Abstract
Previous research indicates that children from lower socioeconomic backgrounds show deficits in aspects of attention, including a reduced ability to filter irrelevant information and to suppress prepotent responses. However, less is known about the neural mechanisms of group differences in attention, which could reveal the stages of processing at which attention deficits arise. The present study examined this question using an event-related brain potential (ERP) measure of selective auditory attention. Thirty-two children aged 3- to 8-years participated in the study. Children were cued to attend selectively to one of two simultaneously presented narrative stories. The stories differed in location (left/right speaker), narration voice (male/female), and content. ERPs were recorded to linguistic and non-linguistic probe stimuli embedded in the attended and unattended stories. Children whose mothers had lower levels of educational attainment (no college experience) showed reduced effects of selective attention on neural processing relative to children whose mothers had higher levels of educational attainment (at least some college). These differences occurred by 100 msec after probe onset. Furthermore, the differences were related specifically to a reduced ability to filter irrelevant information (i.e., to suppress the response to sounds in the unattended channel) among children whose mothers had lower levels of education. These data provide direct evidence for differences in the earliest stages of processing within neural systems mediating selective attention in children from different socioeconomic backgrounds. Results are discussed in the context of intervention programs aimed at improving attention and self-regulation abilities in children at-risk for school failure.
Even before the first day of kindergarten, a child’s academic prospects can be predicted based on characteristics of his or her parents, including their income, occupation, and level of education (e.g., Baydar, Brooks-Gunn, & Furstenberg, 1993; Duncan, Brooks-Gunn, & Klebanov, 1994; Walker, Greenwood, Hart, & Carta, 1994). Collectively, these familial characteristics are considered part of a child’s socioeconomic status (SES), with children from lower socioeconomic backgrounds at-risk for school failure. Indeed, a robust predictor of a child’s classroom grades, standardized test scores, and likelihood of high school graduation is her own mother’s level of education (Baydar et al., 1993; Liaw & Brooks-Gunn, 1994; Walker et al., 1994). These intergenerational cycles of academic underachievement are embedded in a complex system of factors that covary with socioeconomic status, including school and neighborhood characteristics, parenting practices, and exposure to neurotoxins (Brooks-Gunn & Duncan, 1997; Duncan et al., 1994; Jimerson, Egeland, Sroufe, & Carlson, 2000). Several lines of evidence suggest that these and other environmental covariates mediate at least part of the relationship between familial SES and children’s academic outcomes (Brooks-Gunn & Duncan, 1997; Capron & Duyme, 1989; Duncan et al., 1994; Jimerson et al., 2000; Noble, McCandliss, & Farah, 2007; Schiff, Duyme, Dumaret, & Tomkeiwics, 1982).
Although previous research has examined the relationship between SES and broad academic indicators such as graduation rates and standardized test scores, recent research has shifted the focus of the question to whether SES is associated with differences in more specific neurocognitive systems, for example in aspects of language or attention (Farah et al., 2006; Mezzacappa, 2004; Noble et al., 2007; Noble, Norman, & Farah, 2005). It has been proposed that academic underachievement among children from lower socioeconomic backgrounds might be related most strongly to the development of certain foundational skills or neural systems (Mezzacappa, 2004; Noble et al., 2005). Under this hypothesis, atypical development of a foundational skill could have cascading consequences on later development and learning. Thus, if specific neurocognitive deficits could be identified in children from lower socioeconomic backgrounds early in development, it might be possible to develop more focused interventions that target these systems or skills. As such, the long-term goal of this research is the identification of aspects of cognition that could be targeted by interventions for children from lower socioeconomic backgrounds, as part of larger programs of systemic change addressing the negative impacts of lower SES on children’s cognition.
The present research, motivated in part by recent reports of behavioral deficits in aspects of attention as a function of familial SES (Farah et al., 2006; Lipina, Martelli, Vuelta, & Colombo, 2005; Lupien, King, Meaney, & McEwen, 2001; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005), examines the neurobiology of selective auditory attention in children from different socioeconomic backgrounds. Using maternal education as a proxy for SES, the present study examines whether children whose mothers have lower levels of education (defined as no college experience) versus higher levels of education (here defined as some college experience) differ in the effects of sustained, selective auditory attention on early (~100 msec) stages of perceptual processing indexed using event-related brain potentials (ERPs). If differences were observed at this early stage of neural processing, it would provide a possible mechanism whereby deficits in aspects of attention could influence the processing of information in a range of domains.
Attention in children from diverse socioeconomic backgrounds
A handful of recent studies have examined aspects of attention and executive function in children from diverse socioeconomic backgrounds (Farah et al., 2006; Lipina et al., 2005; Lupien et al., 2001; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005). These studies, described in detail below, reveal that children from lower socioeconomic backgrounds experience difficulties with aspects of attention as early as the first year of life (Lipina et al., 2005) that persist at least through early adolescence (Farah et al., 2006). The attention deficits observed appear most linked to tasks that require filtering distracting information, managing response conflict, and regulating behavior.
Even among infants from different socioeconomic backgrounds, differences in attentional precursors can be observed. One recent study (Lipina et al., 2005) tested a group of 280 Argentinian 6- to 14-month-old infants using the A-not-B task, which is associated with later executive functioning skills. The infants from lower socioeconomic backgrounds showed poorer performance on the task relative to their higher SES peers. The authors proposed that these early differences could be a precursor to later difficulties in executive function and attention in children from lower socioeconomic backgrounds.
Indeed, several studies of school-age children report deficits in aspects of attention in children from lower socioeconomic backgrounds. For example, Mezzacappa (2004) assessed a group of 249 children age five- to seven-years-old using the children’s attention network test (ANT; Rueda et al., 2004). Relative to their higher SES peers, children from lower socioeconomic backgrounds showed increased interference from peripheral flanker stimuli, indicating a reduced ability to filter distracting information and manage response conflict. Children from lower socioeconomic backgrounds also showed reduced influences of an alerting cue on accuracy and reaction times, suggesting that children from lower socioeconomic backgrounds might have an overall upregulation of attention vigilance.
A study by Lupien and colleagues (2001) also suggested that attention is compromised in children from lower socioeconomic backgrounds, at least at early ages. In this study, separate comparisons were made of children from higher and lower socioeconomic backgrounds at each of six different age groups, ranging from early elementary school (mean age six years) to late high school (mean age 16 years). Children completed a visual detection task requiring selective attention. In the task, children indicated whether the number “2” appeared in a briefly-presented four-item visual display containing either distractor letters (easy detection) or distractor numbers (difficult detection). In the youngest age group, children from lower socioeconomic backgrounds performed significantly worse than children in the higher SES group on this task. However, this pattern was reversed in children in the oldest age group and absent in children in the middle age groups. These data suggest possible attentional differences associated with SES early in development, though interpretation is confounded by possible differences in letter/number knowledge given the use of the alphanumeric stimuli and probable differences in task difficulty across the age groups.
