Abstract
Phthalates, the most abundantly produced plasticizers, leach out from polyvinyl chloride plastics and disrupt androgen action. Male rats that are exposed to phthalates in utero develop symptoms characteristic of the human condition referred to as testicular dysgenesis syndrome (TDS). Environmental influences have been suspected to contribute to the increasing incidence of TDS in humans (i.e. cryptorchidism and hypospadias in newborn boys and testicular cancer and reduced sperm quality in adult males). In this review, we discuss the recent findings that prenatal exposure to phthalates affects Leydig cell function in the postnatal testis. This review also focuses on the recent progress in our understanding of how Leydig cell factors contribute to phthalate-mediated TDS.
Testicular dysgenesis syndrome
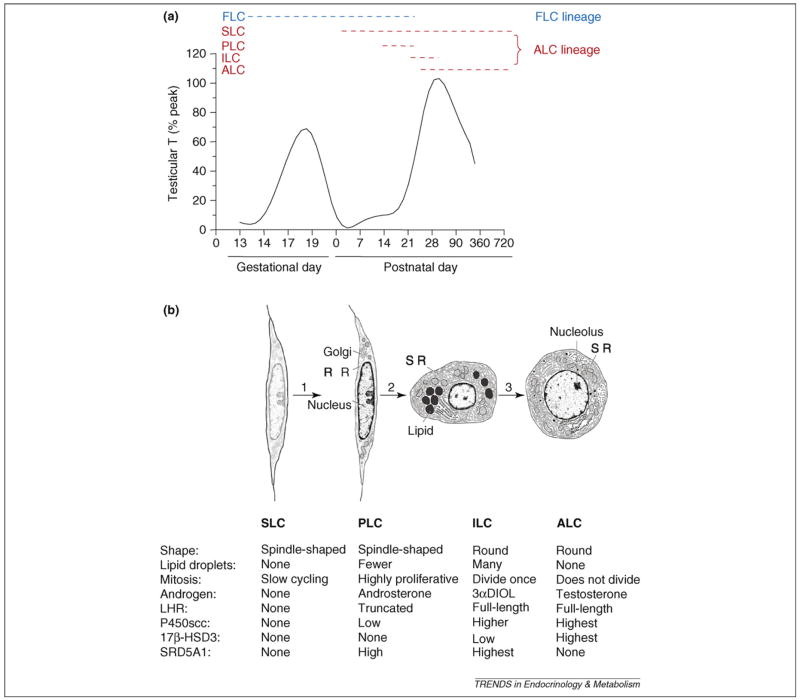
The term ‘testicular dysgenesis syndrome’ (TDS; see Glossary), which was coined in 2001 [1], refers to a spectrum of reproductive disorders that originate in male fetal life. TDS includes cryptorchidism (undescended testes) and hypospadias (abnormal formation of the urethral meatus) in newborn boys and testicular cancer and reduced fertility in adult males. Although the prevalence of TDS has not been established worldwide, the majority of cases of cryptorchidism, hypospadias and testicular cancers are probably linked to TDS [2]. TDS can occur as a result of abnormal Leydig cell function. Testosterone (T), which is produced by Leydig cells, is required for normal testis descent, urethral meatus formation and spermatogenesis. In rodents, there are two populations of Leydig cells in the fetal testis: fetal Leydig cells (FLCs), which involute after birth, with some persisting in the adult testis; and stem Leydig cells (SLCs), which can become adult Leydig cells (ALCs) and are present during the entire lifespan (for a review, see Ref. [3]) (Figure 1). Because TDS originates in fetal life [1], questions have arisen regarding whether factors within the fetal testis affect the ALC population.
Figure 1.
Schematic diagram illustrating the (a) intratesticular testosterone (T) level and (b) cell types in adult Leydig cell lineage during development. Two generations of Leydig cells develop during fetal and pubertal period in rats. (a) The dashed lines show the possible lifespan of each cell type. (b) Distinct cell characteristics in Leydig cell lineage from the commitment of SLC into PLC (1), differentiation of PLC into ILC (2) and further into ALC (3). FLC, fetal Leydig cell; SLC, stem Leydig cell; PLC, progenitor Leydig cell; ILC, immature Leydig cell; ALC, adult Leydig cell; LHR, luteinizing hormone receptor; P450scc, cholesterol side-chain cleavage enzyme; 17β-HSD3, 17β-hydroxysteroid dehydrogenase 3; SRD5A1, 5α-reductase 1; 3αDIOL, 5α-androstane-3α, 17β-diol.
