Abstract
Background
Alterations in cerebrospinal fluid (CSF) tau and β–amyloid peptide 1–42 (Aβ42) levels and rates of cerebral glucose (CMRglu) on fluorodeoxyglucose positron emission tomography (FDG PET) occur years before clinical symptoms of Alzheimer’s disease (AD) become manifest, but their relationship remains unclear.
Objective
To determine whether CSF AD biomarker levels and CMRglu in healthy individuals correlate in brain structures affected early in AD.
Design
Cohort study.
Setting
Alzheimer’s disease research center.
Participants
Twenty individuals without dementia, aged 46 to 83 years.
Interventions
Lumbar CSF sampling and FDG-PET imaging of CMRglu. The CSF Aβ42, tau, and tau phosphorylated at threonine 181 (p–tau181) levels were measured using immunobead–based multiplex assays.
Main Outcome Measures
Correlations between CMRglu and CSF biomarker levels were analyzed via voxel–based and volume–of–interest approaches.
Results
Voxel–based analyses demonstrated significant negative correlations between CSF tau and p–tau181 levels and CMRglu in the posterior cingulate, precuneus, and parahippocampal regions. In contrast, a limited positive correlation was found between CSF Aβ42 levels and CMRglu in the inferior temporal cortex. Volume–of–interest analyses confirmed negative associations between CSF tau and p–tau181 levels and CMRglu in the parietal and medial parietal lobes and a positive association between CSF Aβ42 levels and CMRglu in the parahippocampal gyrus.
Conclusions
In healthy individuals, higher CSF tau and p–tau181 concentrations were associated with more severe hypometabolism in several brain regions affected very early in AD, whereas lower CSF Aβ42 concentrations were associated with hypometabolism only in the medial temporal lobe. This suggests that early tau and Aβ abnormalities may be associated with subtle synaptic changes in brain regions vulnerable to AD. A longitudinal assessment of CSF and FDG–PET biomarkers is needed to determine whether these changes predict cognitive impairment and incipient AD.
INTRODUCTION
Neuropathologic changes in Alzheimer’s disease (AD), including synapse loss, intraneuronal aggregation of hyperphosphorylated tau protein in neurofibrillary tangles (NFTs), and extraneuronal deposition of β–amyloid (Aβ) protein in amyloid plaques (APs) begin years before the onset of clinical symptoms of dementia.1 The relative contributions of altered tau and amyloid metabolism to neurodegeneration in AD remain under investigation. Mutations in the amyloid precursor protein (APP) and presenilin 1 and 2 genes increase the amyloid burden by increasing either APP production or processing of APP to Aβ42 and causing early–onset familial AD. However, it has been consistently demonstrated that NFTs, rather than APs, are more robustly associated with measures of synapse loss and cognitive impairment.2 Relationships among putative antecedent cerebrospinal fluid (CSF) biomarkers and neuropathologic findings in AD are currently a topic of intense investigation.
Concentrations of total tau, tau phosphorylated at threonine 181 (p–tau181) and Aβ42 in CSF may be sensitive biomarkers of incipient NFT and AP formation in AD. CSF tau and p–tau181 concentrations are increased and CSF Aβ42 concentrations are decreased in patients with AD and are associated with higher rates of conversion from mild cognitive impairment (MCI) to AD.3 In addition, we demonstrated that CSF Aβ42 concentrations begin to decline in cognitively healthy individuals who carry the apolipoprotein E*4 (APOE*4) allele (in whom AD onset is accelerated by approximately ten years) decades before the typical age of risk for dementia.4 Although these investigations provide novel insights into the pathogenesis of AD, mechanistic relationships between CSF biomarkers and AD–related neuropathologic features remain to be elucidated in detail.
Neuroimaging uniquely allows non–invasive measurement of biomarkers related to neuronal metabolism and biosynthetic activity in living patients. For example, neuronal fluorodeoxyglucose (FDG) uptake correlates with synaptic activity in the rodent brain5 and, in APP–overproducing transgenic mouse models of AD, FDG uptake is reduced in areas with early AD neuropathologic features.6 In addition, positron emission tomography (PET) imaging of cerebral glucose metabolism (CMRglu) using [18F]–FDG has demonstrated regionally specific reductions in CMRglu in AD.7 That FDG PET imaging of CMRglu is a sensitive biomarker for AD–related neurodegeneration before the onset of clinical disease is supported by seminal human observations published in the 1990’s demonstrating decreased CMRglu in the parietal and posterior cingulate cortices of APOE*4 –positive individuals at increased risk of AD,8,9 in the posterior cingulate cortex of patients with MCI,10 and in the entorhinal cortex of healthy older individuals who later developed progressive cognitive decline.11
Data regarding relationships among cortical CMRglu, synapse loss, NFTs, and APs in AD are scant. De Carli et al12 compared regional CMRglu during life with postmortem NFT and AP counts and synapse loss in 6 patients with AD and found a trend for NFT burden, but not for AP burden or synapse loss, to be associated with frontal, temporal, parietal, anterior cingulate, and primary sensorimotor CMRglu. However, in a similar study of 4 patients with AD, Mielke et al13 observed a negative association between AP burden, but not NFT burden, and CMRglu, and only in a single patient. Findings from imaging studies suggest a dissociation in spatial distribution between traditional neuropathologic markers, such as NFT burden, and cortical hypometabolism in early AD, possibly reflecting degeneration of projecting neurons,14 such that neuropathologic and imaging markers are observed in different neuronal compartments.
