Abstract
Congenital myasthenic syndrome is difficult to diagnose, especially in the neonate when classic myasthenic signs may not be present. Congenital myasthenic syndrome with episodic apnea is a rare cause of recurrent apnea in infancy. We present an infant with nine severe episodes of apnea in the first six months of life who underwent a prolonged evaluation before ptosis was observed, leading to the diagnosis of choline acetyltransferase deficiency, a form of congenital myasthenic syndrome. Midazolam appeared to resolve the apnea on five occasions. The diagnosis was supported by edrophonium testing and repetitive nerve stimulation. Mutation analysis demonstrated compound heterozygous p. T354M and p. A557T mutations, the latter of which is novel. The patient’s respiratory status stabilized on pyridostigmine, and she is ambulatory at 3 years. Pyridostigmine is the primary therapy for choline acetyltransferase deficiency, but the observation of the efficacy of midazolam during this patient’s episodes of apnea is interesting, and bears further study.
Keywords: Choline acetyltransferase, congenital myasthenic syndrome, apnea, apparent life threatening event, benzodiazepines
BACKGROUND
Congenital myasthenic syndrome is caused by mutations in genes encoding pre-synaptic, synaptic, and post-synaptic neuromuscular junction proteins. One subset of congenital myasthenic syndrome is associated with life-threatening episodic apnea. The diagnostic evaluation of infants with apparent life threatening events, including recurrent apnea, can be difficult,[1] especially when the cause is a rare one such as congenital myasthenic syndrome with episodic apnea. Suggestive signs of ptosis, ophthalmoplegia, and bulbar dysfunction, may be absent initially, and family history may be negative. Congenital myasthenic syndrome with episodic apnea is most commonly caused by mutations in the CHAT gene.[2,3] The only known treatments for congenital myasthenic syndrome with episodic apnea are pyridostigmine and respiratory support.
CASE REPORT
A female infant was born after a 39-week gestation complicated by diet-controlled gestational diabetes. Apnea occurred shortly after birth, requiring 24 hours of mechanical ventilation. Evaluation for hypoglycemia, infection, and cardiac disease was negative. A second apneic episode requiring intubation occurred soon thereafter. She was discharged after two weeks.
A week after discharge, she had a third apneic episode, one hour after feeding, requiring rescue breathing, followed by less than 24 hours of mechanical ventilation. A brain MRI was normal. She was diagnosed with severe gastroesophageal reflux and discharged on a proton pump inhibitor. Two weeks later, she developed apnea after a feed. Her mother performed rescue breathing until emergency medical services arrival and positive pressure ventilation continued during transport. In the Emergency Department, midazolam was administered as intubation pre-medication. Immediately upon insertion of the endotracheal tube the patient began to struggle and cry and the tube was withdrawn. After a fifth apneic episode requiring intubation, she underwent Nissen fundoplication and gastrostomy. She was well for two months, but at age four months had a sixth apneic episode after feeding, which again resolved after the administration of midazolam and morphine in preparation for intubation. Later during this hospital admission, a seventh apneic episode resolved with midazolam only. An upper GI demonstrated an intact fundoplication and no evidence of reflux on impedance testing.
At six months, bilateral ptosis was noted. Her mother reported that this had been present for three weeks, worse at the end of the day or with fatigue. Soon after, she had an eighth episode of apnea, again responsive to midazolam alone, and was admitted to the hospital. She had a ninth episode that resolved with midazolam during this admission.
An evaluation for disorders of the neuromuscular junction was performed. Acetylcholine receptor antibodies were negative. An edrophonium test was performed, with administration of 0.1, 0.2, 0.5, and 0.6 mg intravenously. Ptosis clearly improved after the last two doses. The left ulnar compound motor action potential showed no decremental response on 3Hz repetitive stimulation, but after 2 minutes of 20Hz repetitive stimulation, the compound motor action potential decreased from 4.3 to 2.1 mV. The median motor study demonstrated a repetitive compound motor action potential on single stimulation—most likely related to edrophonium administered immediately beforehand.
Pyridostigmine therapy dramatically improved the ptosis. Several mild episodes of grunting at home responded to intramuscular midazolam or enteral lorazepam. At 3 years, she is ambulatory and continues to have mild intermittent ptosis but stable breathing on pyridostigmine 8 mg/kg/day divided into five doses.
