Abstract
Behavioral reactivity to novel stimuli in the first half-year of life has been identified as a key aspect of early temperament and a significant precursor of approach and withdrawal tendencies to novelty in later infancy and early childhood. The current study examines the neural signatures of reactivity to novel auditory stimuli in 9-month-old infants in relation to prior temperamental reactivity. On the basis of the assessment of behavioral reactivity scores at 4 months of age, infants were classified into groups of high negatively reactive and high positively reactive infants. Along with an unselected control group, these groups of temperamentally different infants were given a three-stimulus auditory oddball task at 9 months of age which employed frequent standard and infrequent deviant tones as well as a set of complex novel sounds. In comparison to high positively reactive and control infants, high negatively reactive infants displayed increased amplitude of a positive slow wave in the ERP response to deviant tones compared to standard tones. In contrast, high positively reactive infants showed a larger novelty P3 to the complex novel sounds. Results are discussed in terms of optimal levels of novelty for temperamentally different infants.
Introduction
Behavioral reactivity to novel stimuli in the first half-year of life has been identified as a precursor of approach and withdrawal tendencies to novelty in later infancy and toddlerhood. Specifically, the categorical combinations of high levels of either negative (e.g. fretting, crying) or positive affect (e.g. smiling) and motor activity in response to a battery of novel sensory stimuli at 4 months of age have been respectively related to increased behavioral inhibition to the unfamiliar or higher levels of positive affect and exuberance in later infancy and early childhood (Calkins & Fox, 1992; Calkins, Fox & Marshall, 1996; Fox, Henderson, Rubin, Calkins & Schmidt, 2001b; Kagan & Snidman, 1991). These positive and negative response tendencies seen in early infancy have been conceptualized as temperamental biases towards the expression of approach or withdrawal behaviors, which in turn affect later cognitive and social styles (Fox, Henderson & Marshall, 2001a).
In addition to the many studies examining behavioral reactivity as a key facet of early temperament, there has been increasing interest in assessing individual differences in the reactivity of infants’ physiological systems in response to various kinds of stimulation. Much of this work has examined cardiac measures, such as heart rate or heart rate variability (e.g. Fox & Stifter, 1989; Huffman, Bryan, del Carmen, Pedersen, Doussard-Roosevelt & Porges, 1998; Porges, Doussard-Roosevelt, Portales & Greenspan, 1996) or measures of activity of the hypothalamic-pituitary-adrenal axis (e.g. Gunnar, 1989; Gunnar, Brodersen, Krueger & Rigatuso, 1996) in response to mildly challenging or stressful events. Other related work has examined infant temperament in relation to central nervous system (CNS) activity as indexed by the electroencephalogram (EEG) recorded during quiet attention (Calkins et al., 1996; Fox et al., 2001b). However, very few studies have related differences in temperamental behavioral reactivity to CNS responses to sensory stimulation as indexed by event-related potentials (ERPs). ERP techniques can provide a window into very early stages of stimulus processing and can capture stimulus processing at the level of the millisecond. In this respect, ERP techniques can provide an original perspective on the assessment of reactivity in the context of infant temperament.
One current question in temperament research concerns how novel or changing sensory information is processed by infants and children of different temperaments, especially those who show high levels of behavioral reactivity to novelty (Marshall & Stevenson-Hinde, 2001). In the present study, we examined ERP responses to auditory novelty in groups of 9-month-old infants who differed temperamentally on levels of reactivity to stimulation as observed during a laboratory assessment at 4 months of age. We aimed to address the question of whether differences in behavioral reactivity to novel stimuli measured at 4 months of age were related to electrophysiological responses to novelty measured 5 months later. Towards this goal, we utilized an auditory oddball ERP paradigm designed to capture aspects of the processing of novel changes in an ongoing stimulus train.
ERP studies of reactivity to change and novelty in the auditory domain have often employed oddball paradigms in which the presentation of a frequent, repetitive ‘standard’ stimulus is interspersed with the occasional presentation of a less frequent ‘deviant’ stimulus. The deviant differs from the standard in a given physical characteristic such as duration or frequency. Such paradigms have been used across a diverse range of ages and samples to compare the ERP responses to the standard and deviant stimuli (Näätänen & Alho, 1995). In adults, this comparison typically reveals a more negative-going ERP response to the deviant stimulus than the standard stimulus (the mismatch negativity, or MMN), with this difference peaking between around 100–200 ms after stimulus onset. The MMN reflects a mechanism in primary auditory cortex for detecting and discriminating small changes in an otherwise repetitive stimulus train, and is usually elicited in a passive task in which the subject is not required to attend to or respond to the auditory stimuli (Picton, Alain, Otten, Ritter & Achim, 2000).
While a negative-going MMN has been elicited quite consistently in studies of school-age children (Bar-Haim, Marshall, Fox, Schorr & Gordon-Salant, 2003; Gomes Molholm, Ritter, Kurtzberg, Cowan & Vaughan, 2000; Gomot, Giard, Roux, Barthelemy & Bruneau, 2000), the MMN has been less consistently elicited in studies of infants (He, Hotson & Trainor, 2007; Morr, Shafer, Kreuzer & Kurtzberg, 2002; Novitski, Huotilainen, Tervaniemi, Näätänen & Fellman, 2007; Trainor, McFadden, Hodgson, Darragh, Barlow, Matsos & Sonnadara, 2003). While some researchers have reported a negative-going infant MMN response (e.g. Čeponienė, Hukki, Cheour, Haapanen, Koskinen, Alho & Näätänen, 2000; Cheour, Alho, Čeponienė, Reinikainen, Sainio, Aaltonen & Näätänen, 1998), a number of studies have reported more positive responses to the deviant tone, especially in infants under 1 year of age (He et al., 2007; Leppänen, Eklund & Lyytinen, 1997; Morr et al., 2002; Trainor et al., 2003). For example, Morr et al. (2002) found the ERP to a deviant tone to be more positive than to a standard tone in infants up to 1 year old. The positive-going response occurred as a slow wave in the latency range of 150–300 ms for a small deviance contrast (1000 Hz standard/1200 Hz), and in the range of 200–300 ms for a larger deviance level (1000/2000 Hz). For the smaller deviance level, there was no evidence of a negative-going MMN response. For the larger level of deviance, an MMN-like negativity occurred at frontal and central sites at around 150–160 ms. Since the latency of the MMN decreases with increasing stimulus deviance, Morr et al. (2002) suggested that the larger deviant elicited an MMN earlier than the positive-going slow wave, avoiding the latency overlap that had obscured any MMN to the smaller deviant. Based on this suggestion, Morr et al. (2002) reasoned that the MMN response in the first year of life is easily obscured by the large, slow positivity that may occur in a frequency range overlapping the MMN. Similarly, Kushnerenko, Čeponienė, Balan, Fellman and Näätänen (2002) suggested that in the first year of life, the deviant tones used in oddball tasks elicit a positivity that may obscure or replace an MMN response. While the neural generators of the infant slow wave are not clear, it has been suggested that the infant MMN and positive slow waves are distinct, separable aspects of the infant response to deviant stimuli (Trainor et al., 2003). In support of this argument, it has recently been shown that this slow positivity elicited to the deviant tone can be separated from an overlapping, faster MMN by employing different bandpass filters prior to ERP computation (He et al., 2007). In terms of the functional properties of the infant slow wave, particularly relevant to the current paper is the suggestion of Kushnerenko et al. (2002) that the positive slow wave elicited in auditory oddball tasks with infants is related to the triggering of an attention switch or orienting response to the deviant tone, in contrast to the MMN, which reflects more passive discrimination processes.