In this study, we sought to further elucidate differential neuroanatomical associations between CSF tau and Aβ concentrations and FDG–PET indicators of early neuronal dysfunction in healthy individuals at risk, by virtue of age or APOE genotype, for showing early manifestations of AD pathogenesis uncomplicated by subsequent secondary and tertiary responses to disease activity and neuronal injury. We hypothesized that CSF tau and p–tau or Aβ42 concentrations would robustly correlate with CMRglu in healthy individuals in brain regions affected early in AD. To this end, we measured CSF AD biomarkers and CMRglu in cognitively normal middle–aged and older individuals and applied brain mapping analyses to characterize relationships between CSF tau, p–tau181, and Aβ42 levels and FDG uptake in the brain.
METHODS
PARTICIPANTS
The University of Washington institutional review board approved all the procedures and all the participants provided written informed consent before enrollment into the study. Twenty cognitively normal individuals (11 men and 9 women) were recruited from the University of Washington Alzheimer’s Disease Research Center. Participants were medically healthy, had Mini–Mental State Examination scores of 26 or greater, had Clinical Dementia Rating scores of zero, and had no evidence or history of cognitive or functional decline. For participants older than 50 years, scores on delayed recall were greater than cutoff values set at 1.5 SD below the age–adjusted means for the Wechsler Logical Memory and New York University paragraph recall tests to rule–out mild memory impairment. The mean (SD) age of participants was 61.7 (11.0) years (age range, 46–83 years) at the time of lumbar puncture (LP) for CSF sampling and 64.1 (10.3) years at the time of FDG–PET. The time between LP and FDG–PET was 863 (675) days. Ten participants were positive for the APOE*4 allele.
CSF COLLECTION
Lumbar CSF samples were collected in the morning after an overnight fast using a 24–gauge atraumatic spinal needle (Sprotte; Dyna Medical Corp, London, Ontario, Canada) with the participant in the lateral decubitus position. Samples were sequentially aliquoted and frozen immediately on dry ice at the bedside and were stored at −80°C until assay.
FDG PET IMAGE ANALYSIS
Participants received 10 mCi of [18F]–FDG intravenously and rested in a quiet room during the 30–minute uptake phase. Images were acquired for 5 minutes in 2 dimensional mode and for 15 minutes in 3–dimensional mode using a GE Advance scanner (GE Medical Systems, Milwaukee, Wisconsin) with a subsequent 25–minute 45Ge transmission scan for attenuation correction. The PET image sets were co registered, anatomically standardized to Talairach and Tournoux stereotactic coordinates,15 pixel intensity normalized to global activity, and smoothed using a 3–dimensional Gaussian kernel (2.25 mm SD) to reduce regional variances. Correlations between normalized CMRglu and CSF tau, p–tau181 and Aβ42 levels were calculated on a voxelwise basis, and the correlation coefficients were transformed to Z–scores. Coordinates for which Z values exceeded 3.5 were considered to be statistically significant, controlling the Type I error rate approximately at P=.05 for multiple comparisons.16
To demonstrate the progressive nature of metabolic alterations relative to CSF tau changes, a voxelwise regression analysis was performed to interpolate metabolic maps at different CSF tau levels. These maps were compared with that for a CSF tau level of 0 pg/ml and are expressed as Z values using a variance map generated in the regression analysis. For comparison purposes, CMRglu subtraction images from a separate cohort of 37 patients with clinically diagnosed probable AD and 22 age–matched controls imaged under similar conditions were used to generate Z–score maps of typical AD–related cortical hypometabolism.17
To confirm the findings of the voxelwise analyses, correlations between mean global–normalized CMRglu values and CSF biomarker concentrations were also calculated (using stereotactically–defined volume–of–interest [VOI] analysis) for the frontal, temporal, parietal, medial parietal, anterior cingulate, and posterior cingulate cortices and the parahippocampal gyrus.
