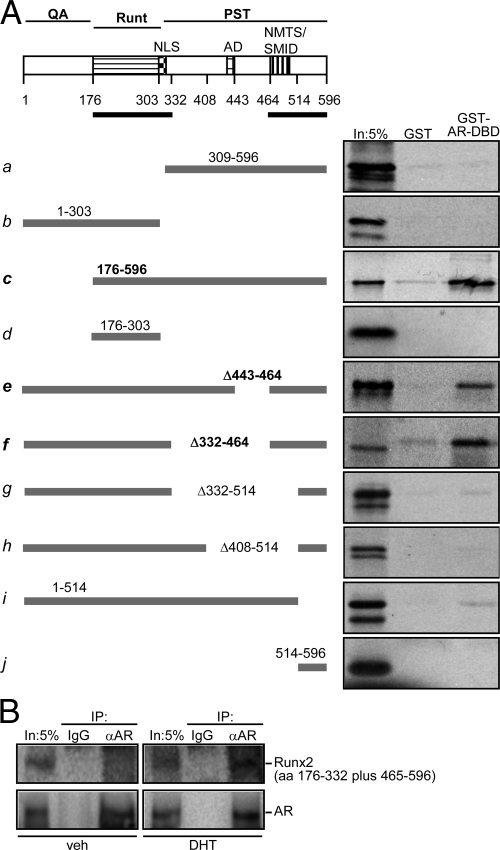
To further dissect the mechanism by which AR inhibits Runx2’s transcriptional activity, we first employed GST pull-down assays to map the Runx2 sequences that mediate the interaction with the AR. AR-DBD, which is this receptor’s domain that contacts Runx2 (Fig. 2B), was used as bait, and the Runx2 fragments illustrated in Fig. 6A were synthesized in vitro as 35S-labeled preys. Because the interaction between Runx2 and ERα was recently mapped to the ERα-DBD and Runx2’s PST domain (51), and given the high similarity between AR-DBD and ERα-DBD, we first tested whether Runx2’s PST domain interacted with AR-DBD. Surprisingly, GST-AR-DBD failed to pull down Runx2’s PST domain (Fig. 6A-a). Similarly, no binding was observed with a fragment representing the QA and Runt domains of Runx2 (Fig. 6A-b). However, a Runx2 fragment containing both the PST and the Runt domains effectively recapitulated the interaction observed with full-length Runx2 (Fig. 6A-c). Like the PST domain, the Runt domain alone also did not bind AR-DBD (Fig. 6A-d). Thus, both the Runt and the PST domains were necessary, but neither one alone was sufficient for the interaction with AR-DBD.
Figure 6.
Mapping of Runx2 sequences required for binding AR-DBD. A, Shown at the top is a block diagram of Runx2 with the glutamine alanine (QA), Runt, and PST domains. Depicted within the PST domain are the nuclear localization signal (NLS), activation domain (AD), and the overlapping NMTS and SMID. The black thick line just below the block diagram represents the minimal Runx2 sequences required for binding AR-DBD as defined in the present study. Thick gray lines below represent a series of 35S-labeled Runx2 fragments used as preys in pull-down assays with GST or GST-AR-DBD as baits. B, Western blot analyses of immunoprecipitates prepared using AR-specific or nonspecific (IgG) antibodies and whole-cell extracts from COS7 cells coexpressing AR and Runx2’s bipartite interaction domain (aa 176–332 and 465–576). In, Input; IP, immunoprecipitation.
To delineate the minimal PST sequence necessary for the interaction with AR-DBD, we tested a series of internal deletions in the PST domain (Fig. 6A-e to -h). Binding was detectable with mutant Δ443-464 (Fig. 6A-e), which retained the activation domain (AD) and the NMTS/SMID domain. Strong interaction was also observed with mutant Δ332-464 despite the absence of AD (Fig. 6A-f). However, the mutants Δ332-514 and Δ408-514 (Fig. 6A-g and -h), which lacked the NMTS/SMID domain did not bind the AR-DBD. Finally, the deletion mutant 1-514 (Fig. 6A-i), which retained both the Runt and the NMTS/SMID domains, but lacked the very C-terminal 82 aa residues of Runx2, showed almost no binding to AR-DBD, suggesting that the 82 C-terminal aa residues are critical for interaction with the AR-DBD. However, this 82-aa fragment did not bind to AR-DBD on its own (Fig. 6A-j). Thus, the Runt domain and the C-terminal part of the PST domain (aa 464-596, from the NMTS/SMID to the C terminus) together represent the minimal Runx2 sequence necessary for binding the AR-DBD (Fig. 6A, black lines under the block diagram). We cloned this bipartite binding sequence, representing aa 176-332 and 465-576, and expressed it in COS7 cells along with AR, followed by Co-IP assay. Western blot analyses of the AR immunoprecipitates confirmed the presence of the Runx2 bipartite interaction domain (Fig. 6B).
