Abstract
In Drosophila, blood development occurs in a specialized larval hematopoietic organ, the lymph gland (LG), within which stem-like hemocyte precursors or prohemocytes differentiate to multiple blood cell types. Here we show that components of the Wingless (Wg) signaling pathway are expressed in prohemocytes. Loss and gain of function analysis indicates that canonical Wg signaling is required for maintenance of prohemocytes and negatively regulates their differentiation. Wg signals locally in a short range fashion within different compartments of the LG. In addition, Wg signaling positively regulates the proliferation and maintenance of cells that function as a hematopoietic niche in Drosophila, the Posterior Signaling Center (PSC), and in the proliferation of crystal cells. Our studies reveal a conserved function of Wg signaling in the maintenance of stem-like blood progenitors and reveal an involvement of this pathway in the regulation of hemocyte differentiation through its action in the hematopoietic niche.
Introduction
Hematopoiesis is a dynamic process that requires a balance between the maintenance of hematopoietic stem cells (HSCs) and their differentiation into different blood cell lineages. The genetic pathways involved in hematopoietic development are conserved across metazoans (reviewed in Evans et al., 2007). The formation of blood cells in Drosophila, as in their vertebrate counterparts, is regulated at the level of multipotent precursors (Mandal et al., 2007). In Drosophila, larval hematopoiesis occurs in a specialized organ called the lymph gland (LG), within which immature stem-like hemocyte precursors or prohemocytes differentiate into three different myeloid cell types called plasmatocytes, crystal cells and lamellocytes (Lanot et al., 2001). It has been previously shown that stem-like precursor cells and the differentiated cells derived from them are located in different and specific compartments of the LG. Hemocyte precursors are maintained in a medial area of the lymph gland termed the medullary zone (MZ) while differentiation of hemocyte lineages occurs in a peripheral region of the gland that has been termed the cortical zone (CZ) (Jung et al., 2005). Analogous to the mammalian hematopoietic system, maintenance of undifferentiated prohemocytes is controlled in part by specialized niche cells located at the posterior end of the LG, in a region named the posterior signaling center (PSC) (Lebestky et al., 2003). PSC cells are specified early in development during embryogenesis from thethird thoracic segment of the cardiogenic mesoderm by the homeobox protein Antp, while the hemocyte precursors of the LG develop from thefirst andsecond thoracic segments. Recent studies have shown that the secreted factor Hedgehog and JAK/STAT signaling mediated by the PSC contribute to the maintenance of prohemocytes within the MZ (Krzemien et al., 2007; Mandal et al., 2007). In addition, expression of the Notch ligand Serrate (Ser) in the cells of the PSC is required for specification of crystal cells of the CZ (Duvic et al., 2002; Lebestky et al., 2003). The identity of additional signaling pathways required for the maintenance of stem-like hemocyte precursors and their role in allowing undifferentiated cells to become competent to differentiate remains unclear. In this study we demonstrate that these processes are partly regulated by the Wingless (Wg) signaling pathway.
The Wg or Wnt/β-catenin signaling pathway has been implicated in multiple developmental processes of metazoans, including cell fate specification, maintenance and proliferation of diverse tissues (Grigoryan et al., 2008; Kalani et al., 2008; Orsulic and Peifer, 1996; Siegfried and Perrimon, 1994; Staal et al., 2008). Alteration of the Wnt/β-catenin pathway also contributes to the etiology of several human cancers, including malignant blood disorders (Deshpande and Buske, 2007; Jauregui et al., 2008; Reya and Clevers, 2005). Wg/Wnt signaling begins through its binding to one or more of its receptors, Frizzled (Fz) and Drosophila Frizzled 2 (DFz2). The key component of the canonical Wg/Wnt pathway β-catenin, known in Drosophila as Armadillo (Arm), is stabilized upon activation of Wg signaling through the Fz receptors. Signaling allows a Disheveled (Dsh)-dependent inactivation of a degradation complex that contains Axin, APC (Adenomatous Polyposis Coli protein) and glycogen synthase kinase 3 (GSK3) known in flies as Shaggy (Sgg). This signaling event prevents Arm degradation and allows it to translocate into the nucleus where together with T-cell factor (TCF) also known as Drosophila Pangolin (Pan), Arm activates transcription of target genes. Variations in intracellular Wg signal transduction occur depending on the interaction between particular Fz receptors and Wg/Wnt ligands (Gordon and Nusse, 2006).
In this study we demonstrate that expression of Wg protein is maintained in stem-like hemocyte precursors of the LG throughout early stages of larval development but withdraws as cells differentiate into hemocytes within the CZ. Analysis of loss and gain of Wg signaling in stem-like precursors indicates that it is required for maintenance of their stem-like identity within the MZ and negatively regulates their differentiation into hemocytes of the CZ through the mediation of both Fz and DFz2 receptors. Wg signaling occurs locally in the different compartments of the lymph gland throughout all developmental stages. In addition to its function in stem-like precursors, DFz2 mediated Wg signaling positively regulates proliferation and maintenance of cells within the niche and crystal cells of the CZ. Our results reveal that DFz2, but not Fz, functions in the maintenance of these cell types. The involvement of the Wg signaling pathway in the regulation of various hemocyte lineages in Drosophila bears similarities to the context-dependent role of Wnt signaling in vertebrate hematopoietic stem cells, and suggests its requirement in the function of the hematopoietic niche.
