Abstract
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma, composed of a monomorphic population of medium-sized B cells with a high proliferation rate and a consistent MYC translocation. Epstein-Barr virus (EBV) has been associated with BL with different frequencies depending on the clinical variant. Kaposi sarcoma–associated herpesvirus, or human herpesvirus 8 (HHV-8), infects a wide range of normal cells, having a well-established role in the pathogenesis of various neoplasms, including Kaposi sarcoma, primary effusion lymphoma, multicentric Castleman disease (MCD) and MCD-associated plasmablastic lymphoma. In secondary immunodeficiencies, such as HIV-1 infection and organ transplantation, HHV-8 is considered an opportunistic pathogen linked to the development of lymphomas in patients with AIDS and HIV+ patients. We studied the association of EBV and HHV-8 by immunohistochemical analysis, in situ hybridization, and polymerase chain reaction in a large number of well-characterized BLs. EBV was present in 45.0% of all BL cases with higher incidence in the pediatric group; most cases were EBV type A. We found no association of BL with HHV-8 in EBV+ BL or in EBV–cases, including the HIV+ BL group.
Keywords: Burkitt lymphoma, Kaposi sarcoma–associated herpesvirus, Human herpesvirus 8, KSHV/HHV-8, LANA protein, HIV, Immunohistochemistry, Polymerase chain reaction, PCR
Burkitt lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma with endemic, sporadic, and immunodeficiency-associated clinical variants composed of a monomorphic population of medium-sized B cells with a high proliferation rate and having a consistent MYC translocation.1,2 Viral infections, in particular Epstein-Barr virus (EBV), have been associated with BL; it is well established that this association occurs with different frequencies depending on the clinical variant.3 EBV is present in the majority of endemic cases of BL and nearly 30% of cases of sporadic BL.1 In Brazil, a high association of EBV with BL has been demonstrated in tropical areas, especially in the northeast region.4,5
Kaposi sarcoma–associated herpesvirus, or human herpesvirus (HHV)-8, is a virus able to infect mammalian cells, including lymphoid cells, dendritic cells, and fibroblasts. Several neoplasms have been associated with HHV-8, including Kaposi sarcoma, primary effusion lymphoma, multicentric Castleman disease (MCD), and MCD-associated plasmablastic lymphoma.6–8
In the context of secondary immunodeficiencies, such as HIV-1 infection and organ transplantation, HHV-8 is considered an opportunistic pathogen that has been linked to the development of lymphoproliferative diseases, including lymphomas and related diseases.9 HHV-8 has also been reported in association with lymphomas in patients with AIDS and HIV+ patients.10 In common variable immunodeficiency, a primary immunodeficiency disorder of unknown etiology, patients have a high risk of B-cell lymphomas; HHV-8 has been identified in at least some of the associated lymphomas and is considered an important factor in their pathogenesis.9 Du et al11 demonstrated monotypic HHV-8+ plasmablasts in MCD and MCD-associated plasmablastic lymphomas.
HHV-8–associated lymphomas have included cases of naive cell origin, germinal center (GC) and post–GC cells, unlike EBV-associated lymphomas, which are generally more restricted to GC or post-GC origin.12,13 HHV-8 and EBV coinfection has been documented in primary effusion lymphoma and in the setting of Castleman disease, typically associated with an immunosuppressed state.14
In the literature, there is scarce information on the association of HHV-8 with BL in HIV+ and HIV− patients, and it is generally limited to data concerning African populations. Lazzi et al,15 in a study of East African patients, evaluated 16 BL cases and detected HHV-8 in nonneoplastic lymphoid cells in 1 case of an HIV− patient with a lymph node partially involved by BL. The molecular analysis in microdissected HHV-8+ cells in this case showed absence of clonality. It is worth mentioning that in this BL case, there was no association with Kaposi sarcoma. In a previous study on HIV-associated malignant lymphomas, also in African patients, Lazzi et al16 analyzed 29 cases of BL, with none showing HHV-8 by polymerase chain reaction (PCR). In 10 cases of African BL, Tao and Ambinder17 did not find HHV-8 or HHV-7 but found HHV-6 in 3 cases studied.
