Abstract
Mammalian MafA/RIPE3b1 is an important glucose-responsive transcription factor that regulates function, maturation, and survival of β-cells. Increased expression of MafA results in improved glucose-stimulated insulin secretion and β-cell function. Because MafA is a highly phosphorylated protein, we examined whether regulating activity of protein kinases can increase MafA expression by enhancing its stability. We demonstrate that MafA protein stability in MIN6 cells and isolated mouse islets is regulated by both p38 MAPK and glycogen synthase kinase 3. Inhibiting p38 MAPK enhanced MafA stability in cells grown under both low and high concentrations of glucose. We also show that the N-terminal domain of MafA plays a major role in p38 MAPK-mediated degradation; simultaneous mutation of both threonines 57 and 134 into alanines in MafA was sufficient to prevent this degradation. Under oxidative stress, a condition detrimental to β-cell function, a decrease in MafA stability was associated with a concomitant increase in active p38 MAPK. Interestingly, inhibiting p38 MAPK but not glycogen synthase kinase 3 prevented oxidative stress-dependent degradation of MafA. These results suggest that the p38 MAPK pathway may represent a common mechanism for regulating MafA levels under oxidative stress and basal and stimulatory glucose concentrations. Therefore, preventing p38 MAPK-mediated degradation of MafA represents a novel approach to improve β-cell function.
Both p38 MAPK and GSK3 regulate MafA protein stability under normal culture conditions, but p38 MAPK is the predominant regulator of MafA stability under oxidative-stress.
Transcription factors binding to the three conserved insulin enhancer elements, A3, RIPE3b/C1-A2, and E1 (PDX-1, MafA, and NeuroD1/BETA2, respectively), are major regulators of insulin gene expression and β-cell function (1,2,3,4,5,6,7,8,9). MafA, a member of the large Maf family of basic leucine zipper transcription factors, was identified as the β-cell-specific factor binding to the RIPE3b/C1-A2 [Maf response element (MARE)] element (9,10,11). Maf factors are important regulators of differentiation (12,13,14,15,16,17,18,19,20). After birth, the loss of MafA in mice results in abnormal islet organization with a gradual reduction in the proportion of β/α-cells and development of impaired glucose tolerance and diabetes (21). This observation demonstrates that MafA regulates β-cell replication/survival and function. Our results suggest that MafA is required for the maturation of β-cells and not for their specification (22,23,24). Differentiation of human embryonic stem cells into glucose-responsive β-cells (25,26) and downstream targets of MafA as important regulators of insulin synthesis and secretion (23) further support such a role of MafA. Thus, strategies to enhance MafA expression could improve β-cell function.
To define approaches that can enhance MafA expression, we characterize here the mechanisms regulating MafA stability in β-cells. Phosphorylation of avian MafA regulates its stability as well as its transcriptional and transforming activities (27,28,29,30). Because mammalian MafA is regulated by glucose, we characterized the role of protein kinases in regulating MafA stability under low and high concentrations of glucose as well as oxidative stress that accompanies chronic hyperglycemia. We demonstrate that p38 MAPK and glycogen synthase kinase 3 (GSK3) regulate MafA stability under basal glucose concentrations, but p38 MAPK, and not GSK3, regulates oxidative stress-mediated degradation of MafA. Our results suggest that inhibiting p38 MAPK can enhance the stability of MafA without affecting its function, and thus this approach may provide new strategies to reverse β-cell dysfunction.
Results
p38 MAPK signaling pathway negatively regulates the stability of MafA
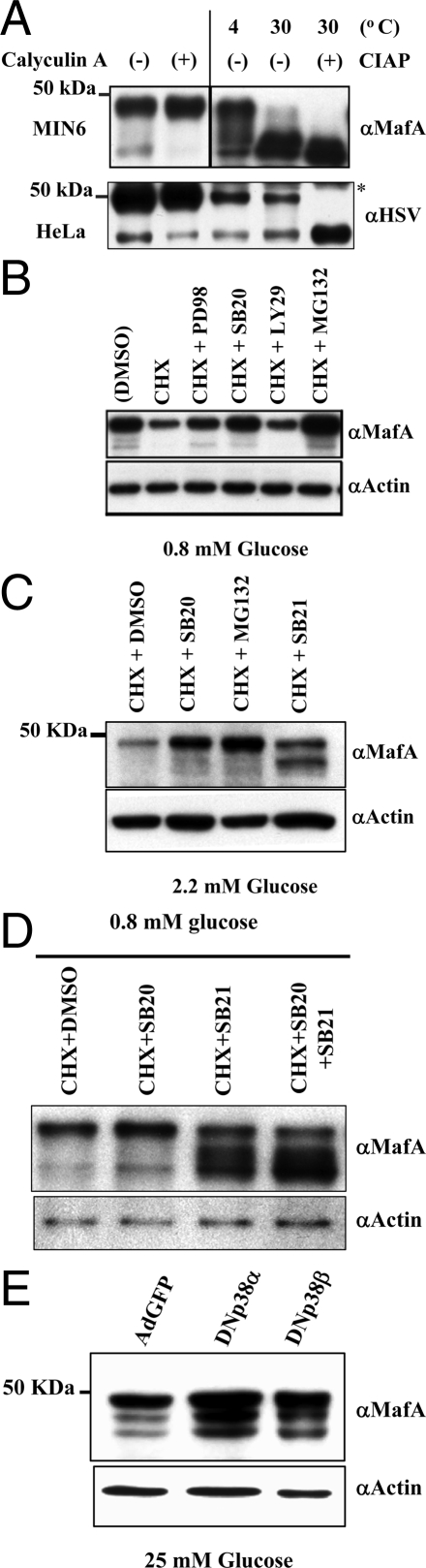
Consistent with both avian MafA (27) and mammalian MafA being phosphoproteins (31,32), immunoblots of MIN6 nuclear extracts with MafA-specific antibody showed two major isoforms of mammalian MafA of relative molecular masses 48 and 40 kDa. Furthermore, nuclear extracts from cells incubated with calyculin A (Ser/Thr phosphatase inhibitor) showed increased levels of the 48-kDa band with a concomitant reduction in 40-kDa isoform (Fig. 1A). Increased pAkt levels in the same extracts demonstrated effective inhibition of Ser/Thr phosphatase activity by calyculin A (data not shown). Incubation of MIN6 extracts at 30 C resulted in dramatic reduction in the 48-kDa band, and addition of calf intestinal alkaline phosphatase (CIAP) to the extracts caused complete loss of the 48-kDa isoform and increased the proportion of 40-kDa MafA. Interestingly, such conversion in the proportion of MafA isoforms in extracts from HeLa cells transfected with the hMAfA-HSV tag plasmid (Fig. 1A, lower panel) was observed only upon addition of CIAP. These results suggest that the 48- and 40-kDa bands represent the major isoforms of MafA and that the endogenous phosphatase in MIN6 reduced the proportion of the 48-kDa band in the absence of CIAP at 30 C. These isoforms were also observed in whole-cell lysates from mouse islets (Fig. 1C). Based on these results, the predicted molecular mass of endogenous MafA (37.5 kDa), and recent studies demonstrating incorporation of 32P in both isoforms of MafA (31,32,33), we suggest that the 48- and 40- kDa bands represent hyper- and hypo-phosphorylated MafA isoforms. Differences in the relative abundance of these isoforms under our experimental conditions are most likely due to the relative abundance of kinases and phosphatases in the cells.
Figure 1.