AR displaces Runx2 from its OSE2 target site at the OC promoter
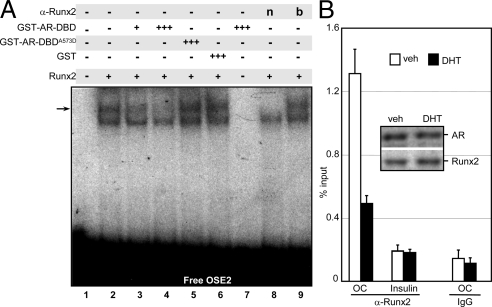
Because Runx2’s Runt domain is necessary for both DNA binding (64) and interaction with the AR-DBD (Fig. 6A), we hypothesized that the AR-DBD prevents Runx2 from binding DNA, accounting for its repression activity. This was initially tested by examining the effect of AR-DBD on the binding of Runx2 to DNA in EMSA with a 32P-labeled OSE2 probe. The source of Runx2 was MC3T3-E1 cell extract, and recombinant GST-AR-DBD was purified using Escherichia coli. Of the two complexes formed, the slow-migrating complex contained Runx2, as it was sensitive to the presence of Runx2 antibodies (Fig. 7A, lane 2 vs. 8), but not to the same antibodies that were denaturated by boiling (Fig. 7A, lane 9). Formation of this Runx2/OSE2 complex was progressively inhibited by increasing amounts of GST-AR-DBD (Fig. 7A, lanes 2–4), but not by GST alone (Fig. 7A, lane 6). GST-AR-DBD itself did not form any complex with the OSE2 probe (Fig. 7A, lane 7), suggesting that it did not inhibit the Runx2/DNA interaction by competing for a common binding site. Unlike GST-AR-DBD, GST-AR-DBDA573D, the interaction of which with Runx2 is impaired (Fig. 2, G and H), failed to inhibit the Runx2/DNA complex formation (Fig. 7A, lane 5). These results strongly suggest competition for the Runt domain as the mechanism underlying AR-mediated repression of Runx2 activity.
Figure 7.
AR-DBD diminishes Runx2’s interaction with its OSE2 target in vitro and in vivo. A, EMSA was performed using 32P-labeled OSE2 probe and MC3T3-E1 whole-cell extracts as the source of Runx2. Where indicated, the binding reaction also contained purified GST, GST-AR-DBD, GST-AR-DBDA573D (see Fig. 2F), or Runx2 specific antibodies, either native (n) or denaturated by boiling (b). The relative amounts of the indicated proteins added to the binding reaction are depicted by + or +++ (see Materials and Methods). Arrow indicates Runx2/OSE2 complex. B, Differentiating d 9 MC3T3E-1 cultures were treated for 4 h with DHT or vehicle and subjected to ChIP assay using anti-Runx2 or nonspecific IgG antibodies. Occupancy was quantified by qPCR of an OC promoter fragment containing the OSE2 site or a fragment of the insulin promoter as control. veh, Vehicle.
To test whether the DNA-binding exclusion model operates in vivo, we investigated whether AR interferes with binding of Runx2 to its chromatin-embedded OSE2 element at the OC promoter in MC3T3-E1 cells. Day 9 cultures, in which Runx2 occupancy at this site is maximal (34), were treated for 4 h with DHT and subjected to Runx2 chromatin coimmunoprecipitation (ChIP) assay. As shown in Fig. 7B, Runx2 occupancy of the endogenous OSE2 site decreased by 2.4-fold in response to DHT treatment. The inhibition of Runx2 occupancy occurred without any significant change in Runx2 protein levels (Fig. 7B, inset). Altogether, the GST pull-down, the EMSA, the immunofluorescence analyses, and the ChIP data suggest that AR binding to Runx2 abrogates its recruitment to target gene promoters. The mutually exclusive interaction of Runx2 with either DNA or AR could account for its repression by androgens.
Discussion
In addition to its physiological functions in the male reproductive system, the AR has important regulatory roles in prostate cancer and bone metabolism. AR’s function has been primarily attributed to transcriptional stimulation of target genes via androgen response elements located in their promoters and enhancers. The strong inhibition of Runx2 by AR, dissected in the present study, may constitute an additional significant mechanism through which androgens execute their functions. Consistent with our in vitro observations, in vivo expression of OC, a classical Runx2 target gene, was inhibited in mice overexpressing AR in osteoblasts (65) and in testosterone-repleted vs. experimentally-hypogonadal men (44), and OC expression was stimulated in AR knockout mice (46). In our studies, the inhibition of Runx2 activity was observed in a variety of cell lines, irrespective of whether the target was a transiently transfected reporter plasmid, a stably integrated reporter, or endogenous target genes. The inhibition of Runx2 did not require AR-induced transcription. Three lines of evidence support this conclusion: 1) the inhibition occurred at concentrations an order of magnitude lower than those inducing transcription; 2) the inhibition was observed with the transcriptionally inactive ARΔAF1 mutant; and 3) the AR-DBD alone, which is the receptor’s domain that interacts with Runx2, was sufficient for inhibition.
