Abstract
Objective
PRO 2000 is a polyanionic microbicide that binds directly to the gp120 envelope protein to inhibit HIV-1 entry. We studied the breadth of PRO 2000 activity against HIV-1 derived from recently transmitted R5 viruses. We also investigated the interaction of this compound with X4 and R5 HIV-1 envelope glycoproteins using an epitope mapping strategy.
Methods
The anti-HIV activity of PRO 2000 against subtype B and C Env-pseudotyped viruses was assessed in saline and cervicovaginal lavage fluid. Competitive binding assays were performed with X4 and R5 monomeric and virus-associated gp120.
Results
PRO 2000 was found to be active against recently transmitted subtype B and C viruses tested in vitro, however, at 1 µg/ml in saline, activity against subtype C was decreased compared to subtype B. Epitope mapping using anti-V3 region antibodies showed that PRO 2000 binds to the V3 region of monomeric and virus-associated X4 gp120 with a higher affinity than to V3 of R5 gp120. In contrast, the interaction of PRO 2000 with the CD4 binding site was similar for both X4 and R5 monomeric and virus-associated gp120.
Conclusions
PRO 2000 has significant activity against recently transmitted viruses, although some activity is lost at low concentrations. Epitope binding studies suggest that this broad activity is due to direct and indirect interactions with multiple gp120 sites rather than V3 binding alone.
Keywords: microbicide, PRO 2000, recently transmitted viruses, HIV-1, cervicovaginal lavage
Introduction
The genetic diversity of the HIV-1 envelope is a major obstacle to the development of anti-HIV vaccines and microbicides. A high polymerase mutation rate, in vivo viral turn-over, and genetic recombination contribute to the extreme envelope diversity within and among viral subtypes. Worldwide, subtypes C and A predominate (50% and 12%, respectively), while in North America, subtype B is most common but consists of only 10% of total transmitted viruses.1 To add to this complexity, major recombinant forms generated by recombination between subtypes also circulate.2
Recent studies monitoring HIV-discordant couples in Africa demonstrate that envelope sequences from recently transmitted subtype A and C viruses exhibit shorter V1–V4 length and fewer glycosylation sites, compared to envelope sequences collected from chronically infected patients.3–5 The homogenous nature of these early HIV-1 variants suggests that recipient infection emanates from a single quasispecies from the chronically infected partner in the majority of cases.6–8 While it is unknown whether the early population represents a selective bottleneck at transmission or amplification, candidate vaginal microbicides and vaccines should target those viruses that are preferentially transmitted.
Several classes of microbicides to prevent the heterosexual transmission of HIV-1 are under investigation.9 Three of the microbicides evaluated in clinical efficacy trials [PRO 2000 (Indevus Pharmaceuticals, Lexington, MA, USA), cellulose sulfate (Ushercell, Polydex Pharmaceuticals, Toronto, ON, Canada and Topical Prevention of Conception and Disease, Chicago IL) and Carraguard (Carraguard/R515, Population Council, New York, NY, USA)], are negatively charged polyanions that interact with the positively charged HIV-1 gp120. There is concern that these compounds bind with greater affinity to the more positively charged V3 loop of CXCR4-tropic (X4) viruses than the V3 loop of CCR5-tropic (R5) viruses.10, 11 In fact, the polyanion dextran sulfate, which did not proceed to efficacy trials, was found to have a higher affinity to X4 gp120 compared to R5 gp120 in binding assays.12
There is renewed concern regarding the activity of polyanions after efficacy trials recently demonstrated that Carraguard and cellulose sulfate did not prevent HIV transmission.13–16 PRO 2000, the remaining polyanionic microbicide in phase 3 trials, has in vitro activity against viruses obtained from individuals chronically infected with subtypes B, A, C, and A/E.17 Our laboratory previously demonstrated that PRO 2000 is active against Env-pseudotyped B viruses in vitro and in cervicovaginal lavage fluid (CVL) and binds to X4 and R5 monomeric gp120 with high binding affinities based on surface plasmon resonance analyses.18, 19
Little is known regarding the extent to which electrostatic interactions of polyanions are influenced by the unique envelope properties associated with recently transmitted viruses. The primary goal of this study was to assess the activity of PRO 2000 against recently transmitted HIV-1 envelopes in the presence or absence of CVL. Secondly, we determined whether functional differences in activity correlated with PRO 2000 binding to specific sites on HIV-1 gp120 using an epitope mapping strategy.