Results
Wg and its receptor are expressed in stem-like hemocyte precursors of the developing lymph gland
Expression of Wg protein is not detected in embryonic lymph glands (Figure 1, A–B′). It is first detected in a majority of cells in the lymph gland upon hatching, during the early first instar (Figure 1, C,C′). In the 2nd instar, prior to the onset of differentiation, Wg is ubiquitiously expressed in the hematopoietic progenitors of the lymph gland (Figure 1, D,D′). The process of cortical zone formation begins in the mid 2nd instar where a few hemocytes at the distal edge of the lymph gland begin to differentiate, expressing maturation markers hemolectin (hml) encoding a homolog of mammalian Von Willebrand factor (Goto et al., 2001), and Peroxidasin (Pxn), a homolog of mammalian heme peroxidase (Nelson et al., 1994). At this stage, Wg begins to withdraw in a number of hemocytes at the distal edge of the LG (Figure 1,E, E′). Among the Wg negative hemocytes there are the ones that initiate expression of a reporter for the hml gene (Figure 1E–F). However withdrawal of Wg expression precedes the expression of the hml marker, and represents the earliest indication of CZ formation described to date. At 72 hours, Wg continues to be expressed at high levels in immature hemocyte precursors but not in the maturing hemocytes of the CZ (Figure 1, G, G′). As an exception, high level of Wg expression is seen in a rare population of hml+ hemocytes of the developing cortical zone, which we determined to be crystal cell precursors (see later for details). By late 3rd instar (108h), a second wave of Wg expression is apparent as mature hml+ hemocytes of the cortical zone re-initiate Wg expression (Figure 1, H, H′). We found that DFz2 is also expressed in all of the cells of the 1st and 2nd instar lymph gland (Figure 1, I–J). In a manner similar to Wg, DFz2 is down regulated during the 2nd and early 3rd instar in hemocytes located at the distal edge of the LG including the hml+ cells (Figure 1, K, K′). Similar analyses showed that Arm and Dsh are also expressed in hemocyte precursors and their expression is down regulated in differentiated cells of the cortical zone (not shown). DFz2 expressing cells are often grouped together in various parts of the developing LG (Figure 1, K, K′) and likely represent cells receiving Wg signal.
Figure 1. Wg protein and its receptor are expressed in immature hemocyte precursors during lymph gland development.
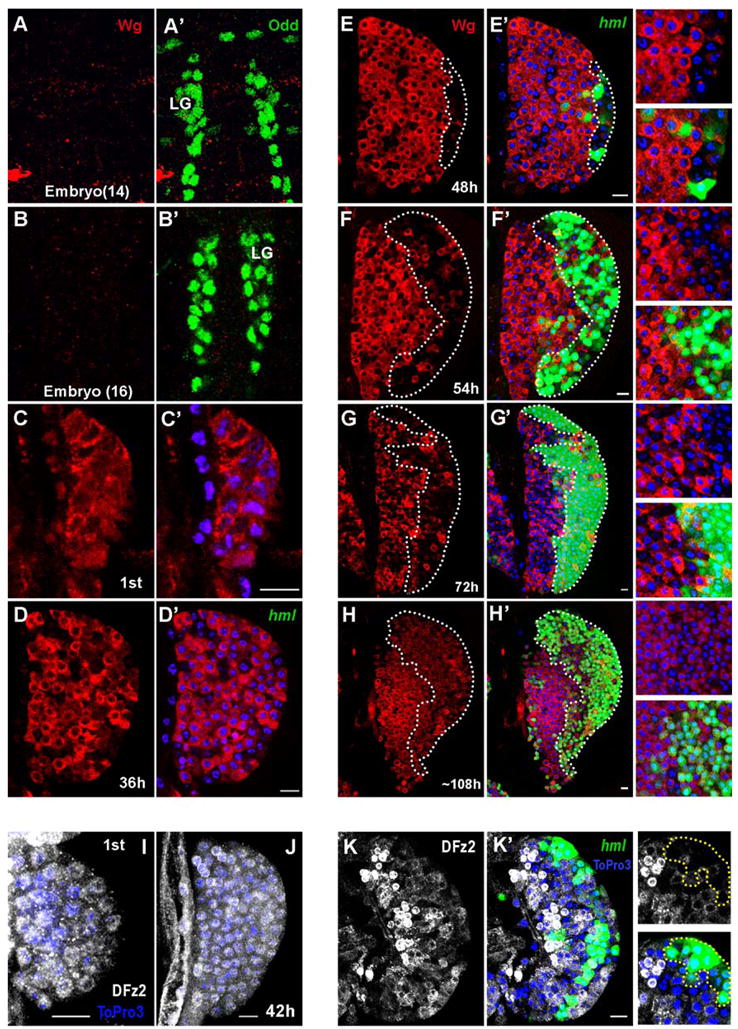
hml-Gal4, UAS-GFP was used to monitor expression of the hml gene. Wg, Odd and DFz2 expression was monitored by antibody staining (color coded in the panels). To visualize nuclei, ToPro-3 (blue) was used. Hours after larval hatching are as indicated in each panel. Scale bar: 10μm.