In addition to the moderately deviant stimuli such as those used in MMN paradigms, some related oddball tasks employ a third category of widely deviant, complex novel stimuli which tend to elicit ERP components that are associated with aspects of an orienting response (Escera, Alho, Winkler & Näätänen, 1998). Specifically, such unexpected, novel infrequent sounds tend to elicit a novelty P3 component in the adult auditory ERP when they are interspersed among simpler standard and deviant tones to which the participant is attending or responding (e.g. Gaeta, Friedman, Ritter & Cheng, 1998). A novelty P3 has also been observed in children using similar attended tasks (e.g. Cycowicz, Friedman & Rothstein, 1996; Gumenyuk, Korzyukov, Alho, Escera, Schroger, Ilmoniemi & Näätänen, 2001; Hogan, Butterfield, Phillips & Hadwin, 2007). In an unattended variation of the above three-stimulus active oddball paradigm, participants are instructed to ignore all the auditory stimuli and focus their attention on an unrelated activity such as reading (e.g. Friedman, Kazmerski & Cycowicz, 1998). ERP analyses from such tasks have shown that deviant tones with a relatively low level of deviance tend to elicit an MMN response, while the complex novel sounds elicit a novelty P3 that is generally smaller than that seen in similar attended tasks (Friedman et al., 1998). Despite the fact that the passive nature of this paradigm is ideal for studying reactivity to auditory novelty in infants, a three-stimulus auditory oddball paradigm has very rarely been employed with infants. In the context of the present study, the closest paradigm is that of Kushnerenko et al. (2002) who used a two-stimulus oddball to elicit a P3 component in a small cross-sectional study of newborns and 2-year-olds using complex novel sounds interspersed among frequent standard tones.
Although auditory ERP responses have been related to social and emotional development in older children (Bar-Haim et al., 2003; Hogan et al., 2007; Woodward, McManis, Kagan, Deldin, Snidman, Lewis & Kahn, 2001) the literature relating temperament and auditory ERP responses during infancy is very sparse. As far as we are aware, no previous study has examined ERP responses to auditory novelty in infants in relation to early temperament. In the current study, we employed a three-stimulus auditory oddball paradigm with 9-month-old infants who had been selected for specific patterns of temperamental reactivity on the basis of a behavioral assessment in earlier infancy. In addition to employing infrequent deviant tones embedded in a train of frequent standard tones, we were interested in using complex novel stimuli as a second level of deviance. We examined the ERP response to these two levels of auditory change in two temperamentally different groups of infants. One group consisted of infants who were motorically active and temperamentally prone to distress when exposed to moderate levels of stimulus novelty at 4 months of age. Prior work has shown an association between this temperamental profile of high negative reactivity and a tendency to be watchful and withdrawn in response to novel stimuli in later infancy and early childhood (Calkins et al., 1996; Fox et al., 2001b). In contrast, the other group of infants had shown positively valenced reactions (e.g. smiling, cooing) when exposed to the same battery of stimuli at 4 months of age. Fox and colleagues focused interest on this latter temperamental category of infants who displayed high levels of motor activity as well as high levels of positively valenced behaviors (e.g. smiling) in response to novel sensory stimulation in early infancy. Compared with infants rated as being more temperamentally negatively reactive, these high positive infants tend to show increased levels of social approach behaviors to unfamiliarity in later infancy and early childhood (Fox et al., 2001b). Regarding these temperamental profiles, there has been particular interest in the physiological correlates of the differences in behavioral reactivity and approach–withdrawal tendencies that characterize the early development of these groups of temperamentally different infants (Fox, Henderson, Marshall, Nichols & Ghera, 2005; Kagan, Reznick & Snidman, 1987; Marshall & Stevenson-Hinde, 2001). The present study is an attempt to add to that literature, using an approach that is relatively novel in the temperament literature –the assessment of electrophysiological responses in relation to changes in an ongoing auditory stimulus train.
Given the recent literature on the infant mismatch response to auditory change in a similar age range (e.g. Morr et al., 2002), the deviant tones were expected to elicit a more positive-going ERP response compared with the response to the standard tones. Based on the findings with infants of Kushnerenko et al. (2002), the complex novel stimuli were also expected to elicit a marked positive component resembling the novelty P3, reflecting the engagement of orienting networks in the brain (Escera et al., 1998). Our main hypothesis concerned whether we would be able to observe differences in these ERP responses to auditory change between the groups of temperamentally high negative and high positive infants. Given the displays of negative affect to moderate levels of stimulus novelty seen in the behavioral assessment of the temperamentally high negative infants at 4 months of age, one candidate hypothesis was that the high negative group of infants would show enhanced physiological reactivity in the ERP to auditory novelty at 9 months of age. However, a more refined approach takes into account the two levels of novelty used in the present study. In particular, we were interested in the possibility that the groups of temperamentally positive and negative infants may show differential ERP responses to the two levels of auditory change, reflecting variation in the levels of novelty at which engagement with a stimulus is promoted. Kagan (1994) proposed that infants and children who are temperamentally prone to negatively valenced reactions to novelty may be particularly vigilant or sensitive to small changes to the stimulus environment. In contrast, children who tend to react to novel situations with approach-related behaviors and positive affect may be less engaged by lower levels of novelty and more engaged by high levels of stimulus deviance (for a similar argument in adults, see Berlyne, 1960). Thus, our working hypothesis was that the two levels of stimulus deviance (the low level of the deviant tone and the high level of the novel sounds) would elicit different ERP profiles between the two temperament groups, with the high negative group showing larger responses to the small level of deviance, and the high positive group showing increased amplitude to the widely deviant novel sounds.
Methods
Behavioral coding at 4 months of age
As part of a larger longitudinal study, a total of 849 4-month-old infants (± 7 days) were assessed for motor and affective reactivity in response to novel sights and sounds in the laboratory setting using a standard battery of tests (Calkins et al., 1996;Fox et al., 2001b; Kagan & Snidman, 1991). Families with young infants were initially contacted by mail using commercially available lists of names and addresses compiled from the birth records of area hospitals. Interested parents completed a brief background survey and were scheduled for laboratory visits with their infants. Families were excluded from further participation if the infant had been born preterm, if the infant had experienced any serious illnesses or problems in development since birth, or if the infant was on any long-term medication.
For the laboratory assessment of temperament at 4 months of age, infants were presented with a battery of novel visual and auditory stimuli which has been commonly used in the literature on early temperamental reactivity to novelty (Calkins et al., 1996; Fox et al., 2001b; Hane & Fox, 2006; Kagan & Snidman, 1991). Infants were assessed in a quiet, alert state and sat in an infant seat during the presentation of two blocks of stimuli. Each block of stimuli consisted of a series of visual presentations followed by a series of auditory presentations. The first series of visual stimuli consisted of three mobiles differing in the number of hanging elements (1, 3, or 6). Each mobile was presented for 20 s, with a 10 s inter-trial interval between presentations. The series of three mobiles was repeated three times for a total of nine trials. Each mobile was displayed approximately 12 inches from the infant’s face. The first series of auditory stimuli consisted of eight short sentences. Each sentence was approximately 6 s in duration, followed by a 2 s inter-trial interval. The sentences were presented in pairs, which differed in the number of voices speaking. The first pair was spoken by a single voice, the second pair by two voices speaking together, the third pair by three voices, and the fourth pair by four voices.