In a series of studies conducted by Farah and colleagues, aspects of attention and executive function were examined in elementary- and middle-school students from different socioeconomic backgrounds. An initial study of 60 African-American first-graders (Noble et al., 2005) found that children from lower socioeconomic backgrounds showed large deficits (Cohen’s d = 0.68) on an executive attention battery that included a go/no-go task, a test of spatial working memory, and false alarm rates on three tasks from the other test batteries. The children from lower socioeconomic backgrounds also tended to have poorer performance on both a dimensional card sort task that required shifting rule sets and a theory of mind task that required taking the perspective of another individual. Similar results were obtained in two subsequent studies. One study (Farah et al., 2006) included 60 middle-school children and reported that adolescents from lower socioeconomic backgrounds had poorer performance on a cognitive control composite including a go/no-go and number Stroop task. A second study (Noble et al., 2007) included a larger sample of 150 first-grade children, in which familial SES was treated a continuous variable. Significant, positive correlations were observed between familial SES and a composite measure of executive function that included a go/no-go task and the NEPSY auditory attention response set task. In addition, in two of the three studies described above, the relationship between SES, executive function, and language was examined (Noble et al., 2007; Noble et al., 2005). In both studies, the relationship between SES and executive function did not persist when performance on standardized measures of language and preliteracy (which also differed between groups) were controlled for statistically. A similar analysis indicated that SES differences in language/preliteracy scores were accounted for partially, but not completely, by differences in children’s attention scores. The authors suggested that this pattern of findings could indicate that differences in language cause secondary deficits in aspects of attention.
Taken together, the results of these studies suggest that children from lower socioeconomic backgrounds have difficulty in some aspects of attention, at least during the early school years, and that these differences might be related to, i.e. co-occur with, cause, or be caused by, performance in other domains, including language and preliteracy. While it has been inferred from these behavioral studies that children from lower socioeconomic backgrounds have deficits in specific neurocognitive systems mediating attentional control and filtering (Farah et al., 2006; Lupien et al., 2001; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005), little direct evidence exists on this point. Yet, such data would be useful in further characterizing the nature of group differences in aspects of attention. That is, these previous studies indicate a behavioral deficit in aspects of attention that are associated with later stages of processing indexed by a late (behavioral) measure of response selection. However, behavioral performance reflects the final output of multiple stages of processing, leaving it unclear whether difficulties in attention arise from earlier (e.g., perceptual) and/or later (e.g., response selection) processing stages. Examining the neural mechanisms of attention can provide greater insight into the nature of group differences on behavioral indices of attention and, as a consequence, into the mechanisms whereby an attention deficit could influence other aspects of cognitive or socioemotional development.
Before proceeding, it is important to note that any investigation into the neural mechanisms of cognitive skills in children from different socioeconomic backgrounds should not be confused with an effort to identify genetic determinants of cycles of poverty and lower educational attainment. Indeed, the brain is highly plastic and modifiable by experience (e.g., Bavelier et al., 2001; Castro-Caldas & Reis, 2003; Pascual-Leone, Armedi, Fregni, & Merabet, 2005; Recanzone, Schreiner, & Merzenich, 1993). Furthermore, the neural mechanisms investigated in the present study have been shown to be modifiable in response to interventions, including training with high-intensity computerized language programs (Stevens, Fanning, Coch, Sanders, & Neville, 2008) or small-group interpersonal reading programs (Stevens, Currin et al., 2008). As such, an understanding of the nature of attention deficits at a more precise level can guide efforts to develop interventions that can target the specific neurocognitive systems that are most in need.
Neural Mechanisms of Selective Attention
Event-related brain potentials (ERPs) have been used to characterize the earliest neural mechanisms of sustained, selected attention in both adults (Hillyard, Hink, Schwent, & Picton, 1973; Hillyard, Woldoff, Mangun, & Hansen, 1987; Woldoff & Hillyard, 1991) and children (Coch, Sanders, & Neville, 2005; Sanders, Stevens, Coch, & Neville, 2006). Over thirty years ago, Hillyard et al. (1973) first used sensory evoked potentials to study selective auditory attention. Subjects attended to rapid tones in one ear and ignored a separate stream of tones (different in pitch) presented in the other ear. The participants’ task was to detect occasional target stimuli in a single ear, forcing them to restrict attention to only one side at a time. ERPs were recorded to standard tone pips occurring in the attended and unattended channel. The first negative component of the evoked potential (N1, 80–110 msec after stimulus onset) was larger to tones when attended relative to when attention was directed away from them to the other ear, indicating attentional modulation of early sensory processing. This early amplification likely resulted from the joint processes of signal enhancement of the attended stimuli and suppression of the competing stimuli presented in the ignored channel. Several subsequent studies replicated and extended this basic ERP signature of selective attention using a variety of different stimuli and paradigms (Hillyard et al., 1973; Hillyard et al., 1987; Woldoff & Hillyard, 1991).
We recently adapted the classic spatial selective auditory attention ERP paradigm to be child-friendly for testing with young elementary-aged and preschool children (Coch et al., 2005; Sanders et al., 2006). Coch, Sanders, and Neville (2005) measured attention in adults and typically developing children aged six- to eight-years. Participants were instructed to listen to one of two narratives, which were played simultaneously from separate speakers located to the left or right of the participant. ERPs were recorded to 100 msec probe stimuli superimposed on the attended and unattended narratives. In both children and adults, probes in the attended story elicited larger amplitude ERPs when attention was directed toward as compared to away from the story. This attentional enhancement began during early sensory processing, approximately 100 msec after probe onset. The similarity in the nature and timing of attentional modulation in adults and children was particularly striking in light of the differences in the underlying morphology of the auditory evoked potential components. Whereas adults displayed an evoked response that included an early positivity (P1) followed by a negative component around 100 msec (N1), children showed a broad positivity from 100–300 msec in response to probe stimuli. This difference in morphology is consistent with developmental research showing that children’s auditory evoked potentials are dominated by a broad, positive response followed by a later negativity, particularly in acoustically crowded environments with short interstimulus intervals between stimuli (Ponton, Eggermont, Kwong, & Don, 2000; Sharma, Kraus, McGee, & Nicol, 1997). We later replicated this work and extended it to children as young as three years of age (Sanders et al., 2006). Similar to six- to eight-year-old children, typically developing three- to five-year-old children also showed a broad positivity in response to probe stimuli that was enhanced with attention by 100 msec after probe onset. However, in three- to five-year olds, the duration of the attention effect extended into the 200 – 300 msec time window, whereas the attention effect in six- to eight-year olds was complete by roughly 200 msec. This extended attention effect in the younger children could reflect a prolonged effect of attention on neural processing and/or variability between or within individual children in the latency of attention effects.
These studies suggest that ERPs can be used to index the early neural mechanisms of selective attention in very young children. Importantly, because ERPs can be recorded continuously and non-invasively, they provide an online index of the neural mechanisms of selective attention without requiring overt behavioral responses. We have used this paradigm to examine the neural mechanisms of sustained, selective attention in young children with specific language impairment (SLI) who, along with children with reading impairment, are reported to have behavioral deficits in aspects of attention (Asbjørnsen & Bryden, 1998; Atkinson, 1991; Cherry, 1981; Sperling, Lu, Manis, & Seidenberg, 2005; Ziegler, Pech-Georgel, George, Alario, & Lorenzi, 2005). Unlike typically developing children, children with SLI do not show ERP evidence of early attentional modulation, even when performing the task as directed (Stevens, Sanders, & Neville, 2006). Further, the deficits in children with SLI are linked specifically to reduced amplification of the neural response to probes in the attended channel (i.e., signal enhancement) rather than difficulties in suppression of responses to probe stimuli in the ignored channel (i.e., distractor suppression) (Stevens et al., 2006). This suggests that the ERP paradigm described above is sensitive to group differences in the neural mechanisms of attention and can be used to index different processes of early, spatial selective attention (signal enhancement and distractor suppression) in young children.
