Abstract
PTH regulates transcription of a number of genes involved in bone remodeling and calcium homeostasis. We have previously shown that the matrix metalloproteinase-13 (MMP-13) gene is induced by PTH in osteoblastic cells as a secondary response through the protein kinase A pathway requiring the runt domain and activator protein 1 binding sites of the proximal promoter. Here, we investigated the changes PTH causes in histone acetylation in this region (which contains the only deoxyribonuclease-hypersensitive sites in the promoter) leading to MMP-13 gene activation in these cells. Chromatin immunoprecipitation experiments revealed that PTH rapidly increased histone H4 acetylation followed by histone H3 acetylation associated with the different regions of the MMP-13 proximal promoter. The hormone also stimulated p300 histone acetyl transferase activity and increased p300 bound to the MMP-13 proximal promoter, and this required protein synthesis. Upon PTH treatment, Runx2, already bound to the runt domain site of the MMP-13 promoter, interacted with p300, which then acetylated histones H4 and H3. The knockdown of either Runx2 or p300 by RNA interference reduced PTH-induced acetylation of histones H3 and H4, association of p300 with the MMP-13 promoter, and resultant MMP-13 gene transcription. Overall, our studies suggest that without altering the gross chromatin structure, PTH stimulates acetylation of histones H3 and H4 via recruitment of p300 to Runx2 bound to the MMP-13 promoter, resulting in gene activation. This work establishes the molecular basis of transcriptional regulation in osteoblasts by PTH, a hormone acting through a G-protein coupled receptor.
PTH stimulates histone acetylation via recruitment of p300 to Runx2 bound to the MMP-13 promoter, resulting in gene activation.
Hormonal regulation of gene expression plays a critical role in most aspects of mammalian development and physiology. In contrast to hormones acting through nuclear receptors, transcriptional activation by peptide hormones is less well understood. For example, PTH, an 84-amino acid hormone, regulates the expression of a large number of genes involved in bone remodeling and calcium homeostasis (1,2). In osteoblasts, PTH acts through its receptor, PTH1R, that is coupled to Gα proteins and the gene activation-signaling pathway has been shown to involve a cascade of events that can start with cAMP, protein kinases (protein kinase A mainly) and expression of immediate early genes (3,4,5).
In the present work, we have investigated the chromatin structure and histone acetylation of the matrix metalloproteinase-13 (MMP-13, collagenase-3) promoter as a way to understand the mechanisms of PTH regulation of transcription activation and control of gene expression in osteoblastic cells. MMP-13 is a member of the large family of MMPs involved in degradation of extracellular matrix components during bone and cartilage remodeling. The MMPs are implicated in several physiological and pathological processes such as normal bone growth and development, wound healing, angiogenesis, and joint destruction during arthritis (6,7). MMP-13 in particular, with a wide substrate specificity, is able to degrade, not only its preferred substrate, type II collagen, but also types I, III, and IV collagens, the cartilage proteoglycan, aggrecan, and other matrix proteins; its expression must therefore be kept under stringent control. Indeed MMP-13 expression has been found to be restricted mainly to areas of bone development and remodeling under normal conditions (8,9).
We and others have shown that PTH is a strong inducer of MMP-13 transcription in vivo (10), in primary rat osteoblasts (11), and in the rat osteoblastic cell line, UMR 106-01 (5,12). In the latter cells, PTH induces transcription of MMP-13 through the protein kinase A (PKA) pathway, but this requires protein synthesis, i.e. it is a secondary response. Analyses of minimal promoter regions in the MMP-13 gene indicated that all the cis-regulatory elements necessary for the PTH response are contained within the first 148 bp upstream of the transcription start site (12,13). Present in that region are consensus binding sites for a number of transcription factors including CAAT enhancer-binding protein, runt domain (RD or Runx), PEA-3, p53, activator protein (AP)-2, AP-1 (12), and Nmp4/CIZ (14); however, the key elements for PTH responsiveness in osteoblasts appear to be the RD-binding site and the AP-1 site, the respective binding factors of which, Runx2 and c-Fos/c-Jun, were shown to act cooperatively and interact physically to induce promoter activation to PTH (12,13,15). The hormone also induces c-fos and c-jun transcription through PKA and cAMP response element-binding protein (CREB) phosphorylation (4,16), and newly synthesized Fos and Jun then occupy the AP-1 site of the MMP-13 promoter (12) and associate with Runx2 already bound to the RD (12,13).
Here, we report that in the proximal promoter of the rat MMP-13 gene, the RD site and the AP-1 site (hence the entire PTH-responsive element) are contained within the only deoxyribonuclease (DNase)-hypersensitive area of the upstream regulatory region of the gene, positioned immediately adjacent to the transcription start site. PTH activation did not alter the existence or the location of these DNase I-hypersensitive sites (DHS), but caused acetylation of histones H3 and H4 by Runx2-mediated recruitment of p300. RNA interference decreasing Runx2 and p300 expression demonstrated the crucial role of Runx2 and p300 in PTH stimulation of histone H3 and H4 acetylation and transcriptional activation of MMP-13 in rat osteoblastic cells.
Results
DNase-hypersensitive sites in the MMP-13 promoter
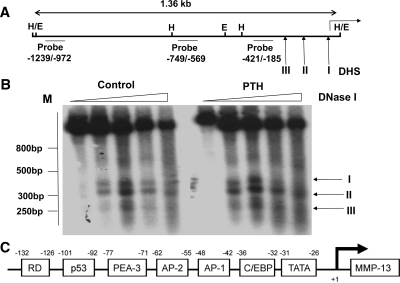
Previous work from this laboratory has identified the RD/Runx site (−132/−126) and the AP-1 site (−48/−42) as essential elements in PTH-induced transcriptional activation of MMP-13 in UMR 106-01 cells and primary rat osteoblastic cells (11,12,13). The two sites, without being adjacent, are sufficiently close to interact physically and, as suggested by strict requirements for specific helical arrangement (13), behave as components of a single nucleoprotein complex. We used the indirect end labeling method to determine the location of DNase I-hypersensitive sites and if PTH alters the structure of chromatin around the MMP-13 proximal promoter to induce transcriptional activation. In both control and PTH-treated cells, Southern blot analysis using hybridization probes derived from MMP-13 promoter −1239/−972 and from −749/−569 yielded strong single bands of 570 and 320 bp, respectively, with HindIII digests and 870–880 bp with EcoRI digests as expected (data not shown) in the absence of DNase I digestion (Fig. 1A). Figure 1B shows the autoradiograms obtained with the hybridization probe −421/−185 after DNase I digestion. In control nuclei, we identified three DHS, one with very strong hybridization intensity, DHS I, at position −36, a second DHS was detected at position −109, and the third DHS, of much weaker intensity, was located at position −183. Collectively from experiments investigating the upstream regulatory region of the MMP-13 promoter to 1.36 kb, analysis of DNase I hypersensitivity suggests that the MMP-13 promoter is, for the most part, organized in nuclease-resistant nucleoprotein structures, but has open regions in the proximal region encompassing the RD and AP-1 binding sites. PTH treatment did not change the location of the DHS, suggesting no PTH-induced change in overall DNase I accessibility despite the substantial evidence showing increased binding of AP-1 factors to the MMP-13 promoter after 30 min and full MMP-13 transcriptional activity by 120 min (5,12). We noticed, nevertheless, small but significant increases in the intensity of hybridization signals for DHS I and DHS II in PTH-treated nuclei, suggesting some relaxation of the chromatin in this region.