The second block of novel stimuli was similar to the first, except that the elements on the mobiles were different, and the auditory stimuli were consonant-vowel stimuli (ma, ga, pa) rather than sentences. The series of three mobiles was presented in an identical fashion as in the first set, for a total of nine trials. Each consonant-vowel stimulus was presented in three consecutive 10 s trials, with 5 s inter-trial intervals. Infants who began to cry during an episode were allowed to cry for a continuous period of no more than 20 seconds, after which the mother was asked to intervene and calm her infant. Once the infant was sufficiently calm, the session was continued. If an infant was unable to continue with a session, scores were prorated for the episodes that the infant missed on the basis of his or her prior responses up to that point. All sessions were videotaped, allowing for the later coding of infant reactivity.
Coding was based on previously described procedures (Calkins et al., 1996; Fox et al., 2001b; Kagan & Snidman, 1991). Specifically, the frequencies of the following behaviors were coded during stimulus presentation: (1) Motor activity, which was coded as arm and leg movements greater than 45 degrees from the resting position, bursts of two or more arm and leg movements, back arches, or hyperextensions of arms and legs; (2) Positive reactivity, coded as smiling or positive vocalizations; and (3) Negative reactivity, coded as fussing, fretting, and crying. Inter-rater reliability was computed on approximately 14% of the sample and Pearson correlations between pairs of raters ranged from .75 to .90.
Behavioral selection of temperament groups at 4 months of age
In line with previous studies of early behavioral reactivity, groups were selected on the basis of infant temperament, reflecting categorical, normative differences in early personality or behavioral style (Kagan, 1994). A control group was recruited which comprised 95 4-month-old infants who were not selected for their behavioral responses at 4 months, and who remained in the longitudinal study as an unselected control group. A further 739 infants were screened at 4 months of age, and infants who scored above specific criteria on the dimensions of motor activity and positive or negative reactivity were selected for follow-up at 9 months of age. The criteria for selection were established based on the median scores of the first 100 of these 739 infants. On the basis of these criteria, two temperament groups were selected: (1) Those above the medians for motor activity and negative reactivity (the ‘high negative’ group, n = 88), and (2) those above the medians for motor activity and positive reactivity (the ‘high positive’ group, n = 66). The high negative and high positive temperament groups were followed up as part of a larger longitudinal study, as were the infants in the unselected control group.
Participants for 9-month ERP collection
Parental informed consent was obtained for all participants and the study was approved by the university Institutional Review Board. At 9 months of age, 178 infants participated in ERP data collection. This total included the first 64 subjects from the unselected control group who had ERP data collected, 67 infants identified as high negative, and 47 infants identified as high positive. The final analyses concerned the 103 infants with usable ERP data (43 control, 32 high negative, and 28 high positive). Data from a total of 75 infants (21 control, 35 high negative, and 19 high positive) were not included in the ERP analyses because of frequent movement artifact that resulted in too few ERP trials (less than 10 trials in any one condition), excessive fussing which caused early termination of the experiment, or technical difficulties with EEG collection. A chi-square performed on the distribution of data loss between the three groups was significant (p < .05), likely reflecting a relatively higher level of data loss in the high negative temperament group. However, a second chi-square analysis involving only the two selected temperament groups (high negative and high positive) was not significant (p > .20).
Stimuli
The experimental design consisted of a passive oddball task using three types of stimuli: 228 standard tones, 36 deviant tones, and 36 unique complex novel sounds. The novel sounds included noises such as a cork popping, a door closing, various animal noises, a bell, whistles, car horns, and other environmental sounds. Each deviant or novel stimulus was preceded by a sequence of 3, 4, or 5 standard stimuli, with the deviant and novel stimuli being presented in a random order. All stimuli were 150 ms in duration and were presented at 75 dB peak SPL at the infant’s ear. The interstimulus interval was 1000 ms (onset to onset). The stimuli were presented through two loudspeakers (situated 2 m either side of the infant) in two blocks, each of 114 standard tones, 18 deviant tones, and 18 novel sounds. The first block used 1000 Hz standard tones and 950 Hz deviant tones (all sine waves), and the second block used the reverse configuration of 950 Hz standard tones and 1000 Hz deviant tones. There was a 20 second pause between blocks, and the two blocks were combined for the ERP analysis. The total duration of stimulus presentation was around 6.5 minutes.
ERP collection and analysis
EEG data in the auditory protocol were collected while participants were quietly distracted by an experimenter with toys and stuffed animals. The EEG was recorded from 14 scalp sites (F3, F4, F7, F8, Fz, C3, C4, P3, P4, Pz, O1, O2, T7 and T8) plus the left and right mastoids using a lycra electrode cap (Electro-Cap International Inc., Eaton, OH) with sewn-in tin electrodes. An anterior midline site (AFz) served as the ground electrode and the EEG was collected referenced to the vertex (Cz). After the cap had been correctly fitted, the scalp underlying each electrode site was gently abraded before electrolytic conducting gel was inserted into the space between the scalp and the electrode. Impedances were measured at each electrode site and were considered acceptable if they were at or below 10 kΩ. All channels were digitized at 512 Hz onto the hard drive of a PC using a 12-bit A/D converter (± 2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI). The EEG signals were amplified by a factor of 5000 using custom bioelectric amplifiers from SA Instrumentation Company (San Diego, CA). Amplifier filter settings for all channels were 0.1 Hz (high-pass) and 100 Hz (low-pass). Prior to the recording of EEG from each participant, a 50 μV 10 Hz signal was input into each of the channels and the amplified signal was recorded for calibration purposes. All further processing was carried out using the EEG and ERP Analysis Systems from James Long Company (Caroga Lake, NY).
Epochs in which the EEG signal exceeded ± 250 μV were excluded from further analysis. The EEG channels were re-referenced in software to an average-mastoids reference, a commonly used reference configuration in developmental ERP studies in the auditory modality. One channel of bipolar vertical EOG was recorded, but there were signal quality issues with this channel in many infants with otherwise usable ERP data (n = 45). For those infants with good EOG data, EOG-EEG propagation factors were computed and were found to be of very small magnitude, indicating that eyeblinks had a very limited effect on the EEG signal. Given this finding, the EOG data were not used in further analysis or processing. Other researchers have also concluded that eyeblink artifacts are less of a problem in infant ERP studies than in adult studies. Even blinks as large as 250 μV have relatively little impact on the infant ERP (Nelson, 1994). After re-referencing to the average-mastoids reference, the EEG signal was subjected to digital filtering between 1 Hz and 15 Hz for the primary analyses. However, a supplemental approach based on He et al. (2007) involved the computation of a second set of ERPs after filtering the re-referenced EEG at 3–15 Hz. As detailed in the results, the ERPs derived using this 3 Hz high-pass cutoff were used in order to examine the relative contributions of a positive slow wave component versus a putative overlapping MMN to the between-group findings. All ERPs were calculated for each stimulus type using a 75 ms prestimulus baseline. The mean number of artifact-free trials for the computation of the ERPs was as follows: 181 for the standard tone (SD = 56), 23 for the deviant (SD = 7) and 22 for the novel sounds (SD = 7), with no differences among the infant temperament or control groups.