Overview of the Present Study
In the present study, 32 children age three- to eight-years completed the ERP measure of sustained, selective auditory attention described above and used in our previous research (Sanders et al., 2006; Stevens et al., 2006). The children were divided into two groups based on their mother’s level of education: 16 children lived with mothers who had completed at least one year of college (higher maternal education) and 16 children lived with mothers who had no college experience (lower maternal education). It was predicted that children in the lower maternal education group would show reduced or absent effects of attention on early stages of neural processing.
Method
Participants
Thirty-two children aged three- to eight-years participated in the present study (range = 3.8 years to 8.7 years, M = 6.1 years, SD = 1.4, 16 girls). Of the participants providing information on race/ethnicity, the majority (90%) were White/Caucasian. All participants met the following criteria for participation in the study: (1) Monolingual English speakers, (2) No history of neurological or language disorders, and (3) Normal hearing, vision, and oral-motor performance on standard screenings. In addition, because our previous research indicated marked deficits on this task in children with specific language impairment (Stevens et al., 2006), only children scoring above the 25th percentile on the receptive language composite were included in the study.
The children in the final sample represent a subset of those reported on previously in our study of 53 typically developing children age three- to eight-years of age (Sanders et al., 2006). This subset of children was selected to maximize differences based on socioeconomic background (see below), while keeping all experimental conditions balanced across the two groups.
Although education and occupation information concerning the mother, father, and any step-parents or guardians was collected using the Hollingshead questionnaire (Hollingshead, 1975), maternal education alone was used as a proxy for SES. This decision was based on the variable family structure observed in the sample of young children. Specifically, whereas all children currently lived with their mother and had done so since birth, the presence and number of years of contact with fathers, step-parents, and other guardians was highly variable. Thus, in order to apply a consistent coding scheme to all children, the mother’s data alone was utilized. As previous research has noted the temporal instability of maternal occupational status and its lack of correlation to children’s cognitive outcomes (Gottfried, Gottfried, Bathurst, Wright Guerin, & Parramore, 2003), only maternal education scores were used. The use of maternal education is consistent with previous research showing that maternal education alone correlates with children’s cognitive outcomes (Baydar et al., 1993; Gottfried et al., 2003; Liaw & Brooks-Gunn, 1994; Noble et al., 2007; Walker et al., 1994).
Thus, children were divided into two groups based on the level of education completed by their mother. “Higher maternal education” was defined as having at least one year of college experience, whereas “lower maternal education” was defined as having completed no more than high school. Of the 16 children in the lower maternal education group, 15 had parents who had completed high school. Of the 16 children in the higher maternal education group, 10 had mothers who had completed partial college (at least one year), four had mothers with four-year degrees, and two had mothers with graduate degrees. Table 1 summarizes the demographic characteristics of each group.
Table 1.
Demographic characteristics of participant groups. None of the differences were statistically significant.
| Higher Maternal Education | Lower Maternal Education | |
|---|---|---|
| N | 16 | 16 |
| # Male | 8 | 8 |
| Race/Ethnicity | 12 White 1 American Indian 1 Hispanic 2 decline to answer |
14 White 1 White/Black 1 decline to answer |
| Age in years (SD) | 6.0 (1.4) | 6.2 (1.3) |
| Receptive Language SS (SD) | 109 (15) | 103 (11) |
Behavioral Testing
All participants completed the receptive language composite from the Clinical Evaluation of Language Fundamentals (CELF; Semel, Wiig, & Secord, 1995). Behavioral and ERP testing occurred in different sessions separated by no more than 35 days.
Materials
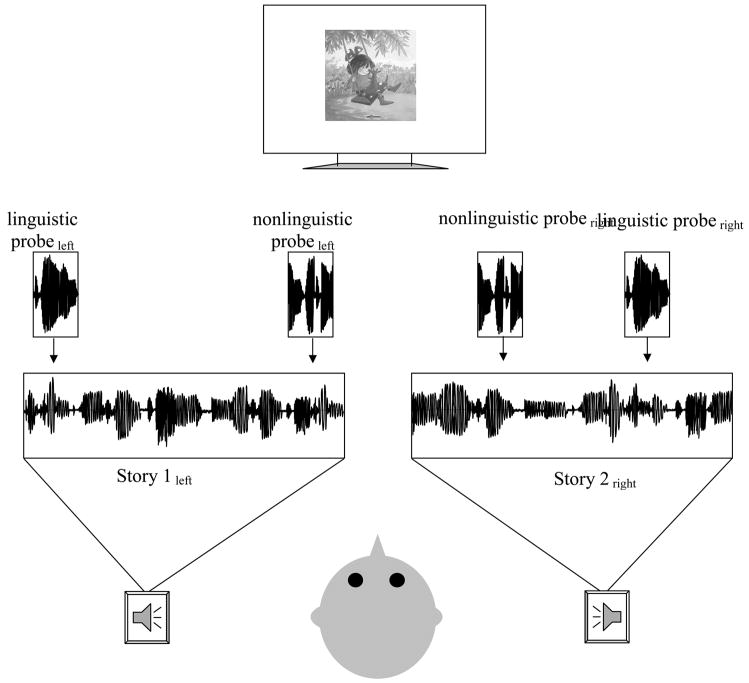
Eight 2.5–3.5 minute stories were digitally recorded (16 bit, 22kHz) using an Electro Voice 1750 microphone connected to a Macintosh computer running a sound-editing program (SoundEdit 16, Version 2). These included four stories from the Blue Kangaroo series (Clark, 1998, 2000, 2002, 2003) and four stories from the Harry the Dog series (Zion & Graham, 1956, 1960, 1965, 1976). The story series were different in style and content and each was recorded by both a male and female narrator, reading in a child directed manner. Pauses were edited to be less than 1 second, and the mean amplitude of the files was normalized to 60 dB SPL (A-weighted). Following editing, stereo files were created that presented one story from each series in a separate channel (right/left speaker). The two audio channels always differed in story series (Harry the Dog or Blue Kangaroo) and narrator voice (male or female). A monitor in front of the participant (57 inches away) presented 2.5 inch scanned images of the story in one of the two channels (corresponding to the attended channel, see Procedure below, and small enough to prevent eye movements). A small green arrow pointing to the left or the right was at the bottom of every image as a reminder of which channel to attend (Figure 1).
Figure 1.
Schematic representation of the experimental paradigm. Children were instructed to attend to the story presented from either the left or right speaker. ERPs were recorded to probe stimuli superimposed on both the attended and ignored narrative.
