Abstract
Glucocorticoid-induced osteonecrosis is a common and dose-limiting adverse event. The goal of this study was to establish a mouse model of glucocorticoid-induced osteonecrosis suitable for testing the effects of different treatment strategies on its frequency. Fourteen murine strains were screened using various glucocorticoids, routes of administration, and diets. Four-week old male BALB/cJ mice were treated with oral dexamethasone for up to 12 weeks either by continuous dosing or by discontinuous dosing, with or without asparaginase. Histopathological features of the distal femurs were examined by light microscopy. Osteonecrotic lesions were characterized by empty lacunae and osteocyte ghosts in trabecular bone surrounded by necrotic marrow and edema. The incidence of dexamethasone induced osteonecrosis in BALB/cJ mice was 40%–45% (4/10 or 5/11) at 12 weeks. The frequency of osteonecrosis trended lower after discontinuous compared to continuous dosing for 12 weeks (8% vs. 45%) (P = 0.06) despite comparable cumulative plasma exposure. Asparaginase hastened the occurrence of osteonecrosis which was observed as early as 4 weeks and the incidence was 50% after 6 weeks. A mouse model of glucocorticoid-induced osteonecrosis was established. Discontinuous was less osteonecrotic than continuous dexamethasone treatment, consistent with the possible benefits of a “steroid holiday” seen in clinical settings. Moreover, asparaginase hastened osteonecrosis, indicating that drugs may interact with glucocorticoids to affect osteonecrosis risk.
Keywords: Glucocorticoid, Osteonecrosis, Mouse Model
Introduction
Glucocorticoids are used extensively to treat diverse diseases, such as rheumatoid arthritis, asthma, and lymphoid malignancies, and as antiemetic, anti-inflammatory, and anticancer agents. There is increasing concern regarding the long-term complications of glucocorticoids, such as osteonecrosis. The incidence of osteonecrosis is as high as 30% in patients with rheumatoid arthritis1–3 and 20% in children treated for ALL.4–6 There is considerable interest in identifying which patients are at highest risk for osteonecrosis, with the long-term goal of modifying regimens to decrease the risk of adverse effects of therapy.
There are likely several mechanisms by which glucocorticoids induce osteonecrosis. These agents cause hyperlipidemia, hypercoagulation, and hypofibrinolysis in the circulatory system.7,8 The pathogenesis of glucocorticoid-induced osteonecrosis may involve intravascular thrombotic occlusion and/or extravascular lipid deposition by fat emboli and/or increased intra-osseous lipocyte size, leading to an increase in bone marrow pressure and intraosseous circulatory disturbances that result in apoptosis followed by necrosis.9–11
Clinical studies have yielded some insights on possible risk factors. In a retrospective analysis, patients who received continuous exposure to dexamethasone had a higher osteonecrosis incidence compared to those who received discontinuous dosing,12 although there were other differences in addition to the glucocorticoid dosing. This has led some centers to incorporate “glucocorticoid holidays” to provide discontinuous or pulsed rather than continuous daily exposure to dexamethasone in an attempt to achieve a safe and effective treatment with decreasing the risk of osteonecrosis.13,14 Comparing among multiple steroid regimens, the frequency of osteonecrosis has varied widely, even with relatively similar glucocorticoid regimens, leading to speculation that other medications (e.g. asparaginase, antimetabolites) might interact to put patients in some trials at higher risk of osteonecrosis than others.15,16 For example, asparaginase could contribute to the risk of osteonecrosis through its effects on thrombosis or on lipid levels.17–20 Genetic polymorphisms in the vitamin D receptor, thymidylate synthase, and plasminogen activator inhibitor 1 (PAI-1) may predispose patients to osteonecrosis,9,21 but confirmation in clinical and preclinical models is needed. Having a murine model to evaluate different schedules of glucocorticoid (with or without interacting drugs), and to evaluate the contribution of genetic variation, would allow for the systematic evaluation of host-related and treatment-related risk factors for the adverse effect.
To date, there has been no mouse model of glucocorticoid-induced osteonecrosis. The lack of prior success may be partly due to the anatomy of a mouse: the lack of body weight concentration on any two limbs differs from humans, an explanation reinforced by the fact that glucocorticoid-induced osteonecrosis has been induced in bipedal animals (e.g. chickens, emus).22–24 Osteonecrosis has been induced in BALB/cJ and C57BL/6J mice after Leishmania amazonensis, a protozoan parasite, was directly injected into the footpad25 or in the tibia of 23 ICR mice.26 However, this intervention is not necessarily helpful in developing a model whose purpose is to mimic the clinical use of glucocorticoids.
Our goal was to establish a murine model of glucocorticoid-induced osteonecrosis by screening strains whose constitutive phenotypes might predispose, and to evaluate the effect of different schedules of glucocorticoid on the development of osteonecrosis.