ERP scoring
The approach to analyzing the waveforms to the standard and deviant tones involved computation of mean amplitudes to each stimulus type over the 100–300 ms window after stimulus onset (for a similar approach, see Morr et al., 2002). Mean amplitudes were best suited for analyses of the ERPs to the tonal stimuli since the slow positivity elicited by the tones (see below) did not have one consistently clear peak. This is a common issue in research using mismatch paradigms, where mean amplitude is preferred over peak amplitude (see Bishop, 2007).
In order to quantify the novelty P3 that was elicited to the novel stimuli (see below), peak amplitude and peak latency were scored for the 100–400 ms range for each participant from the ERP to the novel stimulus. While the analysis of the ERPs to the tonal stimuli centered on the derivation of mean amplitude, peak amplitude was used for the analysis of the responses to the complex novel sounds since these stimuli tended to elicit a clear single peak in individual infants (see also Kushnerenko et al., 2002) rather than the much broader positive slow wave that was seen in the individual ERP responses to the tonal stimuli (see Morr et al., 2002).
ERP responses at the midline sites over frontal, central and parietal scalp regions (Fz, Cz, and Pz, respectively) were used in the analyses. We did not have specific questions about hemispheric asymmetries, so while we show ERP waveforms from a variety of sites (Figures 1–3), we restricted the analysis results to the three midline sites. This approach is derived from the observation that mismatch responses are seen most prominently at the frontal and central midline sites (Bishop, 2007), whereas P3 responses to complex novel stimuli may include parietal components (Friedman, Cycowicz & Gaeta, 2001).
Figure 1.
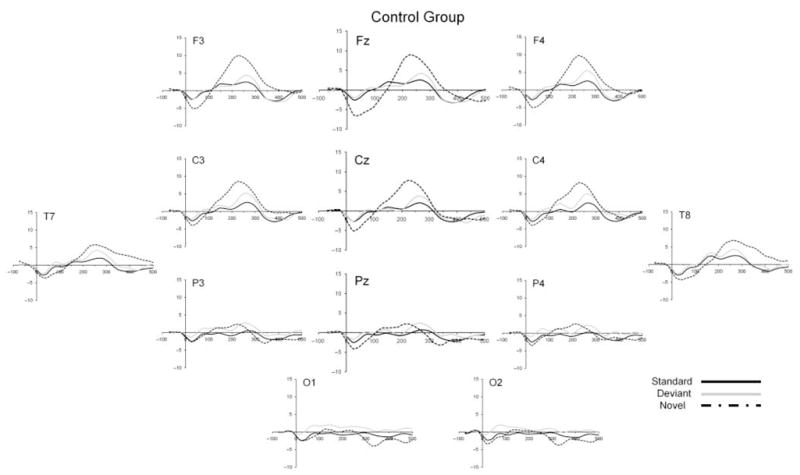
Grand mean ERP waveforms for the standard (solid black line), deviant (solid gray line), and novel (dashed line) stimuli for the control group.
Figure 3.
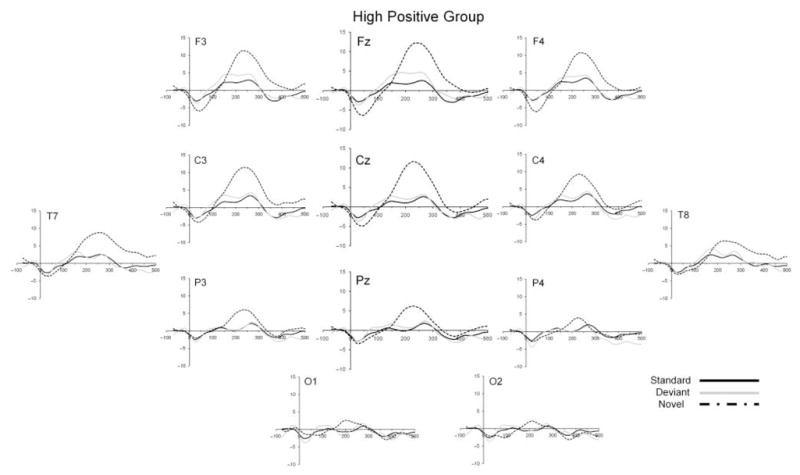
Grand mean ERP waveforms for the standard (solid black line), deviant (solid gray line), and novel (dashed line) stimuli for the high positive temperament group.
Results
ERP morphology
Figures 1–3 show the ERP waveforms for standard, deviant and novel stimuli for each temperament group (control, high negative and high positive) separately. A number of generalizations can be made about the overall morphology of the ERP waveforms to the three types of stimuli. First, all stimuli elicited a small negative component at around 30 ms after stimulus onset. For the standard and deviant tones, this was followed by a broad positive slow wave occurring between 100 and 300 ms which was largest at frontal and central sites. This is consistent with the findings of Morr et al. (2002) who noted a slow positive peak to both the standard and deviant tones within a similar latency window for infants in a similar age range. The complex novel stimulus elicited a prominent positivity peaking at around 200–250 ms, with this component appearing to be largest at frontal sites.
Mean amplitude of standard and deviant tones
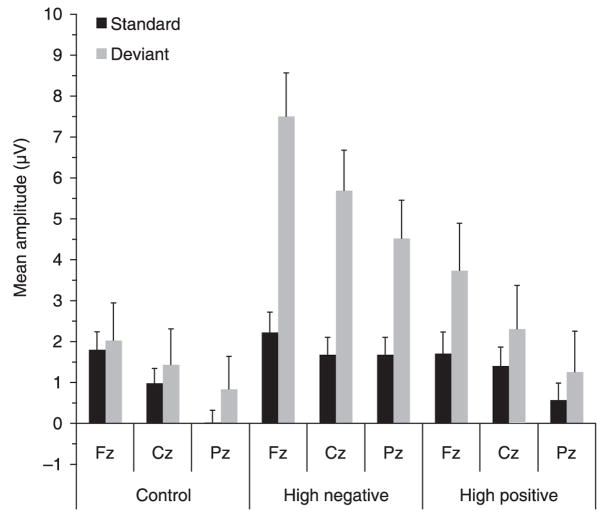
A repeated-measures ANOVA with electrode site (Fz, Cz, Pz) and stimulus type (standard, deviant) as within-subjects factors and temperament group (control, high negative, high positive) as the between-subjects factor was computed for mean amplitude in the 100–300 ms window. All probability values were adjusted using the Greenhouse-Geisser correction. A significant main effect was found for electrode site (F(2, 200) = 33.86, p < .001). Follow-up contrasts showed that mean amplitude at Fz was significantly greater than at Cz (p < .001), which in turn was greater than at Pz (p < .001). A significant main effect for stimulus type was also observed (F(1, 100) = 17.44, p < .001), reflecting an overall increased mean amplitude in response to the deviant tone compared to the standard tone. A significant main effect of temperament group was also found (F(2, 100) = 6.49, p < .01), with post-hoc Scheffé tests showing that the high negative infants tended to show a larger mean amplitude to the standard and deviant tones than the control (p < .01) and high positive (p = .07) infants. The main finding of interest was a significant group × stimulus type interaction effect (F(2, 100) = 5.99, p < .01), which indicated a differential response to the standard and deviant tones between the temperament groups. In separate follow-up repeated-measures ANOVAs within each group, a significant increase in amplitude to the deviant tone relative to the response to the standard tones was found only among the group of high negative infants (F(1, 31) = 19.86, p < .01). No significant difference between mean amplitude to the standard and deviant tones was observed in either the control group (p > .20) or the group of high positive infants (p > .20). Table 1 displays the mean amplitudes for both standard and deviant stimuli for each temperament group for the three analyzed electrode sites (Fz, Cz, Pz) combined, while Figure 4 shows the results in graphical form for each of the three electrode sites separately.
