Abstract
Estrogen receptor-α (Esr1) mediates estrogen action in regulating at all levels of the hypothalamic-pituitary-ovarian axis. Whereas the importance of Esr1 in hypothalamus and pituitary has been demonstrated by loss of fertility in the neuron- and pituitary-specific Esr1 knockout mice, whether Esr1 plays a critical role in the ovary remains to be determined. In the ovary, Esr1 is mainly expressed in the theca/interstitial cells and germinal epithelium and thus is believed to mediate estrogen action in these cells. In this study, we assessed the importance of Esr1 in the ovarian theca cells in regulating female reproduction. The Cre-LoxP approach was used to selectively delete the Esr1 gene in the theca cells, and the reproductive consequence of the deletion was measured. Adolescent theca-specific Esr1 knockout (thEsr1KO) mice (<4 months of age) are fertile and cycling. However, they begin to display an erratic pattern of estrous cycles and become infertile before they reach the age of 6 months. The ovaries of thEsr1KOmice (≥4 months) have fewer corpora lutea but more antral follicles than the age-matching wild-type mice. The numbers of 17-hydroxylase-expressing cells are largely increased in the interstitium of the thEsr1KO mouse ovary. Interestingly, whereas basal levels of serum testosterone and FSH were mildly elevated, LH level was either markedly lower or undetectable in the thEsr1KO mice. When superstimulated by exogenous gonadotropins, thEsr1KO mice released significantly fewer oocytes that wild-type littermates and developed multiple hemorrhagic cysts. Taken together, this study demonstrates that theca Esr1 plays a critical role in regulating female reproduction.
Targeted deletion of Esr1 (ERα) gene in the ovarian theca cell resulted in the elevation of serum testosterone level and the premature loss of fertility.
The foundation of female reproductive physiology has been focused on the development of a healthy follicle capable of responding to the appropriate hormonal stimuli to grow, produce a mature ovum, and subsequently develop into a corpus luteum (1). Estrogen plays an important role in this process, most of the action being mediated by two cognate receptors, estrogen receptor-α (Esr1) and estrogen receptor-β (Esr2). Esr1 and Esr2 exhibit both differential and overlapping tissue distribution as well as significant differences in their ligand-binding and transcriptional properties (2).
In the ovary, Esr2 is mainly expressed in the granulosa cells of growing follicles and has been considered as the predominant ovarian ER form, whereas Esr1 expression is limited to germinal epithelium and theca-interstitial cells. Thus far, three different laboratories have generated at least five different lines of Esr1 knockout mice. Interestingly, however, each of the targeted mouse line displays a different degree of fertility defects (3,4,5,6,7,8,9). In contrast, whereas Esr1 has been considered to be the minor ovarian ER form (8,10,11,12), Esr1 knockout (Esr1KO) mice have been consistently shown to be completely infertile (3,13,14,15). Most interestingly, Esr1KO mice display severe ovarian disorders such as disrupted theca/stromal development, arrest of follicular development at early antral stage, formation of hemorrhagic cysts, and lack of corpora lutea (2,3,13,14,15,16). Similar pathological disorders were observed in the aromatase knockout mice, demonstrating a critical role of Esr1-mediated estrogen action in regulating ovarian function (17,18).
Estrogen regulates female reproduction at all levels of the hypothalamus-pituitary-ovary axis. Recently the tissue-specific gene knockout approach has successfully been applied to demonstrate the Esr1-mediated estrogen action at both hypothalamus and pituitary levels. Deletion of the Esr1 gene at either level resulted in the loss of fertility. In contrast to global Esr1KO mice, milder ovarian defects were seen in the neuron-specific Esr1KO (nEsr1KO) (19) and pituitary-specific Esr1KO (piEsr1KO) mice (20). Folliculogenesis progressed beyond early antral stage without forming hemorrhagic cyst in the ovaries of the nEsr1KO and piEsr1KO mice. Furthermore, upon exogenous gonadotropins stimulation, both nEsr1KO and piEsr1KO mouse ovaries successfully ovulated and formed corpora lutea, indicating that, at some level, the deletion of Esr1 gene in the ovary is directly responsible for the severe ovarian defects seen in the global Esr1KO. We therefore hypothesize that Esr1 plays a critical role at the ovarian level in regulating female reproduction.
In this study, we assessed the reproductive consequence of the ovary-specific deletion of Esr1 gene as a measure of determining the importance of the intraovarian role of Esr1-mediated estrogen action. The floxed Esr1 mouse line that was used for generating piEsr1KO mice and a theca-specific improved form of recombinase (iCre)-expressing mouse line (Cyp17iCre) that we generated recently (21) were used to produce theca-specific ERα knockout (thEsr1KO) mice. Here we report a comprehensive analysis on the reproductive phenotypes of the thEsr1KO.
Materials and Methods
Materials
Pregnant mare’s serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) were purchased from Sigma (St. Louis, MO). Cell strainers were supplied by Becton Dickinson Falcon (Billerica, MA). Media for experiments were obtained from Life Technologies, Inc. (Grand Island, NY). Molecular reagents were purchased from Invitrogen (Invitrogen, Carlsbad, CA).