Figure 1.
Analysis of DNase I-hypersensitive sites in the rat MMP-13 promoter in rat osteoblastic cells. Nuclei isolated from rat osteoblastic osteosarcoma UMR 106-01 cells untreated (control) and treated with rat PTH (peptide 1–34, 10−8 m) for 30 min were digested with increasing amounts of DNase I (0, 20, 40, 80, and 160 U per 107 nuclei); the DNAs were purified and digested with HindIII or with EcoRI. Each sample (30 ug) was separated on 2% agarose gels and characterized by Southern blot analysis. A, Partial restriction map of rat MMP-13 promoter showing the hybridization probes and the positions of the DNase I-hypersensitive sites (DHS I, DHS II, DHS III) relative to the transcription start site. E, EcoRI; H, HindIII. B, Autoradiograms from hybridizations with [α32P-]dCTP-labeled probe −421/−185. The positions of the DNase I-hypersensitive sites (I, II, III) are marked with arrows. C, Schematic representation of the promoter of the rat MMP-13 gene. From right to left are sites for the following consensus sequences: C/EBP, AP-1, AP-2, PEA-3, p53, Runx2 distal. C/EBP, CCAAT enhancer-binding protein.
Rapid increases in histone H4 acetylation at the RD/Runx site in the PTH-activated MMP-13 promoter
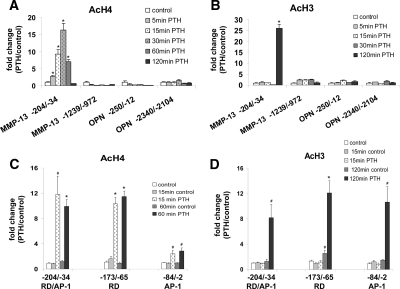
We have identified the presence of nucleoprotein complex(es) in the promoter region immediately adjacent to the MMP-13 transcriptional start site in both control and PTH-treated osteoblastic cells. We next analyzed the status of histone acetylation in this region to identify specific changes in nucleosomal components that are involved in MMP-13 transcription activation. UMR 106-01 cells were treated with control or PTH-containing media, and chromatin immunoprecipitation (ChIP) was carried out using antibodies against diacetylated histone H3 (lysines 9 and 14) or antibodies recognizing multiple acetylated forms of histone H4 (lysines 5, 8, 12, and 16). Under control conditions, there were no differences in histone H3 and histone H4 acetylation between the distal (−1239/−972), the proximal (−204/−34) promoter, and exon 10 (data not shown) regions of the MMP-13 gene with only traces (barely detectable bands on agarose gels) of acetylated histone H3 and histone H4 (an example of this can be seen in Fig. 6B). When cells were treated with PTH, there was no change in the amounts of acetylated histones in the distal upstream region. In the proximal promoter only, we detected 2- to 3-fold increases in acetylated histone H4 as early as 5 min after PTH treatment, with maximum levels of nearly 20-fold reached between 30 and 60 min, returning to near control levels by 120 min (Fig. 2A). In contrast, histone H3 acetylation was stimulated later, to more than 25-fold of control levels at 120 min PTH treatment (Fig. 2B). To show the specificity of PTH-stimulated histone acetylation on the MMP-13 promoter, we have also analyzed the promoter regions of the osteopontin gene, a gene expressed in UMR 106-01 cells but not responsive to PTH (1). There was no alteration in histone acetylation of either the proximal (−250/−12) or the distal (−2340/−2104) promoter regions of the osteopontin gene in response to PTH.
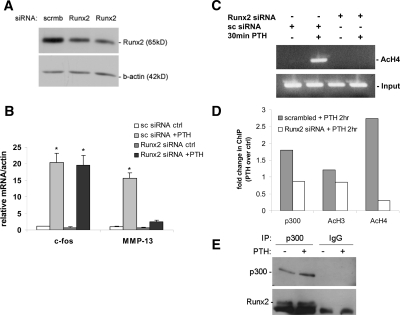
Figure 6.
Knockdown of Runx2 decreases PTH-stimulated histone H4 acetylation, MMP-13 transcription, and recruitment of p300 to the MMP-13 promoter. UMR 106-01 cells were transfected with either 20 nm Runx2 siRNA or control scrambled siRNA for 72 h, treated with rat PTH (peptide 1–34; 10−8 m) and then analyzed by (A) Western blot using Runx2 antibody or β-actin as a loading control, (B) real-time RT-PCR using the c-fos and MMP-13 primers, or ChIP assays using anti-Ac-H4 (C and D), or anti-Ac-H3, or anti-p300; the PCR primers were from the MMP-13 promoter, −204/−34, and the PCR products were separated on 2% agarose gels or real-time PCR was performed and the values normalized to input and expressed as fold-stimulation over the respective control. The input samples (cross-linked and not immunoprecipitated chromatin) were 1/100 of the amount used for immunoprecipitation. E, UMR cells were treated with rat PTH (peptide 1–34; 10−8 m) for 1 h, then p300 immunoprecipitated, and the Western blot reacted with anti-Runx2 showing that Runx2 associates with p300 in these cells. Error bars represent the sd of three independent experiments. *, P < 0.001 compared with the vehicle-treated control. Ac, Acetylated; ctrl, control; IP, immunoprecipitation; scrmb, scrambled.
Figure 2.