CSF BIOMARKER MEASUREMENTS AND APOE*4 GENOTYPING
The CSF total tau, p–tau181, and Aβ42 levels were measured in the 10th milliliter of CSF using immunobead–based multiplex assays (AlzBio3; Innogenetics Inc, Gent, Belgium). Genotyping was performed using a restriction digest method.18
DATA ANALYSIS
Associations between CSF AD biomarker concentrations and age or regional CMRglu values (VOI analyses) were assessed by means of multiple linear regression using a software program (SPSS version 11; SPSS Inc, Chicago, Illinois). Results with probability less than 5% (P<.05) were considered statistically significant.
RESULTS
PET AND CSF AD BIOMARKERS
Voxelwise Analyses
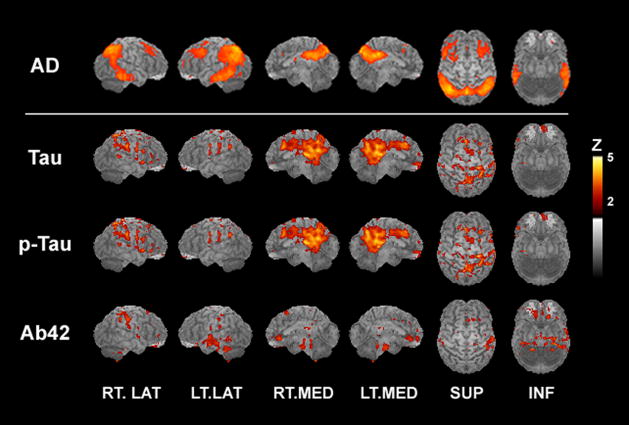
Increasing concentrations of CSF tau, a putative marker of neuronal injury, were associated with more pronounced hypometabolism, a putative marker of synaptic dysfunction, in multiple brain regions known to be affected early in AD. Negative correlations between CSF tau levels and CMRglu were observed for the posterior cingulate gyrus, precuneus/medial parietal lobe, and pulvinar and in the dorsofrontal and parahippocampal gyri and superior parietal lobule (Figure 1 and Table 1). Qualitatively similar results were observed for regional CMRglu correlations with CSF p–tau181 concentrations (Figure 1 and Table 1). Figure 1 shows the typical pattern of regional cortical hypometabolism exhibited by patients with AD and illustrates the close concordance between this pattern and the distribution of negative correlations between CMRglu and CSF tau and p–tau181 levels obtained in the present study.
Figure 1.
Z–score maps of cerebral glucose metabolism (CMRglu) differences between 37 patients with Alzheimer’s disease (AD) and 22 healthy elderly individuals (top row); negative correlations between CMRglu and cerebrospinal fluid (CSF) tau and CSF tau phosphorylated at threonine 181 (p–tau181) levels; and positive correlations between CMRglu and CSF β–amyloid peptide 1 42 (Aβ42) levels in 20 healthy middle–aged and elderly individuals (second, third, and fourth rows, respectively). INF indicates inferior; LT, left; LAT, lateral; MED, medial; RT, right; and SUP, superior. Vertical bar shows image color vs Z–score scale.
Table 1.
Locations of voxels exhibiting significant correlations between CSF AD biomarker concentrations and global normalized CMRglu
| Structure | Z score | Stereotactic Coordinates (mm) |
|---|---|---|
| CSF Tau and CMRglu: Negative Correlations | ||
| Posterior cingulate gyrus | −4.0 | (10, 51, 25) (10, 37, 20) |
| −3.9 | (10, 35, 32) | |
| −3.7 | (10, 35, 29) (10, 37, 25) | |
| −3.5 | (6, 31, 34) | |
| Precuneus | −3.6 | (6, 60, 38) (10, 51, 45) |
| −3.5 | (10, 58, 32) (8, 60, 22) | |
| Pulvinar | −4.0 | (8, 28, 14) (8, 22, 16) |
| −3.9 | (10, 33, 16) (10, 28, 16) | |
| Superior parietal lobule | −3.6 | (30, 51, 50) |
| Postcentral gyrus | −4.3 | (28, 33, 68) |
| Dorsofrontal gyrus | −3.7 | (12, 28, 36) |
| −3.5 | (6, 28, 34) | |
| Parahippocampal gyrus | −3.5 | (3, 33, 4) |
| CSF p–tau181 and CMRglu: Negative Correlations | ||
| Posterior cingulate gyrus | −4.0 | (−10, −51, 25) |
| −3.9 | (10, −40, 18) | |
| −3.8 | (12, −37, 34) (3, −33, 4) | |
| −3.7 | (−10, −40, 22) | |
| −3.6 | (−6, −31, 34) | |
| −3.5 | (−8, −44, 14) | |
| Precuneus | −3.9 | (6, −60, 38) |
| −3.7 | (10, −58, 32) | |
| −3.5 | (−10, −51, 45) | |
| Pulvinar | −3.8 | (10, −33, 16) |
| −3.7 | (10, −26, 16) | |
| Dorsomedial thalamic nucleus | −4.2 | (−8, −22, 16) |
| Dorsofrontal gyrus | −3.5 | (12, 28, 36) |
| Post central gyrus | −4.8 | (−28, −33, 68) |
| CSF Aβ42 and CMRglu: Positive Correlations | ||
| Fusiform gyrus | 3.5 | (35, −37, −27) |
Abbreviations: Aβ42, β amyloid peptide 1–42; AD, Alzheimer disease; CMRglu, cerebral glucose metabolism; CSF, cerebrospinal fluid; FDG–PET, fluorodeoxyglucose positron emission tomography; LP, lumbar puncture; p–tau181, tau phosphorylated at threonine 181.