Role of protein kinases in regulating MafA stability. A, MafA is a phosphoprotein. Anti-MafA-specific antibody recognizes protein bands of apparent molecular mass of 40 and 48 kDa. MIN6 cells were treated with calyculin A (100 nm) or DMSO for 10 min before harvesting. Nuclear extracts (20 μg) were prepared, resolved on 7.5% SDS-PAGE, and immunoblotted with α-MafA antibody. To confirm the phosphorylation of MafA, nuclear extracts (20 μg) from MIN6 cells were incubated for 30 min at 4 or 30 C in the absence or presence of CIAP. Extracts were subsequently resolved on 7.5% SDS-PAGE and immunoblotted with α-MafA antibody. Increased levels of faster migrating MafA bands at 30 C in the absence of CIAP reflects endogenous phosphatase activity. Similar studies were performed using nuclear extracts (20 μg) from HeLa cells transfected with hMafA-HSV tag plasmid that were treated with either calyculin A (100 nm) or DMSO for 10 min before harvesting. Additionally, nuclear extracts (20 μg) from HeLa cells transfected with hMafA-HSV tag plasmid were incubated for 30 min at 4 or 30 C in the absence or presence of CIAP. The expression of hMafA was detected using anti-HSV antibody. *, Nonspecific band. B–E, p38 MAPK and GSK3 regulate MafA stability. B, MIN6 cells were cultured for 12 h with the indicated inhibitors [100 μm PD98059 (PD98), 20 μm SB203580 (SB20), 50 μm LY294002 (LY29), and 10 μm MG-132] in the absence or presence of 50 μm CHX in medium containing 0.8 mm glucose. Cell lysates were resolved on 10% SDS-PAGE and immunoblotted with α-MafA or α-actin antibody. C, Adult mouse islets were cultured for 12 h with 20 μm SB203580, 20 μm SB21673, or 10 μm MG-132 in the presence of 50 μm CHX in medium containing 2.2 mm glucose. Cell lysates were resolved on 10% SDS-PAGE and immunoblotted with α-MafA or α-actin antibody. D, MIN6 cells cultured as in B in the presence of DMSO or indicated kinase inhibitors [20 μm SB203580 (SB20) and 20 μm SB21673 (SB21)]. Cell lysates were resolved on 10% SDS-PAGE and immunoblotted with α-MafA or α-actin antibody. E, MIN6 cells cultured in 25 mm glucose and infected with GFP, DN p38α MAPK, or DN p38β MAPK-expressing adenoviruses. At 48 h after infection, whole-cell lysates were prepared and expression of endogenous MafA and actin were determined by immunoblotting using α-MafA or α-actin antibodies. Representative immunoblots are shown in each case from at least three independent experiments.
Extracts from MIN6 cells cultured in different glucose concentrations showed a concentration-dependent increase in MafA, but the proportion of hyper- and hypophosphorylated isoforms was unchanged (data not shown). However, because phosphorylation of transcription factors can regulate their activity and stability (34), we examined the role of protein kinases in phosphorylating MafA and regulating its stability. After 12 h culture in 0.8 mm glucose (to minimize the effect of glucose on MafA transcription) with or without cycloheximide (CHX) (Fig. 1B), MafA protein was reduced in cells with CHX compared with those without CHX. Inhibition of ERK (PD98059), p38 MAPK (SB203580), or proteasome (MG-132) activity increased the 48-kDa hyperphosphorylated form, with a minor increase in the hypophosphorylated MafA, but inhibition of phosphatidylinositol (PI) 3-kinase (LY294002) had no effect on MafA stability. Similarly, mouse islets cultured for 12 h in 2.2 mm glucose in the presence of p38 MAPK inhibitor or MG-132 had enhanced 48-kDa MafA, whereas inhibiting GSK3 enhanced the 40-kDa isoform, possibly by preventing its phosphorylation to 48-kDa isoform (Fig. 1C). These data show that both p38 MAPK and GSK3 reduce MafA stability in MIN6 cells and mouse islets.
To determine whether SB203580 prevented degradation of only the 48-kDa MafA, we examined its effect in the absence or presence of GSK3 inhibitor SB216763 (Fig. 1D). Both inhibitors individually increased MafA stability; their simultaneous presence further increased the hypophosphorylated MafA. This observation suggests that p38 MAPK mediates degradation of both 48- and 40-kDa isoforms, whereas GSK3-mediated degradation requires hyperphosphorylation of MafA.
SB203580, a relatively specific inhibitor of p38 MAPK, preferentially inhibits both α- and β-isoforms of p38 MAPK (35). We next examined the abilities of these isoforms to regulate MafA stability by immunoblotting whole-cell lysates of MIN6 cells expressing dominant-negative (DN) p38α MAPK, DN p38β MAPK (36), or green fluorescent protein (GFP) for 48 h at 25 mm glucose (Fig. 1E). Both DN p38 MAPK isoforms enhanced endogenous MafA protein expression, by 2.7 ± 0.04-fold (n = 3; P < 0.05) for DN p38 MAPKα and by a similar level (2.75 ± 0.018, n = 3; P < 0.05) for p38 MAPKβ, when normalized to MafA band intensity in extracts from MIN6 cells infected with GFP adenovirus. DN p38 MAPKs also enhanced expression of transfected human MafA (hMafA) in MIN6 cells (data not shown), suggesting that increased MafA in MIN6 cells (Fig. 1E) is due to increased stability of MafA protein and not necessarily due to increased transcription of endogenous MafA. These results demonstrate the ability of p38 MAPK to negatively regulate MafA stability in insulin-producing cells cultured in both high (Fig. 1E) and low (Fig. 1, B–D) concentrations of glucose.
p38 MAPK, but not GSK3, regulates oxidative stress-dependent reduction of MafA
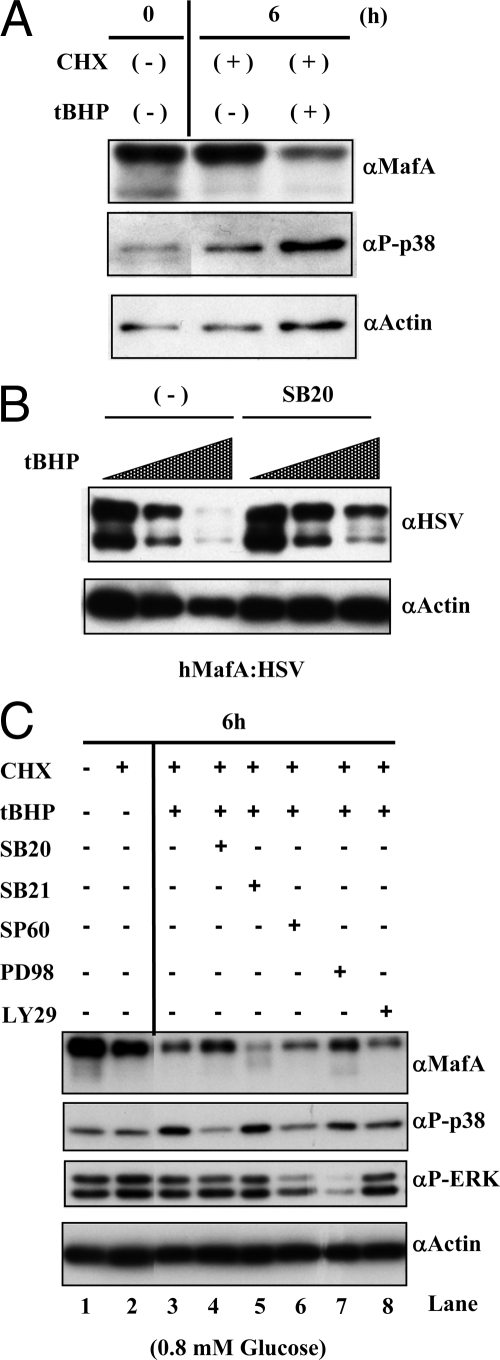
Oxidative stress is a major regulator of p38 MAPK activity (37) and a cause of β-cell dysfunction. We examined whether oxidative stress mediates degradation of MafA via p38 MAPK. Culturing MIN6 cells at 25 mm glucose with CHX resulted in slightly reduced MafA levels and modestly increased active phospho-p38 MAPK (Fig. 2A). Addition of tert-butyl hydroperoxide (tBHP), an inducer of oxidative stress, further reduced MafA stability, and caused a concomitant increase in phospho-p38 MAPK.