Table 1.
Mean amplitude (μV) across the 100 –300 ms window of the ERP to the standard and deviant tones, and peak amplitude (μV) for the response to the complex novel stimuli within the 100– 400 ms window for each temperament group across Fz, Cz, and Pz combined. Standard deviations are indicated in parentheses
| Control (n = 43) | High negative (n = 32) | High positive (n = 28) | |
|---|---|---|---|
| Standard tones | 1.81 (2.6) | 2.22 (2.9) | 1.70 (3.0) |
| Deviant tones | 2.01 (5.3) | 7.49 (7.0) | 3.74 (6.0) |
| Novel sounds | 9.54 (6.2) | 10.98 (4.7) | 13.15 (5.4) |
Figure 4.
Mean amplitude (μV) in the 100–300 ms window at Fz, Cz, and Pz to the standard and deviant tones for the control group, the high negative temperament group, and the high positive temperament group. Error bars indicate +1 SEM.
Relative frequency of positive and negative responses to the deviant
In order to better understand the individual variability in the elicitation of the positive slow wave to the deviant tone, a supplementary analysis was conducted. In this analysis, the frequency of elicitation of a more positive mean amplitude response to the deviant than to the standard (over the 100–300 ms epoch, averaged across Fz, Cz, and Pz) was compared with the frequency of the reverse combination, which was a more positive response to the standard tone than to the deviant tone. Overall, 69 out of 103 infants showed a more positive mean amplitude in the response to the deviant tone compared with response to the standard tone. A chi-square analysis by temperament group was significant (χ2 = 7.00, p < .05), with the high negative group appearing to have a disproportionately large number of infants who showed the more positive response to the deviant tone (see Table 2).
Table 2.
Frequency counts of infants showing more positive responses to the deviant than to the standard tone (deviant > standard) and of infants showing the reverse (standard > deviant) for mean amplitude in the 100–300 ms window. Mean amplitudes were combined across Fz, Cz, and Pz for each temperament group. Frequency counts are shown for ERPs derived using two different high-pass filter settings: 1 Hz and 3 Hz
| 1–15 Hz | 3–15 Hz | |||
|---|---|---|---|---|
| Deviant > Standard | Standard > Deviant | Deviant > Standard | Standard > Deviant | |
| Control | 27 | 16 | 23 | 20 |
| High negative | 27 | 5 | 20 | 12 |
| High positive | 15 | 13 | 13 | 15 |
| Total | 69 | 34 | 56 | 47 |
In a related analysis of frequency counts, we also compared group differences in the elicitation of more positive responses to the deviant tones using a high-pass filter setting of 3 Hz, in contrast to the 1 Hz high-pass setting that was used in the main analysis (for rationale, see above and He et al., 2007). Using this higher filter setting, 56 out of the 103 infants still showed a more positive mean amplitude response to the deviant, but the chi-square test of the distribution among temperament groups was no longer significant (χ2 = 1.58, p > .45; see Table 2). Additionally, ANOVAs using the mean amplitudes derived from the 3–15 Hz data showed that there was no longer a significant interaction of temperament group with stimulus type for the continuous mean amplitudes (in the 100–300 ms window) to the standard and deviant tones.
Peak amplitude and latency for complex novel sounds
A repeated-measures ANOVA with electrode site (Fz, Cz, Pz) as the within-subjects factor and temperament group (control, high negative, high positive) as the between-subjects factor was computed for peak amplitude to the novel sounds. A significant main effect of region was found (F(2, 200) = 55.43, p < .001) with peak amplitude being larger at Fz than Cz (p < .01), which in turn was greater than Pz (p < .001). Importantly, a significant main effect of temperament group was found (F(2, 100) = 3.58, p < .05). Post-hoc Scheffé tests revealed a significant difference in peak amplitude between the high positive and control groups (p < .05) but not between the high positive and high negative groups (p = .32) or between the high negative and control groups (p = .54). Table 1 includes the amplitude of the peak that was elicited in response to the complex novel stimuli for each temperament group for the three analyzed electrode sites combined. In terms of latency, the overall mean latency of the peak elicited to the novel sounds was 239 ms (SD = 70). In a similar ANOVA for latency as was carried out for peak amplitude, the main effect of region approached significance (p < .10), but there was no significant main effect or interaction involving temperament group.
Discussion
In the present study, we examined ERP responses to two levels of auditory stimulus change in groups of temperamentally different infants at 9 months of age. The first level of change consisted of an infrequent deviant tone that differed from a more frequent, repetitive standard tone in its frequency. It is important to note that while the frequency difference between the standard and deviant tones used in the present study was relatively small (1000 Hz/950 Hz), behavioral studies have shown that 9-month-old infants can readily discriminate this difference (e.g. Olsho, Schoon, Sakai, Turpin & Sperduto, 1982; Sinnott & Aslin, 1985). The second level of change consisted of widely deviant, complex novel stimuli that were also interspersed among the train of frequent standard stimuli.
In line with other recent research on the ERP response to tonal changes in infants, we did not find clear evidence of a more negative response to the deviant tone compared with the standard tone. Instead of finding a more negative response that has been reported in newborns and very young infants (e.g. Čeponienėet al., 2000), we replicated several studies that show that the response to a tonal deviant stimulus during the first year of life is characterized by the elicitation of a more positive slow wave to the deviant compared with the response to the standard tone (He et al., 2007; Leppänen et al., 1997; Morr et al., 2002; Novitski et al., 2007; Trainor et al., 2003). Our emphasis is therefore on the functional significance and variability in this positive slow wave, which we see as reflecting orienting processes (see below), rather than on the infant MMN as an indicator of more passive discrimination processes.
The elicitation of the slow positive response to the deviant tone was probabilistic in the sense that not all infants showed a more positive response to the deviant than to the standard. However, one key finding from our analyses is that variation in the appearance of the positive slow wave was significantly associated with individual differences in infant temperament. Specifically, infants previously identified as temperamentally high negative at 4 months of age were more likely to show a positive response to the deviant tone, and as a group showed higher mean amplitude of this positive slow wave. This raises the question of why temperamentally more negative infants may be more likely to show a positive slow wave to the low level of stimulus change that characterized the deviant tone. In order to address this question, it is helpful to view our findings in the context of other studies that have reported positive slow wave responses to deviant stimuli in infant oddball paradigms. Morr et al. (2002) noted a slow positivity to a deviant tone of 1200 Hz (against a 1000 Hz standard) in 8- to 12-month-old infants. These authors made a further observation that when the deviance level was increased (using a 1000 Hz/2000 Hz standard-deviant combination), an earlier, more negative-going response occurred at 150–160 ms in the response to the deviant. Given that MMN latency increases as stimulus deviance decreases, Morr et al. (2002) suggested that small deviance levels may elicit a negative-going MMN response in infants under 1 year old, but that it is obscured by a large slow positivity that occurs in the latency range from around 150 to 300 ms. The notion that a positive-going component could obscure or replace the MMN in infants has also been raised by other researchers in this area. For example, Cheour, Leppänen and Kraus (2000) noted the possibility that in young infants, ‘the MMN is sometimes obscured by other ERP components’, such as a ‘P3-like wave’ (p. 12). Fellman, Kushnerenko, Mikkola, Čeponienė, Leipala and Näätänen (2004), referring to their ERP findings at 6 and 9 months of age, write that ‘the MMN can be diminished or even abolished by the larger amplitude positive difference component’ (p. 296). Kushnerenko et al. (2002) suggested that the presence of this positive component may account for the large between-subjects variability observed in the infant auditory ERP response to deviant stimuli compared with older children and adults.