PTH-stimulated changes in histone H4 and H3 acetylation on the MMP-13 promoter. Cross-linked chromatin prepared from UMR cells, untreated or treated with rat PTH (peptide 1–34, 10−8 m) for various times, was immunoprecipitated with anti-Ac-H4 (A) or anti-Ac-H3 (B) antibodies, and protein-associated DNA was purified by reversal of cross-linking, proteinase K digestion, and phenol/chloroform extraction. ChIP assays were quantitated by real-time PCR using SYBR Green fluorescence and the primers for the promoter regions indicated. Comparison of histone H4 (C) and H3 acetylation (D) levels of the RD/Runx site and of the AP-1 site of MMP-13 promoter. UMR cells, controls at 0, 15, 60, and 120 min and treated with rat PTH (peptide 1–34, 10−8 m) for 15, 60, or 120 min were used for ChIP assays. The primers used for real-time PCR were from MMP-13 proximal promoter regions: primer −204/−34 amplified both the RD site and the AP-1 site, −173/−65 was specific for the RD site, −84/−2 was specific for the AP-1 site. ΔCT values were CT values of sample, normalized with input, minus the normalized CT value from PCR of the sample with β-actin primers, and ΔΔCT values represented the difference between ΔCT of sample and ΔCT of control. ΔCT values were converted to fold change over control by raising 2 to ΔΔCT power. Error bars represent the sd of three independent experiments. # and * , P < 0.005 and P < 0.001 compared with their control samples, respectively. Ac, Acetylated; OPN, osteopontin.
The increases in histone acetylation observed in the PTH-stimulated MMP-13 gene appeared concentrated in the promoter region between −204 and −34, which contains both the RD/Runx site and the AP-1 site. We further dissected that region to compare histone acetylations associated with the RD/Runx site with those around the AP-1 site. ChIP assays were performed using lysates obtained from UMR 106-01 cells treated with control or PTH-containing media at different times. Furthermore, the real time PCR primers used, in addition to those amplifying the entire −204/−34 region, were those specific for the −173/−65 region, which includes the RD/Runx site and not the AP-1 site, and those specific for −84/−2, which contains the AP-1 site and not the RD/Runx site. Analysis of histone H4 acetylation revealed rapid increases after 15 min of PTH treatment lasting until 60 min in the −204/−34 promoter, and this event was mostly restricted to the RD/Runx site (−173/−65) and was much less at the AP-1 site (−84/−2) (Fig. 2C). PTH treatment for 120 min caused acetylation of histone H3 at both the RD/Runx site (−173/−65) and at the AP-1 site (−84/−2) (Fig. 2D).
PTH-stimulated histone H4 and histone H3 acetylation require de novo protein synthesis and occur via the protein kinase A pathway
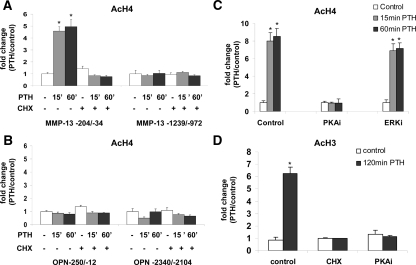
We had previously observed that the PTH induction of MMP-13 transcription required protein synthesis, i.e. is a secondary effect (5), and that maximal transcription did not occur until 2 h after PTH treatment. This was in contrast to the PTH induction of c-fos transcription, which was maximal at 30 min and was a primary response (17) and which we hypothesized was the determinant of the necessary protein synthesis for MMP-13 transcription. We hypothesized that the increase in histone H4 acetylation on the MMP-13 proximal promoter would be a primary response because it occurs rapidly after PTH treatment. To determine this, we pretreated UMR 106-01 cells with the protein synthesis inhibitor, cycloheximide, for 1 h. The cells were then treated with control or PTH-containing media for 15 min and 60 min and subjected to ChIP assays using the acetylated histone H4 antibody. Unexpectedly, the results indicate that in the proximal promoter region −204/−34, PTH-induced histone H4 acetylation was completely abolished by cycloheximide whereas the basal acetylation levels associated with the distal region −1239/−972 remained unaffected (Fig. 3A). Moreover, treatments with cycloheximide at the time of PTH addition inhibited histone H4 acetylation of the proximal promoter as efficiently as preincubating with cycloheximide (data not shown). This, along with the observation that cycloheximide had no effect on acetylation levels of total chromatin (input DNA, data not shown) or on the basal acetylation levels of the PTH nonresponsive osteopontin gene (Fig. 3B) suggests that de novo protein synthesis is required specifically for the changes in the chromatin structure of the proximal promoter region that lead to transcriptional activation of MMP-13. Western blots showed that cycloheximide, for this period of time, had no effect on total histones, total histone acetylation, p300, or Runx2 levels (data not shown).
Figure 3.
PTH-induced increases in histone H4 and H3 acetylation require de novo protein synthesis and the PKA pathway. UMR cells were preincubated with protein kinase inhibitors or cycloheximide for 30 min before examination of PTH-induced increases in histones H4 (A, B, and C) or H3 (D) acetylation. Quantitative real-time PCR was done using primers from the MMP-13 promoter (A, as shown; C and D, −204/−34) to amplify the ChIP DNA samples or the input DNA samples obtained from cross-linked but not immunoprecipitated chromatin or using primers for the osteopontin promoter (B) to amplify the ChIP DNA samples. UMR cells were preincubated with cycloheximide (30 μg/ml), PKA inhibitor (H-89 dihydrochloride, 50 μm) or ERK inhibitor (PD 98059, 50 μm) for 30 min before treatment with rat PTH (peptide 1–34, 10−8 m). Error bars represent the sd of three independent experiments. *, P < 0.001 compared with vehicle-treated control. Ac, Acetylated; CHX, cycloheximide; OPN, osteopontin.
We have previously shown that the PKA signaling pathway is necessary for PTH stimulation of MMP-13 transcription and activation of Runx2 (18). This and many other studies have implicated the PKA pathway in PTH’s regulation of MMP-13 (5,18). Because histone H4 acetylation occurs quite rapidly after PTH treatment and correlates with even faster changes in phosphorylation of Runx2 5 min after PTH treatment, which has been shown to be PKA dependent (18,19), we wished to determine whether this pathway also was responsible for histone acetylation. Thus, UMR 106-01 cells were pretreated with the PKA inhibitor (H-89), followed by PTH treatment and subjected to ChIP assays. The result shows that PTH stimulated histone H4 acetylation in the proximal promoter region −204/−34 at 15 and 60 min, and pretreatment with the PKA inhibitor abolished this effect (Fig. 3C). When cells were pretreated with an ERK inhibitor (PD98059), PTH-stimulated histone H4 acetylation was not affected. Thus, the effect of PTH on the proximal MMP-13 promoter-bound histone H4 acetylation requires the PKA pathway. Similar to histone H4 acetylation, PTH-stimulated (120 min) histone H3 acetylation on the proximal MMP-13 promoter required de novo protein synthesis and the PKA pathway (Fig. 3D).