Environmental endocrine disruptors (EEDs) – chemicals that might interfere with the body’s endocrine system and produce adverse developmental, reproductive, neurological and immune effects in both humans and wildlife [4] – are suspected contributors to TDS because exposure to these agents is associated with reproductive tract anomalies, including cryptorchidism and hypospadias [5]. More than 200 chemicals meet the criteria for classification as EEDs, including compounds such as plasticizers, pesticides, natural plant metabolites, detergents and metals [6]. One class of EEDs is the phthalates (Table 1), the plasticizers for polyvinyl chloride plastics. Recently, attention has been paid to these chemicals because the prenatal exposure of rats to phthalates can cause TDS-like syndrome in male offspring postnatally (for a review, see Refs [7,8]). Epidemiological studies show statistical correlations between serum concentrations of phthalate monoesters, the primary metabolites of phthalates, and the incidence of anomalies such as cryptorchidism and shortened anogenital distance (AGD) [5,9]. Although there are other factors contributing to TDS, such as Sertoli cell and germ cell factors, the focus of this review is on the contribution of the Leydig cell.
Table 1.
Various phthalates and their diverse actions
| Substance name (abbreviation) | Metabolite name (abbreviation) | Actionsa |
|---|---|---|
| Di-(2-ethylhexl) phthalate (DEHP) | Mono-(2-ethylhexyl)-phthalate (MEHP) | Agonists of PPARα and PPARγ (MEHP, MnBuP, MBzP) [35]. |
| Dibutyl phthalate (DBP) | Mono-butyl phthalate (MnBuP) | Leydig cell aggregation (DEHP, DBP) [30,31]. |
| Benzyl butyl phthalate (BBP) | Mono-butyl Benzyl phthalate (MBzP) | Inhibition of T and INSL3 production (DEHP, DBP) [29–31,46–49]. |
| Di-(isononyl) phthalate (DINP) | Mono-isononyl phthalate (MiNP) | |
| Di-(isodecyl) phthalate (DIDP) | Mono-isodecyl phthalate (MiDP) | |
| Di-n-octyl phthalate (DnOP) | Mono-n-octyl phthalate (MnOP) | |
| Di-n-hexyl phthalate (DnHP) | Mono-n-hexyl phthalate (MnHP) | |
| Diethyl phthalate (DEP) | Mono-ethyl phthalate (MEP) |
Actions pertain to all listed phthalates.
Development of Leydig cells
The toxicity of phthalates to males is thought to be age dependent because fetal and prepubertal animals are more sensitive than adult males (for a review, see Ref. [7]). The cellular targets of that toxicity might also vary with age, and it is thought that the Sertoli cell might be more susceptible to damage after exposure during adulthood [10]. Leydig cell steroidogenic capacity serves as a hallmark for phthalate-mediated effects because these compounds, universally categorized as anti-androgens, affect T production. Because phthalates affect Leydig cell development, it is necessary to briefly review the regulation of fetal and postnatal differentiation of Leydig cells (Figure 1b).
Fetal Leydig cells
In rats, the first-generation Leydig cells (FLCs) differentiate from embryonic stem cells between the nascent testis cords, starting on day 12 of gestation (Figure 1a). Shortly after the testis differentiates from the gonad, stem cells undergo lineage commitment and differentiate into mature FLCs that are fully competent for steroidogenesis [11]. They reach their peak of steroidogenic activity just before birth on day 19 of gestation [11] (Figure 1a), and the T secreted is crucial for the development of the penis and sex accessory glands, as well as for testis descent [11]. Testis descent occurs in two phases, of which only the second (inguinoscrotal) phase is androgen-dependent. In the male rat fetus, androgens cause the regression of the cranial suspensory ligament, an androgen-dependent process [12]. However, the first (intra-abdominal) phase is mostly dependent on another FLC-secreted hormone, insulin-like growth factor 3 (INSL3) [12]. A receptor for INSL3, leucine-rich repeat-containing G-protein-coupled receptor 8 (LGR8), is present in the gubernaculum, a ligament attaching the testis to the bottom of the scrotum [13]. INSL3 activates LGR8 to regulate the ligament, thus pulling down the testis [13]. When the INSL3 gene is mutated in mice, initial testis descent does not occur [14]. FLCs involute gradually after postnatal day 7 [11]. Given that the majority of FLCs dedifferentiate or undergo apoptosis after birth [3], they are unlikely to contribute considerably to postnatal steroidogenesis. The effects of phthalates on FLCs, causing hypospadias and cryptorchidism at birth, could lead to TDS.