Linguistic and nonlinguistic probe stimuli were superimposed on the stories in each channel. The linguistic probe was a 100 msec digitized (16 bit, 22 kHz) syllable/ba/spoken in a female voice (different than the female storyteller’s). The non-linguistic probe was an edited version of the/ba/stimulus, in which 15–20 msec segments of the stimulus were reordered to create a buzz-like sound that nonetheless preserved many of the acoustic characteristics of the/ba/. The rise-time of the nonlinguistic and nonlinguistic probes differed. An equal number (N ~ 180 – 206) of linguistic and non-linguistic probes were presented in each channel. The probes were presented randomly every 200, 500 or 1000 msec in one of the two auditory channels.
Procedure
After arrival and a short orientation, a parent or caregiver signed a consent form, and the child provided either verbal or written assent. Before recording data, the participant heard instructions in a practice session that introduced the child to the two voices and probe stimuli. During the practice, children received instruction on attending to a single story while ignoring the distracting story presented in the opposite audio channel. A researcher sat next to the child at all times to monitor behavior, ensure the child remained equidistant between the two speakers, and administer comprehension questions following each story. A camera transmitted the session so other researchers and the caregiver(s) could observe from outside the booth.
Children were instructed to attend selectively to one story, while ignoring the story presented in the opposite audio channel. Half of the children began attending to the right audio speaker, and half of the children began attending to the left audio speaker. Children attended to a total of four narratives, attending twice to the story on the right side and twice to the story on the left side (order either RLLR or LRRL). For each child, the attended narrator and story set remained constant across the four stories. After each story, the experimenter asked the child three basic comprehension questions about the attended story. The comprehension questions always had two alternatives. (A response of “I don’t know” was counted as an incorrect response.) At the end of all four stories, the experimenter asked one general question regarding the unattended stories. The majority of the questions focused on the attended story in order to encourage the child to maintain focus on a single story.
The high and low maternal education groups were matched for all stimulus factors, including attended story, start side, and narration voice.
ERP Recording and Analysis
The electroencephalogram (EEG) was recorded from 29 silver-chloride electrodes (Figure 2) mounted in an elastic cap (Electro-Cap International). Electrodes were also placed horizontally next to each eye and beneath the right eye in order to monitor eye movements and blinks. Online, electrodes were referenced to the left mastoid. Offline, data were referenced to the averaged left and right mastoid. Eye channel impedances were maintained below 10 kΩs, mastoids below 3 kΩs, and all other sites below 5 kΩs.
Figure 2.
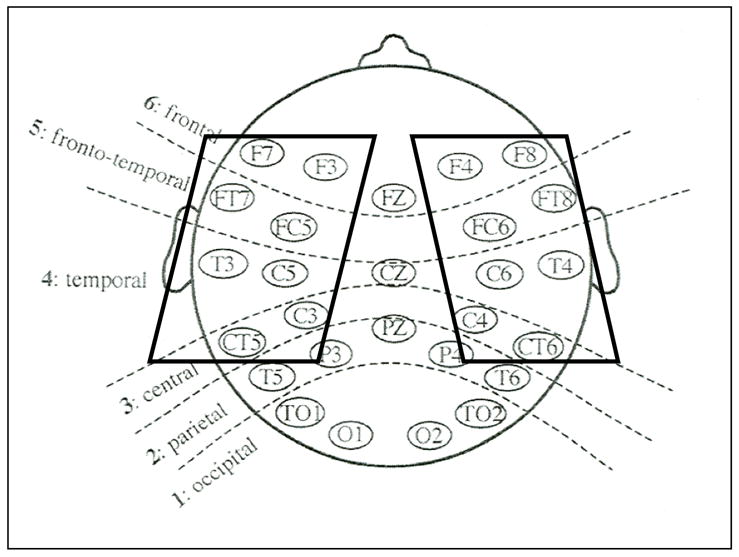
Electrode configuration for ERP recording. The 16 electrodes included in analysis are enclosed in boxes.
The EEG was amplified with Grass 7P511 amplifiers (−3dB cutoff, band pass 0.01 to 100 Hz) and digitized on-line (4 ms sampling rate). Offline, separate ERPs to the four types of probe stimuli (linguistic attended, linguistic unattended, nonlinguistic attended, nonlinguistic unattended) were averaged for each subject at each electrode site over a 500 ms epoch, using a 100 ms pre-stimulus-onset baseline. Individual artifact rejection parameters were selected for each subject on the basis of visual inspection of the raw EEG to identify the smallest amplitude changes associated with eye movements or blinks. Following artifact rejection, there were no differences between maternal education groups in the number of ERP trials available for analysis in any bin, which is an indirect measure of motor and eye movements, largest t (30) < 1, p = .35. All children had at least 78 trials, and on average 163 trials, available for analysis in each of the four bins.
ERP data were analyzed using a 2x2x2 mixed design ANOVA on the mean amplitude of the ERP from 100–200 ms post-stimulus onset, averaged over the anterior four rows of 16 electrodes (F7/8, FT7/8, F3/4, FC5/6, C3/4, C5/6, CT5/6, T3/4; see Figure 2). Within-subject factors included attention (attended/unattended) and probe type (linguistic/nonlinguistic). The between-subject factor was group (higher/lower maternal education). This set of electrodes was selected based on past research using the auditory attention paradigm with both typically developing children (Coch et al., 2005; Sanders et al., 2006) and children with specific language impairment (Stevens et al., 2006). The 100–200 msec time window was selected because, in our ongoing analyses with typically developing children (Sanders et al., 2006) we find a reliable effect of attention for children age 3–5 and 6–8 years during this early time window, although younger children continue to show an effect from 200–300 msec.
Results
Behavioral Testing
On average, standard scores on the CELF receptive language scores were 109 (SD = 15) in the higher maternal education group and 103 (SD = 11) in the lower maternal education group. However, this six point difference in standard scores between groups was not statistically significant, unpaired t (30) = 1.3, P = .2.
ERP Results
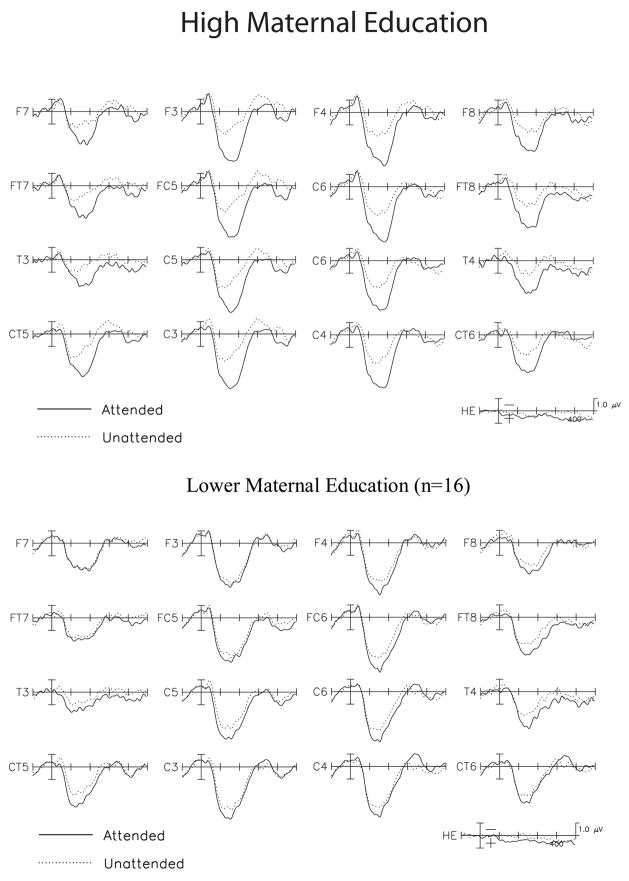
In response to probe stimuli, children in both the higher and lower maternal education groups showed a single, broad positivity peaking around 150 ms post-stimulus onset (see Figure 3). The main effect of group was not significant, F(1, 30) = 1.0, p = .32, nor was the interaction between group and probe type, F(1, 30) < 1, P = .70. Across groups and probe types, the positivity was larger to probes in the attended compared to the unattended channel, main effect of attention F(1, 30) = 52.4, P < .001; interaction between attention and probe type, F(1, 30) < 1, P = .49.