At embryonic stages 14 (A, A′) and 16 (B, B′) Wg protein (red) is not detected in the lymph gland (LG), marked by Odd (green in A′, B′). During the first instar, expression of Wg protein (red in C, C′) initiates in all cells of the LG. Wg (red) is uniformly expressed in the LG during early to mid 2nd instar (D, D′).
During the mid to late 2nd instar, expression of Wg protein (red in E, E′) is down regulated in maturing hemocytes at the distal edge of the LG (outlined by dotted line). At this stage, among Wg-negative hemocytes are cells that express hml (green, E′), marking the onset of hemocyte differentiation in the developing CZ. For clarity, corresponding magnified images are shown on the right side of the panels from E to H.
During early (F, F′) to mid (G, G′) third instar, Wg (red) is not expressed in the maturing hml+ hemocytes of the CZ (F′, G′, green, outlined by dotted line) while its expression is maintained in the prohemocytes of the MZ. A small number of scattered cells within the CZ expressing Wg were later determined to be crystal cells (see Figure 4).
During late 3rd instar, expression of Wg protein (red in H, H′), is maintained in prohemocytes, and in a second wave of expression is turned on in mature hml+ hemocytes (H′, green).
DFz2 protein (gray) is uniformly expressed in immature hemocyte precursors of LG during 1st (I) to mid 2nd (J) instar.
During late 2nd instar, DFz2 (gray) is expressed in immature hemocyte precursors and in a manner similar to Wg, is down regulated in maturing hml+ hemocytes (K, K′; green) of the developing CZ (hml+ cells are outlined by dotted lines in high magnification images on the right panels).
Canonical Wg signal mediated via Fz and DFz2 receptors is involved in the proper maintenance of hemocyte precursors
We assessed the role of Wg signaling by removing function of the Fz and DFz2 receptors using their dominant negative versions. domeless-Gal4 (domeless(dome), encoding an interleukine-like receptor (Brown et al., 2001)), which is expressed in the MZ, was used to express UAS-fzDN and UAS-Dfz2DN in the stem like hemocyte precursors. The double mutant combination gives a slightly smaller LG but otherwise all phenotypic effects are roughly additive over removing each receptor individually. Inactivation of the receptors did not significantly affect the initial differentiation of hemocytes during the 2nd instar (not shown). Later, during early to mid 3rd instar, clusters of dome+ cells are mislocalized to cortical areas of the LG (Figure 2, A–D). Interestingly, the expression of Shotgun (Shg), the Drosophila E-cadherin ortholog expressed as a hallmark indicator of the stem-like hemocyte precursors of the medullary zone, is dramatically down regulated in the mutant background (Figure 2, E–H). We speculate that loss of this important adhesion molecule contributes to the disruption of the proper zonal structure of the LG. Next we analyzed the expression of CZ markers Pxn and P1 in the mutant background. Normally, Pxn is an early marker for differentiated cells of the cortical zone and it is turned off in all but a very small population of dome+ hemocyte precursors at the distal edge of the MZ (Figure 2, I, I′). P1 or Nimrod, an EGF-domain receptor (Kurucz et al., 2007), is a late marker as it is expressed exclusively in terminally differentiated plasmatocytes of the CZ (Figure 2, M, M′). Many of the mislocalized cells in the receptor mutant background express Pxn even though they are dome positive (Figure 2, I–L and I′–L′). The relative number of P1+ hemocytes of the CZ is not significantly changed in these mutant lymph glands except that multiple sites of differentiation of P1+ cells can now be seen in locations that in wild type would be considered “medullary” (Figure 2, M–P′). The increase in double dome+/Pxn+ cells in these mutants indicates that Wg signaling negatively regulates the differentiation of a population of cells undergoing a transition in their fate. The expression data also suggest that Wg functions through Fz and DFz2 receptors. We found that simultaneous inactivation of these receptors significantly down-regulates the level of β-Catenin (Arm) in stem-like hemocyte precursors (not shown), suggesting the involvement of the canonical pathway. In order to explore the possible role of β-Catenin in this process we expressed Wg, as well as activated forms of Arm (ArmΔN and ArmS10) in the MZ using dome-Gal4 as a driver (Figure 3). Constitutive activation of the Wg signal in the hemocyte precursors blocks their ability to differentiate (Figure 3). The cells of the LG maintain expression of dome and fail to differentiate into mature cells of the cortical zone as revealed by using P1 as a marker. Taken together, loss and gain of function phenotypes suggest that the Wg pathway is involved in the proper maintenance of hemocyte precursors and that it negatively regulates the formation of the cortical zone and the population of the differentiated cells that it contains.