None of Runx2’s three major domains is sufficient for interaction with the AR. Instead, both the Runt domain and aa residues 464-596 within the PST domain together were necessary for binding AR. The involvement of the Runt domain raised a plausible mechanism, whereby AR prohibits Runx2 from binding DNA. Evidence in support of such a simple mechanism was provided by EMSA and ChIP assays, where the presence of recombinant AR-DBD or treatment with DHT disrupted Runx2 DNA-binding activity and its genomic recruitment, respectively. Interestingly, the Runx2 domains required for interaction with AR also serve as docking sites for other nuclear components. For example, the Runt domain interacts with Cbfβ (24), and aa residues 464-596 encompass binding sites for the nuclear matrix and numerous coregulatory proteins including SMADs (28) and TLE/Groucho (66). We speculate that these nuclear components may influence the ability of AR to associate with Runx2 and may coordinate between androgen signaling and other pathways that impinge on Runx2. Additionally, posttranslational modifications of the Runt domain or the 464-596 region, e.g. by MAPK (37) may disrupt Runx2’s interaction with and repression by the AR.
Similar to the AR, ERα as well as GR and the vitamin D receptor (VDR) interact with and modulate Runx2 activity (51,55,67). The receptor’s interaction domain was pursued for AR, ERα, and VDR, and was mapped to the zinc finger-containing DBD in each of these cases. The A573D point mutation in AR-DBD and the S51G mutation in VDR-DBD (67), which compromise their respective structural integrity, also prevent their binding to Runx2. Interestingly, despite being highly homologous, each receptor’s DBD recognized a distinct part of Runx2. This is possibly related to the different biological roles assigned for each of the interactions. For example, ERα and AR repress, whereas VDR activates, Runx2 activity (51,67).
The inhibition of Runx2 by AR is potentially important in many aspects of human health and disease, including PCa and bone biology. Like the other two members of the Runt family, Runx2 is implicated in the control of cellular carcinogenesis and plays a role as either tumor suppressor or enhancer in a context-dependent manner (13,68). Conceivably, the pivotal role of AR in PCa progression is mediated, in part, by counteracting the tumor suppressor activity of Runx2 in prostate epithelial cells. The interpretation of AR-mediated Runx2 repression is more difficult in the context of advanced disease, where Runx2 could promote the metastatic phenotype (69). The fact that virtually all PCa tumors retain AR expression suggest that metastatic PCa is less dependent on Runx2 than ER-negative breast cancer metastases, the unopposed Runx2 of which likely promotes the aggressive bone-destructive behavior of these tumors. Alternatively, anti-AR Runx2-protective mechanisms may evolve in advanced PCa, possibly by subcellular or subnuclear compartmentalization of the two transcription factors. The development of new selective AR modulators for the management of PCa and other conditions should take into consideration their influence on Runx2 activity. Unlike their antiandrogen property with respect to AR-stimulated transcription, hydroxyflutamide and casodex mimicked DHT with respect to Runx2 repression (supplemental Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org). It would be interesting to examine the biological activities of future selective AR modulators, which would antagonize transactivation by AR without repressing Runx2.
Because Runx2 knockout experiments established this protein as a master transcription factor required for osteoblast differentiation (1,2), the inhibition of Runx2 by estrogens (51) and androgens (Ref.53 and the present work) may come as a surprise considering the strong protective effect of gonadal steroids on the skeleton (70). A solution for this conundrum is offered by work from two groups, which manipulated Runx2 using other approaches (3,6,7,8). In each of three transgenic lines overexpressing Runx2 in osteoblasts, Matthias and colleagues (7) observed severe bone loss and spontaneous fractures associated with up to 10-fold increased endosteal osteoclast surface. Furthermore, using coculture assays, this group demonstrated that Runx2-overexpressing osteoblasts isolated from the transgenic mice promoted supraphysiological osteoclastogenesis. In line with these results, Komori and colleagues (71) showed that calvarial cells from Runx2-deficient mice fail to support osteoclast differentiation, unless Runx2 is virally restored. More recently, this group transduced normal calvarial osteoblasts with adenoviruses encoding WT or dominant-negative Runx2, which resulted, respectively, in stimulated or repressed differentiation of cocultured osteoclasts (8). These in vitro results lead to the same conclusion as that of Matthias and colleagues (7): overexpression of Runx2 in osteoblasts leads to exaggerated osteocalstogenic signal. In fact, Komori and colleagues (6) were the first to report on increased osteoclast activity at the endosteal surface of transgenic mice overexpressing Runx2 in osteoblasts. Although this early study focused on the defective bone formation, the studies of Runx2 manipulation in vitro and in vivo altogether assign a clear role for Runx2 in regulating osteoblast-derived signals to osteoclasts. Based on these studies, and considering the inhibition of Runx2 by estrogens and androgens, we propose that the proskeletal function of gonadal steroids is executed, in part, by keeping Runx2 in check, thereby restraining osteoblast-mediated osteoclastogenesis. Indeed, increased bone resorption is a hallmark of bone loss that follows sex steroid withdrawal (70).