Generation of thEsr1KO
Two mutant mouse lines were used as founders. The floxed Esr1 (Esr1flox/flox) mouse line, which carries two loxP sites in the introns flanking exon 3 of the Esr1 gene, was used as a target of Esr1 gene excision. This mouse was previously used for generating conventional Esr1KO and piEsr1KO mice (20). The transgenic mouse line, Cyp17iCre, in which an enhanced form of Cre recombinase (iCre) gene expression is driven by a promoter of 17β-hydroxylase a1, was used to selectively delete Esr1 in the theca cells as was previously described (21). Briefly, a female Esr1flox/flox mouse was first bred with a male Cyp17iCre mouse, which resulted in the production of F1 heterozygotes, Esr1flox/wt Cyp17iCre. Then the male F1 Esr1flox/w Cyp17iCre mice were backcrossed with the female Esr1flox/flox mice, which produced four different genotypes including Esr1flox/flox Cyp17iCre, Esr1flox/flox, Esr1flox/wt Cyp17iCre, and Esr1flox/wt. The Esr1flox/flox Cyp17iCre mouse was named thEsr1KO. The genotypes of individual mice were determined by PCR using ear-biopsy DNA. The following primers were used for genotyping the mice: Esr1-P1 (5′-ttg ccc gat aac aat aac at-3′), Esr1-P2F (5′-gtg tca gaa aga gac aat-3′), Esr1-P3 (5′-ggc att acc act tct cct ggg agt ct-3′), Cre-P1 (5′-gga cat gtt cag gga tcg cca ggc g-3′), and Cre-P85 (5′-gtg aaa cag cat tgc tgt cac tt-3′). Primer combinations of Esr1-P1 + Esr1-P3 and Esr1-P2F + Esr1-P3 were used to determine the presence or absence of loxP sequence (flox or wild type) or deletion of exon 3 (Esr1−). Presence of Cre recombinase was determined using primers Cre-P1 and Cre-P85.
Superovulation assay
Two- and 6-month-old mice were injected with 5 IU PMSG and, 48 h later, the mice were additionally injected with 5 IU hCG. One group of mice was euthanized by CO2 overdose 48 h after PMSG injection, and the ovaries were collected for histology. The other group of mice was euthanized 22 h after hCG injection, oocyte-cumulus complexes were retrieved from the ampulla of the oviduct, and the number of ova were counted microscopically using a CKX41 inverted microscope (Olympus, Tokyo, Japan) equipped with a digital camera SN IH045062-H (Olympus). The ovaries were immediately fixed in 10% buffered formalin for later histology. The oocyte-cumulus complexes were further examined before and after cumulus cells were removed using hyaluronidase (80 U/ml) treatment. The quality of oocytes was analyzed according to the standard morphological criteria (22,23).
Fertility assay
For fertility assays, cycling 45- to 50-d-old Esr1flox/flox, Esr1flox/flox Cyp17iCre, and their heterozygote Esr1flox/wtCyp17iCre were individually housed with a proven Esr1flox/flox male. Each cage held one or two females and one male, and their fertility was evaluated by counting the number of litters and litter size per breeding pair. Additionally, variable age-matching female Esr1flox/floxCyp17iCre and male Esr1flox/flox mice were bred for a long-term (6–8 months) fertility assay. At the end of the assay, the mice were killed and the tissues were fixed in 10% buffered formalin. Animal handling procedures were carried out in accordance with the University of Kentucky Animal Care and Use Committee. All mice were maintained on a 14-h light, 10-h dark cycle and given a continuous supply of food and water.
Determination of estrous cyclicity
Using vaginal lavage techniques, the pattern of estrous cycles was determined in three different age groups of mice (2, 4, and 6 months old) for more than 21 d. Vaginal lavage was performed daily, in the mornings at the same time each day, by flushing the vagina with 0.9% sodium chloride. The cell samples were then examined microscopically and scored. Estrus was determined by the presence of cornified cells. Metestrus was scored by the presence of large round cells with an irregular border. A high density of leukocytes indicated the stage of diestrus, whereas small nucleated cells indicated proestrus (24). The cycling data are expressed as either estrus or nonestrus.
Tissue collection, histology, and immunohistochemistry
For all the female mice used in this study, stage of the estrous cycle was determined and recorded before the animals were killed. Animals were deeply anesthetized and perfused intracardially with PBS, followed by 4% paraformaldehyde. Tissues were then embedded in paraffin blocks, serially sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). Embedding and staining procedures were performed by standard protocols. The histology was examined by BX51 microscope (Olympus) equipped with a digital camera and photography was performed using the MagnaFire-SP imaging system (Olympus). Immunohistochemical staining was performed on deparaffinized sections by the linked streptavidin-biotin complex technique using a linked streptavidin-biotin detection kit (Dako, Carpenteria, CA) with 3-amino-9-ethylcarbazole (Dako) as a chromogen. Mouse monoclonal Esr1 antibody (6F11; Novocastra, Newcastle, UK) at a dilution of 1:40, antihuman 17-hydroxylase (Cyp17) antibody (kindly provided by Dr. Alan Conley, University of California, Davis, Davis, CA) and LHβ antibody (provided by the National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA) were used. Autoclave epitope retrieval was performed before the immunohistochemical staining. For counting gonadotroph numbers, serial sections (6 μm thickness; four serial sections per pituitary; two mice per genotype) were stained with LHβ antibody, digital photographs were taken at ×100 (DP71; Olympus) from five randomly chosen areas of a section, and the numbers of LHβ-positive cells were counted on a computer screen.