METHODS
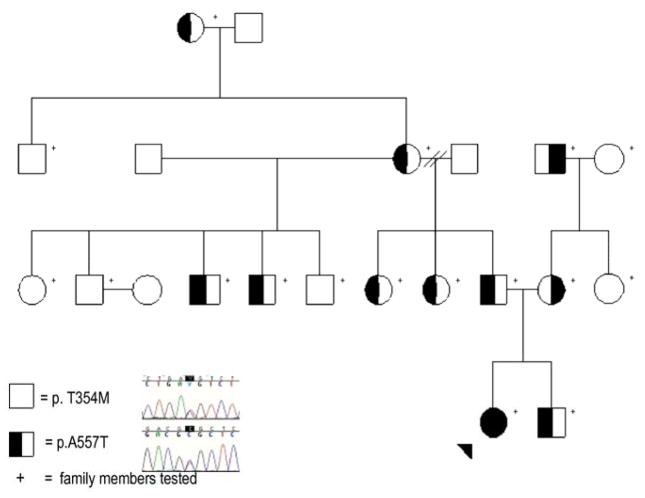
Each participating individual provided a blood or saliva sample after providing informed consent according to an institutionally approved protocol (Fig. 1). PCR primers were determined using ExonPrimer (http://genome.ucsc.edu/) (sequences and PCR conditions available upon request). PCR products were visualized using the eGene HDA-GT12 (now QIAxcel, QIAGEN GmbH, Hilden, Germany), purified using ExoSAP-IT (Amersham, Little Chalfont, UK), and quantified with an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Sequencing was performed on an ABI 3730 (Applied Biosystems, Foster City, CA). Resulting chromatograms were analyzed using Sequencher 4.8 (Gene Codes, Ann Arbor, MI).
Figure 1.
Pedigree of the family under study with illustrative chromatograms. Standard pedigree symbols are used. The arrowhead indicates the proband. The “+” sign indicates individuals who have been studied. The polymorphism inherited from the maternal line is c.1061C>T (p.T354M) in exon 7, extending back at least 2 generations. The polymorphism inherited from the paternal line is c.1669G>A (p.A557T) in exon 12, extending back at least 3 generations. There is no known consanguinity in the family.
RESULTS
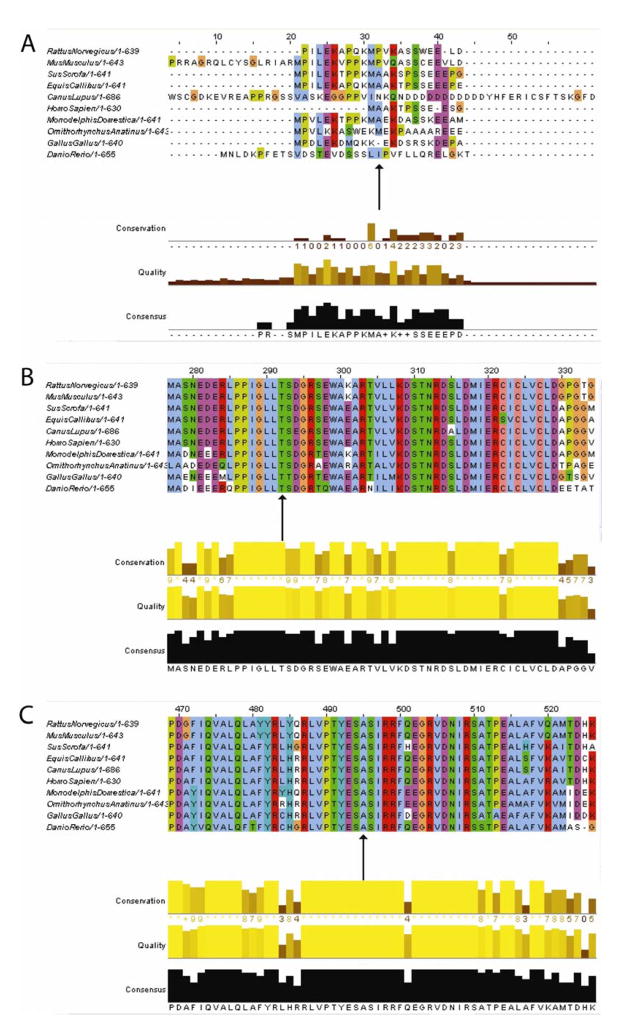
The proband was found to have 3 compound heterozygous base pair changes in the CHAT gene: c.358G>A, c.1061C>T, and c.1669G>A (Fig. 1), corresponding to the following amino acid sequence changes: p.A120T, p.T354M, and p.A557T. The first two polymorphisms were allelic. The c.358G>A (p.A120T) polymorphism was felt to be non-pathogenic based on poor conservation across species (Fig. 2a). The c.1061C>T (p.T354M) polymorphism was not present in 105 ethnically matched controls, corresponded to a change from a polar to a hydrophobic amino acid, and has been previously reported to be a pathogenic mutation.[4] The threonine at position 354 is highly conserved across several species (Fig. 2b). The c.1669G>A (p.A557T) polymorphism was not present in 112 ethnically matched controls and corresponded to a change from a hydrophobic to a polar amino acid. The alanine at position 557 is highly conserved across several species (Fig. 2c). Both amino acid positions are distant from known substrate binding or active conformation sites, but other such amino acid changes in choline acetyltransferase have been determined to be pathogenic. The other evidence above suggests that the c.1061C>T (p.T354M) and c.1669G>A (p.A557T) base pair changes correspond to pathogenic mutations. Further family studies revealed that a number of relatives carry one of the base pair changes (Fig. 1).