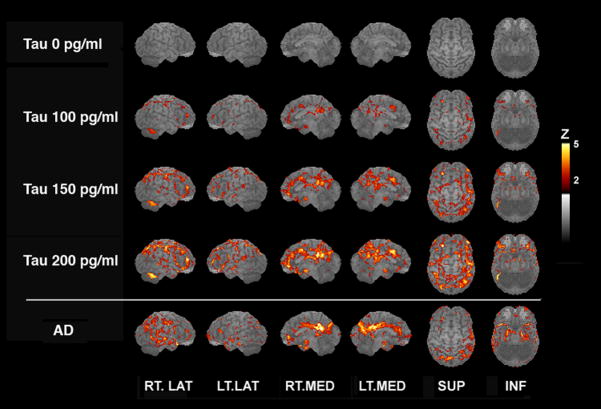
To further characterize the relationship between regional CMRglu and CSF tau concentrations, we constructed predicted Z–score images of cortical CMRglu based on CSF tau concentrations of 0, 100, 150, and 200 pg/ml using regression analysis (Figure 2). The pattern of CMRglu estimated for a CSF tau concentration of 200 pg/ml displayed a high degree of concordance with that exhibited by a patient, not part of the present study, with a clinical diagnosis of AD (Figure 2).
Figure 2.
Z–score maps of voxelwise regression–estimated cerebral glucose metabolism (CMRglu) values corresponding to cerebrospinal fluid (CSF) tau concentrations of 0, 100, 150, and 200 pg/mL and of relative CMRglu (compared with a reference group of elderly control subjects) for a typical patient with Alzheimer’s disease (AD). Views and vertical bar are the same as in Figure 1.
Only 1 brain region displayed a significant association between lower concentrations of CSF Aβ42, a putative marker of increased amyloid sequestration in APs, and hypometabolism, as evidenced by the positive correlation between CMRglu and CSF Aβ42 concentration at a single locus in the fusiform gyrus (Figure 1 and Table 1).
VOI Analyses
Correlations between regional CMRglu and CSF biomarker concentrations are given in Table 2. Controlling for age at LP and time between LP and FDG–PET, there was a significant negative correlation between parietal CMRglu and CSF concentrations of total tau and p–tau181, indicating that higher CSF tau and p–tau181 concentrations were associated with more severe parietal hypometabolism. Negative correlations between parietal CMRglu and CSF tau and p–tau181 levels of similar magnitude were evident in the APOE*4–positive and APOE*4–negative subgroups but did not attain significance, likely due to limited sample size and statistical power. Correlations between parietal CMRglu and CSF tau:Aβ42 and p–tau181:Aβ42 ratios showed a similar pattern of results. In contrast, in the medial parietal/precuneus VOI, CSF p–tau181, but not tau, concentration exhibited a borderline significant (P = .05) negative correlation with CMRglu. The negative correlations with CSF tau and p tau181 were larger in the APOE*4–negative vs APOE*4–positive participants, although not significant in either subgroup. There were also significant negative correlations between medial parietal/precuneus CMRglu and CSF tau:Aβ42 and p–tau181:Aβ42 ratios for all participants combined and for the APOE*4–negative vs APOE*4–positive individuals.
Table 2.