Materials and Methods
Medicine and diet
Dexamethasone sodium phosphate injection solution was purchased from American Pharmaceutical Partners, Inc. (Schaumburg, IL). Methylprednisolone sodium succinate injection solution was purchased from Pharmacia & Upjohn Company (Kalamazoo, MI). Asparaginase (ELSPAR) was purchased from Merck & Co., Inc. (Whitehouse Station, NJ). Sulfamethoxazole/trimethoprim oral suspension and 0.9% sodium chloride were purchased from Baxter Healthcare (Deerfield, IL). Tetracycline was obtained from Sigma (St. Louis, MO). Folic acid–deficient purified diet that contained less than 0.05 ppm residual folic acid and a high-fat diet (with 45% energy from fat) were purchased from TestDiet (Richmond, IN).
Animals
A/J, AKR/J, B6AF1/J, B6C3F1, BALB/cBy, BALB/cJ, BTBR-T+tf/tf, C3H/HeJ, C3HeB/FeJ, C57BL/6J, CB6F1/J, CeH/HeJ, and DBA/2J mice (ages, 1 to 5 months old) were purchased from the Jackson Laboratory (Bar Harbor, ME). NMRI mice were provided by the Animal Resource Center at St. Jude Children’s Research Hospital. All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital.
The ears of all mice were tagged, and the mice were housed 3–5 per cage in standard micro-isolator cages. Food and water were provided ad libitum. Mice received a low-folate diet, or a high-fat diet according to the experimental design. The mice were weighed weekly. The lighting regimen was 12 hours of light to 12 hours of darkness.
Treatments
Screening strategies
Using data from the Mouse Phenome Database of the Jackson Laboratory, we screened 14 strains of mice in pilot experiments on the basis of their known constitutive phenotypes which might predispose to osteonecrosis: A/J, BALB/cJ, DBA/2J (higher platelet counts); AKR/J, C3H/HeJ (higher plasma fibrinogen); B6AF1/J, B6C3F1, BALB/cBy, C3HeB/FeJ, CB6F1/J, NMRI (higher body weight); BTBR-T+tf/tf (lower prothrombin time); and C57BL/6J, CeH/HeJ (lower bone mineral density). Various regimens of oral dexamethasone in the drinking water for up to 15 weeks, methylprednisolone by intraperitoneal injection, and high-fat diets were given to a total of 185 mice from both genders of 14 strains (129 of whom were evaluable at the scheduled time point for histologic examination after 3 to 12 weeks of glucocorticoid therapy), and all bones in the appendages were evaluated histologically. The entire skeleton was evaluated using standard x-ray techniques. Osteonecrosis was identified histologically in a few mice but not radiographically. Osteonecrosis was only observed in male BALB/cJ and C57BL/6J mice on low-folate diets given dexamethasone in the drinking water. In the few animals (only 8 of 129) where osteonecrosis was identified histologically, the only joints affected were distal femurs. Osteonecrosis was not observed in controls receiving water without dexamethasone.
To decrease glucocorticoid induced infections, sulfamethoxazole/trimethoprim (280 mg sulfamethoxazole and 56 mg trimethoprim per 450 mL drinking water, three days a week) and tetracycline (1000 mg/L, seven days a week) were added to the drinking water.27,28 The interindividual coefficient of variation in consumption of drinking water containing dexamethasone has been reported to have a coefficient of variation of ~ 20%.29
Remaining experiments employed male BALB/cJ mice on low-folate diets, antimicrobial prophylaxis, and dexamethasone (or saline control) in the drinking water. In these definitive experiments, we evaluated only the right and left stifle joints, only by histology. A confirmatory experiment was conducted in which twelve mice were assigned to the continuous dexamethasone treatment at 4 mg/L for the first week and 2 mg/L dexamethasone thereafter until week 12.
Continuous vs. discontinuous dexamethasone treatment strategies (Table 1)
Table 1.
Treatment regimens
| Experiment | Group | n | DEX | Treatment period (weeks) |
ASPc | 0.9% Salined |
Frequency of osteonecrosis |
|---|---|---|---|---|---|---|---|
| Continuous | CON DEX | 12 | Yesa | 12 | No | No | 4/10 |
| Control | 5 | No | 12 | No | No | 0/5 | |
|
CON vs. DISC DEX |
CON DEX | 15 | Yesa | 12 | No | No | 5/11 |
| DISC DEX | 15 | Yesb | 12 | No | No | 1/12 | |
| Control | 5 | No | 12 | No | No | 0/5 | |
| DEX+ASP 1 | 10 | Yesa | 4 | Yes | No | 1/9 | |
| Control 1 | 5 | No | 4 | No | Yes | 0/5 | |
| DEX+ ASP | DEX+ASP 2 | 10 | Yesa | 6 | Yes | No | 5/10 |
| Control 2 | 5 | No | 6 | No | Yes | 0/5 | |
| DEX alone | 5 | Yesa | 8 | No | No | 1/5 |
Dexamethasone strategy indicated continuous dexamethasone treatment; 4mg/L dexamethasone in the drinking water was administered during the first week, and then 2mg/L dexamethasone was administered thereafter.