Figure 2.
Effect of oxidative stress on p38 MAPK- and GSK3-mediated regulation of MafA stability. A, Oxidative stress triggers p38 MAPK activation. MIN6 cells were cultured for 6 h in the absence (−) or presence (+) of 50 μm CHX and 100 μm oxidant tBHP in medium containing 25 mm glucose. Cells lysates were resolved on 10% SDS-PAGE. A representative image of immunoblotted gel with α-MafA, α-phospho-p38 or α-actin antibody is shown. B, Inhibiting p38 MAPK pathway can enhance the stability of MafA in the presence of oxidative stress. MIN6 cells transfected with hMafA-HSV were cultured for 2 h in 0, 50, or 200 μm tBHP in the absence or presence of 20 μm SB203580 (SB20). Lysates were prepared and analyzed as above, and a representative image of immunoblotted gel with α-HSV or α-actin antibody is shown. C, p38 MAPK, but not GSK3, regulates MafA stability under oxidative stress. MIN6 cells were cultured for 6 h in 0.8 mm glucose in the absence (−) or presence (+) of 100 μm tBHP, 50 μm CHX, and either 20 μm SB203580 (SB20), 20 μm SB216763 (SB21), 20 μm SP600125 (SP60), 100 μm PD98059 (PD98), or 50 μm LY294002 (LY29). Lysates were analyzed by Western blotting as above. A representative image shows the expression of endogenous MafA, phospho-p38 MAPK, phospho-ERK, and actin.
It is likely that oxidative stress can reduce endogenous MafA protein levels by regulating its stability and transcription. Two distinct approaches were used to demonstrate the effect of oxidative stress on MafA protein stability (Fig. 2, B and C). In the first, MIN6 cells transfected with hMafA expression plasmid and cultured for 16 h at 25 mm glucose were treated with an increasing concentration of tBHP in the absence or presence of SB203580 for 2 h. Because the CMV promoter drives the expression of hMafA, this approach examines the effect of tBHP on MafA protein stability independent of the endogenous MafA promoter. To minimize any effect of oxidative stress on transcription of hMafA affecting the levels of hMafA protein, the study time was kept short (2 h). The presence of 200 μΜ tBHP for 2 h almost completely inhibited hMafA expression (Fig. 2B), and the presence of SB203580 reduced this degradation, demonstrating a role of p38 MAPK in oxidative stress-mediated degradation of MafA.
In the second approach, we examined endogenous MafA stability at low (0.8 mm) glucose concentration in the absence or presence of CHX, tBHP, and inhibitors of p38 MAPK (SB203580), GSK3 (SB216763), JNK (SP600125), ERK (PD98059), or PI 3-kinase (LY294002) (Fig. 2C). Addition of tBHP increased the expression of phospho-p38 MAPK and reduced MafA protein [by 53 ± 6.7% of control (lane 2), P < 0.01] in whole-cell lysates (lane 3 vs. 2). This reduction was prevented by addition of SB203580 (lane 4 vs. 2), which reduced phospho-p38 and increased 48-kDa MafA [77.16 ± 9.4% of control (lane 2), P < 0.05]. As expected (31,32) (Fig. 1, C and D), inhibiting GSK3 reduced 48-kDa MafA and increased 40-kDa (lanes 2 vs. 5) [46.3 ± 9.6% of control (lane 2), P < 0.01]. The rescue of MafA degradation by SB203580 in the presence of tBHP was significantly different from tBHP alone (lane 3 vs. 4, P < 0.05), whereas the GSK3 inhibitor did not prevent MafA degradation under oxidative stress (lane 3 vs. 5, P = 0.29). These results demonstrate that GSK3 can phosphorylate MafA in the presence of oxidative stress but does not regulate its subsequent degradation. Similarly, inhibition of JNK and PI 3-kinase did not rescue oxidative stress-mediated degradation of MafA (compare lane 3 with lanes 6 and 8), whereas inhibition of ERK prevented some degradation (lanes 3 and 7).
These results demonstrate an important role of p38 MAPK in regulating MafA stability in the presence of tBHP/oxidative stress; whereas GSK3 is functional under these conditions, it does not regulate the stability of MafA. These results also support the possibility that p38 MAPK and GSK3 independently regulate degradation of MafA.
Chronic hyperglycemic condition inhibits levels of MafA mRNA and protein
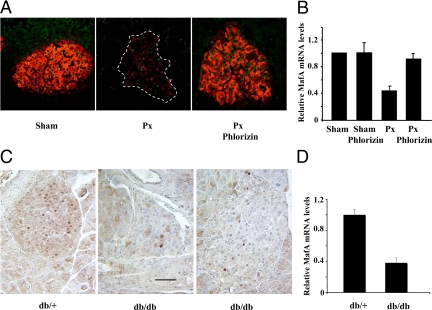
Oxidative stress is increased in rats after 90% pancreatectomy (Px) surgery (38), a model of chronic in vivo hyperglycemia (39). Development of hyperglycemia (250 mg/dl by 3–4 wk), and its reversal by phlorizin treatments have been described earlier (40). Treatment with phlorizin during the third and fourth weeks after Px normalized sugars to levels comparable to that of sham (control) rats. Immunostaining of pancreatic sections showed a dramatic reduction in both the intensity of the staining and the number of MafA-expressing nuclei in Px islets, which was restored upon phlorizin treatment (Fig. 3A). Quantification of MafA protein expression in islets after Px is extremely difficult due to the low number of islets and their reduced MafA expression. To determine whether the reduced MafA expression was due to decreased MafA mRNA expression, real-time RT-PCR was performed on cDNAs from isolated islets. At 4 wk after Px when expression of key β-cell genes was significantly reduced in response to chronic hyperglycemia (40,41), MafA mRNA was significantly reduced (nearly 60%) in Px islets compared with sham islets, and this decrease was completely reversed by phlorizin treatment (Fig. 3B). We also examined MafA expression in another model of chronic hyperglycemia/oxidative stress db/db mice (42). As observed in the Px model, pancreatic sections from db/db mice showed a dramatic reduction in MafA expression compared with db/+ control (Fig. 3C), and the real time RT-PCR results showed approximately 60% reduction in MafA mRNA expression in db/db islets (Fig. 3D). Although one cannot equate RT-PCR and immunostaining results, we observed a dramatic reduction in both the intensity of the staining and the number of MafA-expressing nuclei in both Px and db/db islets that retain nearly 40% of MafA mRNA. These observations suggest a possibility that in vivo oxidative stress may enhance the degradation of MafA protein, raising the likelihood of p38 MAPK regulating this process.
Figure 3.
Effect of chronic hyperglycemia on MafA expression. A, A representative image is shown of pancreatic sections from sham, Px, and Px plus phlorizin rats 4 wk after the surgery (n = 3) stained for MafA (green) and insulin (red). B, Relative expression of MafA mRNA. Pancreatic islets were isolated from rats 4 wk after Px or sham surgery with or without phlorizin treatment as described earlier (40). Total RNA was extracted from the islets, and real-time RT-PCR was performed using MafA and 18S rRNA-specific primers and probes. Results are presented as normalized MafA expression under different conditions relative to the expression in sham samples ± sem from at least three independent experiments. C, Representative images are shown of pancreatic sections from 12-wk-old db/+ and db/db (two images showing different islets) mice stained for MafA (brown). Magnification bar, 50 μm. D, Real-time RT-PCR was performed as described (42) to determine MafA expression in 12-wk-old db/+ and db/db islets. MafA expression was normalized to the expression of cyclophilin, and results are presented as MafA expression in db/db islets relative to its expression in db/+ samples ± sem; n ≥ 3.