Partial Correlations Between Global Normalized CMRglu and CSF AD Biomarker Levelsa
| All Participants | APOE*4–Positive | APOE*4–Negative | ||||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| Parietal lobe VOI | ||||||
| CSF tau | −0.53 | .02 | −0.56 | NS | −0.52 | NS |
| CSF p–tau181 | −0.58 | .01 | −0.60 | NS | −0.55 | NS |
| CSF tau:Aβ42 | −0.55 | .02 | −0.49 | NS | −0.63 | NS |
| CSF p–tau181:Aβ42 | −0.54 | .02 | −0.47 | NS | −0.66 | NS |
| Medial parietal lobe/precuneus VOI | ||||||
| CSF tau | −0.43 | NS | −0.27 | NS | −0.63 | NS |
| CSF p–tau181 | −0.47 | .05 | −0.33 | NS | −0.66 | NS |
| CSF tau:Aβ42 | −0.47 | .048 | −0.28 | NS | −0.75 | .03 |
| CSF p–tau181:Aβ42 | −0.48 | .045 | −0.32 | NS | −0.76 | .03 |
| Parahippocampal gyrus VOI | ||||||
| CSF Aβ42 | 0.54 | .02 | 0.77 | .03 | 0.38 | NS |
| CSF tau:Aβ42 | 0.08 | NS | 0.32 | NS | −0.36 | NS |
| CSF p–tau181:Aβ42 | 0.02 | NS | 0.21 | NS | −0.40 | NS |
Abbreviations: Aβ42, β–amyloid peptide 1–42; AD, Alzheimer disease; APOE*4, apolipoprotein E*4; CMRglu, cerebral glucose metabolism; CSF, cerebrospinal fluid; p–tau181, tau phosphorylated at threonine 181; NS, not significant; VOI, volume of interest.
Controlling for age at lumbar puncture and time between lumbar puncture and fluorodeoxyglucose positron emission tomography.
In the parahippocampal gyrus, there was a positive correlation, controlling for age at LP and time between LP and FDG–PET, between CSF Aβ42 concentrations and global–normalized CMRglu (r = 0.54, P = .02), indicating that lower levels of CSF Aβ42 were associated with more pronounced hypometabolism in this brain region. The positive correlation was restricted to APOE*4 positive vs APOE*4 negative individuals (r = 0.77, P = .03 vs r = 0.38, P = .35).
CSF AD BIOMARKER CONCENTRATIONS, AGE, AND APOE*4 STATUS
The mean (SD) CSF tau concentrations were 45.2 (37.6) pg/mL (range, 13.1–151.8 pg/mL). The mean (SD) CSF p–tau181 concentrations were 51.5 (28.2) pg/mL (range, 30.1–130.3 pg/mL). The CSF Aβ42 concentrations were 142.0 (35.5) pg/mL (rpange, 85.1 193.8 pg/mL). We confirmed previously reported associations between age and CSF AD biomarker concentrations. Age was negatively correlated with CSF Aβ42 level (r = −0.62, P = .004) and positively correlated with CSF tau (r = 0.80, P < .001) and p–tau181 (r = 0.79, P < .001) levels. The negative correlation between age and CSF Aβ42 was present only in APOE*4–positive individuals (r = −0.73, P = .01 vs r = −0.54, P = .14).However, the positive correlations between age and CSF tau and ptau181 levels were significant in APOE*4-positive (r = 0.84, P = .001; r = 0.81, P = .003, respectively) and APOE*4-negative participants (r = 0.78, P = .01; and r = 0.78, P = .01, respectively).
COMMENT
Using voxel based analyses, this study demonstrated, in cognitively intact middle aged and older individuals, that associations between CMRglu and CSF concentrations of tau and p–tau181 exist in brain structures that are consistently affected in AD, especially in the posterior cingulate cortex and precuneus, where very early AD changes are known to occur. These results are generally consistent with pathologic findings of a stronger association between synapse loss and NFT vs AP burden in the brains of patients with AD.