Methods
All envelope-expressing constructs were obtained from the NIH AIDS Research and Reference Reagent Program. Single-cycle HIV-1 viruses were generated using envelopes derived from recent sexually transmitted subtype B and C R5 isolates. Env-pseudotyped single-cycle HIV-1 virus was generated by co-transfecting a single-cycle HIV-1 reporter construct pNL4-3 luc R-E, pCMV delta R8.2 packaging construct and envelope-expressing constructs from recent B or C viruses.3, 20–22 The human astrocytoma cell lines, U87-CD4-CCR5 engineered to express CD4 and CCR5, was used for infection with HIV-1 pseudotyped single-cycle viruses. CVL fluid samples from women with low identifiable risk factors for HIV were obtained as previously described.19
U87-CD4-CCR5 cells were plated in triplicate in a 48 well plate and incubated with PRO 2000 (1, 10 & 100 µg/ml) or DMEM alone for 1 hour followed by infection with HIV-1 Env-pseudotyped viruses for 2 hours. The cells were washed once and cultured in DMEM 10% FCS for 48 hours. CVL diluted 1:2 in serum free media was mixed with PRO 2000 (1,10 & 100 µg/ml) and incubated with U87-CD4-CCR5 cells for 1 hour and HIV-1 pseudotyped B and C viruses were added to the cells for 2 hours. Our previous experiments with herpes simplex virus have shown that there is no difference in the activity of PRO 2000 when preincubated with cells or viruses.23 At 48 hours, U87-CD4-CCR5 cells were lysed in luciferase lysis buffer and cell lysate luciferase was measured in relative light units (RLU) using a luciferase assay system (Promega, Madison, Wisconsin). Background luciferase readings were subtracted from the measured readings for the final results.
To probe the sites of PRO 2000 binding on soluble gp120 monomers, recombinant gp120LAI (Progenics) or gp120JRFL (a gift from the Vaccine Research and Development Branch, Division of AIDS, NIAID; manufactured by Quality Biological) was pre-treated with PRO 2000, captured on ELISA plates coated with sheep antibodies to the C terminus of gp120, and reacted with mAbs specific for V3 (447-52D), the CD4-binding site (b12), or the N-terminus (EH21) of gp120. The background binding was assessed using an irrelevant anti-gp41 mAb. The virus capture assay was performed as previously described with minor modifications.24 Briefly, aldrethiol-2-inactivated pseudovirions bearing HXB2 (gradient-purified) or JRFL (not gradient-purified) envelopes were incubated with PRO 2000 and then added to ELISA plates already coated with capturing mAbs specific for the V3 loop (447-52D), the CD4-binding site (b12), or the C-terminus (670) of gp120. After overnight incubation, the wells were washed and lysed. The p24 concentration in the lysates was measured in a non-commercial ELISA indicating virus binding in the presence of the coating mAbs.
The percent of inhibition by PRO 2000 was calculated relative to the untreated control viruses as previously described.25 ID50 (50% inhibitory dose) and t-tests were calculated using Microsoft Excel. The net V3 loop charge was determined by subtracting negatively charged amino acids from positively charged amino acids.