Figure 2. Wg signaling via Fz and DFz2 receptors is required for the proper maintenance of hemocyte precursors.
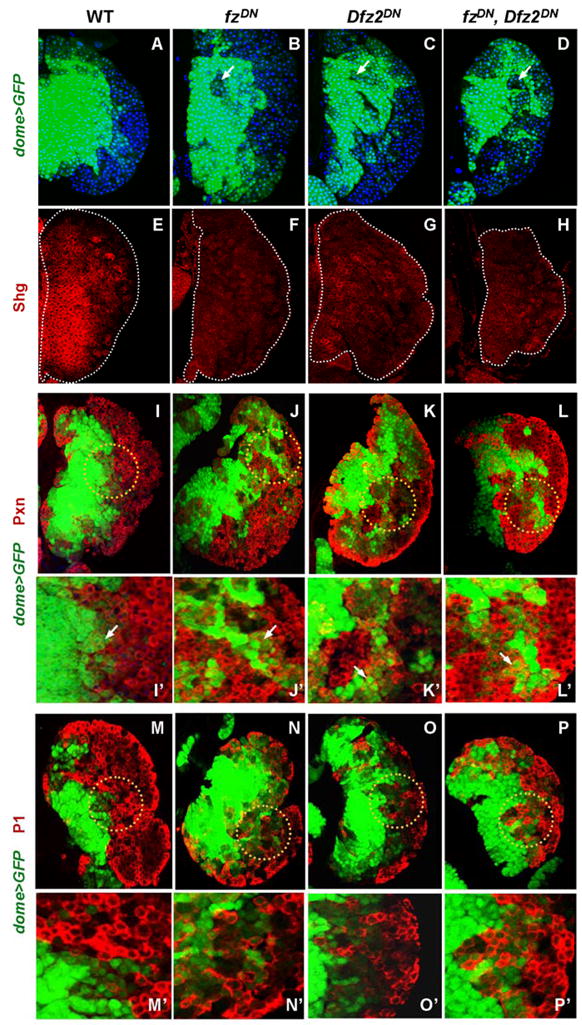
Genotypes (dome+ is an abbreviation for dome-Gal4, UAS-GFP) are listed on the top and drivers and antibodies for detection on the left edge of the panels. Antibodies and the markers are color coded in the appropriate panels. Images represent accrued 6μm confocal sections through the LG. (A–D) Redistribution of dome+ cells in fz/Dfz2 mutants. (A) In wild type (WT), prohemocytes marked by dome (green),are restricted to the medullary area of the LG.
In single mutant (B, C) and in double mutant (D) backgrounds, dome>GFP+ prohemocytes can be found in cortical areas while dome>GFP negative cells often reside inside the MZ compartment (indicated by arrows).
(E–H) Expression of DE-cadherin (Shg) is down regulated in fz/Dfz2 mutants. In WT (E) Shg (red) is expressed in the MZ, while in single (F, G) and double (H) mutants of the receptors, the expression of Shg is significantly reduced (edges of the LG are outlined by a dotted line).
(I–L′) Expression of Pxn, an early maturation marker,is up regulated in dome>GFP+ cells of fz/Dfz2 mutants. In wild type (I, I′), Pxn is expressed in mature hemocytes of the CZ and in rare dome+ hemocyte precursors located at the edge of the MZ (indicated by arrow in I′, which is the magnified area within the dotted circle in I). In single mutants (J, J′ and K, K′) and in the double mutant (L, L′) there is a significant increase in dome+/Pxn+ double positive cells that represent immature hemocytes in a transition state of differentiation (J′ to L′, indicated by arrows).
(M–P′) In fz/Dfz2 mutants the terminal differentiation of plasmatocytes is not affected as revealed by the expression of the late differentiation marker, P1. In WT (M, M′), as well as in single (N, N′ and O, O′) and in double (P, P′) mutants, P1 expression is restricted to mature hemocytes of the CZ. Higher magnification images (M′ to P′) of corresponding areas of LG outlined in M through P are shown. Lymph glands were analyzed at 68h after larval hatching maintained at 29°C.
Figure 3. Constitutive activation of Wg signaling blocks differentiation of hemocyte precursors.