N-terminal domain of MafA is required for regulation of MafA protein stability by p38 MAPK
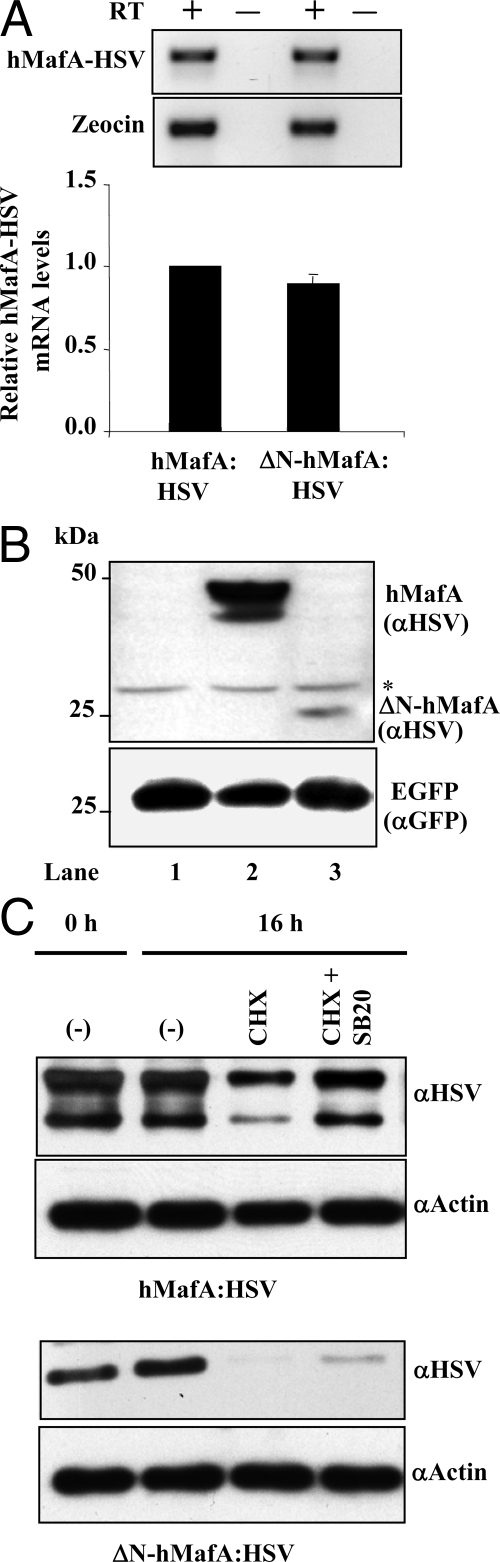
Most phosphorylation sites in MafA are located in its N-terminal domain, and these sites, including some p38 MAPK phospho-acceptor sites (43), are lost in the ΔN-hMafA expression plasmid lacking the N-terminal 137 amino acids (9). To determine whether the deletion of the N-terminal domain of MafA affected its mRNA expression, MIN6 cells were transfected with wild-type hMafA-HSV tag or ΔN-hMafA-HSV tag-expressing plasmids. Real-time RT-PCR analysis was performed using cDNAs obtained from transfected cells. Quantification of herpes simplex virus (HSV)-tagged mRNA expression from wild-type and ΔN-hMafA constructs, normalized to the expression of Zeocin, showed no significant difference in their expression (Fig. 4A). Analysis of PCR products on agarose gel also confirmed the comparable expression of MafA mRNA from these plasmids (Fig. 4A, upper panel). To determine whether the loss of N-terminal domain affected the stability of MafA protein, MIN6 cells were cotransfected with EGFP and either control pcDNA3.1, wild-type hMafA-HSV or ΔN-hMafA-HSV-expressing plasmids. Quantification of HSV-tagged bands in immunoblots, normalized to the expression of GFP protein in the same samples, showed that the expression of ΔN-hMafA protein was significantly lower than that of wild-type hMafA (0.18 ± 0.018% of wild-type control, n = 3; P < 0.01) (Fig. 4B). These observations suggest that the reduction in ΔN-hMafA expression results from its reduced stability and not reduced transcription.
Figure 4.
N-terminal domain of MafA is required for efficient regulation of MafA stability by p38 MAPK. A, MIN6 cells were cotransfected with either hMafA-HSV- or ΔN-hMafA-HSV-expressing plasmid. At 48 h after transfection, cells were harvested and cDNA was prepared from the RNA and used to detect expression of hMafA and Zeocin (control). Real-time RT-PCR was performed using a conserved C-terminal hMafA-HSV primer set that recognizes both wild-type and ΔN-hMafA-HSV. Real-time PCR products after 40 amplification cycles were resolved on 2% agarose gel, and bands corresponding to HSV and Zeocin from a representative ethidium bromide-stained gel is shown (n = 3) (upper panel). The ΔΔCT method was use to quantify real-time RT-PCR results, and CT values for Zeocin were used to normalize hMafA-HSV expression. ΔN-hMafA-HSV mRNA expression is presented as relative to the expression of wild-type hMafA-HSV, mean ± sem from at least three independent experiments (lower panel). B, Whole-cell lysates from MIN6 cells cotransfected with pCMV-EGFP plasmid and pcDNA3.1, hMafA-HSV or ΔN-hMafA-HSV expression plasmids (lanes 1–3, respectively) were resolved on 10% SDS-PAGE and immunoblotted with α-HSV and α-GFP antibodies (n = 3). C, MIN6 cells transfected with hMafA-HSV or ΔN-hMafA-HSV were cultured for 16 h in the absence (−) or presence (+) of CHX or 20 μm SB203580 (SB20) in medium containing 25 mm glucose. Cell lysates were resolved on 10% SDS-PAGE and immunoblotted with α-HSV or α-actin antibodies, and a representative gel is shown (n = 3).
We next examined the effect of p38 MAPK on wild-type and ΔN-hMafA degradation (Fig. 4C). Lysates prepared from MIN6 cells transfected with different expression vectors showed the expected reduced hMafA level in the presence of CHX and its restoration in the presence of p38 MAPK inhibitor (Fig. 4C). ΔN-hMafA was expressed at lower levels than hMafA, and inhibition of p38 MAPK only modestly prevented its degradation. The ability of the p38 inhibitor to rescue degradation of a significant proportion of wild-type hMafA but not of ΔN-hMafA suggests the importance of the N-terminal domain of MafA in p38 MAPK-mediated degradation. These results also demonstrate that the N-terminal domain of MafA plays a role in enhancing the overall stability of MafA.