Adult Leydig cell ontogeny
Three stages of cells have been postulated to be involved in ALC development (Figure 1b). These stages have been described well for the rat [15], and similar stages are thought to occur in other species, including humans [16]. The first recognized cell type in the Leydig cell lineage in the rat, referred to as progenitor Leydig cells (PLCs), is first seen in the testis between postnatal days 10 and 14 (Figure 1). PLCs are similar in appearance to undifferentiated SLCs but express markers of Leydig cells, including P450 cholesterol side-chain cleavage enzyme and a truncated form of luteinizing hormone (LH) receptor (LHR) (for a review, see Ref. [3]). PLCs contain negligible amounts of smooth endoplasmic reticulum (SER), the organelle that houses several steroidogenic enzymes, but are, nonetheless, competent to produce steroids, secreting mainly androsterone [17]. PLCs gradually enlarge and become round cells known as immature Leydig cells (ILCs), which are commonly seen in the testis during days 28 to 56 postpartum. These cells have more SER than PLCs and are able to produce high levels of 5α-reduced androgens, primarily 3α,5α-androstanediol [18]. ILCs undergo a final division and further develop to ALCs by day 56. ALCs are large cells with abundant SER, few lipid droplets and high levels of steroidogenic enzyme activities to produce T. After puberty, there is little proliferation of ALCs [19].
In the adult, LH binding to LHR triggers the cAMP-signaling pathway and a cascade of intercellular events, including increased gene transcription, steroidogenic enzyme activity and T production [20,21]. Lack of LH stimulation results in reduced steroidogenic enzyme activities and Leydig cell atrophy [22]. However, LH stimulation is unlikely to be the initial stimulus for cells to enter the Leydig cell lineage. Evidence for this conclusion comes from the fact that the LHR is truncated in PLCs, resulting in an attenuated response to gonadotropin stimulation. That LH plays a crucial part in the further development of Leydig cells, however, is apparent from studies of gonado-tropin-releasing hormone gene knockout mice, which are deficient in LH. In these mice, Leydig cell numbers are ~10% of control [23]. Moreover, Leydig cells are severely hypoplastic in LHR knockout (LHRKO) mice and do not progress beyond a PLC stage that has attenuated expression of steroidogenic enzymes [24].
Stem Leydig cells
The first identified cell type in the sequence leading to ALCs is LHR- and 3β-hydroxysteroid-dehydrogenase-positive cells that divide a limited number of times in vivo [3]. Until recently, a fundamental unanswered question was whether the ultimate source of PLCs and, thus, of ALCs might be a pool of undifferentiated, self-renewing SLCs. There have been attempts to identify SLCs. In one study [25], putative SLCs from cryptorchid mice were isolated and transplanted into LHRKO mice. Serum T assays revealed notable increases in T levels, indicating that the transplanted cells differentiated to produce at least some Leydig cells. A second study [26] reported the presence of nestin-positive cells in the interstitial compartment of testes and suggested that stem cells ultimately give rise to ALCs. SLCs have been successfully isolated from six-day-old rat testes and can differentiate into ALC lineage when transplanted in adult testes in which ALCs were depleted [27].
Actions of phthalates on Leydig cells
Phthalates show little or limited estrogenic activity [28], and there is a building consensus that phthalates are anti-androgenic. However, phthalates and their mono-phthalate metabolites do not bind to the androgen receptor (AR) in vitro at concentrations of up to 10 μM [29], indicating that phthalates are not direct AR antagonists. In fact, phthalate toxicity toward Leydig cells depends on the dosage and time of exposure during development. Over a wide range of doses – 10–750 mg/kg bis (2-ethylhexyl) phthalate (DEHP) or 500 mg/kg di-n-butyl-phthalate (DBP) – phthalates promote the aggregation of FLCs [30–32]. However, this phenomenon has not been observed in adult testes [30–32]. The mechanism of phthalate-mediated toxicity on Leydig cells, however, remains unclear. One possible mechanism is that phthalate metabolites bind to peroxisome proliferator-activated receptors (PPARs) [33]. The PPAR family contains three subtypes: PPARα, PPARβ and PPARγ [34]. Rat FLCs express PPARα and PPARγ [35]. Phthalates have been known to induce the actions of PPARα and PPARγ [36–38]. The action of phthalates on Leydig cells cannot be explained entirely by a PPARα-mediated pathway, however, because PPARα-knockout mice remain sensitive to phthalate-mediated reproductive toxicity. Another signaling pathway in Leydig cells that might be affected by phthalates is that of the aryl hydrocarbon receptor. In fact, fetal testes from animals treated with phthalates have increased expression levels of aryl hydrocarbon receptor and its downstream gene cytochrome Cyp1b1 [39].