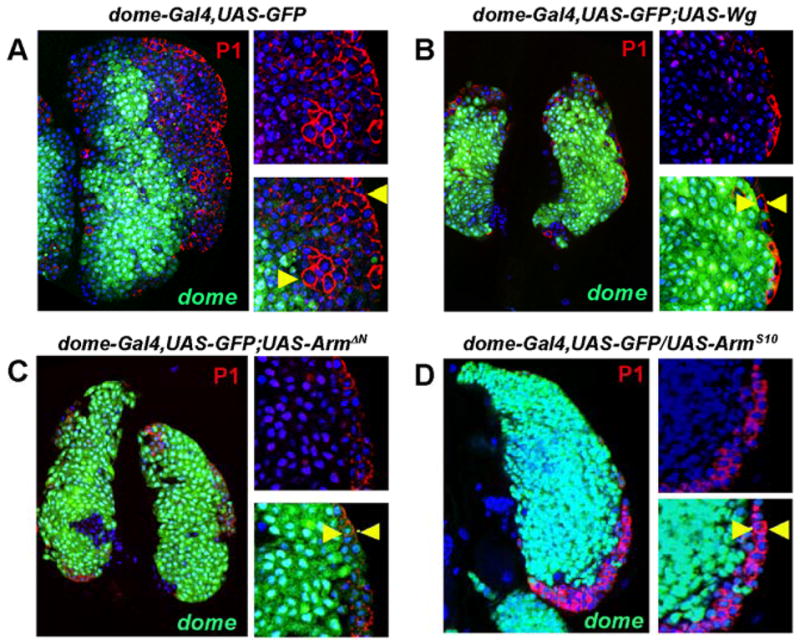
Genotypes are shown on top of corresponding panels. In wild type 3rd instar LG the cortical zone is properly developed and populated by differentiating hemocytes as revealed by staining with the plasmatocyte marker, P1 (A, red). For clarity, magnified images of the LG areas are shown on the right side of the major panels, with the arrowheads demarcating the extent of the CZ. Upon activation of Wg signaling in prohemocytes by overexpression of Wg (B) or expression of the activated forms of D-β-catenin (Arm) (C, D), the development of the CZ is dramatically suppressed. In these mutants the CZ represents only one or two cell layer of P1-positive cells(arrowheads).
Wg/DFz2 signaling is required for the maintenance and proliferation of the hematopoietic niche
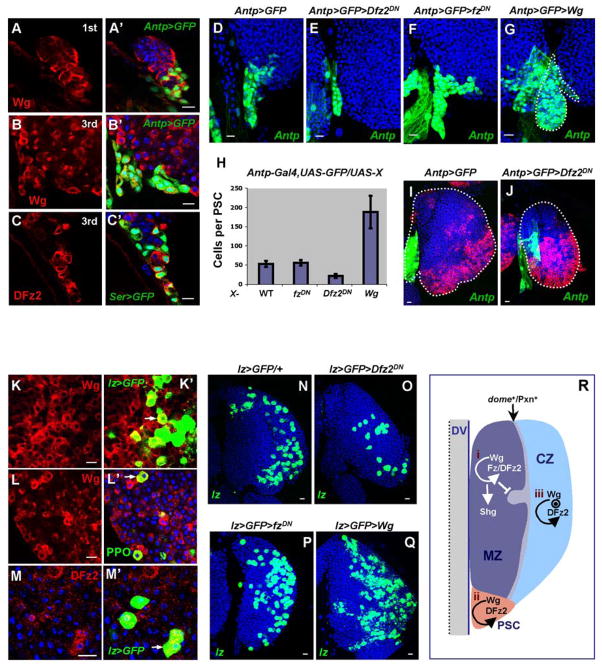
During Drosophila hematopoietic development, the microenvironment (niche) that maintains stem-like precursors of the medullary zone are the cells of the PSC that are specified early in embryonic development by the homeobox protein Antp (Mandal et al., 2007). Function of Antp is required for the maintenance and proliferation of PSC cells during embryonic and larval development. Wg protein is expressed in Antp+ cells of the PSC (Figure 4A, B) along with DFz2, Arm and Dsh (Figure 4, C and not shown). During wild-type larval development, the number of PSC cells increases from 5–8 cells during the 1st instar to approximately 50 cells by the 3rd instar (Mandal et al., 2007). Expression of a dominant negative form of Dfz2 causes significant reduction in the number of PSC cells (Figure 4, D, E, H); no such reduction was found when the dominant negative form of fz was used (Figure 4, F, H). Overexpression of Wg in the PSC causes an increase in the number of PSC cells (Figure 4, G, H). These loss and gain of function analyses suggest a second function of Wg in LG development through its control of PSC cell number. Our previous work has shown that maintenance of the stem-like precursors of the MZ requires the PSC cells functioning as a niche (Mandal et al., 2007). We found that expression of Dfz2DN in the PSC causes a significant reduction in the pool of hemocyte precursors and the overall size of the LG during the 3rd instar (Figure 4, I, J). Thus, Wg signaling is involved both directly by functioning within the precursor population and also indirectly by mediating, through the PSC, the control of stem-like hemocyte number within the lymph gland.
Figure 4. Roles of Wg signaling in controlling proliferation of PSC and crystal cells.
Genotypes are shown on top of each panel (A–G, I, J). Antp-Gal4, UAS-GFP and Ser-Gal4, UAS-GFP are abbreviated as Antp>GFP and Ser>GFP correspondingly, and they both mark cells of the PSC. Antp-Gal4,UAS-GFP;UAS-Dfz2DN is abbreviated as Antp>GFP>Dfz2DN.