We studied the association of EBV and HHV-8 in a large number of well-characterized BL cases in a Brazilian population.
Materials and Methods
Case Material and Clinical Data
We retrospectively obtained 311 cases of BL from the files of Consultoria em Patologia, a large reference consultation service in anatomic pathology located in Botucatu, São Paulo State, Brazil. The study group included all cases of BL with available representative formalin-fixed, paraffin-embedded (FFPE) blocks received in consultation between June 1997 and May 2007. Nodal and extranodal BL cases were included. Clinical data, including sex, age at diagnosis, and tumor location, were obtained from the referring pathologists and oncologists. We reviewed available H&E-stained slides of each case, and representative areas were selected for incorporation into tissue microarrays (TMAs). Morphologic subclassification of the cases was performed according to variants recognized by the World Health Organization classification. 1
TMA Construction
Six TMA blocks were constructed by using a tissue arrayer (Beecher Instruments, Sun Prairie, WI). Each individual case was represented by 3 tumor cores of 0.6 mm that were taken from the original paraffin blocks. Serial sections of 3 µm were cut from the tissue array blocks and used for immunohistochemical analysis. Proper positive and negative control cores for each marker were also included in the array block to provide adequacy of the antibodies used in the immunohistochemical studies as follows: tonsil (CD3, CD10, CD20, bcl-2, bcl-6, and Ki-67), lymphoblastic lymphoma (terminal deoxynucleotidyl transferase), EBV+ Hodgkin lymphoma (EBV latent membrane protein [LMP]-1), and Kaposi sarcoma and HHV-8–AIDS-related non-BL (Kaposi sarcoma– associated herpesvirus/HHV-8).
Immunohistochemical Analysis and In Situ Hybridization
An immunohistochemical study was performed for each TMA using Novolink polymer (Novocastra, Newcastle upon Tyne, England) as the detection system, and an epitope-retrieval method was applied as needed for each specific antibody; diaminobenzidine was the chromogen. Primary antibodies used were anti-CD20, anti-CD3, anti-CD10, anti–bcl-6, anti–Ki-67, anti–bcl-2, anti–EBV-LMP, and anti–HHV-8 latent nuclear antigen (LANA); for this last marker, only cells with a salt-and-pepper granular nuclear pattern of immunostaining were considered positive Table 1.
Table 1.
Primary Antibodies Used for Immunohistochemical Staining in Paraffin Sections
| Antigen | Clone | Dilution | Antigen Retrieval* | Source |
|---|---|---|---|---|
| CD20 | L26 | 1:1,200 | MW, CB | DAKO, Carpinteria, CA |
| CD3 | SP7 | 1:200 | S, CB | NeoMarkers/Lab Vision, Fremont, CA |
| CD10 | 56C6 | 1:100 | S, CB | Novocastra, Newcastle upon Tyne, England |
| bcl-2 | 124 | 1:400 | MW, CB | DAKO |
| bcl-6 | PG-B6P | 1:100 | T + S, TRIS | DAKO |
| Ki-67 | MIB-1 | 1:4,800 | PC, CB | DAKO |
| LANA of human herpesvirus 8 (ORF-73) | LN-53 | 1:30,000 | PC, CB | Advanced Biotechnologies, Columbia, MD |
| TdT | Polyclonal | 1:1,600 | PC, EDTA | DAKO |
| LMP1 | CS1-4 | 1:500 | S, CB | DAKO |
CB, citrate buffer, pH 6; LANA, latent nuclear antigen; MW, microwave oven; ORF, open reading frame; PC, pressure cooker; S, steamer; T, trypsin; TdT, terminal deoxynucleotidyl transferase; TRIS, tris(hydroxymethyl)aminomethane.
Heat-induced epitope retrieval was used.