Figure 3.
Grand average evoked potentials for attended and unattended stimuli, collapsed across linguistic and non-linguistic probes in children in the higher maternal education group (upper panel) and lower maternal education group (lower panel)
Crucial to the hypothesis of the study, the group x attention interaction was significant, F(1, 30) = 13.4, P = .001, indicating that the effect of attention differed for children in the higher and lower maternal education groups (see Figure 3 and Table 2). As the group difference in attention did not vary as a function of probe type (group x attention x probe type, F(1, 30) < 1, P = .63), simple effects tests for the difference between attended and unattended stimuli were conducted for each maternal education group, collapsed across probe type. The means for each group are presented in Table 2. Children in the higher maternal education group showed a larger positivity to probes in the attended versus unattended channel, paired-samples t(15) = 10.0, P < .001. Children in the lower maternal education group also showed attentional modulation during this time window, paired samples t(15) = 2.1, P < .05. However, the magnitude of the effect of attention was significantly larger in the higher than in the lower maternal education group, unpaired t(30) = 3.6, P = .001 (mean effect of attention 1.7 and 0.6 μV for the higher and lower maternal education groups, respectively).
Table 2.
Mean amplitude response (in μV) from 100–200 msec to probe stimuli as a function of attention condition, separately for children in the two maternal education groups. Data are collapsed across the linguistic and nonlinguistic probe types.
| Higher Maternal Education | Lower Maternal Education | |
|---|---|---|
| Attended probes (SD) | 3.2 (1.0) | 2.9 (0.9) |
| * Unattended probes (SD) | 1.4 (0.8) | 2.3 (0.9) |
| * Attention effect, Attended – Unattended (SD) | 1.7 (0.7) | 0.6 (1.1) |
Significant difference, P <.05
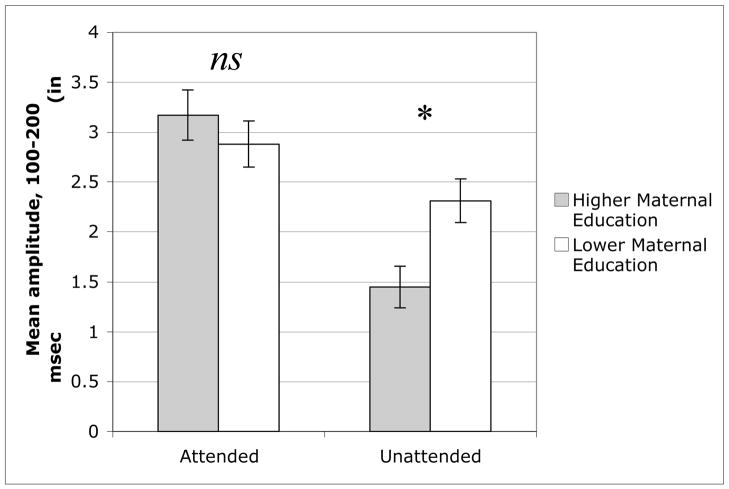
Attentional modulation involves two processes: enhancement of attended stimuli and suppression of distracting, competing stimuli. Following previous research (Stevens et al., 2006), supplemental analyses compared the higher and lower maternal education groups directly on these two processes. If the magnitude of the evoked response to attended and unattended stimuli is taken as an index of each of these processes, respectively, direct comparison of the two groups could identify whether group differences in the magnitude of the attention effect arose primarily from differences in one or both attentional mechanisms. This analysis indicated that whereas the two groups did not differ in response to probe stimuli in the attended channel, independent samples t(30) < 1, P = .40, the groups did differ in response to probe stimuli in the unattended channel, independent samples t(30) = −2.87, P < .01, see Figure 4 and Table 2. The pattern of means revealed that children in the higher maternal education group had a smaller amplitude response to probes in the unattended channel than children in the lower maternal education group, indicating a better ability to suppress the response to distracting information in the unattended channel.
Figure 4.
Mean amplitude response (in μV) to probe stimuli in the attended and unattended channel, separately for children in the higher and lower maternal education groups.
As noted previously, the two groups did not differ significantly in receptive language scores. However, it was still possible that the six point mean difference in receptive language mediated part or all of the relationship between maternal education and attentional modulation. To control for this possibility, a supplemental ANCOVA was conducted that compared the effects of attention on sensorineural processing (Attended – Unattended difference score, collapsed across probe type) across groups, with children’s receptive language standard score included as a covariate. Even when controlling for children’s receptive language scores, the two maternal education groups differed significantly in the effects of attention on sensorineural processing (F (1, 29) = 14.2, P = .001). Receptive language scores did not significantly predict the effect of attention on sensorineural processing (F (1, 29) < 1, P = .36). Thus, the group differences in the effects of attention on sensorineural processing could not be accounted for by differences in receptive language scores between groups, or vice versa.
To explore whether the group differences in early attentional modulation could be explained by on-task performance, responses to the 12 comprehension questions about the attended story were also compared across groups. All children answered at least half of the questions correctly. There were no significant differences between the higher and lower maternal education groups in the number of comprehension questions correctly answered about the attended stories, t (30) = −.17, P = .87, higher maternal education M = 10.1, SD = 1.1, lower maternal education M = 10.2, SD = 0.9. Responses to the single question about the unattended story were at chance levels for both groups, largest one-sample t = 1.6, P = .14, and also did not differ between groups, Fischer’s Exact P = 1.0.
Discussion
In the present study, children whose mothers had lower levels of education (no college experience) showed reduced effects of selective attention on neural processing relative to children whose mothers had higher levels of education (at least some college). These differences were related specifically to a reduced ability to filter irrelevant information (i.e., to suppress the response to ignored sounds) and could not be accounted for by differences in receptive language skill. These data provide direct evidence for differences in the neural systems mediating selective attention in young children from different socioeconomic backgrounds and reveal that such differences affect neural processing within 100 msec of stimulus presentation.