That increased CSF tau and p–tau181 concentrations were associated with reductions in regional CMRglu is consistent with evidence that CSF concentrations of these proteins are increased in association with neuronal damage resulting from a variety of central nervous system insults, including AD, and that synapse loss is strongly associated with NFT burden in the brains of patients with AD.2 In addition, Ceravolo et al19 recently reported negative correlations between CSF tau concentrations and cerebellar–normalized parietal, temporal, occipital and entorhinal/hippocampal CMRglu and between CSF p–tau181 concentrations and bilateral temporal, parietal and entorhinal/hippocampal cerebellar–normalized CMRglu in patients with AD, although it is not clear whether their analyses controlled for age effects on CSF biomarker concentrations. Studies of patients with MCI have also reported negative correlations between CSF tau or p–tau concentrations and “AD–like” (i.e., temporoparietal) cerebrocortical hypometabolism,20 but they did not further describe the specific cortical regions or subcortical gray matter structures in which CMRglu was reduced. In contrast, Okamura et al21 found no correlation between CSF tau levels and cerebellar normalized whole-brain CMRglu in a group of 15 patients with AD. Regarding healthy individuals, Mosconi et al22 reported results similar to our own, finding that middle–aged APOE*4 allele carriers exhibited elevated CSF tau and p–tau231 concentrations and decreased global normalized CMRglu in AD–related brain regions. However, they also found that the magnitude of the p–tau231 effect was more pronounced in APOE*4 carriers with subjective memory complaints compared with APOE*4 carriers without memory complaints or with APOE*4–negative individuals, whereas we found inconsistent effects of APOE*4 allele status on parietal and medial parietal CMRglu correlations with CSF p–tau181 levels and non significant trends for stronger negative associations with CSF tau:Aβ42 and p–tau181:Aβ42 ratios in APOE*4–negative vs APOE*4–positive participants in these brain regions. Similar inconsistent effects of APOE*4 genotype status on CSF tau and p–tau181 levels have been reported in studies of AD,23–25 MCI,26,27 and elderly individuals without dementia.25,27
The neuroanatomical distribution of voxels that exhibit significant negative correlations between CMRglu and CSF tau and p–tau181 levels did not correspond to those associated with early NFT formation in neuropathologic studies, which raises questions regarding their pathophysiologic significance. However, recent research suggests that, as for amyloid, neurotoxic effects of tau may be associated with soluble oligomeric forms of the protein,28 which could account for the lack of spatial concordance between the present results and previous neuropathologic studies.
We also observed negative correlations between CMRglu and CSF tau concentrations for the pulvinar nucleus of the thalamus, a region not typically associated with cognitive function or AD. However, neuroanatomical studies have demonstrated prominent connections between the pulvinar and other midline brain regions involved in cognition and AD, such as the posterior cingulate and inferior parietal region, in nonhuman primates29 and humans.30 In addition, the pulvinar has been implicated in cognitive functions such as visual attention and spatial awareness in humans,31,32 and the pulvinar is a site of NFT and AP deposition in AD,33 supporting a possible important role in cognitive impairment in AD.
Lower CSF Aβ42 concentrations in patients with AD have been ascribed to sequestration of soluble Aβ42 into APs. Consistent with this hypothesis, CSF Aβ42 concentrations were inversely correlated with brain amyloid burden as assessed by PET imaging of [11C]–Pittsburgh compound B (11C–PIB) binding in a study that included healthy elderly individuals and patients with AD.34 The finding that CMRglu and CSF Aβ42 concentrations were positively correlated in the present study is consistent with previous work showing that brain amyloid burden and [11C]–PIB binding are associated with reduced CMRglu.35 In addition, Okamura et al21 also reported positive correlations between CSF Aβ42 levels and cerebellar normalized, age adjusted whole brain and inferior temporal CMRglu in 15 patients with AD. We are not aware of any comparable studies performed with cognitively intact individuals.
Although negative correlations between CSF tau and p–tau181 levels were observed in multiple brain regions, positive correlations between CSF Aβ42 levels and CMRglu were limited to the fusiform gyrus (voxelwise analyses) and the parahippocampal gyrus (VOI analyses). The fact that CMRglu was more robustly associated with CSF tau and p–tau181 vs Aβ42 levels is consistent with neuropathologic findings that NFTs are more robustly correlated with measures of synapse loss in AD than are APs.2
This study is limited by several shortcomings. First, we inferred the presence of incipient neuronal injury and synapse loss based on CMRglu measurements using PET. However, autopsy confirmation of neuronal injury or loss in our still–living participants is not available. Second, we inferred the presence of occult AD–related neurodegenerative processes based on the presence of altered CSF tau and Aβ42 concentrations without autopsy confirmation of NFTs and APs. Evidence that CSF tau concentrations are strongly correlated with brain NFT burden at autopsy is mixed36,37 and although [11C]–PIB binding is inversely correlated with CSF Aβ42 concentrations, pathologic validation of CSF Aβ42 levels as a measure of brain AP burden is, to our knowledge, not available in the literature. Third, we assessed correlations between CSF AD biomarker levels and regional CMRglu at 1 point in time in a single cohort of healthy individuals. Whether the observed correlations will persist or increase in individuals (if any) who develop MCI or AD is unknown. Similarly, whether similar correlations would have been observed in a mixed group of cognitively intact, MCI, and AD individuals is also unknown. Answers to the latter 2 questions may be provided by data from the ongoing Alzheimer’s Disease Neuroimaging Initiative.
In summary, voxel–based and VOI analyses of correlations between regional cortical CMRglu and CSF AD biomarker levels suggest that neuronal injury or loss, reflected in hypometabolism on FDG–PET imaging, is differentially associated with tau and Aβ metabolism in anatomically distinct brain regions in cognitively intact middle aged and early aging individuals. Longitudinal studies of metabolic imaging and CSF AD biomarkers are required to determine whether these initial cross–sectional findings represent prodromal signs of neurodegenerative processes that lead to clinical AD.