Measurement of serum LH, FSH, and testosterone levels
Blood samples for hormone assay were obtained by cardiac puncture at 1500 h on the day of diestrus. RIA for estradiol, testosterone, FSH, and LH were performed following a standard procedure by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core [National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproductive Research) Grant U54-HD28934, University of Virginia, Charlottesville, VA].
mRNA measurement
Total RNA was isolated from 2- and 6-month old mice ovaries, which were collected 22 h after hCG injection. Trizol (Invitrogen) and RNeasy kit (QIAGEN Inc., Valencia, CA) were used for purifying the total RNA following standard procedure following manufacturer’s recommendations. Briefly, Moloney murine leukemia virus reverse transcriptase (Invitrogen; 28025-013) was used for cDNA synthesis. Specific CYP17, aromatase (Cyp19), and steroidogenic acute regulatory protein (StAR) Taqman probe (Applied Biosystems, Foster City, CA) and Taqman gene expression kit was used for quantitative analysis of mRNA, and the PCR was carried out using iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA). As an internal control, glyceraldehyde-3-phosphate dehydrogenase Taqman probe was used. The PCRs were performed using the following conditions: 5 min at 95 C followed by 40 cycles of denaturation (15 sec at 95 C), annealing, and extension (1 min at 60 C) with FAM signal reading after extension. For all time points, triplicates of samples were used for the assay.
Statistical analysis
Statistical significances were assessed using Fisher’s exact test for fertility assay and Student’s two-tailed t test for estrous cycle pattern analysis and serum hormone assay and ΔΔct test for analysis of real-time PCR assay. For all statistical analyses, P < 0.05 was considered significant, if not specifically mentioned.
Results
Generation of theca-specific Esr1KO mice
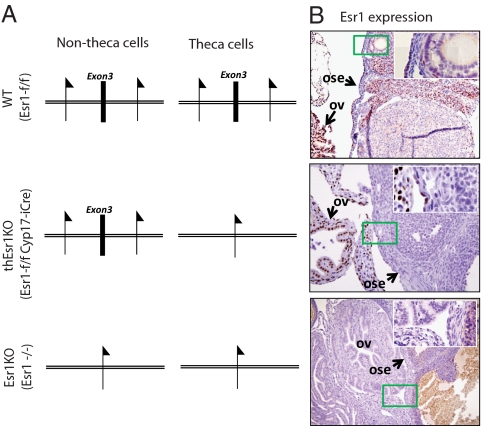
A Cyp17iCre transgenic mouse line that selectively expresses iCre in the theca cells was successively crossed with the floxed Esr1 mouse line that we previously used for generating piEsr1KO mice (20). The genotypes of the resulting offspring were determined by PCR, and the female mice with the genotype of Esr1flox/flox Cyp17iCre were named thEsr1KO (Fig. 1). The deletion of the Esr1 gene in the theca cells of the thEsr1KO mice was demonstrated by the absence of Esr1-positive staining in the theca-interstitial cells, whereas a normal level of Esr1 expression was seen elsewhere including the oviduct (Fig. 1).
Figure 1.
Theca-specific ERα deletion in mice. A, Schematic diagram of targeted deletion of exon 3 (E3) of Esr1 gene. B, Immunohistochemical localization of Esr1 protein expression in the 2-month-old WT (Esr1flox/flox), global Esr1KO (Esr1−/−), and thEsr1KO (Esr1flox/floxCyp17iCre) mouse ovaries and oviducts. Esr1 signal is seen in the nuclei of the theca/interstitial cells and germinal epithelium of WT ovary, whereas the Esr1expression is absent in global Esr1KO mice ovary. Note that Esr1 protein expression is seen in the oviduct (ov) and ovarian surface epithelium (ose) but absent in theca/interstitial cells in the thEsr1KO mice ovary. The inserts are high-power images of the areas boxed in green.