Osteoporosis observed in men with mutations in the ERα or aromatase genes demonstrate that much of the proskeletal effects of androgens in males are mediated by aromatization to estrogens, which then activate ERα (72,73,74). However, the obvious sexual dimorphism of the skeleton suggests an aromatization-independent but AR-dependent role for androgens. The extent of AR vs. ER-dependent contribution of androgens to men’s bone health is a matter of debate (44,75). Studies with AR knockout mice, however, suggest that the two are of approximately equal importance, and that the role of AR is primarily executed by attenuating bone turnover (46). The mechanism mediating this effect of AR in bone is unknown but may very well involve the inhibition of Runx2 as described in the present paper. That androgens protect the skeleton beyond the effects attributable to aromatization may be related to Runx2-independent mechanisms or to different mechanisms employed by AR vs. ERα for interaction with, and inhibition of, Runx2. In this regard, we note that the Runt domain is absolutely necessary for interaction with the AR (present paper), but not with ERα. There is also a clear difference between the sequences within the PST domain that are necessary for Runx2’s interaction with each receptor. For example, the 82 C-terminal aa residues of Runx2 are necessary for interaction with AR but not with ERα (51). Additional observations suggesting a mechanistic difference between AR- and ERα-mediated inhibition of Runx2 include: 1) AR binds to Runx2 stronger than ERα in GST pull-down assays (data not shown); and 2) ERα, but not AR, requires ligand for interaction with Runx2 in co-IP assays.
In summary, we documented inhibition of Runx2 by AR in a variety of cell lines derived from PCa and bone. The inhibition does not require AR transactivation activity, but rather requires direct contact between the two transcription factors, which disrupts Runx2’s DNA binding activity. Inhibition of Runx2 by androgens may constitute a mechanism of hormone carcinogenesis in the initiation of PCa. In bone, it may be critical for limiting turnover, thereby preventing osteoporosis. However, manipulation of Runx2 as a potential therapeutic strategy for either PCa or osteoporosis will be challenging. In the skeleton, the beneficial outcome of inhibiting Runx2, i.e. restrained bone turnover, might be associated with compromised bone formation. In PCa, AR blockade may be beneficial in inhibiting the expression of AR target oncogenes and in stimulating the expression of Runx2 target genes that mediate its tumor suppressor activity; however, the same treatment may cause harm by promoting Runx2 target genes that facilitate the metastatic phenotype. Thus, cell type-dependent and developmentally regulated effects of androgens and estrogens on Runx2 activity must be considered when devising selective receptor modulators for the prevention and treatment of osteoporosis, breast cancer, and PCa.
Materials and Methods
Reagents
DHT and DEX were purchased form Sigma-Aldrich (St. Louis, MO), dissolved in ethanol at a 100 μm stock concentration. Unless otherwise stated, these hormones were applied at a final concentration of 10 nm in 0.001% ethanol as the vehicle. Primary antibodies for Runx2 (sc-10758) and for AR (sc-816 for immunofluorescence, otherwise sc-7305/N-20), as well as horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Flag antibodies were from Sigma-Aldrich. Fluorochrome-conjugated secondary antibodies for confocal microscopy were obtained from Pierce Biotechnology (Rockford, IL). Expression vectors for human AR (pcDNA3.1-AR), human GR (pSG5-GR), and the MMTV-Luc AR reporter were previously described (60), as were the 6XOSE2 Runx2 reporter plasmid and the mouse Runx2 expression vector (51). All other expression plasmids were constructed using standard cloning procedures and confirmed by sequencing. Lentiviral plasmid pHRCMV-Hygro-GFP was kindly provided by Dr. M. Stallcup’s laboratory at University of Southern California, and pHRCMV-Hygro-Flag-AR was constructed by replacing the GFP cassette in pHRCMV-Hygro-GFP with a cDNA encoding human Flag-tagged AR (76).
Cell culture and hormone treatment
The mouse osteoblast cell line MC3T3-E1, its derivative with a stably transfected 6XOSE2-Luc reporter (51), and ROS 17/2.8 cells were maintained in α-MEM supplemented with 10% fetal bovine serum (FBS). COS7 and SaOS-2 cells were maintained in DMEM with 5% FBS. PC3 cells were originally obtained from American Type Culture Collection (Manassas, VA), and a subline described earlier (59) was employed in the present study and maintained in RPMI-1640 and 5% FBS. All cells were incubated at 37 C in a humidified 5% CO2 incubator, and the media was changed every 48 h. For hormone treatment, cells were grown in charcoal-stripped sera (CSS).