Nature of attentional differences
Previous behavioral studies indicate that children from lower socioeconomic backgrounds experience difficulty with selective attention, and particularly in tasks of executive function and in those tasks that require filtering irrelevant information or suppressing prepotent responses (Farah et al., 2006; Lupien et al., 2001; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005). The results from this study help to clarify the nature of these group differences. First, the present data indicate that group differences in sustained selective attention can be traced to early stages of processing (within 100 msec of stimulus presentation). This suggests that group differences in selective attention and filtering arise, at least in part, from differences in attentional modulation of early stages of perceptual processing. Second, the nature of the group difference in early attentional modulation was linked specifically to differences in distractor suppression of stimuli in the unattended channel. Whereas children in the two groups did not differ in response to probe stimuli in the attended channel, children whose mothers had lower levels of educational attainment displayed reduced suppression of distracting information presented in the competing channel. It is interesting to note that the mechanism implicated in attention deficits in children from lower socioeconomic backgrounds (i.e., distractor suppression) is not the same as the mechanism implicated in previous research on attention deficits in children with SLI, who show a deficit in signal enhancement of stimuli in the attended channel (Stevens et al., 2006). Thus, examining the neural systems underlying a particular cognitive ability can identify different mechanisms that give rise to similar impairments in behavioral performance.
To our knowledge, only one other study has compared the neural mechanisms of selective auditory attention in individuals from diverse socioeconomic backgrounds (D'Angiulli, Herdman, Stapells, & Hertzman, in press). Unlike the present research, the study by D’Angiulli and colleagues examined older adolescents age 12–14 years. The adolescents performed a non-spatial auditory attention task, in which they attended to tones at one of two pitches presented binaurally. Participants responded to targets of the attended pitch that were longer in duration. Whereas adolescents from higher SES backgrounds showed attentional modulation of ERPs around 300 msec after stimulus onset and again from ~600–800 msec, adolescents from lower SES backgrounds did not show attentional modulation of the ERPs during either time window. In contrast, only the adolescents from lower socioeconomic backgrounds showed attention effects on theta activity: increased frontal theta activity was elicited by tones in the ignored frequency channel from 200–700 msec after tone onset in children from lower SES backgrounds. Because there were no differences between groups in behavioral performance on the target detection task, the authors suggested that the two groups of adolescents were using different attentional mechanisms to perform the selection task. Thus, in both the present study and the study by D’Angiulli and colleagues, individuals from lower socioeconomic backgrounds exhibit reduced attentional modulation of ERP components.
The present study examined sustained, selective auditory attention using an online measure of neural activity and a task that did not require overt behavioral responses. Indeed, this was one of the primary advantages of using ERPs: the methodology was well-suited to indexing cognitive processes online while minimizing extraneous task demands. In the present study, the children in both the higher and lower maternal education groups were willing and able to complete the task, as indicated by the high overall performance on the comprehension questions about the attended story and the lack of group differences in this measure. At the same time, in the face of these data, it is reasonable to speculate whether there are behavioral consequences of a reduced effect of attention on sensorineural processing.
One possibility, suggested by D’Angiulli and colleagues (in press), is that children from lower socioeconomic backgrounds may use different neural mechanisms to achieve similar behavioral performance on a given task. Another possibility is that group differences in performance do exist, but they only become apparent when processing demands increase. Indeed, previous research has associated the magnitude of the ERP attention effect with improved behavioral performance on detection tasks, as measured by response accuracy, reaction time, and d-prime (Neville & Lawson, 1987; Roder et al., 1999; Squires, Hillyard, & Lindsay, 1973; Teder-Salejarvi & Hillyard, 1998; Teder-Salejarvi, Pierce, Courchesne, & Hillyard, 2005). The possibility that behavioral deficits only arise when task demands are sufficiently difficult could explain why one previous behavioral study of attention found behavioral deficits in children from lower SES backgrounds only in the youngest age group (Lupien et al., 2001).
The present study localizes the timing (100 msec) and mechanism (distractor suppression) of selective attention deficits in children from lower socioeconomic backgrounds. However, the poor spatial resolution of ERPs prevents drawing strong conclusions about the identity of specific neural networks that mediate these group differences. Previous studies (Farah et al., 2006; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005) have speculated that differences in behavioral measures of executive attention and filtering reflect deficits in prefrontal neural systems. It has been noted that the prefrontal cortex shows a protracted time course of development and might be influenced by differences in stress hormones, including cortisol, which are present at higher levels in children from lower SES backgrounds (Lupien, King, Meaney, & McEwen, 2000; Lupien et al., 2001).
Potential implications of an attention deficit
Difficulties suppressing irrelevant information could have profound impacts on a child’s development in other domains. At the most general level, difficulty filtering distracting sounds could render the typical environment poorly suited for learning. For example, an early task for the infant or child is to focus selectively on particular input for further processing, including speech in the environment (Vouloumanos & Werker, 2004). As well, a mechanism similar to selective attention appears to facilitate an early amplification of the neural response to word-initial syllables relative to word-medial syllables, even when matched for acoustic characteristics (Astheimer & Sanders, In press; Sanders & Neville, 2003; Sanders, Newport, & Neville, 2002). Difficulties with selective attention could impede these processes. Likewise, the typical classroom environment, which is replete with auditory and visual distractions, may make it difficult for a child to focus on a teacher’s instructions or an assignment at hand.
Efficient reading, in particular, requires the ability to focus on selective letters, words, or phrases. Previous research indicates that poor readers show an attentional bias for focusing on word-initial or word-final letters when reading. Interventions that train children to focus attention on all individual letters including word medial letters are associated with improvements in decoding skill (McCandliss, Beck, Sandak, & Perfetti, 2003). In addition, when reading new words, children must also be able to suppress prepotent responses based on already-learned words with a similar, but not identical, spelling. For example, when encountering the word cot for the first time, the child must be able to suppress reading the word as cat, a more frequently occurring word that is likely learned earlier in the reading process. Thus, learning to read might be expected to depend upon the ability to focus on relevant dimensions of spelled words, as well as the ability to suppress overlearned responses for early sight words, such that a child can observe and appreciate meaningful differences between newly learned words. Indeed, recent research indicates that preschool measures of aspects of executive function correlate more strongly than IQ with several aspects of academic performance in kindergarten (e.g., Blair & Razza, 2007).
While attention may have a role in these different aspects of learning, it is important to note that the present study was not designed to answer causal questions about the relationship between the neural mechanisms of selective attention and skill in other domains. Indeed, receptive language scores did not show a significant relationship to the attention index when entered as a covariate in the analysis. This lack of a relationship could indicate that attention skills, as measured with this ERP index, are orthogonal to language development within the normal range of abilities represented in the present study (children below the 25th percentile were excluded from the study). However, it is also possible that the attentional index in the present study is poorly suited for correlational analysis. The ERP attention index represents a difference score (Attended – Unattended), which will be less reliable than an index of attention based on either a single measurement or a composite of several different measures tapping the same construct (Bonate, 2000; Zimmerman, 1994). It will take a different type of study to address the question of causality and interrelationships among skills more directly. However, two studies (Noble et al., 2005; 2007) that used composite measures of language and executive function found significant relationships between executive function and language scores. The difference between these previous studies and the current study could be attributed to the different nature of the tasks used and/or the smaller range of language ability represented in the present study, where all children scored at or above the 25th percentile.