thEsr1KO mice are fertile but lose fertility prematurely
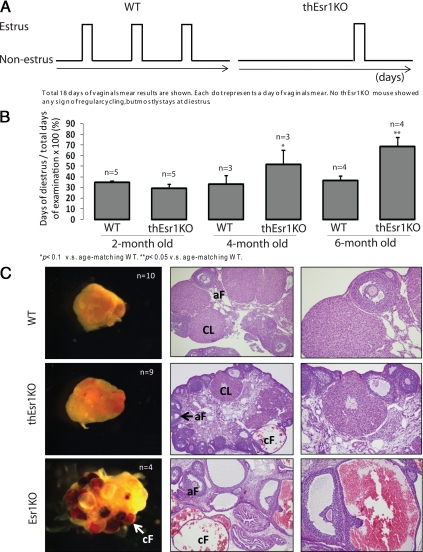
A prolonged preliminary observation on the reproductive activity of the thEsr1KO mice indicated that whereas no noticeable fertility defect was seen in male thEsr1KO mice up to the ages of 6 months, female thEsr1KO showed signs of an age-dependent fertility loss. We therefore compared reproductive activity in different age groups of thEsr1KO mice by pairing them with proven wild-type (WT) males. Whereas no significant difference was found in adolescent (aged 2–3 months) groups, a dramatic decline of fertility was seen in the 4- and 6-month-old thEsr1KO mice compared with wild-type or heterozygote littermates (P < 0.02) (Table 1). The fertility defect in these mice was reflected in the aberrant pattern of estrous cycles. No thEsr1KO mouse showed a normal pattern of cycles when they reached the age of 4 months or older but displayed increased days of diestrus (Fig. 2, A and B). This is in contrast to the cycling pattern of age-matching WT mice, which showed a normal pattern of estrous cycles and were fertile, indicating that the thEsr1KO mice experience a significant change in their reproductive function prematurely.
Table 1.
Effect of theca-specific Esr1 deletion on fertility
| Genotypes | Age (months) | No. of mice used (n) | No. of delivered litters (%) | No. of weaned litters (%) | No. of pups per weaned littera |
|---|---|---|---|---|---|
| Wild typeb | 2 | 23 | 18 (78.2) | 12 (52.2) | 6.5 ± 0.66 |
| 4 | 24 | 19 (79.2) | 15 (62.5) | 7.7 ± 0.63 | |
| 6 | 11 | 10 (90.9) | 7 (63.6) | 9.1 ± 1.38 | |
| thEsr1KO | 2 | 14 | 7 (50.0) | 3 (21.4) | 2.3 ± 1.20 |
| 4 | 10 | 3 (30.0) | 1 (10.0) | 4.0 ± 0.00 | |
| 6 | 10 | 1 (10.0) | 1 (10.0) | 2.0 ± 0.00 |
Number of pups per litter was calculated by the total numbers of weaned pups divided by the numbers of weaned litters; some litters were lost (mostly by carnage by young mother mice) before they reached weaning ages, and therefore the numbers of pups of the litters could not be counted. Fertility of the thEsr1KO mice was significantly affected in the 4- and 6-month (P = 0.015 and 0.0003, respectively) but not in the 2-month-old (P = 0.145) thEsr1KO mice compared with control mice.
Some heterozygotes (Esr1f/wtCyp17iCre) were included in this group; no fertility difference between wild type and heteros were seen.
Figure 2.
Patterns of estrous cycles and ovarian histology of WT (Esr1flox/flox), Esr1KO, and thEsr1KO mouse ovaries. Vaginal smear was performed daily for 3 wk by flushing the vagina with 0.9% sodium chloride. The smears were then examined under the microscope and scored. A, Representative cyclic patterns of 6-month-old WT and thEsr1KO mice. A total of 18 d of vaginal smear results are shown. Each dot represents a day of vaginal smear result. B, Duration of diestrous days in WT and thEsr1KO mice. *, P < 0.1 vs. age-matching WT. **, P < 0.05 vs. age-matching WT. C, Representative ovarian histology. n, Number of mice examined. Ovaries are formalin fixed, paraffin embedded, and stained with H&E. Note that multiple dilated cysts filled with red blood cells in the Esr1KO ovary, whereas no and a hemorrhagic cyst are visible in the WT or thEsr1KO ovaries, respectively. aF, Antral follicle; CL, corpus luteum; cF, cystic follicle.
thEsr1KO mice have decreased ovulatory capacity
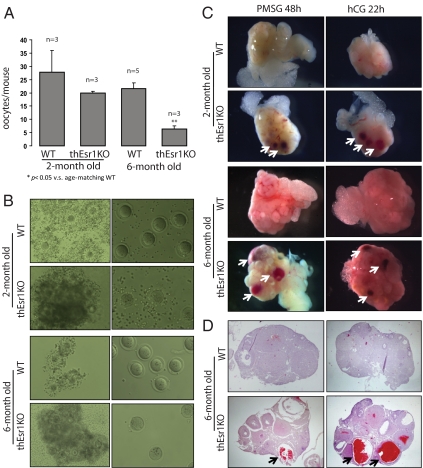
As a measure of assessing the defects in fertility, ovarian phenotypes of 4-month-old WT (Esr1flox/flox), thEsr1KO, and global Esr1KO (ERαKO; Esr1−/−) were examined. Externally, no significant difference was seen between WT and thEsr1KO ovaries, whereas multiple hemorrhagic cysts were apparent in the Esr1KO ovaries (Fig. 2C). Internally, the thEsr1KO ovary showed signs of defects that are intermediate between WT and Esr1KO: reduced numbers of corpora lutea, increased numbers of late preantral and early antral stage follicles, interstitial hypertrophy, and signs of the formation of hemorrhagic cysts but much fewer compared with Esr1KO ovary (Fig. 2C). The ovarian defects of thEsr1KO mice resulted in decreased ovulatory capacity and oocyte quality as was determined by superovulation induction followed by oocyte examination (Fig. 3, A and B). Two- and 6-month-old WT and thEsr1KO mice were injected with PMSG followed by hCG. Twenty-two hours after hCG injection, oocytes were retrieved from oviducts and examined. Whereas the numbers of retrieved oocytes in the 2-month-old thEsr1KO mice were marginally smaller than age-matching WT mice (27 vs. 20 oocytes), significantly fewer numbers of oocytes were released in the 6-month-old thEsr1KO mice than age-matching WT mice (22 vs. six oocytes) (Fig. 3A). Furthermore, whereas oocytes from WT mice were healthy and had well-defined germinal vesicles, the majority of oocytes from thEsr1KO mice displayed signs of degeneration (Fig. 3B). Furthermore, the gonadotropin injection (PMSG alone or PMSG + hCG) resulted in the formation of hemorrhagic follicle in the thEsr1KO ovaries, whereas hemorrhage was rarely seen in the WT ovaries (Fig. 3, C and D).