Perhaps the most thorough examination of the infant positive slow wave seen in mismatch tasks has been through the work of Trainor and colleagues (He et al., 2007; Trainor et al., 2003; Trainor, Samuel, Desjardins & Sonnadara, 2001), who have proposed that the positive slow wave and MMN responses to deviant tones in infancy are functionally distinct and can be separated through varying filter cutoff frequencies prior to ERP computation. Specifically, He et al. (2007) used a high-pass cutoff of 3 Hz to remove the positive slow wave and expose a concurrent MMN response in early infancy (see also Trainor et al., 2001). When this approach was used in the current paper, our main finding of differences between the temperament groups in the ERP to the deviant tone was lost: The high negative group did not differ from the other two groups on the frequency of elicitation of a more positive response to the deviant tone, and the between-groups differences in ERP amplitude were eliminated. Since the 3 Hz high-pass filter would be expected to remove or attenuate the slow positive component elicited in response to the deviant, this pattern of findings suggests that the group differences in the main analyses at the 1 Hz filter setting were likely due to an increased amplitude and rate of elicitation of the positive slow wave in the group of temperamentally negative infants relative to the other groups. In terms of the functional significance of this slow wave, He et al. (2007) point to the suggestion of Kushnerenko et al. (2002) that positive deflections elicited to the deviant stimulus in infants may reflect aspects of an orienting or attention switching process, as a form of P3 response. If this is correct, the large positivity to the deviant stimulus seen in our study may therefore reflect a specific heightened sensitivity of orienting networks late in the first year of life. This sensitivity may be specific to this age period, since the positive component to the deviant has been found to diminish over infancy and into early childhood (Morr et al., 2002) – although it is notable that He et al. (2007) document a decline in the positive slow wave much earlier in infancy. It is also possible that different stimulus characteristics (e.g. ISI, deviance level) may also be an influence in the appearance of the positive slow wave across some studies and not others, a suggestion that needs further investigation.
If indeed there is a functional association between the infant positive slow wave to the deviant stimulus and attention switching or orienting processes, this suggests a particularly intriguing interpretation of our findings. This is that compared to either the control or high positive temperament groups, the temperamentally high negative temperament group showed increased attentional engagement to the change between the standard and deviant tones, as evidenced by the greater elicitation of the positivity to the deviant tone among the high negative group. Given this finding that the positive slow wave response to the deviant stimulus varies with infant temperament, it is possible that temperamental differences may explain at least some of the large individual variability seen in the infant auditory ERP in mismatch paradigms (Kurtzberg, Vaughan, Kreuzer & Flieger, 1995). This possibility is discussed further below in the context of the between-group findings concerning the ERP responses to the novel sounds.
At present, the physiological origins of the infant slow wave elicited to the deviant tone are poorly understood. Trainor et al. (2003) suggest one possibility, which is that the positive slow wave is the result of asynchronous depolarization of neurons in layer IV of the auditory cortex (see discussion in He et al., 2007). The issue of the neural origins of the positive slow wave also relates to an ongoing debate within the temperament literature about the relative roles of top-down versus bottom-up processes in sensory processing. One view states that temperamental influences underlying sensory reactivity to novelty are mediated by higher structures such as the amygdala, which are posited to influence even in very early stages of sensory processing (Woodward et al., 2001). An alternative view proposes that individual differences in early transmission and processing are more strongly determined by inherent variation in the properties of the sensory pathway itself (see Bar-Haim, 2002; Galbraith, 2001). While our findings do not necessarily confirm one viewpoint over the other, they do suggest that groups of temperamentally different infants show differences in electrophysiological indices of reactivity at fairly early stages of novelty processing, and they point to the need for further study to investigate the neural mechanisms involved in the modulation of this reactivity.
As well as showing differences in ERP responses to the deviant tone, the infant temperament groups also showed differing responses to the complex novel stimuli. Across the whole sample, these stimuli elicited a large positivity which tended to be most prominent at frontal electrode sites. In contrast to the findings for the deviant tone, the peak amplitude of the positivity to the novel stimuli was largest for the group of temperamentally high positive infants. Based on prior findings of Kushnerenko et al. (2002), we suggest that there is a resemblance between this component and the novelty P3 which is elicited in similar oddball tasks in older children and adults (Čeponienė, Lepisto, Soininen, Aronen, Alku & Näätänen, 2004; Friedman et al., 2001). If this is correct, the association of the novelty P3 with involuntary attention shifting observed in older children (e.g. Čeponienė et al., 2004;Gumenyuk et al., 2001) and adults suggests the possibility that the high positive temperament group showed an attention switch or a greater degree of orienting to the large degree of change between the standard and complex novel stimuli. In contrast to the high negative group, the high positive group of infants did not show an exaggerated response to the deviant tone, but instead showed a maximal response to the higher level of stimulus change associated with the unexpected, complex novel sounds.
Taken together, the findings suggest differential electrophysiological responses to varying levels of stimulus novelty in groups of temperamentally different infants. Based on the suggestion of Kagan (1994) that temperamentally different infants may have different thresholds for novelty processing, we propose that our ERP findings may contribute to the understanding of the different behavioral reactivity profiles of temperamentally different infants. From an individual differences perspective, Berlyne (1960) hypothesized that novelty preference follows an inverted-U function in relation to the degree of novelty. Specifically, each individual has an optimal level of novelty where curiosity and exploration are maximal. Below this level, curiosity would not be elicited, and above this level, the amount of novelty would overwhelm the individual and preclude engagement with the stimulus. Given the suggestion that the infant positive slow wave observed at frontal sites in response to deviant tones is in fact related to attention switching (Kushnerenko et al., 2002), the more frequent elicitation of this component to the deviant tone in the high negative temperament group may indicate that lower levels of novelty were more likely to elicit an orienting response or involuntary attention switch in this group of infants. This exogenous attention switch could be considered a first step towards ‘engagement’ with a novel stimulus. This interpretation is consistent with the notion that negatively reactive infants and behaviorally inhibited children may be ‘hypervigilant’ to small changes in their environment (Fox et al., 2005). In contrast, the relatively smaller response to the deviant tone and the greater amplitude of the novelty P3 to the widely deviant complex novel stimuli in the high positive group implies that rather than orienting to the lower level of novelty presented by the deviant tone, these infants were more likely to show an attention switch or orienting response to the large deviance level of the novel sounds.