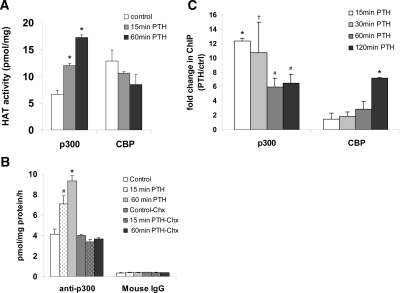
PTH stimulates histone acetyl transferase (HAT) activity, which requires protein synthesis and p300 recruitment to the MMP-13 promoter
The coactivators p300 and cAMP response element-binding protein-binding protein (CBP) possess intrinsic acetyl transferase activity and are implicated in chromatin remodeling of many promoters (e.g. Refs. 20,21,22). Here we measured HAT activity of p300 and of CBP in UMR 106-01 cells treated with PTH for 15 min and 60 min, which displayed high levels of histone H4 acetylation of the MMP-13 promoter at these times. Cell lysates were immunoprecipitated with antibodies against p300 or with antibodies specific for CBP then incubated in the presence of histone H4 peptide and [3H]acetyl coenzyme A as a donor of acetyl groups. Radioactivity associated with the histone H4 substrate was measured by scintillation counting. Nonspecific acetylation for p300 and CBP assays was represented by radioactivity from samples that were immunoprecipitated with mouse IgG or rabbit IgG, respectively. The result shows that there are high endogenous levels of both p300 and CBP-associated HAT activities and PTH treatment for 15 min caused a near doubling of p300 HAT activity with a further stimulation after 60 min (Fig. 4A). We observed no increases in CBP-dependent HAT activity in response to PTH treatment in these cells (Fig. 4A). Addition of 30 μg/ml cycloheximide before PTH entirely abolished the stimulation of p300-dependent HAT activity (Fig. 4B), suggesting that the abolition of histone H4 acetylation (Fig. 3A) by this agent might be due to prevention of the increase in p300 activity.
Figure 4.
PTH stimulates p300 HAT activity, which requires protein synthesis, and also stimulates MMP-13 promoter-bound p300. A, HAT assays were performed as described in Materials and Methods, using lysates (1 mg protein) from UMR cells, control or treated with rat PTH (peptide 1–34, 10−8 m). Whole-cell lysates were immunoprecipitated with anti-p300 antibodies, mouse IgG, anti-CBP-NT antibodies, or rabbit IgG. Results are expressed in picomoles/mg protein/h and represent means ± sem from triplicate samples. B, PTH stimulation of p300 HAT activity requires protein synthesis. HAT assays were performed on lysates from UMR cells treated with cycloheximide (30 μg/ml) at the time of PTH addition and immunoprecipitated with anti-p300 antibodies or mouse IgG. C, ChIP assays using UMR cells, untreated or treated with rat PTH (peptide 1–34, 10−8 m) for various times and immunoprecipitated with anti-p300 antibody. Quantitative real-time PCR was done using MMP-13 promoter primers, −204/−34. Error bars represent the sd of three independent experiments. †, # , and *, P < 0.05, P < 0.005, and P < 0.001 compared with vehicle-treated control, respectively. Chx, Cycloheximide; ctrl, control.
We next determined the level of association of p300 and CBP on the proximal MMP-13 promoter (−204/−34) in response to PTH treatment. UMR 106-01 cells were treated with control or PTH-containing media for 15, 30, 60, and 120 min and subjected to ChIP assays. The amount of p300 bound to the MMP-13 promoter was increased within 15 min of PTH treatment and decreased by 60 min but remained increased compared with control until 120 min (Fig. 4C). CBP associated with the MMP-13 promoter remained unchanged until 120 min. Hence, these results (Fig. 4, A–C) suggest that the rapid PTH-induced stimulation of histone H3 and H4 acetylation on the proximal promoter of MMP-13 might be mediated by recruitment of p300 and an increase in its HAT activity.
Runx2 interacts with p300, and both are required for acetylation of histones H3 and H4 for PTH stimulation of MMP-13 promoter activity
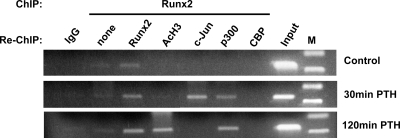
We and others have previously established that Runx2, a bone-specific transcription factor, is associated with the RD/Runx site in both control and PTH-stimulated MMP-13 promoters (10,12,23). Our observations in this study show that PTH caused increases in histone H4 and H3 acetylation on the proximal MMP-13 promoter in this same region (Fig. 2), increased p300 HAT activity (Fig. 4A), and p300 binding to the same proximal MMP-13 promoter (Fig. 4C). Hence, we performed the following experiments to determine whether PTH stimulates Runx2 interaction with p300, and if they interacted, could that account for acetylation of histones on the proximal MMP-13 promoter? UMR 106-01 cells were treated with control or PTH-containing media for 30 and 120 min and subjected to ChIP with antibodies to Runx2 followed by re-ChIP of the Runx2-immunoprecipitated samples with various antibodies to determine whether the proteins were interacting with Runx2 on the same DNA fragment. The results indicate that PTH treatment (30 and 120 min) stimulated recruitment of p300 to the proximal MMP-13 promoter via association with Runx2 (Figs. 5 and 6). Runx2 was also found to be part of the complex with acetylated histone H4 at 30 min PTH treatment (data not shown) and with acetylated histone H3 at 120 min PTH treatment on the proximal MMP-13 promoter (Fig. 5). There was no Runx2 interaction with CBP on the proximal MMP-13 promoter with PTH treatment. We have previously shown that PTH stimulates expression of c-Jun, which is required for MMP-13 promoter activation (10,12), and Runx2 interacts with c-Jun (24). Hence, we have included c-Jun as a positive control for PTH stimulation and Runx2 interaction in the ChIP and re-ChIP experiments. It is interesting that the c-Jun association is transient compared with the prolonged presence of p300. These results (Figs. 5, 4C, and 2) suggest that PTH treatment stimulates promoter-bound Runx2 to recruit p300 resulting in an increase in acetylation of histones H3 and H4.
Figure 5.