Lentiviral particle production and transduction
For packaging, the lentiviral expression plasmids were cotransfected into HEK293T cells along with helper plasmids pMD.G1 and pCMVΔR8.91 (77). Culture media containing viral particles were harvested after 48–72 h and used for transduction of PC3 cells in the presence of 8 μg/ml Polybrene (Millipore Corp., Bedford, MA). After infection with GFP or Flag-AR-expressing lentiviruses, the transduced cells were selected with 50 μg/ml Hygromycin (Invitrogen, Carlsbad, CA).
Transient transfection, luciferase, co-IP, and ChIP
These assays were performed essentially as previously described (34,51). A slight modification was introduced for immunoprecipitation using MC3T3-E1 and PC3-AR cells. These cells were plated in 150-mm dishes in phenol red-free medium supplemented with 5% CSS. The MC3T3-E1 cell culture medium was further supplemented, commencing at confluence, with 50 μg/ml ascorbic acid and 150 mm β-glycerolphosphate.
Quantitative real-time RT-PCR
Total RNA was isolated using Aurum Total RNA kit (Bio-Rad Laboratories, Inc., Hercules, CA) following the manufacturer’s recommendations. Total RNA (1 μg) was reverse transcribed using the Superscript III kit (Invitrogen), and the cDNA was subjected to real-time PCR amplification using iQ SYBR Green Supermix (Bio-Rad). The sequences of primers for real-time PCR amplification are listed in supplemental Table S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org.
GST pull-down assay
Bacterial expression constructs for all GST pull-down bait proteins were derived from pGEX-4T-1 (Amersham Biosciences, Freiburg, Germany) by in-frame fusion of cDNA fragments encoding the indicated protein domains. GST-fusion proteins were overexpressed in E. coli BL21(DE3) cells (Stratagene, Amsterdam, The Netherlands) and purified with the GST purification module (Amersham Biosciences) according to the manufacturer’s protocol. 35S-labeled Runx2 and fragments thereof was produced using TNT T7 Quick Kit from Promega Corp. (Madison, WI) according to the manufacturer’s protocol. The pull-down assay was conducted in interaction buffer containing 20 mm Tris (pH 7.5), 100 mm NaCl (unless otherwise stated), 5 mm MgCl2, 0.01% Nonidet P-40, and Complete protease inhibitor mix from Roche Diagnostics (Indianapolis, IN) (78). Each bait (10 μg) was immobilized on glutathione-S-sepharose beads and then incubated with the indicated 35S-labeled Runx2 or its fragments. The beads were washed five times with the interaction buffer, and the bound Runx2 fragments were eluted with 1× sample buffer and analyzed by SDS-PAGE and autoradiography along with 5% input.
Confocal immunofluorescence microscopy
Cells were grown on 18-mm2 coverslips in six-well plates for 24 h in growth medium with CSS. After transfection and hormone treatment, the cells were fixed with 95% methanol for 15 min and incubated with AR or Runx2 antibodies (1:500) followed by a rhodamine- or fluorescein-conjugated secondary antibody, respectively. Cells were mounted using Vectashield Hard Set mounting medium with 4′,6-diamidino-2-phenylindole (Burlingame, CA) and viewed using an LSM 510 Zeiss confocal microscope (Carl Zeiss, Thornwood, NY) at ×60 magnification. Images were processed using the software program Image J.
FRAP
COS7 cells transiently expressing Runx2-GFP were grown in media with CSS. DHT or vehicle was added to the growth media 1 h before the FRAP experiment. Cells were viewed using an LSM 510 Zeiss confocal microscope at ×60 magnification. A round nuclear section measuring 3.5 μm in diameter was monitored at a wavelength of 488 nm at 4% intensity for 15 sec before a 5-sec photobleaching with an 80% laser power. Images were subsequently captured each second for 1 min at 488 nm (4% intensity) to assess the fluorescence recovery. All data obtained were normalized to the average starting intensity, and data were corrected for loss of background fluorescence. Images were processed using the software program Image J.
EMSA
32P-labeled double-stranded oligonucleotide probe containing the OSE2 site (supplemental Table 1) was prepared as previously described (79) and 100 fmol were incubated on ice for 10 min with 15 μg of MC3T3-E1 whole-cell extract. The incubation buffer contained 20 mm HEPES (pH 7.5), 50 mm KCl, 1 mm MgCl2, 2 mm EDTA, 2.8% glycerol, and 1 μg salmon sperm DNA. Recombinant purified proteins (2.5 or 7.5 μg) and Runx2 antibodies (4 μg) were included in the binding reaction as indicated. Protein-DNA complexes were resolved by PAGE in 6% native gels containing 5% glycerol and 0.25× Tris-borate-EDTA buffer.