Sections from all TMAs were examined for the expression of Epstein-Barr viral early RNA (EBER) by in situ hybridization (ISH) using an EBER-1 probe, consistent with a 30-base oligonucleotide complementary to a portion of the EBER1 gene. Briefly, the slides were deparaffinized, dehydrated, predigested with Pronase (Sigma, St Louis, MO), and hybridized overnight at a concentration of 0.25 ng/µL of probe. After washing, detection was accomplished by using the avidin-biotin immunoperoxidase method with 3′,3-diaminobenzidine as the chromogen, without nickel chloride added for color enhancement. A brown or black color in the nucleus over background levels was considered a positive reaction.5 Appropriate positive (cases of EBV+ BL and EBV+ Hodgkin lymphoma) and negative control (EBV– tissue) cores were also included in the array.
Molecular Study
Molecular analysis for HHV-8 was performed in 32 cases, including 9 known HIV+ BLs and 23 HIV− BLs. The cases in the HIV− BL group were selected in a random manner. DNA isolation was performed as follows: FFPE histologic sections were submitted to deparaffinization by successive xylene baths and dehydration with 100% ethanol. After digestion of the material with Proteinase K at 50°C for 4 to 6 hours, 50 µL of a saturated sodium chloride solution (~6 mol/L) was added, and, after mixing for 30 seconds, the mixture was centrifuged at 4,000 rpm for 15 minutes. The supernatant was removed to a fresh tube, and one-tenth volume of 3 mol/L sodium acetate was added, followed by 2.5 volumes of 100% ethanol. Samples were placed at −20°C overnight and centrifuged at 4°C for 30 minutes at 14,000 rpm. Pellets were washed with 1 mL of 70% ethanol and resuspended in 50 µL of sterile water.18 Size control PCR was performed to investigate the quality of the extracted DNA, using a set of control primers that amplify products by 100, 200, 300, 400, and up to 600 base pairs (bp), depending on the integrity of the extracted DNA.19
The presence of HHV-8 was detected by PCR amplification of a 233-bp fragment from ORF (open reading frame)-26 (capsid antigen). In each 25-µL PCR reaction, 100 ng of DNA, 0.3 µmol/L of 5′ and 3′ oligonucleotide primers (KS330 forward AGCCGAAAGGATTCCACCAT and reverse TCCGTGTTGTCTACGTCCAG), 0.2 mmol/L of deoxynucleoside triphosphate (dNTP), 2.5 µL of 10× PCR buffer, 1.25 U of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA), and 3.0 mmol/L of magnesium chloride were used. PCR was initiated by 1 cycle at 95°C for 2 minutes, followed by 35 cycles of 30 seconds at 93°C, 25 seconds at 60°C, and 30 seconds at 72°C and further followed by a 5-minute extension at 72°C, according to Pak et al.20
Subtyping for EBV, by PCR amplification of the EBNA2 region, was performed in 131 cases. In 3 other EBV+ ISH cases, there was insufficient material in the paraffin block to proceed with PCR. Two primers encompassed this region (E2 up, 5′-AGGCTGCCCACCCTGAGGAT-3′ and E2 low, 5′-GCCACCTGGCAGCCCTAAAG-3′) containing a 16-bp deletion in EBV type A, yielding an amplification product with 170-and 186-bp fragment lengths for types A and B, respectively. In some cases, a seminested reamplification was performed using the E2 up and E2R low (5′-GCTGCCACCTGGCGGAAT-3′) primers, rendering amplification products of 111 and 127 bp for types A and B, respectively. In each 25-µL PCR reaction, 100 ng of DNA, 0.2 µmol/L of 5′ and 3′ oligonucleotide primers, 0.2 mmol/L of dNTP, 2.5 µL of 10× PCR buffer, 1.25 U of Platinum Taq DNA polymerase (Invitrogen), and 1.5 mmol/L of magnesium chloride were used. The mixture was subjected to 35 cycles of amplification (30 seconds at 96°C, 30 seconds at 60°C, and 1 minute at 72°C in a PTC 200 thermocycler [MJ Research, Watertown, MA]). Before cycling, the samples were denatured at 96°C for 2 minutes. After the last cycle, the polymerization step was extended by 10 minutes, according to Araujo et al.4
PCR-amplified products for both studies were analyzed in a 7% polyacrylamide gel with silver staining (5 minutes in a fixative solution, 5% acetic acid and 10% of ethanol; 5 minutes in silver solution, 0.2% silver nitrate in fixative solution; and 5 minutes in developer solution, 3% sodium hydroxide and 0.5% formaldehyde).