Dexamethasone strategy indicated discontinuous dexamethasone treatment; 4 mg/L dexamethasone in the drinking water was given during the first week, and from weeks 2 though 12, the mice were given no dexamethasone during the first 3.5 days of each week and then 4 mg/L for the last 3.5 days of each week.
Asparaginase (7,500 IU/kg) was injected intraperitoneally once a week
0.9% saline (0.1 mL/10 g body weight) was injected intraperitoneally once a week.
Abbreviations: ASP, asparaginase; CON, continuous; DEX, dexamethasone; DISC, discontinuous.
Incidence
Thirty-five 4-week-old male BALB/cJ mice (weight, approximately 13 g) were studied (Table 1). Fifteen mice assigned to the continuous treatment group were given 4 mg/L dexamethasone in their drinking water for the first week and 2 mg/L dexamethasone thereafter until week 12. Fifteen mice assigned to the discontinuous treatment group received 4 mg/L dexamethasone in their drinking water during the first week, and for weeks 2 through 12, the mice were given no dexamethasone during the first 3.5 days of each week and then 4 mg/L for the last 3.5 days of each week. Thus, the cumulative exposure to dexamethasone was identical in the two arms. Five mice served as controls with no dexamethasone. Mice were sacrificed if moribund and date of censoring was documented throughout the experimental period.
Dexamethasone pharmacokinetics /pharmacodynamics study
Sixty 4-week-old male BALB/cJ mice were divided into three groups. Twenty mice were assigned to the continuous and twenty mice to the discontinuous treatment group (Table 1). Twenty mice served as untreated controls. Five mice from each group were sacrificed on days 7, 11, 13, and 15. After anesthesia with 2% isoflurane, 500 to 1000 µL blood was collected by cardiac puncture in the morning from 10:00 a.m. to noon. Plasma was stored at −80°C until assayed.
Dexamethasone and corticosterone were extracted from plasma and quantified by high-performance liquid chromatography (HPLC) using a modification of prior methods.30,31 Plasma (200 µL) was mixed with 100 µL 5% phosphoric acid. Glucocorticoids were extracted into 3 mL ethyl acetate–tertiary methyl butyl ether (50:50 v/v). The upper organic phase was washed with 0.1 mol/L NaOH (750 µL) and evaporated. The residue was reconstituted with 20% methanol (75 µL). The supernatant was injected into the HPLC system with a diode array detector and a 150 mm × 2.00 mm Phenomenex Luna C18 (2) column (3 µm). The mobile phase was 83.75% water, 10% acetonitrile, 6.25% 1-butanol, 0.0985% phosphoric acid, 0.03% triethylamine (v/v) and the rate was 0.2 mL/min at a temperature of 40°C. At 254 nm, the detection limit was 8.73 nM (1.2 pmol on column) for dexamethasone and 2.58 ng/mL (1.0 pmol on column) for corticosterone. The assay was linear from 10.2 to 204 nM and 25–500 ng/mL; these concentrations are the therapeutic range for dexamethasone and normal range for corticosterone in mouse plasma, respectively. For both analytes, the recovery was >85% and the coefficient of variation was <10% for high and low controls.
Combination of continuous dexamethasone and asparaginase treatment
Twenty 4-week old mice assigned to the dexamethasone and asparaginase treatment groups were given 4 mg/L dexamethasone in the first week and 2 mg/L dexamethasone thereafter until week 6. Asparaginase (7,500 IU/kg) was injected intraperitoneally once a week (Table 1). The weekly dose we used is similar to that previously described32 and the human equivalent dose (http://www.fda.gov/cber/gdlns/dose.pdf) is 22,500 IU/m2---a dose comparable to that which is used in some clinical regimens.33 Ten mice were sacrificed at the end of week 4, and another 10 mice were sacrificed at the end of week 6. Ten mice served as vehicle controls (for asparaginase) and received injections of 0.9% saline once a week. Five of the mice in the vehicle control group were sacrificed at week 4, and the remainder sacrificed at week 6. Another five mice were treated with dexamethasone alone until week 8.
Histologic examination
After the pilot experiments involving multiple appendages (see above), tissue samples from definitive experiments were obtained from distal femur only. Bone samples were fixed in 10% neutral buffered formalin overnight, then decalcified in TBD-2 (Thermo Fisher Scientific, Waltham, MA). The specimens were processed routinely and embedded in paraffin. Tissue sections were cut at 4 microns, stained with hematoxylin and evaluated by light microscopy.
Tissue samples were analyzed in a blinded fashion by an experienced veterinary pathologist (KB). Osteonecrosis was defined as the presence of all three of the following criteria: empty lacunae, pyknotic nuclei of ghost osteocytes in the bone trabeculae, and necrosis of the adjacent marrow and stromal elements.8,11,34 All mice that had at least one osteonecrotic lesion in a stifle joint were considered positive for osteonecrosis, whereas those with no osteonecrotic lesions were considered negative for osteonecrosis.