The inference from the argument presented above is that the groups of temperamentally high negative and high positive infants required different levels of novelty in order to elicit a neurophysiological signature of orienting or initial attentional engagement with the stimulus. We suggest that these group differences in novelty processing at 9 months of age are linked to variation in these infants’ optimal level of novelty as assessed in the earlier assessment at 4 months of age. Indeed, the selection procedure at 4 months involved presenting the infant with novel stimuli of moderate intensity. The differences seen in the infants’ behavioral reactions to novelty in this early assessment can be seen as reflecting temperamental approach or withdrawal tendencies, even in the absence of refined motor coordination (Rothbart & Derryberry, 1981). Specifically, the approachful behavioral engagement shown by the high positive infants to the stimuli at 4 months may reflect these infants’ higher optimal levels of novelty and their preference for moderate to high levels of novel stimulation. In contrast, the crying and fretting of the high negative infants to the battery of stimuli at 4 months is likely to be an indication that their optimal level of novelty has been exceeded. The ERP findings provide an intriguing indication that these early differences in reactivity to novelty are related to variation in later infancy in sensory reactivity as indexed by ERP responses to different levels of change in a repetitive auditory stimulus train. An interesting extension of the current study may be to examine the influence of early temperamental reactivity on novelty processing across other tasks and modalities, and at various other age points. For instance, there has been a substantial amount of research in cognitive developmental psychology into infant novelty preferences in the visual domain using variations of the preferential looking paradigm that was originally developed by Fantz (1964). However, very little of this work has examined the relation of temperament to novelty preferences, and the small number of findings have been inconsistent (Fagen & Ohr, 2001). It should be noted that this small number of studies have frequently relied on caregiver report of temperament rather than direct observation, and have not typically carried out selection for temperament groups along the lines of that used in the current study. Given our findings, we believe there is still a good deal of potential for the systematic study of the association between early temperamental behavioral/affective reactivity and novelty preferences in other laboratory tasks assessing infants’ reactions to novelty.
One explanation for the differences in the differential patterning of ERP responses to the complex novel stimuli for the temperament groups concerns the temporal properties of the novelty P3. In a study of adults who had been instructed to ignore a train of auditory stimuli, Friedman et al. (1998) found that the amplitude of the frontal component of the novelty P3 to complex novel stimuli declined as the experiment progressed. Friedman and colleagues suggested that the reduction in amplitude with increased exposure to novel stimuli reflects a diminution of an orienting process: As more novel sounds are presented, they tend to elicit less of an orienting response. Although lack of sufficient numbers of trials precludes an analysis of this possibility in the present study, further studies could investigate whether the frontal positivity to the novel stimuli diminishes less readily for infants with more approachful temperaments (i.e. those in the high positive temperament group). As more novel stimuli were presented in the present study, high positive infants may have been less apt to categorize them into a discrete group of complex novel sounds (see also Friedman et al., 1998). This would have resulted in a sustained orienting response to the novel stimuli for these infants over the course of the entire epoch of stimulus presentation. If the infant positivity to complex novel sounds is indeed related to the novelty P3 in adults, the positivity in the high positive group would be less prone to diminish over time than in the other temperament groups. While long experimental paradigms are difficult to use with infant participants, increasing the number of blocks in the current experimental design may provide some insight into this interesting possibility.
A number of limitations of the current study must be pointed out. First, there was a high degree of data loss, which is typical of studies using ERP techniques in this age range but which constrains the analyzed sample to only a subset of the larger selected sample. In a study such as this one, important information may be lost, especially considering that infants who are highly temperamentally reactive may be less likely to complete the ERP assessment. Indeed, in the current study, the highest degree of data loss was in the group of infants who were classified as negatively reactive at 4 months of age, although this difference was not significant when the positive and negative temperament groups were compared. Second, the number of trials within each condition was relatively low. In part, this reflects the difficulty of carrying out sustained periods of EEG data collection in this age range. While the small numbers of trials clearly elicited the novelty-related components of interest, it is possible that other aspects of the infant auditory ERP (including the MMN) would become clearer over long periods of stimulus presentation. Third, further work is clearly needed, not only to replicate the current results but also to expand knowledge about the function and origin of the infancy ERP components about which we are drawing inferences. In this sense, the current study should be seen as a starting point for further investigation of the relation of early temperament to novelty processing as indexed by electrophysiological reactions to auditory change.
It has previously been proposed that infants and young children who react more negatively to novelty tend to have reduced thresholds of activation in neural circuits underlying the identification and evaluation of novelty (e.g. Kagan, 1994). In support of this hypothesis, there is indeed evidence that this is the case in terms of the short-term reactivity of peripheral physiological systems (Marshall & Stevenson-Hinde, 2001). Through analyzing electrophysiological responses to novelty occurring at relatively early stages of stimulus processing, the current findings suggest an additional perspective on this argument. We raise the possibility that the differential ERP responses in the two temperament groups reflect varying levels of reactivity to novelty, with orienting responses or attention switches more likely to be elicited at different levels of novelty for each group of infants. The finding of differential electrophysiological reactions to varying levels of novelty in temperamentally different infants may provide an important perspective on understanding the basis of later individual differences in behavioral responses to novelty. In this sense, we suggest that the withdrawal responses of behaviorally inhibited older infants and children in response to an unfamiliar person may be related to their efforts to limit their presumed discomfort to a high level of novelty (Thompson & Calkins, 1996). However, such inhibited children may show engagement with stimuli at low levels of novelty which may be uninteresting for more outgoing children (who may have been more positively reactive as infants; Fox et al., 2001b). The latter group of more approachful and socially engaging children may prefer situations in which high levels of novelty are present, and may actively seek engagement with novel stimuli in order to meet that preferential need. While this hypothesis may be intuitively part of theories of approach and withdrawal tendencies in infants and children (e.g. Kagan, 1994), the current study suggests that differences in the processing of novelty can be seen at relatively early stages of sensory processing in temperamentally different infants during the first year of life.
Figure 2.
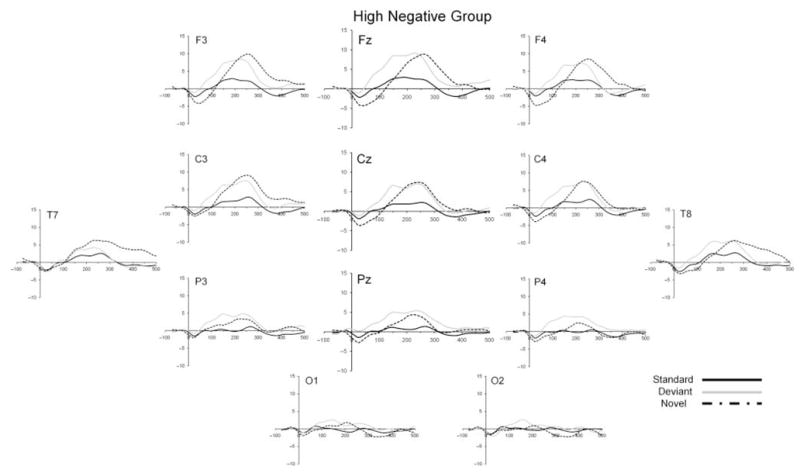
Grand mean ERP waveforms for the standard (solid black line), deviant (solid gray line), and novel (dashed line) stimuli for the high negative temperament group.
Acknowledgments
The authors wish to thank all the families who participated in the study, as well as the students and staff who assisted with laboratory visits and data processing. This work was supported by NIH award 5R37HD017899-19 to NAF.