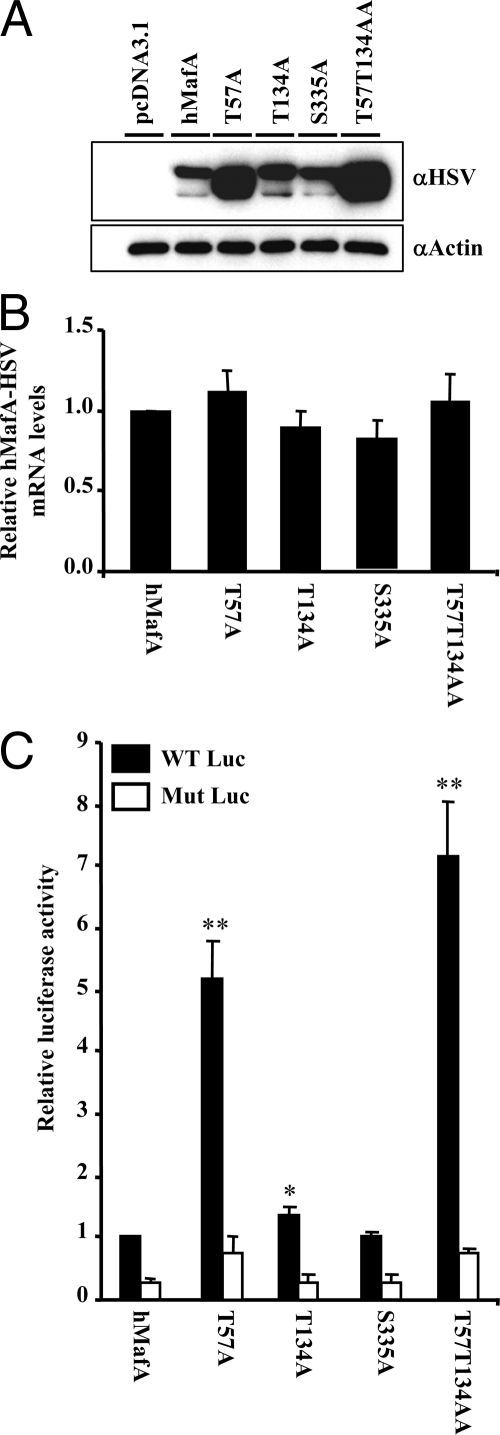
Mutations in known p38 MAPK sites regulate stability and activity of MafA
Using immunoblot and mass spectrometry analyses, three p38 MAPK phospho-acceptor sites threonine (T) 57, T113, and serine (S) 272 were identified in avian MafA (43), which corresponds to T57, T134, and S335 of human MafA. We replaced these phospho-acceptor sites with alanine (A) in hMafA-HSV-tagged expression plasmids. Because our results (Fig. 4) indicated that the N-terminal domain of MafA is important for p38 MAPK-mediated degradation, we also generated a double, mutant T57T134AA. Wild-type hMafA or hMafA expression plasmids with mutations in these sites were cotransfected in HeLa cells with insulin-promoter:luciferase-reporter plasmids, −238 WT LUC (WT Luc) or −109.110m LUC (Mut Luc) [mutated in MafA response element (44)]. Immunoblots using αHSV antibody (Fig. 5A) showed that mutating T57A or T57T134AA phospho-acceptor sites enhanced levels of these hMafA derivatives, but changing S335 had no effect. T134A showed a modest but significant increase in hyperphosphorylated hMafA, whereas T57A caused significant increase in hypophosphorylated MafA. It is important to note that T57 can also be phosphorylated by GSK3, which in the absence of oxidative stress enhances MafA stability (31,32) (Figs. 1C and 2C).
Figure 5.
Role of p38 MAPK phospho-acceptor sites on MafA protein expression and insulin gene transcriptional activation. Designated mutants (T57A, T134A, S335A, or T57T134AA), wild-type hMafA, or pcDNA3.1 expression plasmids were individually cotransfected in HeLa cells along with luciferase reporter plasmids, −238 WT Luc or −109.110m Luc (44). A, Protein expression of either wild-type or different MafA derivatives was examined in HeLa cells. Cell lysates were resolved on 10% SDS-PAGE. A representative gel immunoblotted with α-HSV and α-actin antibodies is shown. B, Relative expression of hMafA-HSV mRNA in HeLa cells transfected with wild-type and different MafA derivatives. Real-time RT-PCR was performed using hMafA-HSV and Zeocin-specific primers as in Fig. 4. Results are presented as expression of different MafA derivatives relative to the expression of wild-type MafA, mean ± sem from at least three independent experiments. C, Relative luciferase activity of hMafA or different mutants (T57A, T134A, S335A, or T57T134AA) cotransfected with either WT Luc (black bars) or Mut Luc (white bars). Results are presented as relative to the activity of wild-type Luc in the presence of hMafA plasmid ± sem for n ≥ 3. *, P < 0.05.
Unlike the effect of mutations in the phospho-acceptor sites on the MafA protein levels, mRNA expression of these MafA derivatives was comparable (Fig. 5B). Luciferase expression in extracts from HeLa cells transfected with T57A and T57T134AA hMafA derivatives showed approximately 5- and 7-fold increases in insulin gene expression (WT Luc); T134A hMafA showed modest but significant induction in insulin expression, whereas S335A mutant had activity similar to wild-type hMafA (Fig. 5C). Most of the increased activity of T57A and T57T134AA derivatives is due to similar 4- to 5-fold increases in the MafA protein levels (Fig. 5A, data not shown). Taken together, these results demonstrate that phospho-acceptor site T57 is important for regulating MafA stability and activity, whereas T134 has a modest effect on these processes.
The presence of either T57 or T134 phospho-acceptor sites is sufficient for p38 MAPK-mediated degradation of MafA
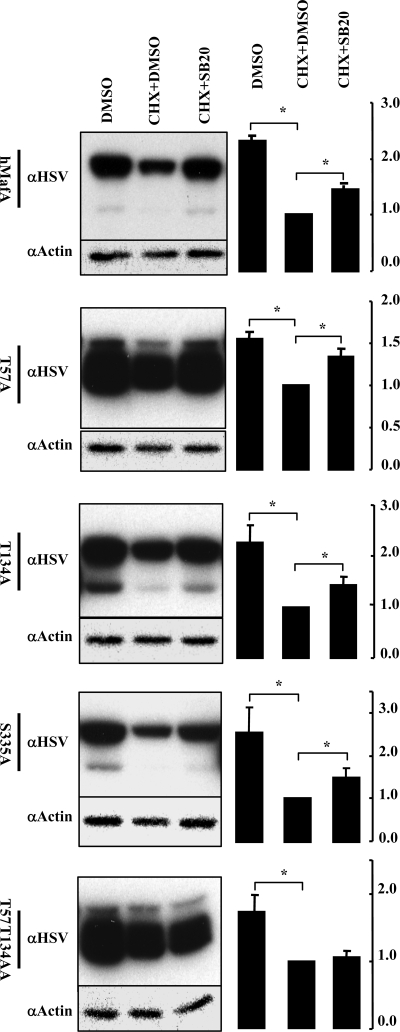
To test whether the increase in MafA stability in T57A and T134A was due to inhibition of phosphorylation of these amino acids by p38 MAPK, we examined the effect of p38 MAPK inhibitor on the stability of these proteins (Fig. 6). As expected (Figs. 1 and 2), inhibition of p38 MAPK activity rescued degradation of wild-type hMafA and, not surprisingly, of S335A mutant. Interestingly, inhibiting p38 MAPK activity still rescued degradation of MafA mutated at either T57 or T134 sites but not that of the T57T134AA double mutant. Thus, phosphorylation at either T57 or T134 is sufficient for p38 MAPK-mediated degradation of MafA, but the prevention of phosphorylation at both sites prevents this action of p38 MAPK. At present, it is unclear whether degradation of MafA results from direct phosphorylation of these two sites by p38 MAPK or whether p38 MAPK phosphorylates a protein in the degradation machinery that recognizes MafA phosphorylated at T57 and/or T134.
Figure 6.
Identification of phospho-acceptor sites essential for p38 MAPK-mediated degradation of MafA. MIN6 cells transfected with wild-type hMafA, T57A, T134A, S335A, or T57T134AA mutants were cultured in 0.8 mm glucose for 12 h in the presence of indicated combination of CHX, DMSO, and 20 μm SB203580 (SB20). Representative gels immunoblotted with α-HSV and α-actin antibodies is shown for each hMafA-HSV expression plasmid. Graphs on the right present results from quantification of band intensities from at least three independent experiments. Results are presented relative to the expression of MafA isoforms in the presence of CHX and DMSO as 1 ± sem. *, P < 0.05.