In wild type, Wg protein (red) is expressed in the PSC adjoining with Antp>GFP+ cells during the 1st (A, A′) through the 3rd (B, B′) larval instar. DFz2 protein (red) is also expressed in the PSC overlapping with a subset of Ser>GFP+ cells in the 3rd instar (C, C′). Inactivation of Dfz2 (compare D and E) but not fz (compare D and F) causes a significant reduction in size of the PSC (green). Overexpression of Wg using Antp-Gal4 causes a significant enlargement in the size of the PSC (G, green). (H) Quantitation of average number of cells per PSC (n=10) in wild-type (WT), mutant receptor, and Wg overexpressed genetic backgrounds as indicated (standard deviations indicated as error bars). (I, J) Defect of PSC caused by lack of DFz2 signaling suppresses development of the LG. In wild type (I) MZ (blue) and CZ (red, as revealed by P1 staining) are properly developed. In LG with a deficit of PSC cells (J) there is a significant reduction in MZ (blue) and the overall size of the LG (margins of the LG are outlined by a dotted line, scale bar: 10μm).
(K–Q) Requirement of Wg/DFz2 signaling in controlling the number of crystal cells.
Wg (red in K, K′) is expressed in a population of lz>GFP+ crystal cells (green in K′, indicated by arrows). Wg is expressed in mature PPO+ crystal cells (green in L′). DFz2 protein (red in M, M′) is expressed in lz>GFP+ crystal cells (green in M′). (N to Q) Genotypes are listed on top of the corresponding panels. Normal numbers of crystal cells in the wild-type LG (N, green). Number of crystal cells in Dfz2 mutant (O, green) is significantly reduced while the number of crystal cells is not affected in fz mutant (P, green). Activation of Wg signaling in crystal cell precursors causes an increase in the number of crystal cells (Q). (R) Multiple roles of Wg signaling in regulating hematopoietic development.
(i) Short range Wg signaling mediated through Fz/DFz2 receptors is involved in maintaining undifferentiated hemocyte precursors in the MZ. Wg positively regulates the expression of Shg and down regulates Pxn expression in hemocyte precursors suppressing development of the cortical zone. Inactivation of Wg signaling promotes hemocyte precursors toward differentiation as evident by an increase in double positive (dome+/Pxn+) transition stage hemocytes.
(ii) Local Wg signaling through the DFz2 receptor is required for maintenance and proliferation of PSC cells and indirectly controls number of prohemocytes.
(iii) Local Wg signaling mediated through the DFz2 receptor, is required for proliferation of crystal cells in the cortical zone.
A late requirement of Wg/DFz2 signaling in controlling crystal cell number
Crystal cells begin to appear in the developing cortical zone during the late 2nd instar and their number gradually rises during the 3rd instar (Jung et al., 2005). The Runx domain protein, Lz, is expressed and is required for the specification of crystal cell precursors (Lebestky et al., 2000). Immunostaining for Wg protein with the crystal cell markers lz-Gal4, UAS-GFP and prophenoloxidase (PPO) revealed that Wg is expressed in a fraction of the lz+ cells (Figure 4, K) and in all of the mature PPO+ crystal cells (Figure 4, L). DFz2 (Figure 4, M), Arm and Dsh (not shown) are also expressed in crystal cells and their precursors during late 2nd to 3rd instars. This is a late expression of Wg signaling components in a differentiated cell type and it is likely to have a function distinct from hemocyte precursor specification. To determine the role of Wg signaling in crystal cell development we expressed components of the pathway using lz-Gal4 as a crystal cell specific driver. Overexpression of Wg protein in lz+ cells causes an increase in the number of crystal cells (Figure 4, N, Q). Inactivation of Dfz2 with a dominant negative version of the receptor causes a significant reduction in the number of crystal cells (Figure 4, N, O). We found that down-regulation of DFz2 signaling reduces crystal cell number to 26 (+/−8) cells compared to 70 (+/−5) cells per LG lobe in wild type. Inactivation of fz activity using a lz-Gal4 driver did not affect crystal cell number (Figure 4, P), suggesting that Wg signaling in crystal cell proliferation is solely mediated through DFz2. We conclude that Wg plays a role in the maintenance of the stem-like hemocyte precursors, the hematopoietic niche and at least one differentiated cell type. For the first function, it requires both Fz and DFz2 receptors whereas the other two functions involve only the DFz2 receptor (Figure 4, R).