Statistical analysis
Fisher’s exact test was used to evaluate the difference in frequency of osteonecrosis among the treated groups. Comparisons of body weight between any two groups were done with the Mann-Whitney U-test. The Wilcoxon rank sum test allowed comparison among groups in the pharmacokinetic/pharmacodynamic study. All statistical analyses were performed using Statistica software (version 8.0; Stata Corporation, College Station, Tex). P values < 0.05 indicated statistical significance.
Results
Dexamethasone alone
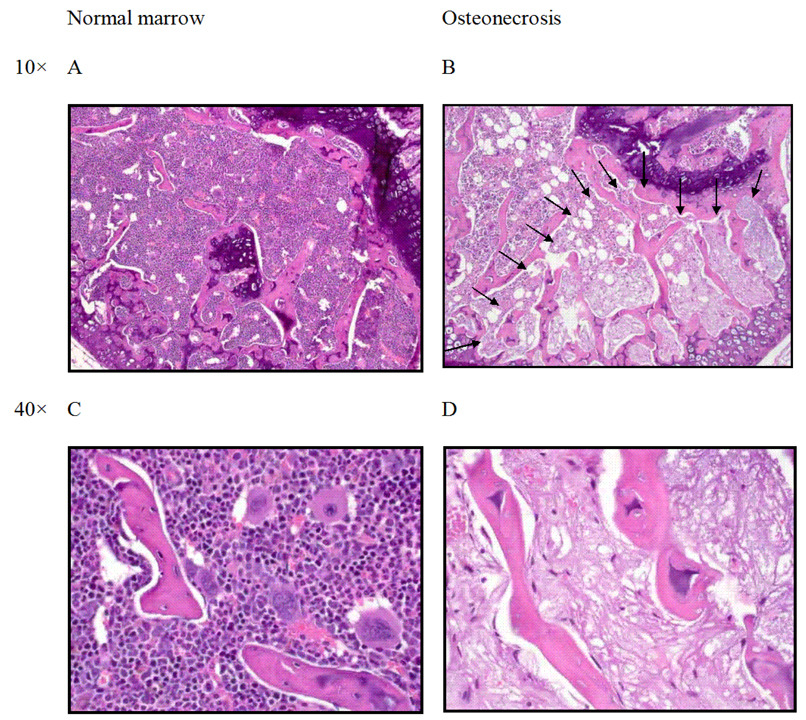
After adjustment of antimicrobial prophylaxis based on the results of the pilot experiments, the survival rate of 12 male BALB/cJ mice treated with continuous dexamethasone was acceptable at 83%. At 12 weeks, osteonecrosis was observed in 4/10 (40%) male BALB/cJ mice by histologic examination (Table 1). In the affected bone (Figure 1), there was segmental necrosis of the marrow components. Spicules of trabecular bone showed empty lacunae and osteocyte ghosts.
Figure 1.
Histologic features of osteonecrosis in a BALB/cJ mouse treated with oral dexamethasone (4 to 2 mg/L). (A,C) Normal architecture of the distal femoral epiphysis with densely cellular hematopoietic elements in the marrow and prominent osteocyte nuclei observed in trabecular bone. (B) Arrows outline segmental osteonecrosis in the distal femoral epiphysis. (D) Higher magnification of osteonecrotic lesion with necrotic marrow elements and empty osteocyte lacunae.
Continuous vs. discontinuous dexamethasone treatment
The mean weights of mice in the continuous (13.7±0.07 g) and discontinuous (14.7±3.1 g) treatment groups were significantly lower than those of mice in the untreated control group (18.1±2.5 g) after just 1 week of treatment (P < 0.01). At later time points (weeks 5 to 12), weight differences compared to untreated controls were generally more pronounced (P < 0.05) with continuous rather than discontinuous treatment; for example, the average weight after 12 weeks of therapy was 31.2±2.3 g for the control group, 18.9±2.0 g for the continuous dexamethasone group, and 22.3±2.2 g for the discontinuous dexamethasone group.