Phthalate-mediated TDS: Leydig cell changes
Effects on fetal Leydig cells
Phthalates, the most abundantly produced plasticizers, leach out from polyvinyl chloride plastics and disrupt androgen action. Animal data indicate a broad spectrum of health outcomes, including TDS, associated with phthalate exposure [40,41]. Important windows of sensitivity to phthalate exposure occur in utero and during lactation, when only FLCs and SLCs are present [42]. There is no consensus on how phthalate exposure affects male reproductive toxicity in humans, probably because of insufficient epidemiological data. In one report from the Agency for Toxic Substances and Disease Registry, investigators could not find a quantitative association between DBP concentration in semen and spermatozoa density. However, a cross-sectional study conducted in Shanghai showed a positive association between the incidence of sperm malformations and DEHP concentration in semen [43]. Recently, a reduction in AGD, an androgen-dependent parameter of male sexual development, was observed in infant boys and associated with increased levels of monobutyl phthalate, monoethyl phthalate, monobenzyl and mono-isobutyl phthalates, the primary metabolites of DBP, diethyl phthalate, and butyl benzyl phthalate in maternal urine samples during late pregnancy [44]. Boys with a short AGD also showed a higher prevalence of cryptorchidism and smaller genital size [9,44]. Phthalate monoester contamination of human breast milk has an inhibitory effect on the postnatal surge of reproductive hormones in newborn boys [5]. These correlations between exposure levels and adverse reproductive health outcomes are apparently preceded by a decrease in FLC function, which results in lower testicular levels of T [45].
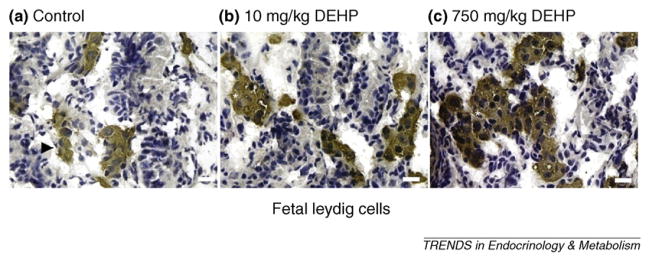
The cryptorchidism and hypospadias at birth can be induced by phthalates in rat models [46]. Apparently, the reduced T and INSL3 production by FLCs could be the cause. Phthalates have been demonstrated to inhibit expression of crucial genes in the steroidogenic pathway, thus leading to lower T production, lower Insl3 mRNA and INSL3 protein expression and reduced FLC numbers [29–31,46–49]. However, whether the disrupted FLC function by phthalates is associated with testicular cancer and reduced fertility at adulthood is far from clear because FLCs might not last to adulthood. One of the persistent effects of disrupted FLC might be the tumor-like FLC aggregation. Phthalates induce FLC aggregation, especially at higher doses (more than 10 mg/kg DEHP or 500 mg/kg DBP) in the central area of the testis (Figure 2), possibly leading to the formation of focal dysgenesis in the postnatal testis, evidenced by aggregated FLCs mingling with other testicular cell types, including Sertoli cells [30–32,50]. Earlier studies classified phthalate-induced FLC aggregation induced by DBP as Leydig cell tumors [51,52]. However, FLC aggregation is not a Leydig cell tumor because FLC number is not changed by DBP exposure [31] and is reduced by DEHP exposure [30]. In rats, the aggregation of FLCs begins just after gestational day 13.5 [48] and becomes more pronounced from gestational day 17 to day 19.5 [48]. Reduced T does not cause FLC aggregation; therefore, other factors are responsible [53]. The induction of FLC aggregation by phthalates could result from signals from other testicular cells, including myoid and Sertoli cells [30]. For example, a dose-dependent increase in leukemia inhibitory factor (Lif) expression induced by DEHP is correlated with FLC cluster size [30]. LIF is also a crucial factor for maintaining the self-renewal and proliferation of stem cells; therefore, increased Lif expression might also be associated with SLCs in ALC lineage, which is discussed below.
Figure 2.
3β-hydroxysteroid dehydrogenase staining of fetal Leydig cells (FLCs). In utero exposure to DEHP at doses of (a) 10 mg/kg per day and (b) 750 mg/kg per day from gestational day 2 to 21 causes significant FLC aggregation compared to vehicle control (a). The white asterisk indicates brown-colored Leydig cell clusters. Bar = 20 μm.