Even if the association between childhood SES and attentional skills represents a causal relationship, the mechanism underlying this relationship is unknown. A number of studies examining other cognitive domains suggest that at least part of the relationship between SES and cognitive outcomes is mediated by environmental differences associated with lower socioeconomic status (Brooks-Gunn & Duncan, 1997; Capron & Duyme, 1989; Duncan et al., 1994; Jimerson et al., 2000; Noble et al., 2007; Schiff et al., 1982). Indeed, the malleability of cognitive outcomes to instructional interventions (Diamond, Barnett, Thomas, & Munro, 2007; McCandliss et al., 2003; Rueda, Rothbart, McCandliss, Saccomanno, & Posner, 2005; Stevens, Fanning et al., 2008; Torgesen et al., 2001) provides one powerful indicator that attention deficits are shaped by environmental factors and amenable to training. Thus, regardless of the pathway underlying this relationship, when there are socioeconomic differences in achievement or cognitive outcomes, it can be asked whether the outcome is amenable to intervention.
Attention interventions
As alluded to previously, if attention represents a core system vulnerable to deficit in children from lower socioeconomic backgrounds, interventions might be designed to target attention skills. In his Principles of Psychology, William James raised the idea of attention training for children, proposing that this would be “the education par excellence” (James, 1890, pg 424, italics original). While James went on to say that such an education is difficult to define and bring about, attention training has recently been implemented in curricula for preschool and school-age children. For example, the Tools of the Mind curriculum, which is based on Vygotskian principles, assists preschool and kindergarten children in developing planning and self-regulation skills (Bodrova & Leong, 2007). The Tools of the Mind curriculum has been used in several federal Head Start programs serving children from lower socioeconomic backgrounds and is reported to improve multiple academic outcomes as well as measures of executive function (Diamond et al., 2007). A separate group of researchers has developed a computerized preschool attention training curriculum that is associated with improvements in IQ scores and a neurophysiological measure of attention (Rueda et al., 2005). Finally, a recent study reports greater effectiveness of a remedial writing intervention for adolescents with dyslexia when the program is preceded by an attention training program (Chenault, Thomson, Abbott, & Berninger, 2006). Prior attention training appears to allow the students to benefit more from the targeted writing intervention. These studies suggest that attention can indeed be trained and that effective interventions exist for improving attention in children of all ages. Furthermore, at least two studies (Rueda et al., 2005; Stevens, Fanning et al., 2008) report that behavioral improvements in attention, nonverbal intelligence, and/or language and preliteracy are accompanied by changes in the neural mechanisms of attention, including the electrophysiological index described above (Stevens, Currin et al., 2008; Stevens, Fanning et al., 2008). These data suggest that modifications in behavior can arise alongside changes in the early neural mechanisms of attention.
In line with previous suggestions (Noble et al., 2005), we hypothesize that interventions targeting attention skills may serve as a force-multiplier, leading to improvements in domains outside of attention. It will be important for future research and intervention studies to investigate the viability of training programs to improve selective attention and self-regulation skills among children from lower socioeconomic backgrounds. Such training programs, if implemented as part of larger efforts of systemic change, may aid in closing the socioeconomic gap in children’s academic achievement. However, such an intervention should not be perceived as a “quick fix” to the impacts of persistent poverty and low socioeconomic status on children’s development. The learning trajectories of children from lower socioeconomic backgrounds, which consistently undershoot those of their peers from higher socioeconomic backgrounds (NAEP, 2005a, 2005b), occur in the context of differences in access to resources (including basic nutrition), home environments, family interaction patterns, and neighborhood and school systems (Brooks-Gunn & Duncan, 1997; Capron & Duyme, 1989; Duncan et al., 1994; Jimerson et al., 2000; Schiff et al., 1982). Thus, while early childhood programs for children in lower socioeconomic backgrounds may include training in foundational systems, including attention regulation and control, to be maximally effective, such interventions should occur within comprehensive programs addressing socioeconomic and class divide.
Conclusions
The present data support previous reports of deficits in selective attention and attentional control, and in particular the filtering of distracting stimuli, among young children from lower socioeconomic backgrounds. These attention deficits impact very early stages of perceptual processing and could have cascading consequences on the development of other skills, including language and reading.
Acknowledgments
This research was supported by NIH/NIDCD grant DC00481 to HJN. We thank Paul Compton and Ray Vukcevich for programming and technical assistance and the members of the Brain Development Lab who assisted with data collection and preprocessing, including Annika Andersson, Jessica Fanning, Petya Ilcheva, Nicole Makarenco, David Paulsen, Lisa Sanders, Lisa Stewart, and Brad Wible.
References
- Asbjørnsen AE, Bryden MP. Auditory attentional shifts in reading-disabled students: Quantification of attentional effectiveness by the Attentional Shift Index. Neuropsychologia. 1998;36(2):143–148. doi: 10.1016/s0028-3932(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Astheimer L, Sanders L. Listeners modulate temporally selective attention during natural speech processing. Biological Psychology. doi: 10.1016/j.biopsycho.2008.01.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. Review of human visual development: Crowding and dyslexia. In: Cronly-Dillon J, Stein J, editors. Vision & visual dysfunction, vol. 13: Vision & Visual Dyslexia. 1991. pp. 44–57. [Google Scholar]
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Corina D, Liu G, et al. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. Journal of Neuroscience. 2001;21(22):8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydar N, Brooks-Gunn J, Furstenberg FF., Jr Early warning signs of functional illiteracy: Predictors in childhood and adolescence. Child Development. 1993;64:815–829. doi: 10.1111/j.1467-8624.1993.tb02945.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza R. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bodrova E, Leong D. Tools of the mind: The Vygotskian approach to early childhood education. 2. Upper Saddle River, NJ: Pearson Education, Inc; 2007. [Google Scholar]
- Bonate P. Analysis of pretest-posttest designs. New York: Chapman & Hall/CRC; 2000. [Google Scholar]
- Brooks-Gunn J, Duncan G. The effects of poverty on children. The Future of Children. 1997;7:55–71. [PubMed] [Google Scholar]
- Capron C, Duyme M. Assessment of effects of socio-economic status on IQ in a full cross-fostering study. Nature. 1989;340:552–554. [Google Scholar]
- Castro-Caldas A, Reis A. The knowledge of orthography is a revolution in the brain. Reading and Writing: An Interdisciplinary Journal. 2003;16:81–97. [Google Scholar]
- Chenault B, Thomson J, Abbott RD, Berninger VW. Effects of prior attention training on child dyslexics' response to composition instruction. Developmental Neuropsychology. 2006;29:243–260. doi: 10.1207/s15326942dn2901_12. [DOI] [PubMed] [Google Scholar]
- Cherry R. Development of selective auditory attention skills in children. Perceptual and Motor Skills. 1981;52:379–385. doi: 10.2466/pms.1981.52.2.379. [DOI] [PubMed] [Google Scholar]
- Clark E. I love you, Blue Kangaroo. Italy: Bantom Doubleday Dell Publishing Group, Inc; 1998. [Google Scholar]
- Clark E. Where are you, Blue Kangaroo? Italy: Random House Children's Books; 2000. [Google Scholar]
- Clark E. It was you, Blue Kangaroo. Italy: Random House Children's Books; 2002. [Google Scholar]