Figure 3.
Superovulation in thEsr1KO mice. Two- and 6-month-old WT and thEsr1KO mice were superovulated using PMSG (5 IU/mouse) and hCG injections (5 IU/mouse), and the numbers of oocytes were retrieved from ampulla at hCG 22 h and examined. A, Number of retrieved oocytes (n, numbers of mice used for each group). *, P < 0.05 vs. age-matching WT mice. B, Oocyte-cumulus complex (left panels) retrieved from the oviducts and cumulus-free oocytes (right panels) after hyaluronidase treatment. Note the well-expanded cumulus mass from WT but dense clumps of cumulus mass from thEsr1KO mice ovary. C, External morphology of ovaries of 2- and 6-month-old WT and thEsr1KO mice 48 h after PMSG and 22 h after hCG injection. Note that blood-filled follicles developed in the thEsr1KO mice ovaries but not the WT. D, H&E staining of 6-month-old WT and thEsr1KO mice ovaries. Note that whereas multiple numbers of corpora lutea are seen in the WT and thEsr1KO mice ovaries, blood filled-cystic follicles are seen only in the thEsr1KO mice. Arrows indicate the hemorrhagic follicles.
thEsr1KO mice have higher serum testosterone and lower LH levels
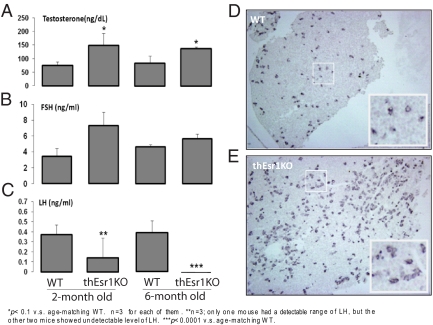
Previous studies (25,3) suggested that ovarian androgen synthesis is negatively regulated by the paracrine action of estrogen, in which estradiol produced by granulosa cells directly acts on Esr1 in the theca cells to suppress Cyp17 transcription and therefore testosterone production. The deletion of Esr1 specifically in the Cyp17-expressing cells of the thEsr1KO mice offers an outstanding experimental condition to test the hypothesis. As an initial step, serum levels of sex steroids and gonadotropins were measured in the 2- and 6-month-old WT and thEsr1KO female mice (Fig. 4, A–C). Whereas no significant difference in estradiol level was seen (5–16 pg/ml range in WT and 5–26 pg/ml range in thEsr1KO mice), testosterone level in the thEsr1KO mice was moderately higher than in the WT mice (Fig. 4A) supporting previous findings. Unexpectedly, however, whereas FSH was marginally higher, LH level was either very low (2 month group) or undetectable (6 month groups) in the thEsr1KO mice (Fig. 4, B and C). Interestingly, pituitary of thEsr1KO mice had increased numbers of gonadotrophs (5.5 LHβ-positive cells per unit area in WT vs. 11.0 in thEsr1KO mice, P < 0.001) with an approximately similar level of LH content in each cell as was determined by immunohistochemistry using LHβ antibody (Fig. 4, D and E).
Figure 4.
Serum hormonal profiles and LHβ expression in the pituitary. A–C, Serum levels of testosterone, FSH, and LH of 2- and 6-month-old WT and thEsr1KO mice (n = 3 for each group). *, P < 0.1 vs. age-matching WT group. **, Among three mice used for the assay, only one mouse had a detectable range of LH, whereas the other two mice showed undetectable level of LH. ***, P < 0.0001 vs. age-matching WT mice. D and E, Immunohistochemical localization of LHβ in the anterior pituitaries of 4-month-old WT and thEsr1KO female mice. Note the presence of the large number of LHβ cells in the pituitary from thEsr1KO female mice compared with the WT pituitary.