Effects on ALC lineage
Although the association of phthalate exposures to human embryos with risk of TDS, including testicular cancer and reduced fertility, would be a difficult epidemiological study to conduct, such a relationship has been shown in animal models of in utero phthalate exposure. In utero exposure to phthalates caused notable reduction of serum T during puberty [54]. Male reproductive tract abnormalities, as well as Leydig cell dysgenesis, were observed at 6, 12 and 18 months of age after in utero exposure to DBP, indicating long-term effects on ALC lineage [55]. One study clearly showed that reduced T production was caused by either decreased steroidogenic enzyme activity or lower cholesterol-transporting protein levels after in utero exposure to DEHP at doses of 234 mg/kg and above [54]. By contrast, ALC volume was increased [54]. The cause for increased ALC numbers after in utero exposure is unclear. However, the mechanism might be similar to prepubertal or pubertal phthalate exposure that causes Leydig cell hyperplasia, hypothesized to be induced by increased estrogen action [56]. Aromatase expression and activity are increased in Leydig cells after postnatal phthalate exposure [56]. This, in turn, could lead to abnormal estrogen action at the testicular level, which is a known cause of Leydig cell hyperplasia and Leydig cell tumors in rodents [57].
One possible explanation for adult-onset TDS after in utero exposure to phthalates is intracellular glucocorticoid excess. Increases in cortisol (in humans) or corticosterone (in rodents) resulting from stress have been shown to predispose offspring to health problems such as hypertension and reproductive dysfunction in adult life [58,59]. For instance, restraint stress applied to pregnant rats resulted in reduced testis weight and a shortened AGD in their male pups at birth [60]. Changes in adult male sexual behavior were also correlated with prenatal dexamethasone exposure [61].
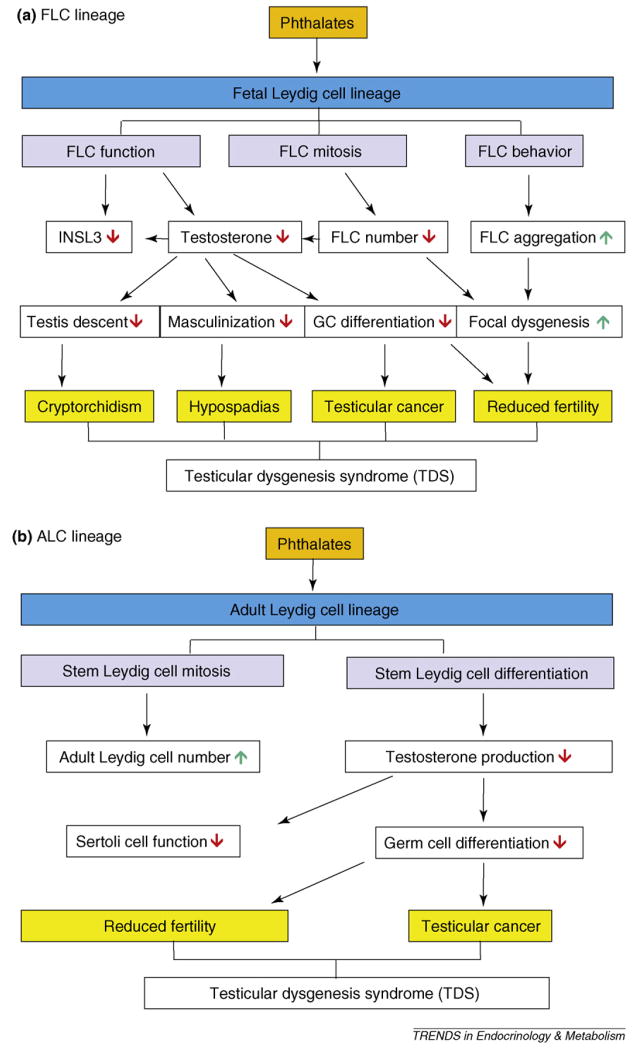
The intracellular concentration of glucocorticoids is controlled by 11β-hydroxysteroid dehydrogenase (11β-HSD). Two isoforms are present in testes. SLCs have abundant 11β-HSD isoform 2 (11β-HSD2), which inactivates glucocorticoids [62]. However, 11β-HSD isoform type 1, a glucocorticoid-activating enzyme, is expressed exclusively in mature ALCs and is not expressed in stem cells or PLCs [62,63]. We have proposed that 11β-HSD2 in SLCs within the testis catalyzes inactivation of glucocorticoids, thus reducing glucocorticoid suppression of ALC development [62]. Experimentally, in utero and lactational exposure to phthalates considerably inhibited 11β-HSD2 gene (Hsd11b2) expression and led to glucocorticoid excess [62]. Therefore, fetal or neonatal imprinting might occur whereby effects can become evident in adulthood. Imprinting can occur through alterations in DNA methylation or histone acetylation. Epigenetic regulation of Hsd11b2 has been demonstrated in humans [64] but has not yet been investigated in rodent SLCs. Our current thinking, then, is that phthalates act on FLCs (Figure 3a) or stem cells of ALC lineage (Figure 3b), thus possibly causing TDS in adult males.