Results
Clinical Features
Of the 311 patients with BL, 221 (71.1%) were male and 90 (28.9%) were female. Of the patients, 149 (47.9%) were 16 years or younger, and 143 (46.0%) were older than 16 years; the age was unknown in 19 cases. The mean age was 23.1 years (range, 2–95 years). Extranodal BL constituted 201 (64.6%) of the cases, and primary lymph node involvement was observed in 99 (31.8%) of the cases; in 11 cases, it was not possible to determine nodal vs extranodal presentation. Extranodal primary disease was found in a higher proportion of pediatric patients (112/149 [75.2%]) than adults (80/143 [55.9%]); 11 cases in the extranodal group did not have age information available.
Morphologic Features
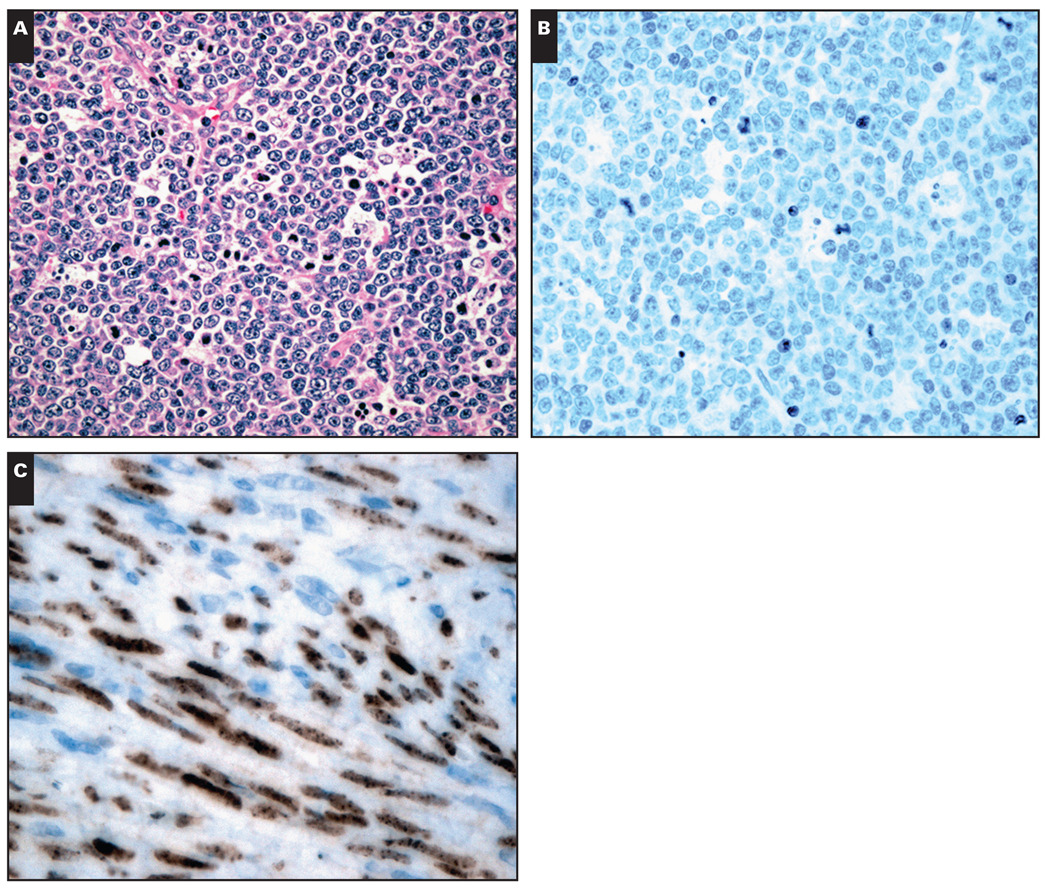
In the original material reviewed to select the areas for TMA, 309 cases of BL showed entirely diffuse architecture, and rare cases exhibited a focal nodular pattern (2 cases). Most of the cases showed cytologic features of the “classic” subtype (271/311 [87.1%]) Image 1A, 28 (9.0%) of the cases showed histologic features of atypical BL, and 12 (3.9%) showed plasmacytoid differentiation.