Supplementary Material
Acknowledgments
We thank Dr. W. L. Gerald and Dr. H. I. Scher (Memorial Sloan Kettering Cancer Center, New York, NY) for providing clinical expression microarray data; Dr. A. B. Houtsmuller (Erasmus University Medical Centre, Rotterdam, The Netherlands), Dr. R. J. Matusik (Vanderbilt Prostate Cancer Center, Nashville, TN), Dr. E. F. Need (University of Adelaide, Adelaide, Australia), Dr. G. Karsenty (Columbia University, New York, NY), and Dr. J. Westendorf (Mayo Clinic, Rochester, MN) for plasmids. We also thank our colleagues at University of Southern California: Dr. M. Stallcup for reagents; Drs. M. Chen and Y. P. Hou for expertise with lentiviral transduction; members of the Frenkel and Coetzee laboratories for helpful suggestions; and J. Cogan and Y. Shi for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants DK071122 and AR047052 (to B.F.) and CA109147 (to G.A.C.); by W81XWH-05-1-0025 (to B.F.) and W81XWH-07-1-0067 predoctoral grant (to O.K.) from the Department of Defense; by YI02 (to G.B.) from the Prostate Cancer Foundation of Australia; and by awards from the Prostate Cancer Foundation (to G.A.C.). S.K.B was supported by California Community Foundation Grant from the Arthritis Foundation Southern California Chapter. G.B. holds a National Health and Medical Research Council of Australia C.J. Martin Biomedical Fellowship. B.F. is the holder of the J. Harold and Edna L. LaBriola Chair in Genetic Orthopaedic Research at University of Southern California. The experiments were conducted in facilities supported by NIH/National Cancer Institute Grant P30 CA 014089-30 and constructed with support from Research Facilities Improvement Program Grant C06 (RR10600-01, CA62528-01, and RR14514-01) from the NIH/National Center for Research Resources.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 23, 2009
Abbreviations: aa, Amino acid; AAT, androgen ablation therapy; AF1, activation function 1; AR, androgen receptor; ChIP, chromatin immunoprecipitation; co-IP, coimmunoprecipitation; CSS, charcoal-stripped sera; DBD, DNA-binding domain; DEX, dexamethasone; FBS, fetal bovine serum; FRAP, fluorescence recovery after photobleaching; GFP, green fluorescent protein; GR, glucocorticoid receptor; GST, glutathione-S-transferase; LBD, ligand-binding domain; MMTV, mouse mammary tumor virus; NMTS, nuclear matrix targeting signal; NTD, N-terminal domain; OC, osteocalcin; OSE2, osteoblast specific element 2; PCa, prostate cancer; PSA, prostate-specific antigen; PST, proline-serine-threonine; SMAD, Sma- and Mad- related protein; SMID, SMAD interaction domain; VDR, vitamin D receptor; WT, wild type.
References
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T 1997 Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ 1997 Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T 2000 Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem 275:8695–8702 [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Komori T 2005 Role of Runx proteins in chondrogenesis. Crit Rev Eukaryot Gene Expr 15:243–254 [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G 1997 Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T 2001 Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 155:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P 2002 High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol 22:6222–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Z, Yoshida CA, Furuichi T, Amizuka N, Ito M, Fukuyama R, Miyazaki T, Kitaura H, Nakamura K, Fujita T, Kanatani N, Moriishi T, Yamana K, Liu W, Kawaguchi H, Nakamura K, Komori T 2007 Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn 236:1876–1890 [DOI] [PubMed] [Google Scholar]
- Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J 2008 Haploinsufficiency of Runx2 results in bone formation decrease and different BSP expression pattern changes in two transgenic mouse models. J Cell Physiol 217:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, Stein GS, Gerstenfeld LC 2004 Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res 64:4506–4513 [DOI] [PubMed] [Google Scholar]
- Javed A, Barnes GL, Pratap J, Antkowiak T, Gerstenfeld LC, van Wijnen AJ, Stein JL, Lian JB, Stein GS 2005 Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci USA 102:1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth K, Cameron ER, Neil JC 2005 The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer 5:376–387 [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Pande S, Pratap J, Gaur T, Grigoriu S, Ali SA, Stein JL, Lian JB, van Wijnen AJ, Stein GS 2007 Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc Natl Acad Sci USA 104:19861–19866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB 1997 Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem 66:1–8 [DOI] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G 1996 Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452 [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G 2007 Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes BL, Ducy P, Sijbers AM, Hendriks JM, van Someren EP, de Jong NG, van den Heuvel ER, Olijve W, van Zoelen EJ, Dechering KJ 2006 Microarray analysis on Runx2-deficient mouse embryos reveals novel Runx2 functions and target genes during intramembranous and endochondral bone formation. Bone 39:724–738 [DOI] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, Zwingman C, Kidess A, Stricker S, Mundlos S 2007 Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2(−/−) mouse model. Gene Expr Patterns 7:102–112 [DOI] [PubMed] [Google Scholar]