Figure 3.
Phthalates affect (a) fetal Leydig cell (FLC) lineage and (b) adult Leydig cell (ALC) lineage after in utero exposures, causing testicular dysgenesis syndrome. FLC, fetal Leydig cell; INSL3, insulin-like growth factor 3; GC, germ cell; ↓ = decrease or inhibition; ↑ = increase or stimulation.
Future directions
In recent years, progress has been made in our understanding of the prevalence of human TDS. Geographical analysis of these symptoms shows that environmental factors, rather than genetic factors, are the most likely causes of TDS. Exposure of pregnant rats to phthalates has been suggested as a useful animal model for human TDS because the testicular and other changes in phthalate-exposed rats have been reported in human TDS. Although multiple changes occur in fetal testicular cells including FLCs, Sertoli cells and germ cells, the changes in FLCs are apparently the direct causes of TDS-like syndromes such as hypospadias and cryptorchidism. Human evidence of a link between phthalate exposure and TDS is difficult to obtain. However, accumulating human epidemiological data point to a relationship between adverse fetal development and phthalate exposure.
The mechanism of phthalate action has not been well understood. Additional studies using transgenic animal models will be useful to determine the consequences after in utero phthalate exposure. Epigenetic studies also might show whether phthalates affect fetal imprinting. Endocrine disruptors have resulted in epigenetic reprogramming in several documented cases (for a review, see Ref. [65]), and similar phenomena of adult-onset reproductive disorders after fetal exposure to environmental toxicants have been reported [66]. Therefore, it is likely that phthalates could also be associated with epigenetic modifications of testicular genes.
Acknowledgments
This review was written in memory of Dr Matthew P. Hardy for his scientific contribution to this area. We thank Chantal M. Sottas for technical assistance. Supported in part by National Institute of Environmental Health Sciences R01 ES10233 to M.P.H. and R-S.G.
Glossary
- Adult Leydig cell
a terminally differentiated Leydig cell formed during puberty. Adult Leydig cells have an extensive smooth endoplasmic reticulum, a distinct rim of heterochromatin in the nucleus, a prominent nucleolus and few lipid droplets. They primarily produce testosterone
- Androgen
a steroid hormone that functions in the development of male sex organs, maintenance of male characteristics and spermatogenesis. In males, testosterone is the primary androgen that is produced mainly by Leydig cells
- Aromatase
an enzyme in the cytochrome P450 superfamily, the function of which is to convert androgens into estrogens
- Cryptorchidism
a condition of incomplete testicular descent. The condition might be unilateral or bilateral. The testicles normally descend into a scrotum to optimize sperm production
- Environmental endocrine disruptor
a chemical that might interfere with the body’s endocrine system to produce adverse developmental, reproductive, neurological and immune effects in humans and wildlife
- Focal dysgenesis
a small area of a seminiferous tubule that is unable to produce sperm
- Fetal Leydig cell
a terminally differentiated Leydig cell formed in the fetus. Fetal Leydig cells have an extensive smooth endoplasmic reticulum and, in the rat, many lipid droplets. They produce testosterone and insulin-like growth factor 3
- Hypospadias
a condition of the defected urethra in the male newborn that involves an ectopically located urinary meatus (opening)
- Immature Leydig cell
a cell intermediate between a progenitor and adult Leydig cell. Immature Leydig cells are morphologically similar to fetal Leydig cells. However, immature Leydig cells primarily produce 5α-reduced androgens
- Inguinoscrotal testicular descent
the descent of testis into the area where the abdomen and thigh meet and further inside the protuberance of skin and muscle containing the testicle
- Leydig cell
a testosterone-producing cell in the interstitium of the testis
- Phthalate
a chemical compound added to plastics to increase their flexibility. They are mainly used to soften polyvinyl chloride plastics
- Progenitor Leydig cell
a cell that is produced by the commitment of a stem Leydig cell to the Leydig cell lineage. Progenitor Leydig cells remain fibroblastic in appearance but produce Leydig cell-specific markers such as 3β-hydroxysteroid dehydrogenase
- Restraint stress
a stress produced by immobilization of animals
- Sertoli cell
a cell in the seminiferous tubule of the testis, the function of which is to support the development of sperm (germ cells in the male)
- Spermatogenesis
the process by which undifferentiated germ stem cells develop into mature spermatozoa
- Stem Leydig cell
the founder of the Leydig cell lineage. A stem Leydig cell is unique in the Leydig cell lineage, in that it divides to produce two daughter cells with different fates