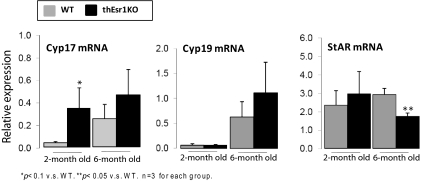
No dramatic difference of steroidogenic enzyme gene expressions but highly populated theca cells in the interstitium of thEsr1KO ovary
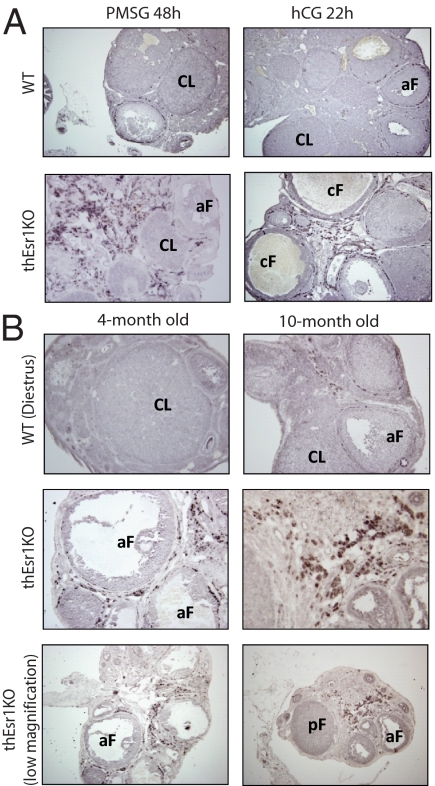
To further investigate the cause of the elevation of testosterone level in the thEsr1KO mice, the expression patterns of steroidogenic enzymes involved in the androgen synthesis were analyzed. To overcome any existing physiological differences that may affect steroidogenic gene expression at the time of assay, a superovulation regimen was used for the assay. Two- and 6-month-old thEsr1KO mice and their littermates were induced superovulation by PMSG and hCG injections. Twenty-two hours after hCG injection, mRNA expression levels of Cyp17, aromatase (Cyp19), and StAR were measured by quantitative PCR using total RNA extracted from whole ovarian homogenates (Fig. 5). Whereas not dramatically different, Cyp17 mRNA expression levels were higher in thEsr1KO mice in both age groups. No significant difference in Cyp19 mRNA expression was seen between the two genotypes, whereas the expression levels were elevated in the aged (6 month) groups. StAR mRNA levels were not different in the 2-month group, but the level was significantly lower in the thEsr1KO mice of the aged group. After finding out that there was not a dramatic difference of the key steroidogenic gene mRNA expressions, we tested whether there was a change in the number of testosterone-producing cells (Cyp17 positive cells). An immunohistochemical examination on the superovulated 6-month group of mouse ovaries revealed that thEsr1KO ovaries were heavily populated with Cyp17-positive cells (Fig. 6A). Interestingly, the majority of the theca cells in the thEsr1KO ovaries were found in interstitial areas in contrast to the WT ovary in which Cyp17-positive cells are mostly localized in the theca interna of preovulatory follicles. To determine whether the increase of theca cells is a general phenotype of thEsr1KO mice, we then examined the ovaries of younger groups (4 months old) and older groups (10 months old) on the day of diestrus when the Cyp17 expression was supposed to be minimal. As was in the gonadotropin-injected mouse ovaries, more cyp17-positive cells were seen in the ovaries of thEsr1KO than WT mice (Fig. 6B).
Figure 5.
Expression of steroidogenic enzyme gene mRNA after superovulation induction. Two- and 6-month-old WT and thEsr1KO mice were superovulated using PMSG (5 IU/mouse) and hCG injections (5 IU/mouse), and ovarian mRNA expression levels were measured at 22 h after hCG injection by quantitative RT-PCR (n = 3 for each group). *, P < 0.1 vs. WT mice; **, P < 0.05 vs. WT mice.
Figure 6.
Population of Cyp17-positive cells after gonadotropin stimulation. A, Ovaries of 6-month-old WT and thEsr1KO mice were collected 48 h after PMSG and 22 h after hCG injection, formalin fixed, paraffin embedded, and stained with anti-Cyp17 antibody. Note that the Cyp17-positive cells (dark brown color) are heavily populated in the ovaries of 6-month-old thEsr1KO mice. The majority of the theca cells in the thEsr1KO ovaries were found in interstitial areas in contrast to the WT ovary in which Cyp17-positive cells are mostly in the theca interna of preovulatory follicles. B, Ovaries of 4- and 10-month-old WT and thEsr1KO mice were examined on the day of diestrus. Large numbers of Cyp17-positive cells were seen in the ovaries of thEsr1KO than WT mice. aF, Antral follicle; CL, corpus luteum; cF, cystic follicle; pF, preantral follicle.
Discussion
It has long been speculated that estrogen plays critical roles in the ovary. However, due to the complexity of the mechanism of estrogen action and the near-ubiquitous expression of estrogen receptors, it has been challenging to determine the intraovarian role of estrogen. In this study, we determined the physiological significance of Esr1 expression in the theca cells by using a tissue-specific gene knockout approach. By cross-breeding of floxed Esr1 mice and a newly generated Cyp17iCre mouse line, theca cell-specific deletion of Esr1 gene was achieved. Reproductive outcome, pattern of estrous cycles, ovarian morphology, and ovulatory response to gonadotropin stimulation of the thEsr1KO mice in comparison with WT mice were used as a measure of the significance of the Esr1 expression in the theca cell.