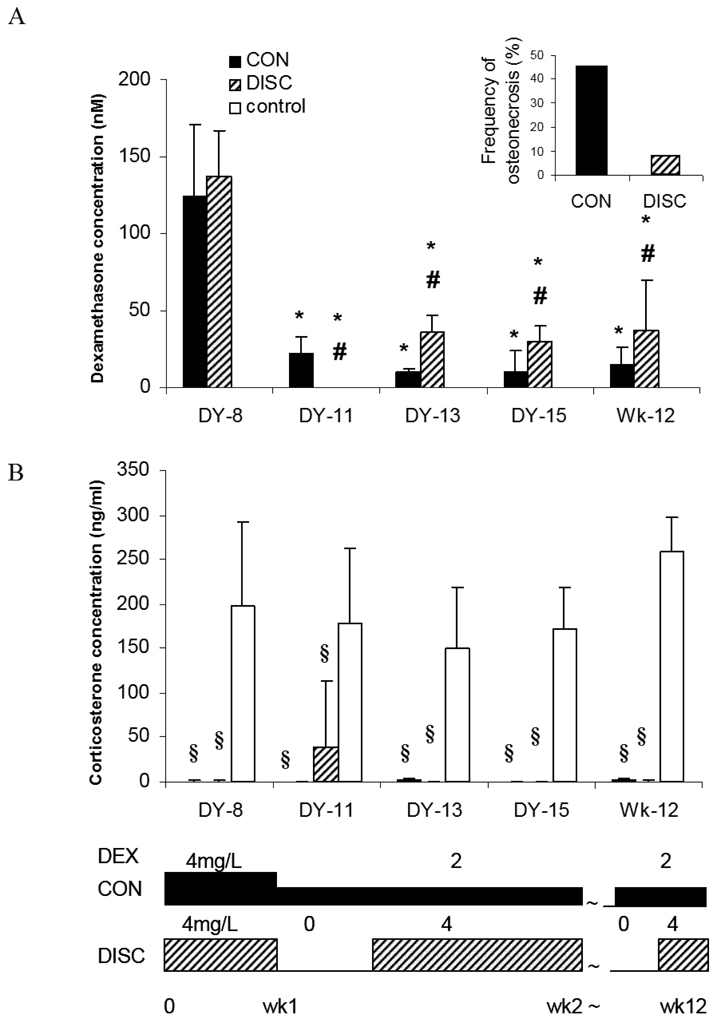
At 12 weeks, osteonecrosis was observed in 5 of 11 (45%) mice in the continuous treatment group compared to 1 of 12 (8%) mice in the discontinuous treatment group (P=0.06) (Table 1, Figure 2). Focal osteonecrotic lesions were found in the femoral bone of treated mice, and no such lesions were found in any mouse in the control group (no dexamethasone treatment).
Figure 2.
Frequency of osteonecrosis with continuous (CON) vs discontinuous (DISC) dexamethasone dosing. Dexamethasone and corticosterone plasma levels during the continuous and discontinuous treatments. (A) Dexamethasone levels decreased after 1 week of treatment in the continuous and discontinuous treatment groups. Dexamethasone levels in the continuous treatment group were less than half those in the discontinuous treatment group after day 13. No dexamethasone concentrations were detected in the control group. (B) Corticosterone levels in the continuous and discontinuous treatment groups were suppressed by the treatment with dexamethasone, whereas corticosterone levels in the discontinuous treatment group partially recovered on day 11, which was the fourth day after dexamethasone use had been discontinued [*, P < 0.05 for the difference from day 8; #, P < 0.05 for the difference between different treatment strategies (continuous vs. discontinuous) on the same day; §, P < 0.05 for the difference between treatment strategies (continuous and discontinuous) and controls]. Abbreviations: DEX, dexamethasone; DY, day; Wk, week.
Dexamethasone pharmacokinetics and pharmacodynamics
At day 7, as expected, plasma dexamethasone levels did not differ (P=0.5) between the discontinuous and continuous arms, as up to that point, treatment was identical. Consistent with autoinduction of dexamethasone clearance, in both treatment arms, the dexamethasone plasma levels at each time point after day 13 were significantly lower (P<0.001) than the corresponding levels on day 7 (Figure 2).
At day 13, day 15, and at the end of therapy (week 12), dexamethasone plasma levels in the discontinuous treatment group were approximately 3-fold greater than those (P<0.05) in the continuous group (average of 34.4 vs 11.5 nM), with daily doses of dexamethasone being 2-fold greater in the discontinuous than in the continuous group (Figure 2A). This is consistent with slightly greater auto-induction of dexamethasone clearance in the continuous vs. discontinuous arms. Using a trapezoidal rule to estimate the cumulative (12 week) area under the curve, the 12 week exposure in the discontinuous treatment arm was 2259 nM·d, which is comparable to (and not less than) that in the continuous treatment arm (1995 nM·d). Thus, the discontinuous exposure arm actually had slightly higher systemic exposure than the continuous arm, even through the frequency of osteonecrosis trended lower (P=0.06) in the discontinuous arm.
As expected, dexamethasone levels were not detected in the discontinuous treatment group on day 11 (3.5 days after the dexamethasone had been removed from the drinking water), nor in the untreated control group at any time point (Figure 2A).
Plasma corticosterone was completely suppressed after one week of dexamethasone exposure compared to untreated controls (P<0.001) (Figure 2B). On day 11, the corticosterone levels in the discontinuous treatment group indicated that the mice had partially recovered from glucocorticoid-induced hypothalamic-pituitary-adrenal (HPA) suppression, but these levels were still significantly lower than those in the control groups (P<0.05) (Figure 2B). Corticosterone remained completely suppressed (<2.5 ng/mL) at day 11 in the continuous group. Thus, the discontinuous arm had less adrenal suppression than the continuous arm, consistent with the lower incidence of osteonecrosis.
Combination of dexamethasone and asparaginase
To attempt to hasten the onset of osteonecrosis, and to recapitulate the drug stresses for osteonecrosis that are observed clinically in patients, we determined the frequency of osteonecrosis in mice receiving asparaginase plus dexamethasone.