Discussion
Mammalian MafA is a phosphoprotein that regulates insulin gene expression and glucose-responsive insulin secretion. Here we demonstrate that MafA’s phosphorylation by different protein kinases differentially regulates its stability. Consistent with other recent results (31,32), we show that inhibiting GSK3 activity causes accumulation of hypophosphorylated MafA. In contrast, p38 MAPK regulates degradation of both hyper- and hypophosphorylated MafA, and inhibiting p38 MAPK activity in both high- and low-glucose conditions prevents degradation of MafA. Importantly, we demonstrate that p38 MAPK, but not GSK3, regulates MafA stability under oxidative stress, a major mediator of β-cell dysfunction. We show that simultaneous blocking of phosphorylation of both reported p38 MAPK targets (T57 and T134) in the N-terminal domain prevents p38 MAPK-mediated degradation of MafA. Therefore, we suggest that p38 MAPK is an important regulator of MafA stability under both physiological and pathological conditions.
There is general agreement that MafA has multiple phosphorylation sites that can be phosphorylated by kinases such as p38 MAPK, ERK1/2, and GSK3 (27,28,31,32,43). However, the relative importance of these kinases, their sites of phosphorylation, and the effects of these phosphorylations still need characterization. Phosphorylation of MafA is implicated in regulating its stability, transcriptional activation, and transforming ability. Increased phosphorylation of MafA enhances its degradation (28,31,32), whereas the requirement of phosphorylation in transcriptional activation and function varies depending on the experimental setup (27,28,29,30,31,32,43). We show that T57A, T134A, and T57 T134AA double mutant significantly induce insulin gene expression as compared with wild-type MafA, but S335A mutant does not (Fig. 5). We further demonstrate that this induced insulin gene expression possibly resulted from increased stability of these proteins. Thus, the loss of phosphorylation at either T57 or T134 is not detrimental to MafA’s ability to activate some of its targets.
In contrast, avian MafA with mutations in all three known p38 MAPK sites (T57, T113, and S272) did not induce the lens differentiation program or activate its downstream targets (43). The cause of this discrepancy is unclear. It is likely that at least some effects of T57A (Fig. 5) or the triple mutant (43) are the result of inhibiting phosphorylation of not only T57 but also T53 and S49 by GSK3 (31,32). Interestingly, the effect of inhibiting GSK3-mediated MafA phosphorylation on its transcriptional activity also differs based on the experimental systems. A mutation in GSK3 priming site (S65) in avian MafA inhibits transcriptional activation in some studies (27,29) but not in others (28,30). Similarly, mutations in GSK3 (S49, T53, T57, and S61) and priming kinase (S65) sites of mammalian MafA resulted in either a modest increase (31) or inhibition (32) of transcriptional activity. Demonstration that both phosphorylated and unphosphorylated MafA can induce gene expression (32) suggests that the relative levels of these isoforms and interacting cofactors may contribute to some of the observed differences in these studies. Our finding that T57A mutant can activate gene expression (Fig. 5) is consistent with the results from Han and colleagues (31). These data demonstrate that phosphorylation of at least some phospho-acceptor sites on the MafA is not essential for activation of insulin gene expression. This observation suggests that inhibiting p38 MAPK activity will enhance the stability of MafA without inhibiting its activity.
Ubiquitination plays an important role in degradation of MafA (31,32), and lysines targeted for ubiquitination are located in its C-terminal domain (33), whereas the N-terminal domain contains the majority of p38 MAPK (Figs. 4–6) and GSK3 sites (31,32). Because ΔN-hMafA is less stable than hMafA, our results also suggest the presence of signals in the N-terminal domain that enhance MafA stability (Fig. 4). Increasing evidence supports a role of GSK3 in regulating ubiquitination and degradation of MafA (31,32,33). GSK3 converts hypophosphorylated MafA to the hyperphosphorylated form required for its ubiquitination. Consistent with these results, we observed that GSK3 inhibitor enhanced the levels of hypophosphorylated MafA under low glucose (Fig. 1C). However, inhibition of p38 MAPK predominantly increases hyperphosphorylated MafA isoforms (Figs. 1 and 3–6), suggesting that the mechanisms regulating degradation of MafA via GSK3 and p38 MAPK are different. Of the known p38 MAPK sites [T57, T113, and S335 (43)], only simultaneous mutations of both T57 and T134 amino acids (Fig. 6) prevented p38 MAPK-mediated degradation, suggesting that phosphorylation at either phospho-acceptor sites by p38 MAPK is sufficient to trigger degradation of MafA. Because inhibiting p38 MAPK does not significantly alter migration of MafA in SDS-PAGE, we cannot resolve between the possibilities that p38 MAPK phosphorylates the protein degradation machinery enabling it to recognize MafA phosphorylated at either T57 or T134 or that p38 MAPK directly phosphorylates these sites and thereby triggering degradation of MafA.
One of our major findings is that p38 MAPK mediates oxidative stress-dependent degradation of MafA. Oxidative stress can induce p38 MAPK activity and has been implicated in triggering β-cell dysfunction. We previously demonstrated a link between hyperglycemia in 90% Px rats with oxidative stress and β-cell dysfunction (38). Here we report that both MafA mRNA and protein expression are down-regulated in this model as well as in another model of hyperglycemia and oxidative stress, db/db mice (42). Additionally, enhanced degradation of MafA protein by oxidative stress results from increased activity of p38 MAPK (Fig. 2), which is consistent with the ability of antioxidant treatment to prevent degradation of MafA in HIT-T15 cells chronically cultured in high glucose (45). These observations suggest a physiological role of p38 MAPK in degradation of MafA in response to increased oxidative stress. Interestingly, inhibiting GSK3 activity in the presence of oxidative stress did not prevent MafA degradation but prevented phosphorylation of the remaining MafA (Fig. 2C). Thus, in our experimental conditions, oxidative stress did not prevent the ability of GSK3 to phosphorylate MafA but selectively inhibited its subsequent degradation. Hence, we suggest that p38 MAPK is the more important regulator of MafA stability under oxidative stress/diabetic milieu. Even so, transgenic mice expressing constitutively active GSK3 in β-cells showed reduced β-cell proliferation and mass as well as impaired glucose tolerance (46) but only after 3 months. Because overexpression of DN MafA for 24 h can impair glucose-stimulated insulin secretion (23), it is likely that the impairments seen in transgenic mice are secondary to the reduced Pdx-1 expression and not a direct effect of GSK3 on MafA expression.
We present evidence that p38 MAPK and GSK3 regulate MafA stability by independent pathways. It is important to note that one of the p38 MAPK targets, T57, also mediates GSK3-dependent degradation of MafA (31). So, it will be challenging to decipher the precise mechanisms by which p38 MAPK and GSK3 regulate T57-dependent degradation of MafA. It is possible that under basal conditions, GSK3 is the predominant regulator of MafA stability, but under oxidative stress, p38 MAPK becomes the major regulator of this process. Because of its ability to regulate degradation of both hyper- and hypophosphorylated MafA under both basal and high glucose conditions, as well as oxidative stress, p38 MAPK may be a better target than GSK3 for therapeutic intervention to overcome β-cell dysfunction.
Materials and Methods
Plasmids
HSV-tagged hMafA and ΔN-hMafA that lacks the N-terminal domain (1–137 amino acids) have been described (9). The QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to construct plasmids with mutations in phospho-acceptor sites as described earlier (47).