Image 1.
A, Classical Burkitt lymphoma in an HIV+ patient. Prominent starry-sky pattern (H&E, ×200). B, Negative immunodetection for human herpesvirus-8 (latent nuclear antigen [LANA]-1) (×200). C, Kaposi sarcoma (positive control sample) showing positive nuclear immunostaining for LANA (×400).
Immunohistochemical Analysis and ISH
All cases had an immunophenotype consistent with BL, as described in the World Health Organization classification.1 CD20 and CD10 were positive in all cases; bcl-6 positivity was found in 250 cases (80.4%) and was more frequent in the pediatric than in the adult population. bcl-2 was negative in all cases.
ISH for EBV showed 134 positive cases (45.0%), the majority in the pediatric population (76 [56.7%]) and 52 (38.8%) in patients older than 16 years. In 6 EBV+ BL cases, the age was unknown. In the pediatric group, 39 (51%) were EBV+; in the adult group, 36.3% were EBV+. In 13 cases, ISH for EBV was inconclusive because there was insufficient neoplastic tissue available in the cores.
None of the cases showed nuclear granular positivity for LANA protein with HHV-8 antibody Image 1B. LMP-1 expression was not observed in any of the cases. All positive control samples for LMP-1 and LANA protein showed the expected immunostaining reactivity Image 1C.
Molecular Analysis
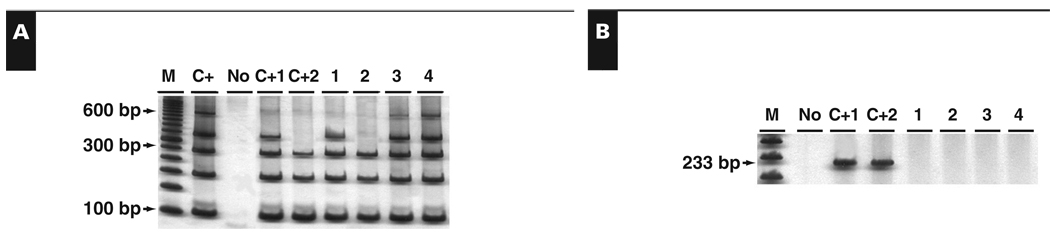
Molecular analysis for HHV-8 was performed in 32 cases, including 9 known HIV+ and 23 HIV− BLs; 19 were EBV−, and the other 13 cases presented EBV infection. No BL case had HHV-8 viral DNA, including cases of sporadic BL and HIV-associated BL Image 2.
Image 2.
Human herpesvirus (HHV)-8 DNA detection by polymerase chain reaction (PCR). A, Size control PCR. B, HHV-8–specific PCR. C+, DNA extracted from peripheral blood; C+1, positive HHV-8 Kaposi sarcoma; C+2, positive HHV-8–AIDS-related non-Burkitt lymphoma; M, DNA molecular weight marker; No, DNA absence; 1–2, HIV+ Burkitt lymphoma cases; 3–4, HIV– Burkitt lymphoma.
EBV molecular subtyping analysis showed that 96 (73.3%) of 131 cases were EBV type A. The rest were type B (27 [20.6%]). It is worth noting that in 8 cases (6.1%), the DNA obtained from the FFPE blocks had less than 100 bp, giving inconclusive results for the EBV subtyping PCR analysis. No case showed both EBV subtypes in the same sample.