Compared to untreated controls, the mean weight losses of mice treated with dexamethasone plus asparaginase were 4.56 ± 3.6 g at 4 weeks and 4.47 ± 3.42 g at 6 weeks (P < 0.01). No significant difference in weight was observed between mice treated with the combination of dexamethasone plus asparaginase compared to dexamethasone alone.
Only one mouse died during the experimental period (the death occurred on day 11 in the dexamethasone plus asparaginase treated group). Osteonecrotic lesions were not observed in any mouse in the vehicle control group. Osteonecrosis was observed in 1 of 9 (11%) dexamethasone plus asparaginase -treated mice at week 4 and in 5 of 10 (50%) at week 6 (P=0.06 for asparaginase plus dexamethasone vs control). Osteonecrotic lesions were found focally in the epiphyseal area of the distal femur. Osteonecrosis was found in 1 of 5 (20%) mice receiving 8 weeks treatment with dexamethasone alone.
Discussion
To our knowledge, there has been no prior mouse model of glucocorticoid-induced osteonecrosis. Unlike rabbits, chickens, or humans, mice more evenly distribute their weight over four limbs; as weight-bearing is considered a major cofactor in glucocorticoid-induced osteonecrosis, this may account for the history of difficulty in producing a murine model. Using an approach where we screened murine strains having at least one likely predisposing phenotypic characteristic, and by providing strong supportive care to minimize early toxic deaths, we have established a mouse model of glucocorticoid-induced osteonecrosis and shown its utility for clinically relevant therapeutic questions.
The histology of the lesions we observed is similar to what has been described in humans 35 and in non-murine animal models.10,11 Histopathologic features of osteonecrosis were seen in the epiphyseal area of the distal femur, and histologic characteristics were similar to those described previously: diffuse empty lacunae were present, and dead or dying osteocytes were surrounded by necrotic bone marrow with or without repair tissue.10,11,34
One of the most important steps in building the model was developing an effective antimicrobial prophylaxis. Initial attempts utilizing 7-day-per-week trimethoprim/sulfamethoxazole (primarily for pneumocystis prophylaxis) resulted in more than half the mice being lost, mostly to infection. By changing the regimen to 3-day-per-week trimethoprim/sulfamethoxazole (which maintains the efficacy of daily pneumocystis prophylaxis but alters the normal gut flora less), and by expanding coverage with addition of tetracycline, the mice were able to be maintained up to and beyond 12 weeks of therapy with a low level of infectious mortality. In the absence of other concurrent agents (such as asparaginase), this prolonged treatment period (≥ 8 weeks) with glucocorticoids was required to induce osteonecrosis.
The model immediately proved useful for testing one of the most clinically relevant questions in use of glucocorticoids in rheumatoid arthritis and in childhood leukemia: does a strategy of using discontinuous rather than continuous dosing of dexamethasone decrease the risk of osteonecrosis? We found that the incidence of osteonecrosis tended to be lower (P=0.06) with discontinuous than with continuous treatment. Although this difference did not reach statistical significance at an alpha level of 0.05, our post-hoc power to detect a difference of an 8% vs 45% incidence of osteonecrosis between the two treatments was low with this sample size; it would have been necessary to study 24 evaluable mice per treatment arm to have 80% power to detect this difference at the alpha = 0.05 level.
Corticosterone levels reflected a greater pharmacodynamic systemic effect of continuous dexamethasone compared to the discontinuous arm; corticosterone was completely suppressed with continuous use but even a few days “holiday” from dexamethasone resulted in a partial recovery of plasma corticosterone (Figure 2). These findings in our murine model are consistent with clinical reports that cortisol levels were lower in renal transplant recipients developing osteonecrosis after glucocorticoid use than in those who did not develop osteonecrosis.36 Thus, discontinuous dexamethasone may be less osteonecrotic than continuous dexamethasone (8% in the discontinuous vs 45% in the continuous dexamethasone group) by allowing partial recovery from adrenal suppression during the few days each week off dexamethasone. Moreover, our pharmacokinetic data proved that the murine plasma dexamethasone levels were in the same range as are achieved clinically (10–200 nM).37 Interestingly, the overall cumulative systemic exposure was similar in the discontinuous and continuous groups, although if anything, slightly higher with discontinuous exposure. We attribute the slightly higher dexamethasone levels in the discontinuous group to the fact that dexamethasone’s autoinduction of its own clearance was less pronounced with intermittent exposure than in the continuously exposed group. In any case, despite at least comparable systemic exposure, the discontinuous arm was less osteonecrotic.
Another way in which this murine model recapitulated characteristics of the clinical setting is that osteonecrosis appeared to be hastened by the concurrent use of asparaginase, which resulted in osteonecrosis at as early as 4 weeks and was comparable in frequency after 6 weeks of dexamethasone plus asparaginase as it was after 12 weeks of dexamethasone alone. We hypothesize that asparaginase may potentiate dexamethasone because of asparaginase’s inhibition of protein synthesis, possible regulation of dexamethasone’s pharmacokinetics, alterations in lipid metabolism, or a combination of all of these effects. Hanada et al also reported that l-asparaginase–induced coagulopathy may be a plausible cause of osteonecrosis.16 The use of asparaginase in combination with dexamethasone significantly alters serum lipid profiles in children with ALL,19,38 and variability in the use of this agent is likely one of the features that affects variability in the frequency of glucocorticoid-associated osteonecrosis in childhood ALL regimens.