- Barski A, Frenkel B 2004 ChIP display: novel method for identification of genomic targets of transcription factors. Nucleic Acids Res 32:e104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregizer S, Barski A, Gersbach CA, García AJ, Frenkel B 2007 Identification of novel Runx2 targets in osteoblasts: cell type-specific BMP-dependent regulation of Tram2. J Cell Biochem 102:1458–1471 [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Zaidi SK, Cascino JE, Kahler R, van Wijnen AJ, Lian JB, Yoshida M, Stein GS, Li X 2002 Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol Cell Biol 22:7982–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev RA, Dong YF, Sampson E, Zuscik MJ, Schwarz EM, O'Keefe RJ, Rosier RN, Drissi MH 2008 Runx2-mediated activation of the Bax gene increases osteosarcoma cell sensitivity to apoptosis. Oncogene 27:3605–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder TM, Jensen ED, Westendorf JJ 2005 Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 75:213–225 [DOI] [PubMed] [Google Scholar]
- Kundu M, Javed A, Jeon JP, Horner A, Shum L, Eckhaus M, Muenke M, Lian JB, Yang Y, Nuckolls GH, Stein GS, Liu PP 2002 Cbfβ interacts with Runx2 and has a critical role in bone development. Nat Genet 32:639–644 [DOI] [PubMed] [Google Scholar]
- Sierra J, Villagra A, Paredes R, Cruzat F, Gutierrez S, Javed A, Arriagada G, Olate J, Imschenetzky M, Van Wijnen AJ, Lian JB, Stein GS, Stein JL, Montecino M 2003 Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity. Mol Cell Biol 23:3339–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Voss AK, Chowdhury K, Gruss P 2000 Querkopf, a MYST family histone acetyltransferase, is required for normal cerebral cortex development. Development 127:2537–2548 [DOI] [PubMed] [Google Scholar]
- Gasperowicz M, Otto F 2005 Mammalian Groucho homologs: redundancy or specificity? J Cell Biochem 95:670–687 [DOI] [PubMed] [Google Scholar]
- Afzal F, Pratap J, Ito K, Ito Y, Stein JL, van Wijnen AJ, Stein GS, Lian JB, Javed A 2005 Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J Cell Physiol 204:63–72 [DOI] [PubMed] [Google Scholar]
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R 2001 TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 20:2254–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock M, Otto F 2005 Control of RUNX2 isoform expression: the role of promoters and enhancers. J Cell Biochem 95:506–517 [DOI] [PubMed] [Google Scholar]
- Sudhakar S, Li Y, Katz MS, Elango N 2001 Translational regulation is a control point in RUNX2/Cbfa1 gene expression. Biochem Biophys Res Commun 289:616–622 [DOI] [PubMed] [Google Scholar]
- Bae SC, Lee YH 2006 Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene 366:58–66 [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Javed A, Choi JY, van Wijnen AJ, Stein JL, Lian JB, Stein GS 2001 A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin gene. J Cell Sci 114:3093–3102 [DOI] [PubMed] [Google Scholar]
- Pregizer S, Baniwal SK, Yan X, Borok Z, Frenkel B 2008 Progressive recruitment of Runx2 to genomic targets despite decreasing expression during osteoblast differentiation. J Cell Biochem 105:965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Yasui N, Ito K, Huang G, Fujii M, Hanai J, Nogami H, Ochi T, Miyazono K, Ito Y 2000 A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci USA 97:10549–10554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Xiao G, Jiang D, Franceschi RT 2007 Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol 176:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT 2000 MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem 275:4453–4459 [DOI] [PubMed] [Google Scholar]
- Qiao M, Shapiro P, Kumar R, Passaniti A 2004 Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J Biol Chem 279:42709–42718 [DOI] [PubMed] [Google Scholar]
- Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, Santerse AB, Schröder FH, van der Kwast TH 1994 Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 144:735–746 [PMC free article] [PubMed] [Google Scholar]
- Sadi MV, Walsh PC, Barrack ER 1991 Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer 67:3057–3064 [DOI] [PubMed] [Google Scholar]
- Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang C 1992 Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol 147:798–803 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ 2007 Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 21:2855–2863 [DOI] [PubMed] [Google Scholar]
- Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS 2003 Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 88:204–210 [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C 2004 Androgens and bone. Endocr Rev 25:389–425 [DOI] [PubMed] [Google Scholar]
- Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S 2003 Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA 100:9416–9421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed F, Khosla S 2005 Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Klein RF 1995 Osteoporosis in men. Endocr Rev 16:87–116 [DOI] [PubMed] [Google Scholar]