The Cyp17-iCre driven deletion of Esr1 gene lead to sex-dependent fertility outcome: whereas the majority of female thEsr1KO mice lose fertility before they reach the ages of 6 months, male counterpart (Leydig cell-specific Esr1KO; LeyEsr1KO) mice do not show any apparent sign of fertility loss up to the ages of 6 months (data not shown). This sex-dependent reproductive performance indicates that Esr1 plays a role critically related to fertility regulation in the ovary but not the testis. This finding is not surprising because it was shown the fertility defect in the global Esr1KO male mice were not caused by testicular defects but epididymal malfunction (15). However, considering the fact that Esr1 is expressed in the Leydig cells of the testis (26,27,28) and aging global Esr1KO mice displayed testicular hypotrophy (15), future analysis on LeyEsr1KO mouse testis is warranted.
In the case of females, thEsr1KO mice are born fertile but gradually lose fertility leading to near complete infertility by the age of 6 months, when the age-matching WT females maintain their fertility at the highest level (Table 1). The loss of fertility in the thEsr1KO female mice is accompanied by the loss of estrous cyclicity (Fig. 2, A and B), a physiological presentation of cyclic changes of circulating estrogen levels (29). It is presumed, therefore, that the steroidogenic activity is altered in the thEsr1KO mice. In fact, the main functional role of ovarian theca cells is to synthesize androgens (testosterones and androstenedione) that in turn serve as the substrate of Cyp19 aromatase for estrogen production in the granulosa cells. Whereas comparison of steroid levels at different stages of estrous cycles in cycling WT and thEsr1KO mice would be a desirable way to determine the role of Esr1 in theca cell steroidogenesis, this approach was not applicable due to the acyclic nature of the thEsr1KO mice (Fig. 2, A and B). Therefore, alternatively, the serum levels of steroids on the mornings of diestrus were measured along with gonadotropin levels. Concurrent with the previous reports (2,25), moderately elevated estradiol, testosterone (Fig. 4A), and FSH (Fig. 4B) levels were seen. Surprisingly, however, LH level was significantly lower in the thEsr1KO or undetectable (Fig. 4C). Whereas the present study did not find the direct cause of the gradual loss of fertility in the thEsr1KO female mice, both the aberrant levels of steroids and gonadotropins are believed to be responsible. In regard to the lower basal level of circulating LH, we found, unexpectedly, increased numbers of gonadotrophs with approximately similar level of LH content in each cell in the pituitary in the thEsr1KO pituitary as determined by immunohistochemistry using anti-LHβ antibody (Fig. 4, D and E), indicating that secretion of LH is compromised by the Esr1 deletion in the theca cells but not the hormone synthesis. The potential cause of defect in LH secretion is currently under investigation.
Unexpectedly, ovaries of the gonadotropin-injected thEsr1KO mice developed hemorrhagic follicles, whereas such response was not induced in the WT ovaries (Fig. 3, C and D). Whereas hemorrhagic cysts were seen in not all but some unstimulated thEsr1KO ovaries, all of the gonadotropin-injected mouse ovaries had the hemorrhagic follicles, demonstrating that the gonadotropin is the direct cause of the hemorrhagic response in the thEsr1KO mouse ovary. Whereas a cohort of previous studies showed that either the global deletion of aromatase or Esr1 resulted in the formation of hemorrhagic cyst formation (3,16,18,31), chronic elevation of LH has been suggested to be the primary cause of the polycystic ovarian phenotype (31). Our finding that thEsr1KO mice had markedly lower serum LH level and displayed hemorrhagic response to gonadotropin treatment is in support of the previous suggestion. It is, however, worth noting that no such response was seen in the WT mouse ovary, indicating that deletion of Esr1 in the theca cells may predispose the ovary to be vulnerable to form hemorrhagic cysts on gonadotropin hyperstimulation via a mechanism to be determined.
Previous studies suggested that theca cell androgen synthesis is negatively regulated by paracrine action of estrogen, in which androgens synthesized by theca cells are converted into estrogens in the granulosa cells, which in turn directly acts on Esr1 in the theca cells to suppress Cyp17 expression, a rate-limiting enzyme for androgen production, and therefore reducing androgen production (16,32). The theca-specific deletion of Esr1 gene in the thEsr1KO mouse offers an ideal model system for testing the suggested mechanism because of intact Esr1-mediated estrogen action elsewhere but not in the theca cells. The mechanism being supported, it was expected that the thEsr1KO ovary would have a higher content of the Cyp17 transcripts and testosterone level than WT counterpart. The levels of Cyp17 mRNA expression in the WT and thEsr1KO ovaries were therefore compared under superovulation paradigm because it is well established that PMSG increases but hCG decreases Cyp17 mRNA expression. In general, Cyp17 transcript content was higher in the thEsr1KO ovaries with as high as 5-fold more Cyp17 mRNA expression seen in the 2-month-old mice (Fig. 5). Taken together with the elevated testosterone level in the thEsr1KO mice (Fig. 4A), these results seemingly supported the hypothesis until an immunohistological examination found an unexpectedly large number of theca cells (Cyp17 positive cells) in a thEsr1KO ovary. Examination of a limited number of ovaries indicated that this change occurs as early as 2 months of age. Subsequent analysis found patches of Cyp17-positive theca cells mostly in the interstitium of both PMSG/hCG-injected immature and adult thEsr1KO mouse ovaries (Fig. 6). These findings suggest that theca Esr1 not only regulates Cyp17 expression but also may control proliferation/distribution of the theca cells in the ovary. A theca cell culture system is proposed to be used for further determination on the role of estrogen-Esr1 in Cyp17 gene expression and theca cell proliferation.