When screening the murine strains, we used two diets to attempt to promote the occurrence of osteonecrosis: a low-folate diet and a high-fat diet. We previously reported that in children with ALL, the thymidylate synthase low-activity 2/2 enhancer repeat genotype is associated with the risk of osteonecrosis.21 This suggested that exposure to methotrexate, which is an antifolate drug that results in a chronic state of folate insufficiency in children with ALL and in patients with rheumatoid arthritis, could contribute to glucocorticoid-induced osteonecrosis, consistent with the finding that hyperhomocysteinemia (also associated with low folate) has been linked with the risk of osteonecrosis.9,39 Accordingly, we utilized a low-folate diet in our definitive experiments, and the results of this model may have more applicability to clinical contexts in which folate is low (such as patients with arthritis or leukemia receiving methotrexate). We also tested a high-fat diet, with the notion that this might facilitate glucocorticoid-induced adipogenesis in bones and could predispose the mice to microthrombi and osteonecrosis; however, we did not observe any osteonecrosis among mice on a high-fat diet.
The BALB/cJ strain was chosen for study because of a significantly higher platelet count than other strains (Mouse Phenome Database of the Jackson Laboratory: http://phenome.jax.org/pub-cgi/phenome/mpdcgi). Because thrombocytosis has been associated with thrombosis, and at least some osteonecrosis may have a thrombotic etiologic component, we considered thrombocytosis a possible risk factor. However, it is possible that other as yet unknown phenotypes could have also contributed to the predisposition of BALB/cJ mice to osteonecrosis.
We have developed a reliable and reproducible mouse model of dexamethasone-induced osteonecrosis, with confirmation that the pharmacokinetics and pharmacodynamics reflect clinically relevant exposures. This model reflects several characteristics of clinical glucocorticoid-induced osteonecrosis, including the importance of dosing schedule and drug interactions. This model can be used to further optimize glucocorticoid regimens to minimize their risk of osteonecrosis.
Acknowledgements
This study was supported by NCI grants (# CA 51001 and CA 21765), a Center of Excellence grant from the State of Tennessee, the American Lebanese Syrian Associated Charities (ALSAC) and the Phelan Foundation.
We thank Erick Vasquez and Markus Morgenstern for laboratory, Julia Cay Jones for helping with scientific editing, Wenjian Yang and Mark Wilkinson for computing assistance, and the ARC Histology laboratory for assistance with tissue collection and sectioning.
References
- 1.van Vugt RM, Sijbrandij ES, Bijlsma JW. Magnetic resonance imaging of the femoral head to detect avascular necrosis in active rheumatoid arthritis treated with methylprednisolone pulse therapy. Scand J Rheumatol. 1996;25:74–76. doi: 10.3109/03009749609069211. [DOI] [PubMed] [Google Scholar]
- 2.Zizic TM. Osteonecrosis. Curr Opin Rheumatol. 1991;3:481–489. doi: 10.1097/00002281-199106000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Tsurko VV, Ivanova MM, Tokmachev I. Corticosteroid therapy and aseptic bone necrosis in patients with rheumatoid arthritis. Ter Arkh. 1995;67:71–74. [PubMed] [Google Scholar]
- 4.Burger B, Beier R, Zimmermann M, et al. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)--experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–225. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 5.Mattano LA, Jr., Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children's Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 6.Arico M, Boccalatte MF, Silvestri D, et al. Osteonecrosis: an emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88:747–753. [PubMed] [Google Scholar]
- 7.Smith RW, Margulis RR, Brennan MJ, et al. The influence of ACTH and cortisone on certain factors of blood coagulation. Science. 1950;112:295–297. doi: 10.1126/science.112.2907.295. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Hirano K, Tsutsui H, et al. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin Orthop Relat Res. 1995:235–243. [PubMed] [Google Scholar]
- 9.Glueck CJ, Freiberg RA, Fontaine RN, et al. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Miyanishi K, Yamamoto T, Irisa T, et al. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology (Oxford) 2001;40:196–201. doi: 10.1093/rheumatology/40.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Irisa T, Sugioka Y, et al. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. doi: 10.1002/art.1780401119. [DOI] [PubMed] [Google Scholar]
- 12.Mattano L, Sather HN, La MK, et al. Modified dexamethasone (DXM) reduces the incidence of treatment-related osteonecrosis (ON) in children and adolescents with higher risk acute lymphoblastic leukemia (HR ALL): a report of CCG-1961 (Abstract 777) Blood. 2003;102:221a. [Google Scholar]