- Bland R 2000 Steroid hormone receptor expression and action in bone. Clin Sci (Lond) 98:217–240 [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C 2002 Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA 99:13498–13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, Stallcup MR, Coetzee GA, Frenkel B 2008 Modulation of Runx2 activity by estrogen receptor-α: implications for osteoporosis and breast cancer. Endocrinology 149:5984–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan T, Pasco JA, Kotowicz MA, Nicholson GC, Morrison NA 2002 Alleles of RUNX2/CBFA1 gene are associated with differences in bone mineral density and risk of fracture. J Bone Miner Res 17:1527–1534 [DOI] [PubMed] [Google Scholar]
- Kawate H, Wu Y, Ohnaka K, Takayanagi R 2007 Mutual transactivational repression of Runx2 and the androgen receptor by an impairment of their normal compartmentalization. J Steroid Biochem Mol Biol 105:46–56 [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G 1995 Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 15:1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning YM, Robins DM 1999 AML3/CBFα1 is required for androgen-specific activation of the enhancer of the mouse sex-limited protein (Slp) gene. J Biol Chem 274:30624–30630 [DOI] [PubMed] [Google Scholar]
- Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, Stein JL, Van Wijnen AJ, Wang YL, Stein GS 2002 Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J Cell Sci 115:4167–4176 [DOI] [PubMed] [Google Scholar]
- Stein GS, van Wijnen AJ, Stein JL, Lian JB, Montecino M, Choi J, Zaidi K, Javed A 2000 Intranuclear trafficking of transcription factors: implications for biological control. J Cell Sci 113:2527–2533 [DOI] [PubMed] [Google Scholar]
- Yeung F, Law WK, Yeh CH, Westendorf JJ, Zhang Y, Wang R, Kao C, Chung LW 2002 Regulation of human osteocalcin promoter in hormone-independent human prostate cancer cells. J Biol Chem 277:2468–2476 [DOI] [PubMed] [Google Scholar]
- Buchanan G, Craft PS, Yang M, Cheong A, Prescott J, Jia L, Coetzee GA, Tilley WD 2004 PC-3 cells with enhanced androgen receptor signaling: A model for clonal selection in prostate cancer. Prostate 60:352–366 [DOI] [PubMed] [Google Scholar]
- Jia L, Shen HC, Wantroba M, Khalid O, Liang G, Wang Q, Gentzschein E, Pinski JK, Stanczyk FZ, Jones PA, Coetzee GA 2006 Locus-wide chromatin remodeling and enhanced androgen receptor-mediated transcription in recurrent prostate tumor cells. Mol Cell Biol 26:7331–7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL 2004 Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol 164:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Kakushi H, Tohkin M 1994 Androgens directly stimulate mineralization and increase androgen receptors in human osteoblast-like osteosarcoma cells. Biochem Biophys Res Commun 204:905–911 [DOI] [PubMed] [Google Scholar]
- Wiren KM, Zhang X, Chang C, Keenan E, Orwoll ES 1997 Transcriptional up-regulation of the human androgen receptor by androgen in bone cells. Endocrinology 138:2291–2300 [DOI] [PubMed] [Google Scholar]
- Bäckström S, Wolf-Watz M, Grundström C, Härd T, Grundström T, Sauer UH 2002 The RUNX1 Runt domain at 1.25A resolution: a structural switch and specifically bound chloride ions modulate DNA binding. J Mol Biol 322:259–272 [DOI] [PubMed] [Google Scholar]
- Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ 2004 Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology 145:3507–3522 [DOI] [PubMed] [Google Scholar]
- Javed A, Guo B, Hiebert S, Choi JY, Green J, Zhao SC, Osborne MA, Stifani S, Stein JL, Lian JB, van Wijnen AJ, Stein GS 2000 Groucho/TLE/R-esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J Cell Sci 113:2221–2231 [DOI] [PubMed] [Google Scholar]
- Paredes R, Arriagada G, Cruzat F, Villagra A, Olate J, Zaidi K, van Wijnen A, Lian JB, Stein GS, Stein JL, Montecino M 2004 Bone-specific transcription factor Runx2 interacts with the 1α,25-dihydroxyvitamin D3 receptor to up-regulate rat osteocalcin gene expression in osteoblastic cells. Mol Cell Biol 24:8847–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron ER, Neil JC 2004 The Runx genes: lineage-specific oncogenes and tumor suppressors. Oncogene 23:4308–4314 [DOI] [PubMed] [Google Scholar]
- Pratap J, Lian JB, Javed A, Barnes GL, van Wijnen AJ, Stein JL, Stein GS 2006 Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev 25:589–600 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Enomoto H, Shiojiri S, Hoshi K, Furuichi T, Fukuyama R, Yoshida CA, Kanatani N, Nakamura R, Mizuno A, Zanma A, Yano K, Yasuda H, Higashio K, Takada K, Komori T 2003 Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-κ B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2−/− mice by RANKL transgene. J Biol Chem 278:23971–23977 [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER 1997 Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D 1997 Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15:871–875 [DOI] [PubMed] [Google Scholar]
- Kim JH, Yang CK, Heo K, Roeder RG, An W, Stallcup MR 2008 CCAR1, a key regulator of mediator complex recruitment to nuclear receptor transcription complexes. Mol Cell 31:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Chan KY, Scharf KD, Nover L 2007 Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem 282:3605–3613 [DOI] [PubMed] [Google Scholar]
- Luppen CA, Smith E, Spevak L, Boskey AL, Frenkel B 2003 Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res 18:1186–1197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.