Results
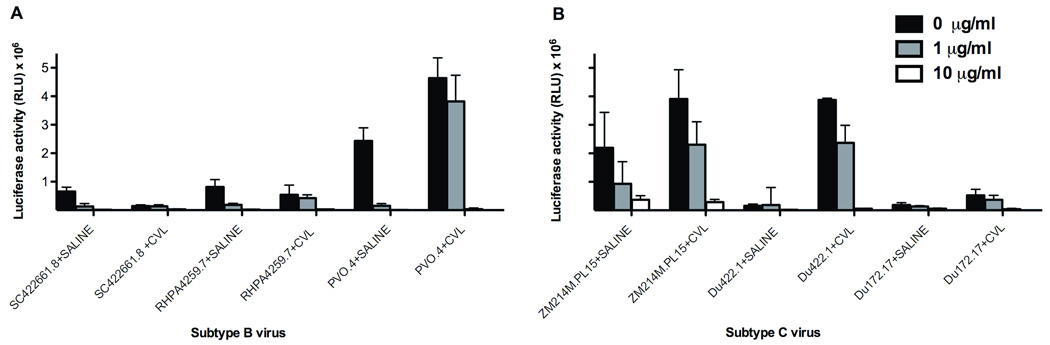
Figure 1 shows the activity of PRO 2000 in saline and CVL against recently transmitted subtype B and C viruses. The highest concentration of 100 µg/ml of PRO 2000 completely inhibited infection of U87-CD4-CCR5 cells mediated by envelopes derived from recently transmitted B and C viruses (data not shown). At 10 µg/ml of PRO 2000, subtype B viruses were inhibited by an average of 98% and subtype C viruses by 81% (p=0.15). In contrast, a concentration of 1 µg/ml of PRO 2000 inhibited subtype B viruses by 83% and subtype C viruses by only 41% (p=0.01).
Fig. 1. Activity of PRO 2000 in saline and in the presence of cervicovaginal lavage fluid (CVL).
Saline or CVL from healthy donors was mixed with 1, 10, or 100 µg/ml of PRO 2000 for 5 min then incubated with U87-CD4-CCR5 cells for 1 h at 37°C, followed by 2 h infection with a HIV-1 pseudotyped subtype B or subtype C from recently infected patients. The cells were washed with DMEM and cultured in fresh medium for 48 h. Single-cycle HIV-1 infection was determined by lysing cells to measure luciferase production. The concentration of 100 µg/ml of PRO 2000 inhibited infection of U87-CD4-CCR5 cells mediated by envelopes derived from recently transmitted B and C viruses in saline and CVL. Data represent the mean +/− SD for virus tested. Each condition was tested in triplicate. RLU, relative light units.
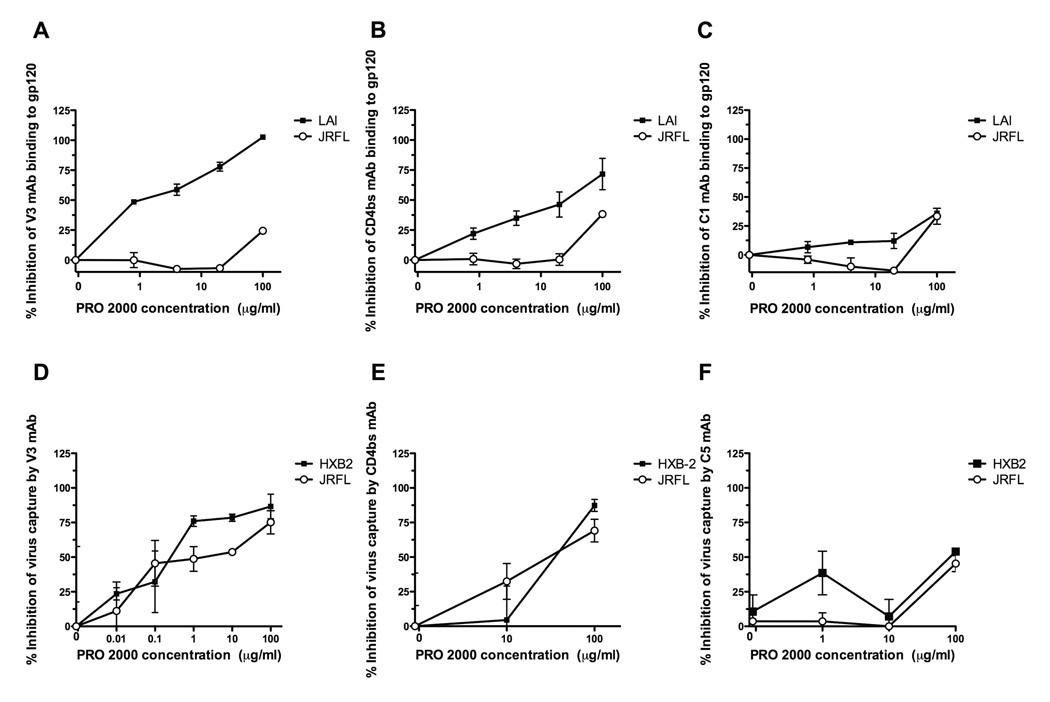
In the presence of CVL, PRO 2000 markedly inhibited subtype B and C viruses to the same extent at concentrations of 10 µg/ml and 100 µg/ml (data not shown), but the inhibitory activity decreased at 1 µg/ml against some of the strains. Specifically, at 1 µg/ml, subtype B virus was inhibited by an average of 15% (as compared to 83% inhibition in saline, p=0.01) and subtype C was inhibited by an average of 36% (as compared to 41% inhibition in saline, p=0.51). In Figure 2, the blocking sandwich ELISA shows that PRO 2000 inhibited the binding of mAb to the V3 loop of X4-tropic gp120LAI in a dose-dependent manner with an ID50 of 1.0 µg/ml (Figure 2A). PRO 2000 also blocked the binding of anti-CD4-binding site mAb to gp120LAI, with an ID50 of 16.8 µg/ml (Figure 2B). However, PRO 2000 had little effect on the binding of anti-V3 mAb or CD4-binding site mAb to the R5-tropic gp120JRFL (ID50 of >100 µg/ml). The compound had little or no effect on the binding of mAb to the C1 region in the N-terminus of gp120 from X4-tropic or R5-tropic strains (Figure 2C). Hence, PRO 2000 specifically inhibited the binding of mAbs to the V3 loop and the CD4-binding sites of X4-tropic gp120LAI.