- Testicular dysgenesis syndrome
a condition that originates in the fetus and includes cryptorchidism (undescended testes) and hypospadias (abnormal formation of the urethral meatus) in newborn boys and testicular cancer and reduced fertility in adult males
- Testicular cancer
a disease in which cells become malignant in one or both testicles
References
- 1.Skakkebaek NE, et al. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 2.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 3.Ge RS, Hardy MP. Regulation of Leydig cells during pubertal development. In: Payne AH, Hardy MP, editors. The Leydig Cell in Health and Disease. Humana Press; 2007. pp. 55–70. [Google Scholar]
- 4.Carlsen E, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Main KM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman JM, et al. Endocrine-disrupting chemicals: prepubertal exposures and effects on sexual maturation and thyroid activity in the female rat. A focus on the EDSTAC recommendations. Crit Rev Toxicol. 2000;30:135–196. doi: 10.1080/10408440091159185. [DOI] [PubMed] [Google Scholar]
- 7.Ge RS, et al. Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol. 2007;23:366–373. doi: 10.1016/j.reprotox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Swan SH, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster PM, et al. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of di-n-pentyl phthalate. Toxicol Appl Pharmacol. 1982;63:120–132. doi: 10.1016/0041-008x(82)90031-x. [DOI] [PubMed] [Google Scholar]
- 11.O’Shaughnessy PJ, et al. The foetal Leydig cell –differentiation, function and regulation. Int J Androl. 2006;29:90–95. doi: 10.1111/j.1365-2605.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- 12.Emmen JM, et al. Involvement of insulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryptorchidism. Endocrinology. 2000;141:846–849. doi: 10.1210/endo.141.2.7379. [DOI] [PubMed] [Google Scholar]
- 13.Scott DJ, et al. Characterization of the rat INSL3 receptor. Ann N Y Acad Sci. 2005;1041:13–16. doi: 10.1196/annals.1282.003. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann S, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 15.Benton L, et al. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- 16.Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol. 2001;168:213–216. doi: 10.1677/joe.0.1680213. [DOI] [PubMed] [Google Scholar]
- 17.Ge RS, Hardy MP. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–3795. doi: 10.1210/endo.139.9.6183. [DOI] [PubMed] [Google Scholar]
- 18.de Santa Barbara P, et al. Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev Dyn. 2000;217:293–298. doi: 10.1002/(SICI)1097-0177(200003)217:3<293::AID-DVDY7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Hardy MP, et al. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- 20.Hakola K, et al. Dose and time relationships of intravenously injected rat recombinant luteinizing hormone and testicular testosterone secretion in the male rat. Biol Reprod. 1998;59:338–343. doi: 10.1095/biolreprod59.2.338. [DOI] [PubMed] [Google Scholar]
- 21.Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- 22.Manova K, et al. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- 23.Baker PJ, et al. Differentiation of adult-type Leydig cells occurs in gonadotrophin-deficient mice. Reprod Biol Endo. 2003 doi: 10.1186/1477-7827-1-4. ( www.rbej.com) [DOI] [PMC free article] [PubMed]
- 24.Zhang FP, et al. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145:1453–1463. doi: 10.1210/en.2003-1049. [DOI] [PubMed] [Google Scholar]
- 25.Lo KC, et al. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology. 2004;145:4011–4015. doi: 10.1210/en.2003-1729. [DOI] [PubMed] [Google Scholar]
- 26.Davidoff MS, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge RS, et al. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci U S A. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris CA, et al. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parks LG, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 30.Lin H, et al. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc Natl Acad Sci U S A. 2008;105:7218–7222. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahood IK, et al. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- 32.Mahood IK, et al. Cellular origins of testicular dysgenesis in rats exposed in utero to di(n-butyl) phthalate. Int J Androl. 2006;29:148–154. doi: 10.1111/j.1365-2605.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 33.Gazouli M, et al. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology. 2002;143:2571–2583. doi: 10.1210/endo.143.7.8895. [DOI] [PubMed] [Google Scholar]
- 34.Lemberger T, et al. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 35.Schultz R, et al. Expression of peroxisome proliferator-activated receptor alpha messenger ribonucleic acid and protein in human and rat testis. Endocrinology. 1999;140:2968–2975. doi: 10.1210/endo.140.7.6858. [DOI] [PubMed] [Google Scholar]