Cell culture and reagents
MIN6 cells (passages 15–35) were grown in DMEM containing 10% fetal bovine serum (FBS) or in glucose-free DMEM (Invitrogen, Carlsbad, CA) containing 10% dialyzed FBS (Invitrogen) and the indicated concentrations of glucose (Sigma Chemical Co., St. Louis, MO). HeLa cells were grown in DMEM containing 10% FBS. Calyculin A (Cell Signaling Technology, Danvers, MA), CHX (Sigma), actinomycin D (Sigma), MG-132 (Calbiochem, San Diego, CA), SB203580 (Calbiochem), SB216763 (Tocris, Ellisville, MO), PD98056 (Calbiochem), SP600125 (Calbiochem), LY294002 (Calbiochem), and tBHP (Sigma) or dimethylsulfoxide (DMSO) were used at the indicated concentrations.
Animal studies
Sprague Dawley rats (Taconic Farms, Germantown, NY) weighing approximately 100 g underwent 90% Px or sham surgery as described (39). For reversal of chronic hyperglycemia, 2 wk after surgery, phlorizin was injected ip twice a day (0.8 g/kg · d). At 4 wk after surgery, rats were anesthetized, and pancreas and islets were removed as described (40) and used for MafA staining and RT-PCR analysis, respectively. Some of the samples were from previous studies (40,41), whereas others were obtained after the same protocols. Twelve-week-old db/db mice and their age-matched lean db/+ littermates were taken from the Garvan Institute breeding colony, and the experiments were performed as described earlier (42). All animal protocols were approved by the Animal Care Committees of the institutions (Joslin Diabetes Center and Garvan Institute/St. Vincent’s Hospital) where the experiments were conducted.
Isolation of mouse islets and culture
Mouse islets were isolated using collagenase digestion and cultured in RPMI 1640 medium (Cell-Gro) as described (48). After overnight culture, islets of similar sizes were handpicked and cultured in 60-mm dishes for 12 h in 2.2 mm glucose in the absence or presence of different inhibitors. Isolation of db/db and db/+ islets and subsequent preparation of RNA and cDNAs were performed as described (42).
Immunostaining
Frozen sections of rat pancreas were fixed in 10% formalin for 20 min at −20 C and immunostained (22) with primary antibodies (1:100) guinea pig antiinsulin (Linco, St. Charles, MO) and rabbit anti-MafA antibody (22). Confocal images were taken on a Zeiss LSM410 (Zeiss, Thornwood, NY). Results were based on at least three independent samples.
Pancreases from 12-wk-old db/db and db/+ mice were fixed in 4% paraformaldehyde and embedded in paraffin. After deparaffinization, antigen retrieval was performed in 10 mm citrate buffer (pH 6.0) by microwaving. Sections were incubated overnight with MafA antibody (1:300), and the ABC elite kit was used for amplification of signals according to the manufacturer’s protocol. Diaminobenzidine was used for visualization of signals for 10 min, followed by dehydration. Images were taken on an Olympus BH-2 microscope; all the sections were stained and photographed in parallel with identical settings. Final images were compiled using Adobe Photoshop.
Transfection and adenoviral transduction of cells
MIN6 or HeLa cells were transfected with 8 μg pcDNA3.1, hMafA-HSV, ΔN-hMafA-HSV, or GFP using Lipofectamine (Invitrogen) as described (44), resulting in approximately 50% transfection efficiency in both cell types. Indicated effectors were added 16 h after transfection (time 0 h) and cultured for additional time. MIN6 cells were infected with adenoviruses expressing DN p38 MAPKα (DN p38α), DN p38 MAPKβ (DN p38β) (36), or GFP (control) and harvested 48 h later.
Luciferase assay
HeLa cells were cotransfected using the Lipofectamine reagent with pcDNA3.1, hMafA-HSV or its derivatives (ΔNhMafA-HSV, T57A, T134A, S335A, or T57T134AA), expression plasmids along with insulin-promoter:luciferase reporter plasmids, −238 WT luciferase (WT LUC) or −109.110m mutant luciferase (MUT-LUC) (44), and pRL-null. Cellular extracts were collected after 48 h, and luciferase assays were performed (Promega, Madison, WI). The firefly luciferase activity from WT LUC or MUT LUC was normalized to the Renilla luciferase activity of the cotransfected pRL-null plasmid. Each experiment was performed with several independent plasmid preparations (n > 3).
Preparation of nuclear extracts and total cell lysates
Nuclear extracts of MIN6 and HeLa cells were obtained using NucBuster protein extraction kit (Novagen, San Diego, CA). Nuclear extracts from MIN6 or transfected HeLa cells (20 μg) were incubated for 30 min at 4 or 30 C in the absence or presence of CIAP (New England Biolabs, Ipswich, MA) for in vitro phosphatase treatment. Nuclear extracts were also prepared from these cells 10 min after the treatment with 100 nm calyculin A, a Ser/Thr phosphatase inhibitor, or DMSO. Total cell lysates were prepared by lysing cells on ice in RIPA buffer containing protease inhibitors; lysates were cleared by centrifugation.
Immunoblot analysis
All primary antibodies for immunoblot analyses were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), except anti-MafA (22), anti-HSV tag (Abcam, Cambridge, MA), anti-phospho p38 (Cell Signaling Technology), anti-phospho-ERK (Cell Signaling Technology), anti-GFP (Clontech, Palo Alto, CA), and secondary antibodies (Bio-Rad Laboratories, Hercules, CA). Total cell lysates and nuclear extracts were boiled for 5 min in the presence of β-mercaptoethanol and resolved by 7.5 or 10% SDS-PAGE, and Western blots were performed as described (22). Representative images from n ≥ 3 independent experiments are shown.
RT-PCR
For Px experiments, real-time PCR was performed with MafA-specific primers and probes; 18S rRNA was used as internal control. Results were quantified using the ΔΔCT method and presented relative to expression in sham islets ± sem. Real-time PCR with db/db and db/+ islets were performed as described (42) using MafA-specific primers, and the results are presented relative to db/+ islets ± sem.
In experiments using transfected cells with HSV-tagged MafA expression plasmids, real-time PCR was performed to detect expression of HSV-tagged MafA and the Zeocin gene present on the same plasmid but driven by a different promoter. Primers that selectively recognize HSV-tagged MafA (in all of the MafA derivatives) were used to determine the expression of different MafA derivatives, and their expression was normalized to the expression of Zeocin in the same cDNAs (n ≥ 3 independent experiments). Primers used for the study were MafA 5′-CTTCAGCAAGGAGGAGGTCATC, MafA 3′-GCGTAGCCGCGGTTCTT, MafA-HSV 5′-GAGCGGGACCTGTACAAGGAGAAA, MafA-HSV 3′-TGGCTGGCAACTAGAAGGCACAGT, Zeocin 5′-GACGACGTGACCCTGTTCATC, and Zeocin 3′-GGCTGCTCGCCGATCTC.
Statistical analysis
Results are expressed as means ± sem. Paired Student’s t test was used, and P values <0.05 were considered statistically significant.
Acknowledgments
We thank Dr. J.-I. Miyazaki (Osaka, Japan) for MIN6 cells. We thank Drs. J.-C. Jonas and G. Xu for their contributions to the 90% partial pancreatectomy experiments, Jennifer Lock for islet isolation, and Dr. Yibin Wang for dominant-negative p38 viruses. We thank Therese Salameh for technical assistance.