Fig. 2. PRO 2000 competitively inhibits gp120 mAb binding to X4 and R5 viruses.
In Figure 2A–2C, PRO 2000 inhibited the binding of mAbs to the V3 loop and the CD4-binding site, but had little effect on the mAb binding to the N-terminus of gp120. The capacity of PRO 2000 to inhibit mAb binding to soluble gp120 was tested in a blocking sandwich ELISA. Soluble gp120LAI or gp120JRFL (1 µg/ml) was treated with the designated concentrations of PRO 2000 and captured onto ELISA plate with sheep anti-C terminus of gp120. The binding of mAbs to the V3 (2A), CD4-binding site (2B), and N-terminal C1 region (2C) of PRO 2000-treated gp120 was detected by alkaline phosphatase-conjugated secondary anti-human IgG antibody. MAb binding to gp120 in the absence of PRO 2000 was considered to be 100%; the OD405 values ranged from 1.0 to 2.9, depending on the combinations of gp120 and mAbs tested. Background levels (0% binding) were determined from wells reacted with an irrelevant mAb control. Data from one of two experiments are shown. In Figure 2D and 2E, PRO 2000 inhibited the binding of mAbs to V3 and the CD4-binding site of gp120 expressed on HIV-1 virions. Virus capture assay was performed to assess the inhibitory effect of PRO 2000 on mAb binding to intact HIV pseudovirions. Pseudovirions bearing envelope of HXB2 (50 ng p24/ml) or JRFL (100 ng p24/ml) isolates were treated with varying concentrations of PRO 2000 and captured by V3 mAb 447-52D (2D), CD4-binding site mAb b12 (2E), or mAb to the C-terminal C5 region (2F) coated on ELISA plates. The amount of p24 associated with the captured virions was determined by non-commercial p24 ELISA. The quantity of virions captured in the absence of PRO 2000 was considered as 100% (typically 40–100 pg p24/ml for HXB2 and 100–200 pg p24/ml for JRFL). Background levels were obtained from wells containing no p24 or virions or from wells in which virions were captured with an irrelevant mAb. The data shown are from one of 3 or 4 repeated experiments.