Discussion
The spectrum of HHV-8–associated lymphoid tumors is different from that associated with EBV.21,22 HHV-8 is closely associated with Kaposi sarcoma but is associated with only a few categories of lymphoproliferative diseases, mostly occurring in HIV-infected patients, with primary effusion lymphoma (PEL), a nonsolid B-cell lymphoma, having the most significant association. Extranodal marginal zone lymphoma, multiple myeloma, Kikuchi disease, and hemophagocytic syndrome are lymphoproliferative disorders with a variable and generally infrequent association with HHV-8.6,23–27 In contrast, the evidence strongly supports a role for EBV in the pathogenesis of a wide spectrum of human lymphoid malignancies, including B- and T-cell lymphomas, natural killer neoplasms, and Hodgkin lymphoma and nonhematologic tumors such as nasopharyngeal carcinoma and gastric tumors.1,12,13
In BL, EBV has been demonstrated in up to 100% of the endemic form and in only 15% to 30% of sporadic cases in the United States.1 In some parts of the world (North Africa and South America), the incidence is intermediate between true sporadic and endemic variants.1 In Brazil, there are limited data about the frequency of EBV in cases of BL. Previous studies showed that the frequency of EBV in cases of Brazilian BL varied from 58% to 87%.4,5,28–30 It is important to emphasize that in the 5 previous studies on BL in Brazil, all EBV+ cases were described in the pediatric population only. Our study on BL showed 134 EBV+ cases (45.0%), with 76 cases (56.7%) occurring in patients in the age group of 16 years or younger and 52 cases (38.8%) in patients older than 16 years, which represents 36.4% of the adult group. To the best of our knowledge, this is the first study in Brazil reporting the frequency of EBV in adult cases of BL. Our overall results place Brazil as a country with intermediate association of BL with EBV.1
EBV strains can be categorized into 2 types (A and B), and different geographic prevalence of these strains has been observed.4 By studying the EBNA-2 gene, we determined that 96 (73.3%) of 131 cases contained type A EBV and 27 (20.6%) contained type B EBV. The simultaneous presence of both types of EBV in the same tumor was not observed in any sample. This pattern, with a predominance of type A, is similar to that observed in sporadic cases and previous studies of Brazilian BL4,31 and different from the pattern described in BL occurring in Equatorial Africa.32 LMP-1 was negative according to the highly restricted pattern of EBV latent proteins observed in BL.33
HHV-8–associated lymphomas include cases of naive cell origin, GC and post–GC cells, unlike EBV-associated lymphomas, which are of GC or post-GC origin.12,13 Moreover, HHV-8 and EBV coinfections have been documented in the setting of primary effusion lymphoma and Castleman disease, typically associated with an immunosuppressed state.14,34,35
The biologic heterogeneity of AIDS–non-Hodgkin lymphoma is highlighted by their histogenetic differences. HHV-8–associated lymphomas, which often develop in persons with advanced AIDS, present predominantly as PEL. HHV-8 has also been recently detected in solid extracavitary-based lymphomas.36–38 The HHV-8–associated solid lymphomas are unusual lymphomas that occur more specifically in HIV+patients, are extracavitary and arise in nodal and/or extranodal sites, and sometimes are associated with coinfection with EBV; histologically, they usually display PEL-like morphologic features and a plasma cell–related phenotype.
Lazzi et al,15,16 in different studies of African patients (East Africa), found no case with tumoral lymphoid cells infected by HHV-8 in 16 and 29 BL cases, respectively. Tao and Ambinder17 studied HHV-8, HHV-7, and HHV-6 DNA in 10 cases of BL in Africa and found only HHV-6 in 3 of their cases.
Molecular identification of HHV-8 is known to produce false-positive and false-negative results.34 The latter is thought, in part, to be related to HHV-8 sequence variation, which can range up to 35% in certain regions of the viral genome. On the other hand, LANA immunohistochemical studies are thought to be a more reliable marker of HHV-8 infection. In the present study, we found no evidence for HHV-8 in BL, including cases with HIV+ status using immunohistochemical and molecular methods. We were, however, able to confirm the association of EBV with BL, found in 45.0% of the cases of BL in the present series. In no case was coinfection of EBV and HHV-8 found.
Acknowledgments
Supported by grants 5R01CA082274 and 5R01CA112217 from the National Cancer Institute (Dr Harrington) and the AIDS Malignancy Consortium (National Cancer Institute), Bethesda, MD.