Discussion
Our results demonstrate a requirement for Wg signaling in hematopoiesis, adding to the role of the Notch, Hedgehog, Jak/STAT and Ras/Raf/MAPK pathways in various aspects of blood development in Drosophila (reviewed in Evans et al., 2007). It is known that the Wg/Wnt signaling pathway is required for hematopoietic development in mammals and its deregulation is involved in leukemogenesis in humans (Reya and Clevers, 2005; Staal et al., 2008). Although a large number of studies have explored its function, the role of Wnt signaling in mammalian hematopoiesis remains unresolved due to the often conflicting results obtained from in vitro, in vivo and misexpression experiments (reviewed in Staal and Sen, 2008). Independent studies of β-catenin knock out in mice have shown reduced self-renewal capacity of HSCs (Zhao et al., 2007), while other studies have suggested that Wnt signaling may still occur in HSCs lacking both β- and γ-catenins (Jeannet et al., 2008; Koch et al., 2008). Loss of Wnt signaling derived from the endosteal HSC niche inhibits the ability of HSCs to reconstitute an immune system due to loss of quiescence (Fleming et al., 2008). It is not clear from these studies if the defects in reconstitution are due to engraftment problems or stem cell exhaustion. In our studies we took advantage of Drosophila genetics to help clarify the role of Wg signaling in hematopoiesis. The lymph gland allows an in vivo model system in which to visualize the dynamic interactions of Wg with other pathways and dissect the genetic networks involved in the maintenance of stem-like blood progenitors.
As in vertebrates, hematopoietic development in Drosophila is controlled at the level of multipotential stem-like cells that are maintained and eventually differentiate into various mature hemocyte lineages (Evans et al., 2007; Jung et al., 2005). Our time course studies show that Wg and other components of the pathway are withdrawn from cells upon their differentiation but maintained in stem-like hemocyte precursors. Loss and gain of function analyses indicate that Wg signaling operates through a short-range mechanism in hemocyte precursors, where local concentrations of Wg negatively control their differentiation. It is important to note that this function of Wg signaling is later than its requirement for promoting development of cardiogenic mesoderm during early embryogenesis (Mandal et al., 2004), as dome-Gal4 and Wg expressions in LG overlap only during larval instar. The function of Wg signaling in stem-like hemocyte precursors is conserved, as the loss of Wnt3a or β-catenin in mammalian HSCs significantly impairs their self-renewal, while activation of the pathway exhausts HSCs through a block in differentiation that increases their self-renewal (Fleming et al., 2008; Kirstetter et al., 2006; Reya et al., 2003). We have identified Drosophila E-Cadherin as a target of Wg function in prohemocytes, where it likely contributes to proper zonation of the LG. Control of E-Cadherin expression by the Wnt/Wg pathway has been shown in other invertebrate and vertebrate tissues, indicating a conservation of this function (Wang et al., 2007; Wodarz et al., 2006). The observed deficiency in DE-cadherin expression in stem-like progenitors lacking Wg signaling may provide an additional similarity to the lack of engraftment of mammalian HSCs exposed to a Wnt-deficient niche.
During hematopoietic development, Wg signaling operates locally in different compartments of the LG. Importantly, Wg is required for maintaining proper development of the PSC, the hematopoietic niche. This indicates that Wg signaling controls maintenance of hematopoietic progenitors by a dual mechanism, a direct cell autonomous function in prohemocytes and an indirect regulation that depends on its role in the development of the niche. An analogous mechanism has been shown in the mouse system, where a microenvironment lacking Wnt signaling fails to maintain HSCs in a quiescent state, reducing their long term reconstituting activity (Fleming et al., 2008).
The maintenance of stem-like hemocyte precursors is mediated by integration of a number of signaling pathways. In addition to the intrinsic function of the Wg pathway in prohemocytes, other niche generated extrinsic signals, such as Hh and the JAK/STAT pathways, participate in this process (Krzemien et al., 2007; Mandal et al., 2007; Martinez-Agosto et al., 2007). Wg/Wnt signaling function is important in intestinal stem cells of Drosophila and mammals (Fevr et al., 2007; Takashima et al., 2008), as well as in mammalian HSCs, T and B cells (Staal et al., 2008). It is clear that the functions of Wg signaling in hematopoietic processes of Drosophila are conserved during mammalian hematopoiesis.
Previous studies have suggested that the function of Fz and DFz2 receptors can be either redundant or distinct depending on the signaling context (Bhanot et al., 1999; Gordon and Nusse, 2006). In this regard, we found that function of Fz receptors is redundant in prohemocyte maintenance while only DFz2 is utilized for Wg signaling in cells of the hematopoietic niche and the crystal cells.
Our ability to observe the dynamic pattern of wingless expression across developmental stages established its in vivo role in the maintenance of stem-like progenitors. The advantage of being able to manipulate individual cell populations within each compartment of the lymph gland permitted us to dissect the direct vs. indirect effects of Wg on blood progenitors. This versatility in analysis emphasizes the advantages of Drosophila blood development as a model of hematopoiesis in which to further investigate the complex events of Wg signaling and its intricate interaction with other signaling networks during hematopoietic development.