PTH causes Runx2 association with other proteins at the MMP-13 proximal promoter (−204/−34). Chromatin prepared from UMR cells, untreated and treated with rat PTH (peptide 1–34, 10−8 m) for 30 min or 120 min was immunoprecipitated with anti-Runx2, after which the immune complexes were collected then diluted with ChIP dilution buffer and resubjected to ChIP assays with anti-Runx2, anti-Ac-H3, anti-c-Jun, anti-p300-CT, or anti-CBP-NT antibodies. DNA was analyzed by PCR with primers to −204/−34 of the MMP-13 proximal promoter, and PCR products were separated on 2% agarose gels. Ac, Acetylated.
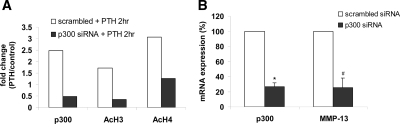
To further confirm the role of Runx2 and its recruitment of p300 for histone acetylation and MMP-13 gene activation in response to PTH treatment, we performed RNA interference experiments. UMR 106-01 cells were transiently transfected with either scrambled small interfering RNA (siRNA) or Runx2 siRNA, followed by PTH treatment and subjected to Western blot analysis (Fig. 6A) and real time RT-PCR analysis (Fig. 6B). siRNA duplexes (20 nm) for Runx2 caused approximately 70% inhibition of Runx2 protein in both control and PTH-treated UMR 106-01 cells (Fig. 6A). PTH stimulated c-fos and MMP-13 mRNA expression, and knockdown of Runx2 expression by Runx2 siRNA abolished only the PTH stimulation of MMP-13 in these cells (Fig. 6B). Thus, Runx2 is required only to activate the MMP-13 gene, and not the c-fos gene (the latter does not have an RD in its promoter). Scrambled siRNA treatment did not change endogenous Runx2 protein levels (Fig. 6A) and did not affect c-fos mRNA levels (Fig. 6B), demonstrating that Runx2 siRNA interference was specific to knockdown only of Runx2 expression.
To determine the importance of Runx2 for histone H3 and H4 acetylation for PTH-stimulated MMP-13 promoter activation, UMR 106-01 cells transiently transfected with either scrambled siRNA or Runx2 siRNA were subjected to ChIP assays using the acetylated H3, H4, and p300 antibodies. The results in Fig. 6, C and D, show the data from ChIP assays using the proximal MMP-13 promoter primers. Runx2 siRNA abolished the PTH-stimulated promoter-bound histone H4 acetylation and decreased the recruitment of p300 (Fig. 6, C and D). In addition, immunoprecipitation of p300 demonstrated its association with Runx2 (Fig. 6E) whereas the ChIP-ReChIP experiment showed the recruitment of p300 to Runx2 on the MMP-13 promoter (Fig. 5).
To determine the importance of p300 for histone H3 and H4 acetylation and PTH-stimulated MMP-13 promoter activation, UMR 106-01 cells were transiently transfected with either scrambled siRNA or p300 siRNA. The cells were then treated with control or PTH-containing media for 120 min and subjected to ChIP assays using the antibodies to acetylated H3 and H4 with the proximal MMP-13 promoter primers. The results show that PTH stimulated the binding of p300 to the proximal MMP-13 promoter, at the same time as histone H3 and H4 acetylation, whereas knockdown of p300 reduced the PTH-stimulated binding of p300 to the promoter concomitant with decreased acetylation of histones H3 and H4 (Fig. 7A). The knockdown of p300 subsequently decreased MMP-13 mRNA expression in these rat osteoblastic cells (Fig. 7B). Thus, these results (Figs. 6 and 7) suggest that Runx2 plays a crucial role in PTH-dependent MMP-13 gene activation by recruiting p300 leading to stimulation of acetylation of histone H3 and H4 by p300 by its HAT activity.
Figure 7.
Knockdown of p300 decreases PTH-stimulated histone H3 and H4 acetylation and MMP-13 transcription. UMR 106-01 cells were transfected with either 20 nm p300 siRNA or control scrambled siRNA for 72 h, untreated or treated with rat PTH (peptide 1–34; 10−8 m) for 2 h, and then analyzed by (A) ChIP assays using Ac-H3, Ac-H4, or p300 antibodies. Quantitative real-time PCR was done using the −204/−34 MMP-13 promoter primers and (B) real-time RT-PCR for mRNA abundance using the p300 and MMP-13 primers. Error bars represent the sem of three independent experiments. # and *, P < 0.005 and P < 0.001 compared with the scrambled siRNA control, respectively. Ac, Acetylated.
Discussion
We have previously identified the minimal PTH-responsive region for activation of MMP-13 expression in rat osteoblastic cells as being within the first 148 bp upstream of the transcriptional start site and have established that interaction between the AP-1 site (−48/−42) and the RD/Runx site (−132/−126) is essential and that the proteins binding to these sites appear to interact (12,13). In this study we have investigated the chromatin structure and histone H4 and H3 acetylation of the MMP-13 promoter to identify the changes enabling interaction between the two essential sites and how PTH regulates this process. Interestingly, our DNase I-hypersensitive studies show that nuclease accessibility is restricted to small regions, i.e. all within the proximal promoter region containing the AP-1 site and the RD/Runx site. This observation is consistent with our previous data showing that disruption of helical phasing between the AP-1 site and the RD/Runx site inhibited PTH-induced promoter activation (13). PTH treatment did not alter the position of the DHS, suggesting that nucleosome dissolution or sliding is not part of the PTH stimulation of transcription of this gene, and the chromatin changes required for MMP-13 activation must involve changes in structural components within the nucleosome.
PTH is a strong inducer of MMP-13 transcription in postnatal calvariae in vivo (10) and in primary rat osteoblasts in culture (11), although in both cases there is a normal increase in basal expression of the enzyme through development or differentiation. We have shown that the same response elements are necessary for both PTH stimulation of transcription in primary osteoblasts and promoter expression in vivo (11,25). Because the gene becomes normally expressed in bone or in primary osteoblasts, the gene must be derepressed in these processes. This may be in contrast to the UMR cells in which the gene seems always to be in a repressed state until treatment with agents acting through the PKA pathway like PTH. In this way, there may be some differences between the UMR cells and the events occurring in primary osteoblasts.