We next examined whether PRO 2000 binds to the same sites on native HIV-1 envelope expressed on intact virions. MAb-mediated virus-capture assays were performed using pseudovirions bearing X4-tropic (HXB2) or R5-tropic (JRFL) envelopes that were pre-treated with titrated doses of PRO 2000. PRO 2000 potently inhibited the capture of HXB2 virions by anti-V3 mAb 447-52D (Figure 2D), with an ID50 of 0.4 µg/ml. In contrast to the results observed with soluble gp120JRFL monomers, PRO 2000 blocked V3 mAb-mediated capture of R5-tropic JRFL, although the blocking activity was weaker compared to the X4 results as indicated by a higher ID50 of 15 µg/ml. When the CD4-binding site mAb b12 was used to capture the virions, PRO 2000 had similar inhibitory effects on both X4-tropic HXB2 and R5-tropic JRFL, the ID50 for the two viruses were 59 and 61 µg/ml, respectively (Figure 2E). The compound had little or no effect on the binding of mAb to the C5 region in the C-terminus of the gp120 from X4-tropic or R5-tropic strains (Figure 2F). These data indicate that PRO 2000 binds the V3 loop and inhibits the CD4-binding site of gp120 from X4-tropic and R5-tropic viruses when presented in the context of whole virion.
Discussion
The purpose of this study was to assess the activity and mechanism of action of PRO 2000 against clinically relevant HIV-1 envelopes. This is the first study demonstrating activity of PRO 2000 against recently transmitted viruses, an issue critical to assessing candidate microbicides. In the presence of CVL, PRO 2000 at a concentration of 10 µg/ml inhibited subtype B and C infection by >90%, but at 1 µg/ml, the activity was decreased. Recent in vitro data demonstrate that two other polyanionic microbicides, cellulose sulfate and Carraguard, which failed to prevent HIV transmission in clinical trials, may actually enhance HIV infection at low concentrations (between 1–10 µg/ml).25, 26
If the effective working concentration of PRO 2000 is narrow, modest decreases in activity in CVL could have important clinical implications. Recent studies highlight the need for much higher inhibitory concentrations in vivo than in vitro. In the cervical explant model, while 10 µg/ml of PRO 2000 inhibited HIV (Bal) infection by 70%, 1 µg/ml inhibited infection by only 25%.27 In macaques, in vivo protection requires the use of inhibitor concentrations that are several orders of magnitude greater than active in vitro concentrations.28 The effective concentration of a microbicide also depends on the actual concentration in the genital mucosa. A bioavailability study found that while the concentration of PRO 2000 0.5% in women’s cervicovaginal secretions was at the target concentration of 25 µg/ml 2 hours after the first dose, five of 12 women did not achieve this concentration after six daily doses.29 Thus, small shifts in activity in vitro combined with dilutional effects and pharmokinetics in the normal genital tract may have significant clinical implications. While our data show that PRO 2000, at low concentrations in saline, has less activity against subtype C viruses compared to subtype B viruses, it is difficult to predict the clinical implications of these differences because the in vivo inhibitory range of PRO 2000 has not been determined in human or non-human primate models. An ongoing phase 3 trial of PRO 2000 may define the clinical implications of these in vitro differences.