Figure 2.
Cross-species amino acid sequence matching suggests that (a) alanine at position 120 is not well-preserved, while (b) threonine at position 354 and (c) alanine at position 557 are highly conserved. Arrows indicate the amino acids of interest. Graphs generated at http://www.ebi.ac.uk/Tools/clustalw2/index.html.
DISCUSSION
This infant’s presentation with multiple severe episodes of apnea in the first six months of life is typical of choline acetyltransferase deficiency.[2,3] One of her two compound heterozygous mutations is novel, as well as her apparent response to midazolam during acute episodes of apnea. The prolonged course of this patient before diagnosis is also instructive, and suggests that congenital myasthenic syndrome with episodic apnea should be considered in the evaluation of apparent life threatening events when more common causes have been excluded.
Congenital myasthenic syndrome with episodic apnea is most commonly caused by mutations in the CHAT gene,[2,3] and less commonly by mutations in SCN4A[5] and RAPSN.[6] The CHAT gene encodes the protein choline acetyltransferase, which is a pre-synaptic protein involved in the vesicular packaging of acetylcholine before release. The mutations identified to date, including the current ones, have mostly been scattered throughout the gene, with no apparent “hotspots”.
The apparent response of her apnea to midazolam on multiple occasions seems counter-intuitive, since high doses of benzodiazepines may cause respiratory depression. One case report of congenital myasthenic syndrome with episodic apnea describes respiratory arrest requiring mask ventilation following rectal diazepam administration after a convulsion.[3] In four of our patient’s apneic episodes, midazolam was the only medication administered, but a causal relationship between administration of this medication and her arousal cannot be proven with the data obtained.
Benzodiazepines are predominantly centrally-acting GABA agonists that are not known to act directly on the neuromuscular junction. However, patients sedated with midazolam sometimes demonstrate surprising resistance to upper airway obstruction.[7] Apnea induced by midazolam may be associated with respiratory arousals,[7] and small doses of midazolam appear to trigger an increase in cardiac sympathetic activity,[8] suggesting that midazolam at low doses may have a stimulatory effect on the cardiac and respiratory systems.
Once the diagnosis of choline acetyltransferase deficiency is established, treatment with pyridostigmine should be initiated. It is not prudent to recommend therapy of this disorder with midazolam at this time given the risks involved, but if such patients require a benzodiazepine for endotracheal intubation or other established indications, clinicians should be alert to any unexpected observations such as ours.
Acknowledgments
The authors thank the patient’s family for their assistance and cooperation.
FUNDING
This study was funded by NINDS K08 NS048180 (PBK). DNA sequencing was performed by the Molecular Genetics Core Facility at Children’s Hospital Boston, supported by NIH P30 HD18655. LMK is an investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043–1048. doi: 10.1136/adc.2003.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohno K, Tsujino A, Brengman JM, Harper C, Bajzer Z, Udd B, Beyring R, Robb S, Kirkham FJ, Engel AG. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci U S A. 2001;98:2017–2022. doi: 10.1073/pnas.98.4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byring RF, Pihko H, Tsujino A, Shen XM, Gustafsson B, Hackman P, Ohno K, Engel AG, Udd B. Congenital myasthenic syndrome associated with episodic apnea and sudden infant death. Neuromuscul Disord. 2002;12:548–53. doi: 10.1016/s0960-8966(01)00336-4. [DOI] [PubMed] [Google Scholar]
- 4.Barisic N, Muller JS, Paucic-Kirincic E, Gazdik M, Lah-Tomulic K, Pertl A, Sertic J, Zurak N, Lochmuller H, Abicht A. Clinical variability of CMS-EA (congenital myasthenic syndrome with episodic apnea) due to identical CHAT mutations in two infants. Eur J Paediatr Neurol. 2005;9:7–12. doi: 10.1016/j.ejpn.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, Cannon SC, Engel AG. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A. 2003;100:7377–82. doi: 10.1073/pnas.1230273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno K, Engel AG, Shen XM, Selcen D, Brengman J, Haper CM, Tsujino A, Milone M. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–85. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton JR, Ward DS, Karan S, Voter WA, Palmer L, Varlese A, Rackovsky O, Bailey P. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–1164. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Win NN, Fukayama H, Kohase H, Umino M. The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth Analg. 2005;101:97–102. doi: 10.1213/01.ANE.0000156204.89879.5C. [DOI] [PubMed] [Google Scholar]