References
- 1.Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 2.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 3.Cogliatti SB, Novak U, Henz S, et al. Diagnosis of Burkitt lymphoma in due time: a practical approach. Br J Haematol. 2006;134:294–301. doi: 10.1111/j.1365-2141.2006.06194.x. [DOI] [PubMed] [Google Scholar]
- 4.Araujo I, Foss HD, Bittencourt A, et al. Expression of Epstein-Barr virus-gene products in Burkitt’s lymphoma in Northeast Brazil. Blood. 1996;87:5279–5286. [PubMed] [Google Scholar]
- 5.Bacchi MM, Bacchi CE, Alvarenga M, et al. Burkitt’s lymphoma in Brazil: strong association with Epstein-Barr virus. Mod Pathol. 1996;9:63–67. [PubMed] [Google Scholar]
- 6.Li CF, Ye H, Liu H, et al. Fatal HHV-8–associated hemophagocytic syndrome in an HIV-negative immunocompetent patient with plasmablastic variant of multicentric Castleman disease (plasmablastic microlymphoma) Am J Surg Pathol. 2006;30:123–127. doi: 10.1097/01.pas.0000172293.59785.b4. [DOI] [PubMed] [Google Scholar]
- 7.Fakhari FD, Jeong JH, Kanan Y, et al. The latency-associated nuclear antigen of Kaposi sarcoma–associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest. 2006;116:735–742. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140:13–24. doi: 10.1111/j.1365-2141.2007.06879.x. [DOI] [PubMed] [Google Scholar]
- 9.Wheat WH, Cool CD, Morimoto Y, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma–associated herpes virus/human herpesvirus type 8–positive solid lymphomas. J Mol Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du MQ, Liu H, Diss TC, et al. Kaposi sarcoma–associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97:2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 12.Frank D, Cesarman E, Liu YF, et al. Posttransplantation lymphoproliferative disorders frequently contain type A and not type B Epstein-Barr virus. Blood. 1995;85:1396–1403. [PubMed] [Google Scholar]
- 13.Küppers R, Klein U, Hansmann ML, et al. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 14.Hernández JL, Gómez-Román J, Ramos-Estébanez C, et al. Human herpesvirus and Epstein-Barr virus coinfection in localized Castleman disease during pregnancy. Haematologica. 2005;90 suppl ECR35. [PubMed] [Google Scholar]
- 15.Lazzi S, Bellan C, Amato T, et al. Kaposi’s sarcoma–associated herpesvirus/human herpesvirus 8 infection in reactive lymphoid tissues: a model for KSHV/HHV-8–related lymphomas? Hum Pathol. 2006;37:23–31. doi: 10.1016/j.humpath.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Lazzi S, Ferrari F, Nyongo A, et al. HIV-associated malignant lymphomas in Kenya (Equatorial Africa) Hum Pathol. 1998;29:1285–1289. doi: 10.1016/s0046-8177(98)90258-1. [DOI] [PubMed] [Google Scholar]
- 17.Tao Q, Ambinder RF. Lack of Kaposi’s sarcoma-associated virus (KSHV) and detection of human herpes virus 6 and 7 by PCR in African Burkitt’s lymphoma from HIV-negative patients [letter] Hum Pathol. 1999;30:1269–1270. doi: 10.1016/s0046-8177(99)90051-5. [DOI] [PubMed] [Google Scholar]
- 18.Howe JR, Klimstra DS, Cordon-Cardo C. DNA extraction from paraffin-embedded tissues using a salting-out procedure: a reliable method for PCR amplification of archival material. Histol Histopathol. 1997;12:595–601. [PubMed] [Google Scholar]