To further understand the mechanism of PRO 2000 activity against R5 and X4 viruses, we developed a binding assay to measure PRO 2000 binding to the V3 and CD4-binding regions of monomeric and native envelope of R5 and X4 viruses. As expected, there was a preferential affinity of PRO 2000 to the V3 loop of monomeric and virion-associated X4 gp120 compared to R5 gp120. The increased binding activity most likely reflects the interaction of PRO 2000 with the more positively charged V3 loop on X4 viruses, compared to R5 viruses. This differential activity was more striking against the monomers compared to the virion-associated envelopes suggesting that structural differences of the intact virion alter the exposure of cationic sites. In contrast to the differential effect on the V3 loop, PRO 2000 had equivalent activity against the CD4-binding site in the context of R5 and X4 on whole viruses, which confirms published data that PRO 2000 interferes with HIV-1 binding to CD4.30 However, PRO 2000 inhibition of CD4-binding site mAb b12 binding was much more pronounced on the intact virion than on the monomeric gp120. It is possible that PRO 2000 may not directly bind to the CD4-binding site; instead, the binding to the V3 loop could sterically interfere with mAb access of the CD4-binding site on the native envelope trimers. Previous studies have demonstrated that the V1/V2 domain can mask the V3 loop on the adjacent gp120 subunit of the envelope trimers and prevent anti-V3-mediated HIV-1 neutralization.31 More recent data on the structure of the envelope trimer further illustrates the close intra-molecular proximity of the V3 loop to the CD4-binding site.32 Given this proximal relationship, when PRO 2000 binds the V3 loop, it may sterically inhibit mAb binding to the CD4-binding site on the adjacent gp120 subunit of the native envelope trimers. Thus, the broad in vitro activity of PRO 2000 against R5 viruses may be partially explained by indirect interactions with the envelope regions outside of the V3 loop.
This study detected differential binding of PRO 2000 to X4 and R5 viruses, and differences in activity against recently transmitted subtype B and C viruses at low concentrations. Given the extraordinary genetic diversity of HIV-1, it is essential that candidate microbicides demonstrate broad spectrum activity against the most clinically relevant isolates prior to progressing to clinical trials.
Acknowledgments
PRO 2000 was obtained from Indevus Pharmaceuticals. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: the recently transmitted envelopes (SC422661 clone 8, PVO clone 4, pRHPA4259 clone 7, Du172 clone 17, Du422 clone 1 and ZM214 M. PL15), pCMV delta R 8.2 and pNL4-3 luc R-E. MAbs 447-52D, 847D, and 670 were provided by Dr. Susan Zolla-Pazner, b12 was obtained from Dr. Dennis Burton via the NIH AIDS Reagent Repository, and EH21 was provided by Dr. James Robinson. Cervicovaginal lavage fluid (CVL) was obtained in conjunction with Dr. Marla J. Keller (Albert Einstein College of Medicine, Bronx, NY).
Sponsorship: The work was funded by NIH U19 HD48733 (Mary E. Klotman) and the Doris Duke Charitable Foundation (Darpun D. Sachdev).
Funding disclosure
The work was funded by NIH U19 HD48733 (Mary E. Klotman) and the Doris Duke Charitable Foundation (Darpun D. Sachdev).
Footnotes
Disclosure of prior presentations
Data partially presented previously at IDSA 2007, October 4–7, 2007 in San Diego, CA and published as “Zerhouni-Layachi B, Dhawan D, Ortigoza M, Profy A, Tuen M, Hioe C, Klotman ME. The Candidate Microbicide PRO 2000 has Differential Binding to X4 and R5 gp120 but Broad Antiviral Activity Against Diverse HIV-1 Subtypes. Abstract 881, 2007.”
Data partially presented previously at the 14th Conference on Retroviruses and Opportunistic Infections (CROI 2007), February 25–28, 2007 in Los Angeles, CA and published as “Dhawan D, Zerhouni-Layachi B, Hioe C, Klotman ME. Epitope Mapping of the Candidate Microbicide PRO 2000 against HIV gp120. Abstract 997, 2007.”
Data partially presented previously at XVI International AIDS Conference, August 13–18, 2006 in Toronto, Canada and published as “Dhawan D, Zerhouni-Layachi B, Klotman ME. The candidate polyanionic microbicide, PRO 2000, inhibits entry mediated by diverse HIV-1 envelopes. XVI International AIDS Conference, Toronto, ThPE0019, 2006.”