Acknowledgments
Funding/Support: This material is based upon work supported, in part, by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs; by grants R01 NS045254, P50 AG05136, R01 AG023185, R01 AG05131 and K08 AG023670 from the National Institutes of Health; and by the National Alzheimer’s Coordinating Center.
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Karina Wirch, BS, recruited and scheduled participants for FDG PET; Barbara Lewellen, CNMT, performed imaging data coordination; and University of Washington PET technologists performed imaging studies.
Author Contributions: Dr Petrie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Petrie, Cross, Raskind, Peskind, and Minoshima. Acquisition of data: Petrie, Galasko, Schellenberg, Peskind, and Minoshima. Analysis and interpretation of data: Petrie, Cross, Raskind, Peskind, and Minoshima. Drafting of the manuscript: Petrie, Galasko, and Minoshima. Critical revision of the manuscript for important intellectual content: Petrie, Cross, Galasko, Schellenberg, Raskind, Peskind, and Minoshima. Statistical expertise: Petrie, Cross, and Minoshima. Obtained funding: Petrie, Galasko, Schellenberg, Raskind, Peskind, and Minoshima. Administrative, technical and material support: Cross, Galasko, Schellenberg, Raskind, Peskind, and Minoshima. Study supervision: Peskind and Minoshima.
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer–related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. Medline:1759558. [DOI] [PubMed] [Google Scholar]
- 2.Gomez–Isla T, Hollister R, West H et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41(1):17–24. doi: 10.1002/ana.410410106. Medline:9005861. [DOI] [PubMed] [Google Scholar]
- 3.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow–up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. Medline:16488378. [DOI] [PubMed] [Google Scholar]
- 4.Peskind ER, Li G, Shofer J et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid β– amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63(7):936–939. doi: 10.1001/archneur.63.7.936. Medline:16831961. [DOI] [PubMed] [Google Scholar]
- 5.Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985;82(17):6010–6013. doi: 10.1073/pnas.82.17.6010. Medline:3862113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiman EM, Uecker A, Gonzalez–Lima F et al. Tracking Alzheimer’s disease in transgenic mice using fluorodeoxyglucose autoradiography. Neuroreport. 2000;11(5):987–991. doi: 10.1097/00001756-200004070-00018. Medline:10790869. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl DE. Imaging local brain function with emission computed tomography. Radiology. 1984;150(3):625–631. doi: 10.1148/radiology.150.3.6607481. Medline:6607481. [DOI] [PubMed] [Google Scholar]
- 8.Reiman EM, Caselli RJ, Yun LS et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. Medline:8592548. [DOI] [PubMed] [Google Scholar]
- 9.Small GW, Mazziotta JC, Collins MT et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942–947. Medline:7884953. [PubMed] [Google Scholar]
- 10.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. Medline:9225689. [DOI] [PubMed] [Google Scholar]
- 11.de Leon MJ, Convit A, Wolf OT et al. Prediction of cognitive decline in normal elderly subjects with 2 [18F]fluoro 2 deoxy D glucose/positron emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98(19):10966–10971. doi: 10.1073/pnas.191044198. Medline:11526211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCarli C, Atack JR, Ball MJ, et al. Post mortem regional neurofibrillary tangle densities but not senile plaque densities are related to regional cerebral metabolic rates for glucose during life in Alzheimer’s disease patients. Neurodegeneration. 1992;1:113–121. [Google Scholar]
- 13.Mielke R, Schroder R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer’s disease. Acta Neuropathol. 1996;91(2):174–179. doi: 10.1007/s004010050410. Medline:8787151. [DOI] [PubMed] [Google Scholar]
- 14.Minoshima S, Cross DJ, Foster NL, Henry TR, Kuhl DE. Discordance between traditional pathologic and energy metabolic changes in very early Alzheimer’s disease: pathophysiological implications. Ann N Y Acad Sci. 1999;893:350–352. doi: 10.1111/j.1749-6632.1999.tb07852.x. Medline:10672264. [DOI] [PubMed] [Google Scholar]
- 15.Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med. 1994;35(9):1528–1537. Medline:8071705. [PubMed] [Google Scholar]
- 16.Worsley KJ, Marrett S, Neelin P, Evans AC. A unified statistical approach for determining significant signals in location and scale space images of cerebral activation. In: Myers R, Cunningham VJ, Bailey D, Jones T, editors. Quantification of Brain Function Using PET. San Diego, CA: Academic Press; 1996. pp. 327–333. [Google Scholar]