- 36.Corton JC, Lapinskas PJ. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci. 2005;83:4–17. doi: 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- 37.Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 38.Lampen A, et al. Teratogenic phthalate esters and metabolites activate the nuclear receptors PPARs and induce differentiation of F9 cells. Toxicol Appl Pharmacol. 2003;188:14–23. doi: 10.1016/s0041-008x(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 39.Lovekamp-Swan T, et al. Dual activation of PPARα and PPARγ by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201:133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 40.Ahamed S, et al. Signal transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Mol Carcinog. 2001;30:88–98. doi: 10.1002/1098-2744(200102)30:2<88::aid-mc1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Foster PM, et al. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7:231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 42.Ema M, et al. Critical period for adverse effects on development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy. Toxicol Lett. 2000;111:271–278. doi: 10.1016/s0378-4274(99)00192-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YH, et al. Phthalate exposure and human semen quality in Shanghai: a cross-sectional study. Biomed Environ Sci. 2006;19:205–209. [PubMed] [Google Scholar]
- 44.Marsee K, et al. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpe RM. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol Lett. 2001;120:221–232. doi: 10.1016/s0378-4274(01)00298-3. [DOI] [PubMed] [Google Scholar]
- 46.Fisher JS, et al. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 47.Wilson VS, et al. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Hutchison GR, et al. The origins and time of appearance of focal testicular dysgenesis in an animal model of testicular dysgenesis syndrome: evidence for delayed testis development? Int J Androl. 2007;31:103–110. doi: 10.1111/j.1365-2605.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 49.McKinnell C, et al. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di (n-Butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- 50.Mahood IK, et al. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect. 2007;115 (Suppl 1):55–61. doi: 10.1289/ehp.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shono T, Suita S. Reasonable explanation for both the antiandrogenic mechanism of DBP and DBP-induced Leydig cell hyperplasia in prenatally DBP-treated rats. Toxicol Appl Pharmacol. 2000;164:336. doi: 10.1006/taap.1999.8867. [DOI] [PubMed] [Google Scholar]
- 52.Mylchreest E, et al. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 53.Scott HM, et al. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- 54.Culty M, et al. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol Reprod. 2008;78:1018–1028. doi: 10.1095/biolreprod.107.065649. [DOI] [PubMed] [Google Scholar]
- 55.Barlow NJ, et al. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32:79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- 56.Akingbemi BT, et al. Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. Proc Natl Acad Sci U S A. 2004;101:775–780. doi: 10.1073/pnas.0305977101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosland MC. Hormonal factors in carcinogenesis of the prostate and testis in humans and in animal models. Prog Clin Biol Res. 1996;394:309–352. [PubMed] [Google Scholar]
- 58.Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997;60:1213–1221. doi: 10.1016/s0024-3205(96)00611-x. [DOI] [PubMed] [Google Scholar]
- 59.Seckl JR, et al. Placental 11 beta-hydroxysteroid dehydrogenase and the programming of hypertension. J Steroid Biochem Mol Biol. 1995;55:447–455. doi: 10.1016/0960-0760(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 60.Dahlof LG, et al. Influence of maternal stress on the development of the fetal genital system. Physiol Behav. 1978;20:193–195. doi: 10.1016/0031-9384(78)90072-0. [DOI] [PubMed] [Google Scholar]
- 61.Ward IL, Reed J. Prenatal stress and prepuberal social rearing conditions interact to determine sexual behavior in male rats. Behav Neurosci. 1985;99:301–309. doi: 10.1037//0735-7044.99.2.301. [DOI] [PubMed] [Google Scholar]
- 62.Han L, et al. In utero and lactational exposures to diethylhexyl-phthalate affect two populations of leydig cells in male Long-Evans rats. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.072975. ( www.biolreprod.org) [DOI] [PMC free article] [PubMed]
- 63.Phillips DM, et al. Corticosteroid 11β-dehydrogenase in rat testis. Endocrinology. 1989;125:209–216. doi: 10.1210/endo-125-1-209. [DOI] [PubMed] [Google Scholar]
- 64.Alikhani-Koopaei R, et al. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–S10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- 66.Newbold RR, et al. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–1363. [PubMed] [Google Scholar]