- 13.Kroot EJ, Huisman AM, Van ZJ, et al. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: a pilot study. Ann N Y Acad Sci. 2006;1069:300–306. doi: 10.1196/annals.1351.028. [DOI] [PubMed] [Google Scholar]
- 14.Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methylprednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004;31:1083–1087. [PubMed] [Google Scholar]
- 15.Hui L, Wiernik PH. Avascular necrosis of bone after adult acute lymphocytic leukemia treatment with methotrexate, vincristine, L-asparaginase, and dexamethasone (MOAD) Am J Hematol. 1996;52:184–188. doi: 10.1002/(SICI)1096-8652(199607)52:3<184::AID-AJH8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Hanada T, Horigome Y, Inudoh M, et al. Osteonecrosis of vertebrae in a child with acute lymphocytic leukaemia during L-asparaginase therapy. Eur J Pediatr. 1989;149:162–163. doi: 10.1007/BF01958270. [DOI] [PubMed] [Google Scholar]
- 17.Haskell CM, Canellos GP, Leventhal BG, et al. L-asparaginase toxicity. Cancer Res. 1969;29:974–975. [PubMed] [Google Scholar]
- 18.Priest JR, Ramsay NK, Bennett AJ, et al. The effect of L-asparaginase on antithrombin, plasminogen, and plasma coagulation during therapy for acute lymphoblastic leukemia. J Pediatr. 1982;100:990–995. doi: 10.1016/s0022-3476(82)80536-2. [DOI] [PubMed] [Google Scholar]
- 19.Parsons SK, Skapek SX, Neufeld EJ, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89:1886–1895. [PubMed] [Google Scholar]
- 20.Steinherz PG. Transient, severe hyperlipidemia in patients with acute lymphoblastic leukemia treated with prednisone and asparaginase. Cancer. 1994;74:3234–3239. doi: 10.1002/1097-0142(19941215)74:12<3234::aid-cncr2820741224>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Cui Q, Wang GJ, Su CC, et al. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997:8–19. [PubMed] [Google Scholar]
- 23.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Conzemius MG, Brown TD, Zhang Y, et al. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J Orthop Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 25.breu-Silva AL, Calabrese KS, Cupolilo SM, et al. Histopathological studies of visceralized Leishmania (Leishmania) amazonensis in mice experimentally infected. Vet Parasitol. 2004;121:179–187. doi: 10.1016/j.vetpar.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki K, Itakura C. Aseptic necrosis of bone in ICR mice. Lab Anim. 1988;22:51–53. doi: 10.1258/002367788780746601. [DOI] [PubMed] [Google Scholar]
- 27.Walzer PD, Foy J, Steele P, et al. Activities of antifolate, antiviral, and other drugs in an immunosuppressed rat model of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1992;36:1935–1942. doi: 10.1128/aac.36.9.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walzer PD, Powell RD, Jr., Yoneda K. Experimental Pneumocystis carinii pneumonia in different strains of cortisonized mice. Infect Immun. 1979;24:939–947. doi: 10.1128/iai.24.3.939-947.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Healey MC. The immunosuppressive effects of dexamethasone administered in drinking water to C57BL/6N mice infected with cryptosporidium parvum. J Parasitol. 1993;79:626–630. [PubMed] [Google Scholar]
- 30.Ling S, Jamali F. Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci. 2003;6:246–251. [PubMed] [Google Scholar]
- 31.Mehvar R, Dann RO, Hoganson DA. Simultaneous analysis of methylprednisolone, methylprednisolone succinate, and endogenous corticosterone in rat plasma. J Pharm Biomed Anal. 2000;22:1015–1022. doi: 10.1016/s0731-7085(00)00253-3. [DOI] [PubMed] [Google Scholar]
- 32.Reiff A, Zastrow M, Sun BC, et al. Treatment of collagen induced arthritis in DBA/1 mice with L-asparaginase. Clin Exp Rheumatol. 2001;19:639–646. [PubMed] [Google Scholar]
- 33.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 34.Kabata T, Kubo T, Matsumoto T, et al. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology (Oxford) 2005;44:1233–1237. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]
- 35.Sissons HA, Nuovo MA, Steiner GC. Pathology of osteonecrosis of the femoral head. A review of experience at the Hospital for Joint Diseases, New York. Skeletal Radiol. 1992;21:229–238. doi: 10.1007/BF00243063. [DOI] [PubMed] [Google Scholar]
- 36.Rico H, Gomez-Castresana F, Cabranes JA, et al. Increased blood cortisol in alcoholic patients with aseptic necrosis of the femoral head. Calcif Tissue Int. 1985;37:585–587. doi: 10.1007/BF02554910. [DOI] [PubMed] [Google Scholar]
- 37.Richter O, Ern B, Reinhardt D, et al. Pharmacokinetics of dexamethasone in children. Pediatr Pharmacol (New York) 1983;3:329–337. [PubMed] [Google Scholar]
- 38.Halton JM, Nazir DJ, McQueen MJ, et al. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998;83:379–384. [PubMed] [Google Scholar]
- 39.Wilcken DE. MTHFR 677C-->T mutation, folate intake, neural-tube defect, and risk of cardiovascular disease. Lancet. 1997;350:603–604. doi: 10.1016/S0140-6736(05)63320-X. [DOI] [PubMed] [Google Scholar]