The association between histone acetylation and gene activation and, conversely, histone deacetylation and repression has long been recognized (26,27). More recently hyperacetylation restricted to specific promoter regions has been observed for several active genes. For example, transcriptional activation of the ε-globin gene is associated with histone H3 hyperacetylation at the TATA-proximal nucleosome (28). Increased acetylated histone H3 in the proximal promoter has been reported in steroidogenic acute regulatory gene activation (29). In the transcriptionally active osteocalcin gene, the promoter region between the vitamin D3 enhancer and basal promoter exhibited the highest levels of acetylated histone H4 (30). In the present study, we found increased acetylated histone H4 at the RD/Runx site of the MMP-13 promoter during the first hour of PTH activation (Fig. 2A). After 2 h of PTH treatment, there was increased acetylated histone H3 throughout the entire proximal promoter (Fig. 2B). This selective acetylation of histone proteins by PTH could be due to selective recruitment of coactivators (such as p300, CBP) associated with the HAT activities and/or sequestration of corepressors (such as histone deacetylases) associated with deacetylase activities at different sites of the proximal MMP-13 promoter region at different times. We have found in another study that histone deacetylase 4 is associated with Runx2 at the RD site of the MMP-13 promoter under control conditions and is released by PTH treatment (Shimizu E., N. Selvamurugan, J. J. Westendorf, E. N. Olson, and N. C. Partridge, manuscript submitted).
The requirement for de novo protein synthesis and PKA signaling for PTH stimulation of histone H3 and H4 acetylation on the proximal MMP-13 promoter (Fig. 3) suggests the recruitment of newly synthesized factors (such as the AP-1 factors), cofactors, and posttranslational modification of Runx2 on the promoter. The requirement for de novo protein synthesis for PTH stimulation of acetylation of histone H3 and H4 is specific for the MMP-13 promoter because neither PTH nor cycloheximide treatment had an effect on total levels of histone H3 and H4 acetylation, as found by Western blot analysis (data not shown). It is also interesting that the PTH-induced increase in p300 HAT activity requires protein synthesis. This is not due to changes in total Runx2 or p300 but could indicate newly synthesized p300 is recruited to the MMP-13 promoter, or alternatively, some other as yet unknown protein is required. The impressive work from Gannon’s group (31,32) has shown that the cyclical recruitment of estrogen receptor (ER)α and associated transcription factors to the pS2 promoter also involves protein degradation and requires replacement by newly synthesized proteins.
Runx2 has been shown to be a key factor in expression of several genes involved in bone formation, and the present data (Fig. 6B) confirm that it is essential for MMP-13 gene expression. The MMP-13 promoter-bound Runx2 interacted with p300 only after PTH addition (Fig. 5), strongly suggesting involvement of PTH-induced posttranslational modifications capable of causing conformational changes in Runx2 as we have determined from a number of our other papers (12,18,19). Runx2 activity can be regulated by posttranslational modifications such as acetylation or phosphorylation, which may allow Runx2 to interact with transcriptional cofactors. Our data show recruitment of p300 proteins within the proximal promoter of the MMP-13 gene (Fig. 4C), and the ChIP-ReChIP and siRNA experiments (Figs. 5 and 6) implicate recruitment by Runx2. Because PTH-stimulated Runx2 activity requires PKA and its PKA consensus sequence, the protein undergoes phosphorylation after PTH stimulation by PKA (18,19), and the PKA signaling pathway is required for histone H4 and H3 acetylation (Fig. 3, C and D); phosphorylation of Runx2 may be one of the primary events recruiting p300 for subsequent histone H4 and H3 acetylation that leads to MMP-13 gene activation by PTH. It is evident from the p300 siRNA experiment that knockdown of p300 expression in rat osteoblastic cells reduced acetylated histone H3 and H4 bound to the MMP-13 promoter (Fig. 7A). This suggests that p300 may be responsible for modification of chromosomal proteins (such as histones H3, H4) on the MMP-13 promoter that lead to subsequent recruitment and/or sequestration of other factors and cofactors resulting in MMP-13 transcription. The possibility of p300 being recruited, not directly by Runx2, but instead, by another transcription factor interacting with Runx2 at the RD/Runx site, was made unlikely by the results of the siRNA experiments showing reduction in p300 and of histone H4 acetylation on the MMP-13 promoter of Runx2-depleted cells (Fig. 6D).
Based on this work, we propose a model for MMP-13 activation by PTH in rat osteoblastic cells (UMR 106-01). Upon PTH stimulation, PKA is activated leading to phosphorylation of Runx2 protein bound at the RD site of the MMP-13 promoter (18,19), resulting in recruitment of p300 and subsequent histone H4 acetylation, followed by histone H3 acetylation. This, in turn, would perhaps allow binding of newly formed AP-1 factors (i.e. c-Jun) at the AP-1 site. Thus, both early and later stages of PTH treatment would allow entry and exit of transcriptional factors and cofactors, resulting in maximal MMP-13 transcription initiation.
Materials and Methods
Cell culture
Rat osteoblastic UMR 106-01 cells were maintained in Eagle’s MEM supplemented with nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% fetal bovine serum (FBS). Cells were plated at 5 × 106 cells/100-mm dish, grown in this medium for 2 d, and transferred to medium containing 2% FBS for 60 min before treatment with 10−8 m rat PTH (peptide 1–34). Cycloheximide, H-89 dihydrochloride, and PD 98059 were from Calbiochem (La Jolla, CA) and were used at final concentrations of 30 μg/ml, 50 μm, and 50 μm, respectively. All of these agents were dissolved in dimethylsulfoxide and diluted 1:1000 into medium.
DNase I hypersensitivity studies
DNase I hypersensitivity analyses were performed according to the indirect end-labeling method (33). Nuclei were isolated using the Nuclei EZ PREP kit from Sigma Chemical Co. (St. Louis, MO), resuspended in cold digestion buffer (10 mm Tris-HCl, pH 7.5; 10 mm NaCl; 6 mm MgCl2; 0.05 mm CaCl2; 0.25 m sucrose; and 0.5 mm dithiothreitol) and incubated at 37 C for 10 min with increasing amounts of DNase I (Amersham Biosciences, Piscataway, NJ). The reaction was stopped by addition of 1 vol of 25 mm EDTA, 1% sodium dodecyl sulfate, and 200 μg/ml proteinase K followed by incubation at 56 C for 3 h. DNA was purified by phenol/chloroform extraction and ethanol precipitation, digested with EcoRI or with HindIII, and subsequently analyzed by 2% agarose gel electrophoresis and Southern blotting. The hybridization probes were generated by PCR in the presence of [α32P]dCTP (Amersham Biosciences) using the following primers (cf. Fig. 1A):
Probe −1239/−972: forward, 5′-GCTAACACTTCATTGTGATGA-3′; reverse, 5′-CTACTTCTCAACTTCAATGCT-3′
Probe −749/−569: forward, 5′-TACAGTGTAAGGCCCTAGGCA-3′; reverse, 5′-TGGAATGCAGCCACTTCAATG-3′
Probe −421/−185: forward, 5′-GCTGAGAACTCATCAGCCTA-3′; reverse, 5′-GGCATATCAAAACGCATCTG-3′
Probe −204/−34: forward, 5′-CAGATGCGTTTTGATATGCC-3′; reverse, 5′-AATAGTGATGAGTCACCACTT-3′
PCR conditions involved preincubation at 95 C for 5 min, 30 cycles of 45-sec denaturation at 94 C, 45-sec annealing at 58 C, and 1-min elongation at 72 C with final incubation at 72 C for 5 min.