- Clark E. What shall we do, Blue Kangaroo? Italy: Random House Children's Books; 2003. [Google Scholar]
- Coch D, Sanders L, Neville H. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- D'Angiulli A, Herdman A, Stapells D, Hertzman C. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. doi: 10.1037/0894-4105.22.3.293. in press. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett W, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G, Brooks-Gunn J, Klebanov P. Economic deprivation and early childhood development. Child Development. 1994;65:296–318. [PubMed] [Google Scholar]
- Farah M, Shera D, Savage J, Betancourt L, Giannetta J, Brodsky N, et al. Childhood poverty: Specific associations with neurocognitive development. Brain Research. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Gottfried A, Gottfried A, Bathurst K, Wright Guerin D, Parramore M. Socioeconomic status in children's development and family environment: Infancy through adolescence. In: Bornstein M, Bradley R, editors. Socioeconomic status, parenting, and child development. Mahwah: NJ: Lawrence Erlbaum Associates; 2003. pp. 189–207. [Google Scholar]
- Hillyard SA, Hink R, Schwent V, Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Woldoff M, Mangun G, Hansen J. Mechanisms of early selective attention in auditory and visual modalities. The London Symposia, EEG supplement. 1987;39:317–324. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status, Unpublished manuscript. Yale University; New Haven, Connecticut: 1975. [Google Scholar]
- James W. Principles of psychology. New York: Henry Holt and Co; 1890. [Google Scholar]
- Jimerson S, Egeland B, Sroufe A, Carlson B. A prospective longitudinal study of high school dropouts: Examining multiple predictors across development. Journal of School Psychology. 2000;38:525–549. [Google Scholar]
- Liaw F, Brooks-Gunn J. Cumulative familial risks and low-birthweight children's cognitive and behavioral development. Journal of Clinical Child Psychology. 1994;23:360–372. [Google Scholar]
- Lipina S, Martelli M, Vuelta B, Colombo J. Performance on the A-not-B task of Argentinian infants from unsatisfied and satisfied basic needs homes. Interamerican Journal of Psychology. 2005;39:49–60. [Google Scholar]
- Lupien SJ, King S, Meaney M, McEwen B. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- McCandliss B, Beck I, Sandak R, Perfetti C. Focusing attention on decoding for children with poor reading skills: Design and preliminary tests of the Word Building intervention. Scientific Studies of Reading. 2003;7:75–104. [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- NAEP. The nation's report card: Mathematics. Washington, DC: U.S. Department of Education Institute of Education Sciences NCES 2006–453; 2005a. [Google Scholar]
- NAEP. The nation's report card: Reading. Washington, DC: U.S. Department of Education Institute of Education Sciences NCES 2006–451; 2005b. [Google Scholar]
- Neville H, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. II. Congenitally deaf adults. Brain Research. 1987;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Noble K, McCandliss B, Farah M. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K, Norman MF, Farah M. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Armedi A, Fregni F, Merabet L. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clinical Neurophysiology. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Recanzone G, Schreiner C, Merzenich M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. Journal of Neuroscience. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, Teder-Salejarvi W, Sterr A, Rosler F, Hillyard SA, Neville H. Improved auditory spatial tuning in blind humans. Nature. 1999;400:162–166. doi: 10.1038/22106. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss B, Halparin J, Gruber D, Lercari L, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart M, McCandliss B, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the USA. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L, Neville H. An ERP study of continuous speech processing. I. Segmentation, semantics, and syntax in native speakers. Cognitive Brain Research. 2003;15:228–240. doi: 10.1016/s0926-6410(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Sanders L, Newport E, Neville H. Segmenting nonsense: An event-related potential index of perceived onsets in continuous speech. Nature Neuroscience. 2002;5:700–703. doi: 10.1038/nn873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L, Stevens C, Coch D, Neville H. Selective auditory attention in 3- to 5-year-old children: An event-related potential study. Neuropsychologia. 2006;44:2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schiff M, Duyme M, Dumaret A, Tomkeiwics S. How mcuh could we boost scholastic achievement and IQ scores? A direct answer from a French adoption study. Cognition. 1982;12:165–196. doi: 10.1016/0010-0277(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals (CELF-3) 3. San Antonio: The Psychological Corporation: Harcourt Brace & Co; 1995. [Google Scholar]
- Sharma AS, Kraus N, McGee T, Nicol T. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalography and Clinical Neurophysiology. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis F, Seidenberg M. Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Squires K, Hillyard S, Lindsay P. Vertex potentials evoked during auditory signal detection: Relation to decision criteria. Perception and Psychophysics. 1973;14:265–272. [Google Scholar]
- Stevens C, Currin J, Paulsen D, Harn B, Chard D, Larsen D, et al. Kindergarten children at-risk for reading failure: Electrphysiological measures of selective auditory attention before and after the Early Reading Intervention [Abstract] Cognitive Neuroscience Society 2008 [Google Scholar]
- Stevens C, Fanning J, Coch D, Sanders L, Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: Electrophysiological evidence from language-impaired and typically developing children. Brain Research. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Sanders L, Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Teder-Salejarvi W, Hillyard S. The gradient of spatial auditory attention in free field: An event-related potential study. Perception & Psychophysics. 1998;60:1228–1242. doi: 10.3758/bf03206172. [DOI] [PubMed] [Google Scholar]
- Teder-Salejarvi W, Pierce K, Courchesne E, Hillyard S. Auditory spatial localization and attention deficits in autistic adults. Cognitive Brain Research. 2005;23:221–234. doi: 10.1016/j.cogbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Torgesen J, Alexander A, Wagner R, Rashotte C, Voeller K, Conway T. Intensive remedial instruction for children with severe reading disabilities: Immediate and long-term outcomes from two instructional approaches. Journal of Learning Disabilities. 2001;34:33–58. doi: 10.1177/002221940103400104. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Werker J. Tuned to the signal: The privileged status of speech for young infants. Developmental Science. 2004;7:270–276. doi: 10.1111/j.1467-7687.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Walker D, Greenwood C, Hart B, Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Development. 1994;65:606–621. [PubMed] [Google Scholar]
- Woldoff M, Hillyard SA. Modulation of early auditory processing during selective listenting to rapidly presented tones. Electroencephalography and Clinical Neurophysiology. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- Ziegler J, Pech-Georgel C, George F, Alario F, Lorenzi C. Deficits in speech perception predict language learning impairment. Proceedings of the National Academy of Science. 2005;102:14110–14115. doi: 10.1073/pnas.0504446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. A note on interpretation of formulas for the reliability of differences. Journal of Educational Measurement. 1994;31:143–147. [Google Scholar]
- Zion E, Graham M. Harry the dirty dog. United States: HarperCollins Children's Books; 1956. [Google Scholar]
- Zion E, Graham M. Harry and the lady next door. United States: HarperCollins Children's Books; 1960. [Google Scholar]
- Zion E, Graham M. Harry by the sea. United States: HarperCollins Children's Books; 1965. [Google Scholar]
- Zion E, Graham M. No roses for Harry! United States: HarperCollins Children's Books; 1976. [Google Scholar]