- 19.van Dongen JJM, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 20.Pak F, Pyakural P, Kokhaei P, et al. HHV-8/KSHV during the development of Kaposi’s sarcoma: evaluation by polymerase chain reaction and immunohistochemistry. J Cutan Pathol. 2005;32:21–27. doi: 10.1111/j.0303-6987.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 21.Carbone A, Gloghini A, Vaccher E, et al. KSHV/HHV-8 associated lymph node based lymphomas in HIV seronegative subjects: report of two cases with anaplastic large cell morphology and plasmablastic immunophenotype. J Clin Pathol. 2005;58:1039–1045. doi: 10.1136/jcp.2005.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone A. KSHV/HHV-8 associated Kaposi’s sarcoma in lymph nodes concurrent with Epstein-Barr virus associated Hodgkin lymphoma. J Clin Pathol. 2005;58:626–628. doi: 10.1136/jcp.2004.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith KJ, Skelton HG. Human herpesvirus 8 DNA sequences in pemphigus: the role of the virus in oncogenic and autoimmune manifestations [letter] Arch Dermatol. 1998;134:751. doi: 10.1001/archderm.134.6.751. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki S, Iino T, Nakamura M, et al. Detection of human herpesvirus-8 in peripheral blood mononuclear cells from adult Japanese patients with multicentric Castleman’s disease. Br J Haematol. 2003;120:471–477. doi: 10.1046/j.1365-2141.2003.04120.x. [DOI] [PubMed] [Google Scholar]
- 25.Fredricks DN, Martin TM, Edwards AO, et al. Human herpesvirus 8 and sarcoidosis. Clin Infect Dis. 2002;34:559–560. doi: 10.1086/338406. [DOI] [PubMed] [Google Scholar]
- 26.Marioni G, Marchese-Ragona R, Marino F, et al. MALT-type lymphoma and Warthin’s tumour presenting in the same parotid gland. Acta Otolaryngol. 2004;124:318–323. doi: 10.1080/00016480310015263. [DOI] [PubMed] [Google Scholar]
- 27.Tedeschi R, Luostarinen T, De Paoli P, et al. Joint Nordic prospective study on human herpesvirus 8 and multiple myeloma risk. Br J Cancer. 2005;93:834–837. doi: 10.1038/sj.bjc.6602751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez MI, Bhatia K, Barriga T, et al. Molecular epidemiology of Burkitt’s lymphoma from South America: differences in breakpoint location and Epstein-Barr virus association from tumors in other world regions. Blood. 1992;79:3261–3266. [PubMed] [Google Scholar]
- 29.Sandlund JT, Fonseca T, Leimig T, et al. Predominance and characteristics of Burkitt lymphoma among children with non-Hodgkin lymphoma in northeastern Brazil. Leukemia. 1997;11:743–746. doi: 10.1038/sj.leu.2400609. [DOI] [PubMed] [Google Scholar]
- 30.Klumb CE, Hassan R, De Oliveira DE, et al. Geographic variation in Epstein-Barr virus–associated Burkitt’s lymphoma in children from Brazil. Int J Cancer. 2004;108:66–70. doi: 10.1002/ijc.11443. [DOI] [PubMed] [Google Scholar]
- 31.Chen WG, Chen YY, Bacchi MM, et al. Genotyping of Epstein-Barr virus in Brazilian Burkitt’s lymphoma and reactive lymphoid tissue: type A with a high prevalence of deletions within the latent membrane protein gene. Am J Pathol. 1996;148:17–23. [PMC free article] [PubMed] [Google Scholar]
- 32.Young LS, Yao QY, Roponey CM, et al. New type B isolate of Epstein-Barr virus from Burkitt’s lymphoma and normal individuals in endemic areas. J Gen Virol. 1987;68:2853–2862. doi: 10.1099/0022-1317-68-11-2853. [DOI] [PubMed] [Google Scholar]
- 33.Rezk SA, Weiss LM. Epstein-Barr virus–associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–1304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Carbone A, Gloghini A. AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol. 2005;130:662–670. doi: 10.1111/j.1365-2141.2005.05613.x. [DOI] [PubMed] [Google Scholar]
- 35.Burmeister T, Schwartz S, Horst HA, et al. Molecular heterogeneity of sporadic adult Burkitt-type leukemia/lymphoma as revealed by PCR and cytogenetics: correlation with morphology, immunology and clinical features. Leukemia. 2005;19:1391–1398. doi: 10.1038/sj.leu.2403847. [DOI] [PubMed] [Google Scholar]
- 36.Carbone A, Gloghini A. HHV-8–associated lymphoma: state-of-the-art review. Acta Haematol. 2007;117:129–131. doi: 10.1159/000097459. [DOI] [PubMed] [Google Scholar]
- 37.Du MQ, Diss TC, Liu H, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 38.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406–1412. [PubMed] [Google Scholar]