- 17.Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer’s disease. Lancet. 1994;344(8926):895. doi: 10.1016/s0140-6736(94)92871-1. Medline:7916431. [DOI] [PubMed] [Google Scholar]
- 18.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. Medline:2341813. [PubMed] [Google Scholar]
- 19.Ceravolo R, Borghetti D, Kiferle L et al. CSF phosporylated TAU protein levels correlate with cerebral glucose metabolism assessed with PET in Alzheimer’s disease. Brain Res Bull. 2008;76(1–2):80–84. doi: 10.1016/j.brainresbull.2008.01.010. Medline:18395614. [DOI] [PubMed] [Google Scholar]
- 20.Fellgiebel A, Scheurich A, Bartenstein P, Muller MJ. FDG–PET and CSF phospho–tau for prediction of cognitive decline in mild cognitive impairment. Psychiatry Res. 2007;155(2):167–171. doi: 10.1016/j.pscychresns.2006.12.002. Medline:17531450. [DOI] [PubMed] [Google Scholar]
- 21.Okamura N, Arai H, Higuchi M et al. Cerebrospinal fluid levels of amyloid β–peptide1–42, but not tau have positive correlation with brain glucose metabolism in humans. Neurosci Lett. 1999;273(3):203–207. doi: 10.1016/s0304-3940(99)00644-8. Medline:10515194. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi L, De Santi S, Brys M et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. Medline:17720148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galasko D, Chang L, Motter R et al. High cerebrospinal fluid tau and low amyloid β42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55(7):937–945. doi: 10.1001/archneur.55.7.937. Medline:9678311. [DOI] [PubMed] [Google Scholar]
- 24.Kanai M, Shizuka M, Urakami K et al. Apolipoprotein E4 accelerates dementia and increases cerebrospinal fluid tau levels in Alzheimer’s disease. Neurosci Lett. 1999;267(1):65–68. doi: 10.1016/s0304-3940(99)00323-7. Medline:10400250. [DOI] [PubMed] [Google Scholar]
- 25.Sunderland T, Mirza N, Putnam KT et al. Cerebrospinal fluid β–amyloid 1–42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE ε4 allele. Biol Psychiatry. 2004;56(9):670–676. doi: 10.1016/j.biopsych.2004.07.021. Medline:15522251. [DOI] [PubMed] [Google Scholar]
- 26.Buerger K, Teipel SJ, Zinkowski R et al. Increased levels of CSF phosphorylated tau in apolipoprotein E ε4 carriers with mild cognitive impairment. Neurosci Lett. 2005;391(1–2):48–50. doi: 10.1016/j.neulet.2005.08.030. Medline:16165272. [DOI] [PubMed] [Google Scholar]
- 27.Herukka SK, Helisalmi S, Hallikainen M, Tervo S, Soininen H, Pirttila T. CSF Aβ42, Tau and phosphorylated Tau, APOE ε4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28(4):507–514. doi: 10.1016/j.neurobiolaging.2006.02.001. Medline:16546302. [DOI] [PubMed] [Google Scholar]
- 28.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54(3):197–201. doi: 10.1016/j.neures.2005.11.009. Medline:16406150. [DOI] [PubMed] [Google Scholar]
- 29.Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol. 1985;232(2):219–228. doi: 10.1002/cne.902320207. Medline:3973091. [DOI] [PubMed] [Google Scholar]
- 30.Leh SE, Chakravarty MM, Ptito A. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. Int J Biomed Imaging [serial online] 2008 January 02;:1–5. doi: 10.1155/2008/789539. Medline:18274667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125(pt 2):350–360. doi: 10.1093/brain/awf032. Medline:11844735. [DOI] [PubMed] [Google Scholar]
- 32.LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10(2):613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. Medline:2303863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuljis RO. Lesions in the pulvinar in patients with Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53(2):202–211. doi: 10.1097/00005072-199403000-00012. Medline:8120542. [DOI] [PubMed] [Google Scholar]
- 34.Fagan AM, Mintun MA, Mach RH et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. Medline:16372280. [DOI] [PubMed] [Google Scholar]
- 35.Klunk WE, Engler H, Nordberg A et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. Medline:14991808. [DOI] [PubMed] [Google Scholar]
- 36.Buerger K, Ewers M, Pirttila T et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(pt 11):3035–3041. doi: 10.1093/brain/awl269. Medline:17012293. [DOI] [PubMed] [Google Scholar]
- 37.Engelborghs S, Sleegers1 K, Cras P et al. No association of CSF biomarkers with APOEε4, plaque and tangle burden in definite Alzheimer’s disease. Brain. 2007;130(pt 9):2320–2326. doi: 10.1093/brain/awm136. Medline:17586559. [DOI] [PubMed] [Google Scholar]