Footnotes
This study was supported by National Institutes of Health (NIH) Research Grant DK060127 and American Diabetes Association (ADA) Grant 7-08-RA-146 (to A.S.); National Health and Medical Research Council of Australia Grant 427616 (to D.R.L.). T.K., I.E.K., and W.N. were supported in part by postdoctoral fellowships from The Adler Foundation, NIH Institutional Training Grant (NIH T32 DK07260), and Juvenile Diabetes Research Foundation (3-2005-74), respectively. We acknowledge the Diabetes Endocrinology Research Center (NIH DK-36836)-supported Media and Advanced Microscopy (Histology and Confocal facilities) Cores at the Joslin.
Present address for T.K.: Department of Internal Medicine II, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 30, 2009
Abbreviations: CHX, Cycloheximide; CIAP, calf intestinal alkaline phosphatase; DMSO, dimethylsulfoxide; DN, dominant negative; FBS, fetal bovine serum; GFP, green fluorescent protein; GSK3, glycogen synthase kinase 3; hMafA, human MafA; HSV, herpes simplex virus; PI, phosphatidylinositol; Px, pancreatectomy; tBHP, tert-butyl hydroperoxide.
References
- Ohlsson H, Thor S, Edlund T 1991 Novel insulin promoter- and enhancer-binding proteins that discriminate between pancreatic α- and β-cells. Mol Endocrinol 5:897–904 [DOI] [PubMed] [Google Scholar]
- Karlsson O, Edlund T, Moss JB, Rutter WJ, Walker MD 1987 A mutational analysis of the insulin gene transcription control region: expression in β-cells is dependent on two related sequences within the enhancer. Proc Natl Acad Sci USA 84:8819–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Tsai MJ 1991 Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem 266:16708–16714 [PubMed] [Google Scholar]
- Sharma A, Stein R 1994 Glucose-induced transcription of the insulin gene is mediated by factors required for B-cell-type-specific expression. Mol Cell Biol 14:871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T 1993 IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR 1993 Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol 7:1275–1283 [DOI] [PubMed] [Google Scholar]
- Miller CP, McGehee Jr RE, Habener JF 1994 IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J 13:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Stellrecht CM, Tsai MJ 1995 Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev 9:1009–1019 [DOI] [PubMed] [Google Scholar]
- Olbrot M, Rud J, Moss LG, Sharma A 2002 Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA 99:6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H 2002 MafA is a glucose-regulated and pancreatic β-cell-specific transcriptional activator for the insulin gene. J Biol Chem 277:49903–49910 [DOI] [PubMed] [Google Scholar]
- Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R 2003 Members of the large Maf transcription family regulate insulin gene transcription in islet β-cells. Mol Cell Biol 23:6049–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH 1999 Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci USA 96:3781–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara M, Kawauchi S, Kobayashi M, Ogino H, Takahashi S, Yasuda K 2001 Isolation, characterization, and expression analysis of zebrafish large Mafs. J Biochem (Tokyo) 129:139–146 [DOI] [PubMed] [Google Scholar]
- Ho IC, Hodge MR, Rooney JW, Glimcher LH 1996 The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell 85:973–983 [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Frampton J, Graf T 1996 MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell 85:49–60 [DOI] [PubMed] [Google Scholar]
- Eichmann A, Grapin-Botton A, Kelly L, Graf T, Le Douarin NM, Sieweke M 1997 The expression pattern of the mafB/kr gene in birds and mice reveals that the kreisler phenotype does not represent a null mutant. Mech Dev 65:111–122 [DOI] [PubMed] [Google Scholar]
- Manzanares M, Cordes S, Kwan CT, Sham MH, Barsh GS, Krumlauf R 1997 Segmental regulation of hoxb-3 by kreisler. Nature 387:191–195 [DOI] [PubMed] [Google Scholar]
- Reza HM, Yasuda K 2004 Roles of Maf family proteins in lens development. Dev Dyn 229:440–448 [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M 1999 Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem 274:19254–19260 [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS 2000 Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development 127:307–317 [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S 2005 MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A 2006 A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol 293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB 2007 MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Rowan S, Salameh T, Maas RL, Bonner-Weir S, Sell SM, Sharma A 2008 Preferential reduction of [beta] cells derived from Pax6-MafB pathway in MafB deficient mice. Dev Biol 314:443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE 2006 Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE 2008 Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- Benkhelifa S, Provot S, Nabais E, Eychène A, Calothy G, Felder-Schmittbuhl MP 2001 Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol 21:4441–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi H, Ogino H, Kageyama Y, Yasuda K 2003 The stability of the lens-specific Maf protein is regulated by fibroblast growth factor (FGF)/ERK signaling in lens fiber differentiation. J Biol Chem 278:537–544 [DOI] [PubMed] [Google Scholar]
- Pouponnot C, Sii-Felice K, Hmitou I, Rocques N, Lecoin L, Druillennec S, Felder-Schmittbuhl MP, Eychène A 2006 Cell context reveals a dual role for Maf in oncogenesis. Oncogene 25:1299–1310 [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Kataoka K, Vogt PK 2003 MafA has strong cell transforming ability but is a weak transactivator. Oncogene 22:7882–7890 [DOI] [PubMed] [Google Scholar]
- Han SI, Aramata S, Yasuda K, Kataoka K 2007 MafA stability in pancreatic β-cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol 27:6593–6605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocques N, Abou Zeid N, Sii-Felice K, Lecoin L, Felder-Schmittbuhl MP, Eychène A, Pouponnot C 2007 GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol Cell 28:584–597 [DOI] [PubMed] [Google Scholar]
- Guo S, Burnette R, Zhao L, Vanderford NL, Poitout V, Hagman DK, Henderson E, Ozcan S, Wadzinski BE, Stein R 2009 The stability and transactivation potential of the mammalian MafA transcription factor are regulated by serine 65 phosphorylation. J Biol Chem 284:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ 2000 Regulation of transcription factor function by phosphorylation. Cell Mol Life Sci 57:1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JM, Cobb MH 2002 Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci 23:40–45 [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross Jr J, Brown JH, Hans J, Chien KR 1998 Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem 273:2161–2168 [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM 2002 Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23:599–622 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, Groff A, Ferran C, Bonner-Weir S, Sharma A, Weir GC 2002 Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to β-cell survival during chronic hyperglycemia. Diabetes 51:413–423 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Trent DF, Weir GC 1983 Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest 71:1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC 1999 Chronic hyperglycemia triggers loss of pancreatic β-cell differentiation in an animal model of diabetes. J Biol Chem 274:14112–14121 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC 2002 Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J Biol Chem 277:10912–10921 [DOI] [PubMed] [Google Scholar]
- Kjørholt C, Akerfeldt MC, Biden TJ, Laybutt DR 2005 Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of β-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 54:2755–2763 [DOI] [PubMed] [Google Scholar]
- Sii-Felice K, Pouponnot C, Gillet S, Lecoin L, Girault JA, Eychène A, Felder-Schmittbuhl MP 2005 MafA transcription factor is phosphorylated by p38 MAP kinase. FEBS Lett 579:3547–3554 [DOI] [PubMed] [Google Scholar]
- Harrington RH, Sharma A 2001 Transcription factors recognizing overlapping C1-A2 binding sites positively regulate insulin gene expression. J Biol Chem 276:104–113 [DOI] [PubMed] [Google Scholar]
- Harmon JS, Stein R, Robertson RP 2005 Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem 280:11107–11113 [DOI] [PubMed] [Google Scholar]
- Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA 2008 Mice with β-cell overexpression of glycogen synthase kinase-3β have reduced β-cell mass and proliferation. Diabetologia 51:623–631 [DOI] [PubMed] [Google Scholar]
- Nishimura W, Salameh T, Kondo T, Sharma A 2005 Regulation of insulin gene expression by overlapping DNA-binding elements. Biochem J 392:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Lock J, Xu G, Bonner-Weir S, Weir GC 2005 Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia 48:2074–2079 [DOI] [PubMed] [Google Scholar]