One of the most unique phenotypes caused by theca-specific Esr1 knockout, however, was that the ovary had more numbers of large preantral and early antral stage follicles, whereas less number of oocytes was released from thEsr1KO ovary, (Figs. 2 and 3), indicating that the Esr1 may play a critical role in antral stage folliculogenesis. The possibility of Esr1 in the theca cell proliferation and differentiation is being sought. Taken together, the present study demonstrates that the theca Esr1 plays a critical role in regulating female reproduction.
Acknowledgments
We are grateful to Ms. Katie Gieske and Dr. Phillip Bridges for the technical assistance and critical comments.
Footnotes
This work was supported by National Institutes of Health/National Center for Research Resources Grant P20 RR15592 (to C.K.) and the University of Kentucky Start-Up Fund (to C.K.).
Disclosure Summary: The authors have nothing to declare.
First Published Online May 7, 2009
Abbreviations: Cre, cAMP response element; Cyp17, 17-hydroxylase; Cyp19, aromatase; Esr1, estrogen receptor-α; Esr2, estrogen receptor-β; Esr1KO, Esr1 knockout; hCG, human chorionic gonadotropin; H&E, hematoxylin and eosin; iCre, theca-specific improved form of recombinase; nEsr1KO, neuron-specific Esr1KO; piEsr1KO, pituitary-specific Esr1KO; PMSG, pregnant mare’s serum gonadotropin; StAR, steroidogenic acute regulatory protein; thEsr1KO, theca-specific Esr1 knockout; WT, wild type.
References
- Richards JS 1980 Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev 60:51–89 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS 2003 Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS 2005 In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER) αand ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 146:2817–2826 [DOI] [PubMed] [Google Scholar]
- Yang P, Wang J, Shen Y, Roy SK 2004 Developmental expression of estrogen receptor (ER) α and ERβ in the hamster ovary: regulation by follicle-stimulating hormone. Endocrinology 145:5757–5766 [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M 2008 Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA 105:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F 2000 Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol 165:359–370 [DOI] [PubMed] [Google Scholar]
- Mowa CN, Iwanaga T 2000 Developmental changes of the oestrogen receptor-α and -β mRNAs in the female reproductive organ of the rat—an analysis by in situ hybridization. J Endocrinol 167:363–369 [DOI] [PubMed] [Google Scholar]
- Méndez MC, Chávez B, Echeverría O, Vilchis F, Vázquez Nin GH, Pedernera E 1999 Evidence for estrogen receptor expression in germ cell and somatic cell subpopulations in the ovary of the newly hatched chicken. Cell Tissue Res 298:145–152 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS 2001 Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Ann NY Acad Sci 948:1–8 [DOI] [PubMed] [Google Scholar]
- Couse JE, Mahato D, Eddy EM, Korach KS 2001 Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev 13:211–219 [DOI] [PubMed] [Google Scholar]
- Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS 1999 Targeted disruption of the estrogen receptor-α gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology 140:2733–2744 [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER 1998 Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK 2000 An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology 141:2614- 2623 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C 2008 Pituitary gonadotroph estrogen receptor-α is necessary for fertility in females. Endocrinology 149:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges PJ, Koo Y, Kang DW, Hudgins-Spivey S, Lan ZJ, Xu X, DeMayo F, Cooney A, Ko C 2008 Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor α (Esr1) from the ovary and testis. Genesis 46:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaldi F, Rienzi L 2008 Morphological selection of gametes. Placenta 29(Suppl B):115–120 [DOI] [PubMed] [Google Scholar]
- Xia P 1997 Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod (Oxford, UK) 12:1750–1755 [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E 2005 Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673 [DOI] [PubMed] [Google Scholar]
- Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP 1996 Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51:159–186; discussion 186–158 [PubMed] [Google Scholar]
- Lamb DJ 1995 Genes involved in testicular development and function. World J Urol 13:277–284 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA 2002 Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–881 [PubMed] [Google Scholar]
- Hess RA, Bunick D, Bahr J 2001 Oestrogen, its receptors and function in the male reproductive tract—a review. Mol Cell Endocrinol 178:29–38 [DOI] [PubMed] [Google Scholar]
- Tanaka Y 1962 [Histochemical change of the vaginal smear, epithelium and the endometrium of the mouse during the sex cycle]. J Jpn Obstet Gynecol Soc 14:273–282 [PubMed] [Google Scholar]
- Huynh K, Jones G, Thouas G, Britt KL, Simpson ER, Jones ME 2004 Estrogen is not directly required for oocyte developmental competence. Biol Reprod 70:1263–1269 [DOI] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS 1999 Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-α knockout mouse. Endocrinology 140:5855–5865 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]