ChIP assays
ChIP analyses were performed using a modification of the Upstate Biotechnology, Inc. (UBI; Lake Placid, NY) procedure. Briefly, DNA and associated proteins were cross-linked by incubating cells in medium with 2% FBS containing 1% formaldehyde at 37 C for 10 min. Cells were washed in ice-cold PBS containing 1:250 dilution of protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and 1 mm phenylmethylsulfonylfluoride, scraped, sonicated to generate 400- to 1000-bp DNA fragments and then diluted 10-fold. Two-milliliter aliquots, equivalent to 5 × 107–108 cells, were precleared at 4 C for 1 h with 80 μl of a salmon sperm DNA/protein A-agarose 50% gel slurry and immunoprecipitated at 4 C overnight using the following antibodies from UBI: antiacetylated histone H3, antiacetylated histone H4, anti-p300-CT or anti-CBP-NT. Immune complexes were collected, washed sequentially in low-salt, high-salt, lithium chloride followed by two washes in Tris/EDTA buffers. Protein-DNA complexes were eluted with ChIP elution buffer (1% sodium dodecyl sulfate and 50 mm NaHCO3) and heated at 65 C for 4 h to reverse cross-linking. In Re-ChIP experiments, the immunoprecipitated complexes obtained by ChIP with anti-Runx2/PEBP2α (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were eluted by incubation for 30 min in ChIP elution buffer containing 1:250 dilution of protease inhibitor cocktail and 1 mm phenylmethylsulfonylfluoride and then diluted 10-fold and subjected again to the ChIP procedure. Supernatant obtained from sonicated chromatin without antibody was used as the input control. After treatment with proteinase K for 1 h at 45 C, the DNA was purified and resuspended in 50 μl Tris-EDTA buffer, and 2-μl aliquots (triplicate samples for each treatment group) were used for PCR. PCR primers used for analysis of the MMP-13 gene were the following:
−1239/−972: forward, 5′-GCTAACACTTCATTGTGATGA-3′; and reverse, 5′-CTACTTCTCAACTTCAATGCT-3′
−204/−34: forward, 5′-CAGATGCGTTTTGATATGCC-3′; and reverse, 5′-AATAGTGATGAGTCACCACTT-3′
−173/−65: forward, 5′-AGAGATGCCCTAATTTTCCATTTC-3′; and reverse, 5′-GTGTTCACTTCCTAGTGAGT-3′
−84/−2: forward, 5′-ACTCACTAGGAAGTGAACAC-3′; and reverse, 5′-TCCCAGGGCAAGCATTCTCTA-3′
The rat osteopontin gene primers used were the following.
−2340/−2104: forward, 5′CAAGGTTGCAGACACTGAAAG-3′; and reverse, 5′-CATTTCTAGAAGGGTGACAGG-3′
−250/−12: forward, 5′-GCTCTTCCAGGATTCTAAATG-3′; and reverse, 5′-CCTCCCAGAATTTAAACGCTG-3′
PCR amplification conditions were those described above.
Quantitative analysis of histone acetylation and promoter-bound proteins was performed by real-time PCR according to the ABI protocol using the above primers and detection of PCR products by increase in SYBR green fluorescence over a no-template PCR control. Data are expressed as CT values or threshold number of cycles required for fluorescence above control. CT values for each sample were first corrected by subtracting the CT value of the respective input sample and then normalized to ΔCT values by subtracting the CT values obtained from amplification with rat β-actin primers. Relative differences among the treatment groups were determined using the ΔΔCT method outlined by ABI where ΔΔCT represents the difference between ΔCT of the sample and the ΔCT of the corresponding appropriate control. ΔΔCT values were converted to fold differences over control by raising 2 to the ΔΔCT power.
siRNA treatment and real-time RT-PCR analysis
UMR 106-01 cells were transfected at 40–60% confluence using 20 nm siRNA duplexes specific to Runx2 (Santa Cruz Biotechnology), p300 (Ambion, Inc., Austin, TX), or control scrambled siRNA duplexes. After 72 h, cells were treated with PTH (peptide 1–34) and then harvested for Western blots and ChIP analyses or for RT-PCR analyses. RNA extraction and real-time RT-PCR were performed as previously described (1) using the following primers:
Runx2: forward, 5′-TAAAGTGACAGTGGACGGTCCC-3′; and reverse, 5′-TGCGCCCTAAATCACTGAGG-3′
exon 10 MMP13: forward, 5′-ATACAGTATCTGGAGTAATCG-3′; and reverse, 5′-CAATTCTTCAATGTGGTTCCA-3′
c-Fos: forward, 5′-CTGCCTCTTCTCAATGACCCTG-3′; and reverse, 5′-GCCGGAAACAAGAAGTCATCAA-3′
HAT assay
HAT assays were performed using standard methods (34,35). Briefly, UMR 106-01 cell lysates were immunoprecipitated using anti-p300-CT, a mouse monoclonal antibody (UBI), or anti-CBP-NT of rabbit origin (UBI) and for nonspecific HAT activity, mouse or rabbit IgG. Immune complexes were collected with protein G agarose beads, washed and incubated (30 C for 30 min) with 30 μl of a HAT assay cocktail containing 2 μg (66.6 μm) of histone H4 peptide and 100 μm acetyl coenzyme A-0.25 μCi/μl [3H]acetyl coenzyme A (Amersham Biosciences). The reaction mixture was then blotted onto P81 (Whatman, Piscataway, NJ) filter papers and radioactivity was measured by liquid scintillation counting.
Statistical analysis
Almost all experiments were repeated at least three times, and significance was assessed using Student’s t test.
Acknowledgments
We thank Stephen Jefcoat for technical assistance and Dr. Joseph Fondell for critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants DK 47420 and DK 48109 (to N.C.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 7, 2009
Abbreviations: AP, Activator protein; CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; CREB, cAMP response element-binding protein; DHS, DNase I-hypersensitive site; DNase, deoxyribonuclease; ER, estrogen receptor; FBS, fetal bovine serum; HAT, histone acetyl transferase; MMP-13, matrix metalloproteinase-13; PKA, protein kinase A; RD, runt domain; siRNA, small interfering RNA.