References
- 1.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. Aids. 2006 Oct 24;20(16):W13–W23. doi: 10.1097/01.aids.0000247564.73009.bc. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008 Apr 10;358(15):1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Salazar-Gonzalez JF, Derdeyn CA, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006 Dec;80(23):11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004 Mar 26;303(5666):2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 5.Overbaugh J, Kreiss J, Poss M, et al. Studies of human immunodeficiency virus type 1 mucosal viral shedding and transmission in Kenya. J Infect Dis. 1999 May;179 Suppl 3:S401–S404. doi: 10.1086/314792. [DOI] [PubMed] [Google Scholar]
- 6.Chohan B, Lang D, Sagar M, et al. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1–V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol. 2005 May;79(10):6528–6531. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blish CA, Nedellec R, Mandaliya K, Mosier DE, Overbaugh J. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. Aids. 2007 Mar 30;21(6):693–702. doi: 10.1097/QAD.0b013e32805e8727. [DOI] [PubMed] [Google Scholar]
- 8.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan D, Keller M, Klotman ME. Topical microbicides: the time has come. AIDS Read. 2006 Mar;16(3):144–148. 155-148, 161; discussion 148, 156-147. [PubMed] [Google Scholar]
- 10.Schols D, Pauwels R, Desmyter J, De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology. 1990 Apr;175(2):556–561. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- 11.Callahan LN, Phelan M, Mallinson M, Norcross MA. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J Virol. 1991 Mar;65(3):1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulard M, Lortat-Jacob H, Mondor I, et al. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol. 2000 Feb;74(4):1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Dec 6;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 14.van de Wijgert JH, Shattock RJ. Vaginal microbicides: moving ahead after an unexpected setback. Aids. 2007 Nov 30;21(18):2369–2376. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 15.Ramjee G. Microbicides in HIV prevention. Future HIV Therapy. 2007 Jul;1:161–170. [Google Scholar]
- 16.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008 Jul 31;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 17.Dezzutti CS, James VN, Ramos A, et al. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob Agents Chemother. 2004 Oct;48(10):3834–3844. doi: 10.1128/AAC.48.10.3834-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scordi-Bello IA, Mosoian A, He C, et al. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob Agents Chemother. 2005 Sep;49(9):3607–3615. doi: 10.1128/AAC.49.9.3607-3615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller MJ, Zerhouni-Layachi B, Cheshenko N, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006 Jan 1;193(1):27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005 Aug;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkus ME, Limbach K, Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247–266. doi: 10.1016/0042-6822(90)90294-2. 517-263. [DOI] [PubMed] [Google Scholar]
- 23.Cheshenko N, Keller MJ, MasCasullo V, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004 Jun;48(6):2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorny MK, Williams C, Volsky B, et al. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J Virol. 2006 Jul;80(14):6865–6872. doi: 10.1128/JVI.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turville SG, Aravantinou M, Miller T, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS ONE. 2008;3(9):e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao W, Richards C, Hamer D. Enhancement of HIV infection by cellulose sulfate. AIDS Res Hum Retroviruses. 2008 Jul;24(7):925–929. doi: 10.1089/aid.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher PS, Wallace GS, Mesquita PM, Shattock RJ. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology. 2006;3:46. doi: 10.1186/1742-4690-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005 Nov 3;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 29.Lacey CJ, Wright A, Weber JN, Profy AT. Direct measurement of in-vivo vaginal microbicide levels of PRO 2000 achieved in a human safety study. Aids. 2006 Apr 24;20(7):1027–1030. doi: 10.1097/01.aids.0000222075.83490.ca. [DOI] [PubMed] [Google Scholar]
- 30.Rusconi S, Moonis M, Merrill DP, et al. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob Agents Chemother. 1996 Jan;40(1):234–236. doi: 10.1128/aac.40.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol. 2006 Jul;80(14):7127–7135. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008 Sep 4;455(7209):109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]