Experimental procedures
Genetics
UAS-wg, UAS-armS10, lz-Gal4,UAS-GFP, was obtained from Bloomington Drosophila Stock Center. UAS-Dfz2GPI(UAS-Dfz2DN) and UAS-armΔN was gifted from T. Tabata (Sato et al., 2006). UAS-fzDN transgenic flies were obtained from R.W. Carthew (Zhang and Carthew, 1998). We used several Gal4 lines that are expressed in different compartments of lymph glands: Antp-Gal4, Ser-Gal4, dome-Gal4 (Mandal et al., 2007), and hmlΔ-Gal4 (Sinenko and Mathey-Prevot, 2004). For control of Gal4/UAS experiments (Brand and Perrimon, 1993), larvae from crosses of a particular Gal4 line and w1118 were used. To restrict transgene expression to a specific stage of development we utilized the Gal80TS technique (McGuire et al., 2003).
Immunohistochemistry
Monoclonal antibodies against Wg (4D4) and Arm (N2 7A1 ARMADILLO) were obtained from the Drosophila Hybridoma Bank. Rabbit antibodies against DFz2N and mouse anti-Shg were gifted by V. Budnik (Mathew et al., 2005) and V. Hartenstein (Mandal et al., 2007) respectively. Hemocyte-specific monoclonal antibodies P1 (anti-Nimrod) and L1 were obtained from I. Ando (Kurucz et al., 2007); mouse anti-Pxn was a gift from J.H. Fessler (Nelson et al., 1994); and rabbit anti-PPO2 from F. Kafatos (Muller et al., 1999). To visualize cell nuclei we performed DNA staining utilizing the dye ToPro3 (Molecular Probes). Cy3- or FITC- labeled anti-mouse, -rat, and -rabbit secondary antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. Lymph gland immunostaining was performed as previously described (Lebestky et al., 2000) with the following modifications. To stage larvae, crosses were set up in grape juice agar plates and hatching 1st instar larvae were transferred to small Petri dishes with regular food medium at 25°C or 29°C and allowed to grow. Dissected larvae with attached LG were fixed with 4% methanol-free formaldehyde (Polysciences, Inc) for 20min at room temperature. For immunostaining with anti-Wg antibodies we used a PBS-TBSA solution consisting of PBS with 0.1% Tween-20 (Fisher Scientific) and 1% BSA (Roche).
Acknowledgments
We thank J.D. Axelrod, R. Nusse, T. Tabata, G. Struhl, and R.W. Carthew for sharing numerous Drosophila stocks. We acknowledge V. Budnik, T. Uemura and V. Hartenstein for anti-Dsh, anti-DFz2N and anti-Shg antibodies and we thank I. Ando, F. Kafatos and J.H. Fessler for hemocyte specific monoclonal antibodies. We are grateful to the Bloomington Drosophila Stock Center at Indiana University and the Kyoto Drosophila Genetic Resource Center for sharing numerous fly stocks. We thank the Developmental Studies Hybridoma Bank at the University of Iowa for making accessible a number of monoclonal antibodies. We thank all colleagues in the Banerjee lab for valuable discussions during these studies. This work was supported by an NIH grant (5R01 HL067395) to U.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- Deshpande AJ, Buske C. Knocking the Wnt out of the sails of leukemia stem cell development. Cell Stem Cell. 2007;1:597–598. doi: 10.1016/j.stem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol. 2002;12:1923–1927. doi: 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Sinenko SA, Mandal L, MartinezAgosto JA, Hartenstein V, Banerjee U, Rolf B. Advances in Developmental Biology. Elsevier; 2007. Genetic Dissection of Hematopoiesis Using Drosophila as a Model System; p. 259. [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Goto A, Kumagai T, Kumagai C, Hirose J, Narita H, Mori H, Kadowaki T, Beck K, Kitagawa Y. A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. Biochem J. 2001;359:99–108. doi: 10.1042/0264-6021:3590099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui MP, Sanchez SR, Ewton AA, Rice L, Perkins SL, Dunphy CH, Chang CC. The role of beta-catenin in chronic myeloproliferative disorders. Hum Pathol. 2008;39:1454–1458. doi: 10.1016/j.humpath.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of -catenin and {gamma}-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of - and {gamma}-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. Embo J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. Cell-cell signalling: Wingless lands at last. Curr Biol. 1996;6:1363–1367. doi: 10.1016/s0960-9822(96)00731-2. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci. 2006;9:67–75. doi: 10.1038/nn1604. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Mathey-Prevot B. Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 2004;23:9120–9128. doi: 10.1038/sj.onc.1208156. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Wang L, O’Leary H, Fortney J, Gibson LF. Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood. 2007;110:3334–3344. doi: 10.1182/blood-2007-01-068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Stewart DB, Nelson WJ, Nusse R. Wingless signaling modulates cadherin-mediated cell adhesion in Drosophila imaginal disc cells. J Cell Sci. 2006;119:2425–2434. doi: 10.1242/jcs.02973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Carthew RW. Interactions between Wingless and DFz2 during Drosophila wing development. Development. 1998;125:3075–3085. doi: 10.1242/dev.125.16.3075. [DOI] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]