References
- Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC 2003 Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem 278:19723–19731 [DOI] [PubMed] [Google Scholar]
- Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC 2007 Determination of parathyroid hormone’s dual effects on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem 282:33086–33097 [DOI] [PubMed] [Google Scholar]
- Chase LR, Aurbach GD 1970 The effect of parathyroid hormone on the concentration of adenosine 3′,5′-monophosphate in skeletal tissue in vitro. J Biol Chem 245:1520–1526 [PubMed] [Google Scholar]
- Pearman AT, Chou WY, Bergman KD, Pulumati MR, Partridge NC 1996 Parathyroid hormone induces c-fos promoter activity in osteoblastic cells through phosphorylated cAMP response element (CRE)-binding protein binding to the major CRE. J Biol Chem 271:25715–25721 [DOI] [PubMed] [Google Scholar]
- Scott DK, Brakenhoff KD, Clohisy JC, Quinn CO, Partridge NC 1992 Parathyroid hormone induces transcription of collagenase in rat osteoblastic cells by a mechanism using cyclic adenosine 3′,5′-monophosphate and requiring protein synthesis. Mol Endocrinol 6:2153–2159 [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR 1997 Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99:1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE 1996 Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 97:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack S, Vallon R, Schmidt J, Grigoriadis A, Tuckermann J, Schenkel J, Weiher H, Wagner EF, Angel P 1995 Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ 6:759–767 [PubMed] [Google Scholar]
- Mattot V, Raes MB, Henriet P, Eeckhout Y, Stehelin D, Vandenbunder B, Desbiens X 1995 Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J Cell Sci 108:529–535 [DOI] [PubMed] [Google Scholar]
- Porte D, Tuckermann J, Becker M, Baumann B, Teurich S, Higgins T, Owen MJ, Schorpp-Kistner M, Angel P 1999 Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene 18:667–678 [DOI] [PubMed] [Google Scholar]
- Winchester SK, Selvamurugan N, D'Alonzo RC, Partridge NC 2000 Developmental regulation of collagenase-3 mRNA in normal, differentiating osteoblasts through the activator protein-1 and the runt domain binding sites. J Biol Chem 275:23310–23318 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Chou WY, Pearman AT, Pulumati MR, Partridge NC 1998 Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem 273:10647–10657 [DOI] [PubMed] [Google Scholar]
- D'Alonzo RC, Selvamurugan N, Karsenty G, Partridge NC 2002 Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation. J Biol Chem 277:816–822 [DOI] [PubMed] [Google Scholar]
- Shah R, Alvarez M, Jones DR, Torrungruang K, Watt AJ, Selvamurugan N, Partridge NC, Quinn CO, Pavalko FM, Rhodes SJ, Bidwell JP 2004 Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab 287:E289–E296 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Brown RJ, Partridge NC 2000 Regulation of collagenase-3 gene expression in osteoblastic and non-osteoblastic cell lines. J Cell Biochem 79:182–190 [DOI] [PubMed] [Google Scholar]
- Tyson DR, Swarthout JT, Partridge NC 1999 Increased osteoblastic c-fos expression by parathyroid hormone requires protein kinase A phosphorylation of the cyclic adenosine 3′,5′-monophosphate response element-binding protein at serine 133. Endocrinology 140:1255–1261 [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Scott DK, Brakenhoff KD, Quinn CO, Partridge NC 1992 Parathyroid hormone induces c-fos and c-jun messenger RNA in rat osteoblastic cells. Mol Endocrinol 6:1834–1842 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Pulumati MR, Tyson DR, Partridge NC 2000 Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor α1. J Biol Chem 275:5037–5042 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Shimizu E, Lee M, Liu T, Li H, Partridge NC 2009 Identification and characterization of Runx2 phosphorylation sites involved in matrix metalloproteinase-13 promoter activation. FEBS Lett 583:1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu TK, Palhan VB, Wang Z, An W, Cole PA, Roeder RG 2000 Activator-dependent transcription from chromatin in vitro involving targeted histone acetylation by p300. Mol Cell 6:551–561 [DOI] [PubMed] [Google Scholar]
- Lu H, Pise-Masison CA, Fletcher TM, Schiltz RL, Nagaich AK, Radonovich M, Hager G, Cole PA, Brady JN 2002 Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template. Mol Cell Biol 22:4450–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam WH, Taylor MH, Tanner KG, Denu JM, Goodman RH, Smolik SM 2002 The acetyltransferase activity of CBP is required for wingless activation and H4 acetylation in Drosophila melanogaster. Mol Cell Biol 22:3832–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Porte D, Munz C, Angel P 2001 AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem 276:20029–20038 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Partridge NC 2000 Constitutive expression and regulation of collagenase-3 in human breast cancer cells. Mol Cell Biol Res Commun 3:218–223 [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Jefcoat SC, Kwok S, Kowalewski R, Tamasi JA, Partridge NC 2006 Overexpression of Runx2 directed by the matrix metalloproteinase-13 promoter containing the AP-1 and Runx/RD/Cbfa sites alters bone remodeling in vivo. J Cell Biochem 99:545–557 [DOI] [PubMed] [Google Scholar]
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD 1996 Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843–851 [DOI] [PubMed] [Google Scholar]
- Grunstein M 1997 Histone acetylation in chromatin structure and transcription. Nature 389:349–352 [DOI] [PubMed] [Google Scholar]
- Gui CY, Dean A 2001 Acetylation of a specific promoter nucleosome accompanies activation of the ε-globin gene by β-globin locus control region HS2. Mol Cell Biol 21:1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LK, Stouffer RL, Strauss 3rd JF 2001 Quantitative analysis of the hormone-induced hyperacetylation of histone H3 associated with the steroidogenic acute regulatory protein gene promoter. J Biol Chem 276:27392–27399 [DOI] [PubMed] [Google Scholar]
- Shen J, Montecino M, Lian JB, Stein GS, Van Wijnen AJ, Stein JL 2002 Histone acetylation in vivo at the osteocalcin locus is functionally linked to vitamin D-dependent, bone tissue-specific transcription. J Biol Chem 277:20284–20292 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Métivier R, Reid G, Gannon F 2006 Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Rep 7:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C 1980 The 5′ ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature 286:854–860 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK 2003 Small molecule modulators of histone acetyltransferase p300. J Biol Chem 278:19134–19140 [DOI] [PubMed] [Google Scholar]
- Kundu TK, Wang Z, Roeder RG 1999 Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Mol Cell Biol 19:1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]