References
- Bar-Haim Y. Introversion and individual differences in middle ear acoustic reflex function. International Journal of Psychophysiology. 2002;46:1–11. doi: 10.1016/s0167-8760(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA, Schorr EA, Gordon-Salant S. Mismatch negativity in socially withdrawn children. Biological Psychiatry. 2003;54:17–24. doi: 10.1016/s0006-3223(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Conflict, arousal, and curiosity. New York: McGraw-Hill; 1960. [Google Scholar]
- Bishop DV. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychological Bulletin. 2007;133:651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. The relations among infant temperament, security of attachment, and behavioral inhibition at twenty-four months. Child Development. 1992;63:1456–1472. [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Čeponienė R, Hukki J, Cheour M, Haapanen ML, Koskinen M, Alho K, Näätänen R. Dysfunction of the auditory cortex persists in infants with certain cleft types. Developmental Medicine and Child Neurology. 2000;42:258–265. doi: 10.1017/s001216220000044x. [DOI] [PubMed] [Google Scholar]
- Čeponienė R, Lepisto T, Soininen M, Aronen E, Alku P, Näätänen R. Event-related potentials associated with sound discrimination versus novelty detection in children. Psychophysiology. 2004;41:130–141. doi: 10.1111/j.1469-8986.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Cheour M, Alho K, Čeponienė R, Reinikainen K, Sainio K, Altonen O, Näätänen R. Maturation of mismatch negativity in infants. International Journal of Psychophysiology. 1998;29:217–226. doi: 10.1016/s0167-8760(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Cheour M, Leppänen PHT, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 2000;111:4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Rothstein M. An ERP developmental study of repetition priming by auditory novel stimuli. Psychophysiology. 1996;33:680–690. doi: 10.1111/j.1469-8986.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Fagen JW, Ohr PS. Learning and memory in infancy: habituation, instrumental conditioning, and expectancy formation. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the infant. New York: Guilford; 2001. pp. 233–273. [Google Scholar]
- Fantz RL. Visual experience in infants: decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Fellman V, Kushnerenko E, Mikkola K, Čeponienė R, Leipala J, Näätänen R. Atypical auditory event-related potentials in preterm infants during the first year of life: a possible sign of cognitive dysfunction? Pediatric Research. 2004;56:291–297. doi: 10.1203/01.PDR.0000132750.97066.B9. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ. The biology of temperament: an integrative approach. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001a. pp. 631–646. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001b;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Stifter CA. Biological and behavioral differences in infant reactivity and regulation. In: Kohnstamm GA, Bates JE, editors. Temperament in childhood. Chichester: John Wiley; 1989. pp. 169–183. [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Ritter W, Cheng J. An event-related potential study of age-related changes in sensitivity to stimulus deviance. Neurobiology of Aging. 1998;19:447–459. doi: 10.1016/s0197-4580(98)00087-6. [DOI] [PubMed] [Google Scholar]
- Galbraith GC. From brainstem to cortex: neurobiologic research provides keys to the riddles, mysteries and enigmas of brain dysfunction. Clinical Neurophysiology. 2001;112:721–723. doi: 10.1016/s1388-2457(01)00511-9. [DOI] [PubMed] [Google Scholar]
- Gomes H, Molholm S, Ritter W, Kurtzberg D, Cowan N, Vaughan HGJ. Mismatch negativity in children and adults, and effects of an attended task. Psychophysiology. 2000;37:807–816. [PubMed] [Google Scholar]
- Gomot M, Giard MH, Roux S, Barthelemy C, Bruneau N. Maturation of frontal and temporal components of mismatch negativity (MMN) in children. NeuroReport. 2000;11:3109–3112. doi: 10.1097/00001756-200009280-00014. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Alho K, Escera C, Schroger E, Ilmoniemi RJ, Näätänen R. Brain activity index of distractibility in normal school-age children. Neuroscience Letters. 2001;314:147–150. doi: 10.1016/s0304-3940(01)02308-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Studies of the human infant’s adreno-cortical response to potentially stressful events. In: Lewis M, Worobey J, editors. New directions for child development. Vol. 45. San Francisco, CA: Jossey-Bass; 1989. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: normative changes and individual differences. Child Development. 1996;67:877–889. [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological Science. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- He C, Hotson L, Trainor LJ. Mismatch responses to pitch change in early infancy. Journal of Cognitive Neuroscience. 2007;19:878–892. doi: 10.1162/jocn.2007.19.5.878. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Butterfield EL, Phillips L, Hadwin JA. Brain response to unexpected novel noises in children with low and high trait anxiety. Journal of Cognitive Neuroscience. 2007;19:25–31. doi: 10.1162/jocn.2007.19.1.25. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Kagan J. Galen’s prophecy: Temperament in human nature. New York: Basic Books; 1994. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. American Psychologist. 1991;46:856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG, Kreuzer JA, Flieger KZ. Developmental studies and clinical applications of mismatch negativity: problems and prospects. Ear and Hearing. 1995;16:105–117. doi: 10.1097/00003446-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Čeponienė R, Balan P, Fellman V, Näätänen R. Maturation of the auditory change detection response in infants: a longitudinal ERP study. NeuroReport. 2002;13:1843–1848. doi: 10.1097/00001756-200210280-00002. [DOI] [PubMed] [Google Scholar]
- Leppänen PH, Eklund KM, Lyytinen H. Event-related brain potentials to change in rapidly presented acoustic stimuli in newborns. Developmental Neuropsychology. 1997;13:175–204. [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral inhibition: physiological correlates. In: Crozier WR, Alden LE, editors. International handbook of social anxiety. Chichester: John Wiley; 2001. pp. 53–76. [Google Scholar]
- Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear and Hearing. 2002;23:118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Alho K. Mismatch negativity – a unique measure of sensory processing in audition. International Journal of Neuroscience. 1995;80:317–337. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of first-year recognition memory. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 269–313. [Google Scholar]
- Novitski N, Huotilainen M, Tervaniemi M, Näätänen R, Fellman V. Neonatal frequency discrimination in 250–4000-Hz range: electrophysiological evidence. Clinical Neurophysiology. 2007;118:412–419. doi: 10.1016/j.clinph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Olsho L, Schoon C, Sakai R, Turpin R, Sperduto V. Auditory frequency discrimination in infancy. Developmental Psychology. 1982;18:721–726. [Google Scholar]
- Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: different water in the same river. Auditory and Neurootology. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal ‘brake’ predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D. Development of individual differences in temperament. In: Lamb M, Brown A, editors. Advances in developmental psychology. Vol. 1. Hillsdale, NJ: Erlbaum; 1981. pp. 37–86. [Google Scholar]
- Shafer VL, Morr ML, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear and Hearing. 2000;21:242–251. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Sinnott JM, Aslin RN. Frequency and intensity discrmination in human infants and adults. Journal of the Acoustical Society of America. 1985;78:1986–1992. doi: 10.1121/1.392655. [DOI] [PubMed] [Google Scholar]
- Thompson RA, Calkins SD. The double-edged sword: emotional regulation for children at risk. Development and Psychopathology. 1996;8:163–182. [Google Scholar]
- Trainor LJ, McFadden M, Hodgson L, Darragh L, Barlow J, Matsos L, Sonnadara R. Changes in auditory cortex and the development of mismatch negativity between 2 and 6 months of age. International Journal of Psychophysiology. 2003;51:5–15. doi: 10.1016/s0167-8760(03)00148-x. [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Samuel SS, Desjardins RN, Sonnadara RR. Measuring temporal resolution in infants using mismatch negativity. NeuroReport. 2001;12:2443–2448. doi: 10.1097/00001756-200108080-00031. [DOI] [PubMed] [Google Scholar]
- Woodward S, McManis M, Kagan J, Deldin P, Snidman N, Lewis M, Kahn V. Infant temperament and the brainstem auditory evoked response in later childhood. Developmental Psychology. 2001;37:533–538. [PubMed] [Google Scholar]