Abstract
Background and Aims
Differences in acquired mutations in colon and rectal tumors may account for differences in risk factors. In this study, we examined similarities and differences in somatic alterations in colon and rectal tumors.
Methods
Cases were identified from two large population-based case-control studies of colon cancer and rectal cancer. We sequenced exons 5 to 8 of the p53 gene and codons 12 and 13 of the Ki-ras gene to identify tumor mutations. Micro-satellite instability was determined based on BAT26 and TGFβRII analysis; CpG Island Methylator Phenotype was determined based on having two or more of the following markers methylated p16, MLH1, MINT1, MINT2, and MINT31.
Results
p53 mutations were observed in 39.7 percent of proximal, 51.0 percent of distal, and 46.6 percent of rectal tumors; Ki-ras mutations were observed in 36.0 percent of proximal, 26.9 percent of distal and 30.5 percent of rectal tumors. While 40.9 percent of proximal tumors were considered CpG Island Methylator Phenotype positive (having 2 or more of 5 markers methylated), only 12.9 percent of distal and 11.9 percent of rectal tumors were CpG Island Methylator Phenotype positive. Likewise, microsatellite instability was observed in 23.7 percent of proximal and only 3.8 percent of distal and 2.0 percent of rectal tumors. Over 50 percent of distal colon or rectal tumors had only one acquired mutation, while only 35.1 percent of proximal tumors had one mutation. The most common single mutation for colon and rectal tumors was p53 followed by Ki-ras mutations.
Conclusions
Our findings suggest unique mutational pathways are involved in the development of most colorectal tumors. Proximal colon cancers are more likely to have microsatellite instability, CpG Island Methylator Phenotype, and Ki-ras mutations, while rectal and distal colon tumors are more likely to have a p53 mutation than proximal colon tumors. Overall, rectal and distal colon tumors share similar mutational frequencies than do proximal colon tumors.
Keywords: Colon cancer, Rectal cancer, p53, Ki-ras, CpG Island Methylator Phenotype, microsatellite instability, Distal, Proximal
Introduction
The belief that colorectal cancer is a heterogeneous disease stems from several avenues of observation. Numerous studies have shown that risk factors associated with colon and rectal cancers differ by tumor site 1-6. For instance, although physical activity has been consistently associated with reduced risk of colon cancer, associations are not consistent for rectal cancer 4, 7, 8; obesity appears to influence risk of colon but not rectal cancer 5, 9. Dietary associations influence risk differently based on tumor site 10, 11. Furthermore, men are more likely to be diagnosed with rectal tumors than women. People diagnosed with rectal cancer are slightly younger than those diagnosed with colon cancer despite the fact that reported family history of colorectal cancer is less common among people diagnosed with rectal cancer than with colon cancer 1. Familial syndromes such as HNPCC, APC, and attenuated APC cluster in the proximal area of the colon rather than the distal colon 12-14. These syndromes are rare and combined estimates of their occurrence range from one to five percent of cases 15. Evaluation of colon tumor biomarkers shows that microsatellite instability and CpG island methylator phenotype (CIMP) are predominately characteristics of tumors that are more proximally located 16, 17. Additionally, it has been suggested that distal and rectal tumors are similar in occurrence of p53 mutations 18.
Colon tumor mutations also have been associated with specific diet and lifestyle factors, studies of rectal cancer specifically are generally lacking. Studies have documented unique associations between smoking cigarettes and MSI and CIMP in colon tumors 19, 20, dietary intake has been associated with specific Ki-ras mutations and p53 mutations 21-23, and alcohol use has been associated with MSI tumors 24. Given the associations between diet and lifestyle factors and colon tumors, it is possible that differences in prevalence of tumor mutations observed for colon and rectal cancers contribute to differences in observed risk factors for these two disease sites.
The purpose of this report is to describe the tumor characteristics from two population-based collections of colon and rectal tumors that include information on p53, Ki-ras, MSI, CIMP, and BRAF mutations. We provide details of specific tumor marker distribution based on tumor site and gender. While previous studies have shown differences in some of these mutations by site, this large population-based study adds to and confirms other reports.
Methods
Comparison data come from participants identified for a case-control study of colon cancer or from a case-control study of rectal cancer conducted in the Kaiser Permanente Medical Care Program of Northern California (KPMCP) and the state of Utah. All eligible cases within these defined areas were identified and recruited for the study which involved a detailed in-person interview and a blood draw. Case eligibility was determined by the Surveillance Epidemiology and End Results (SEER) Cancer Registries in Northern California and in Utah. To be eligible for these studies, participants had to be between 30 and 79 years of age at time of diagnosis, English speaking, mentally competent to complete the interview, could not have had previous colorectal cancer 25, and could not have known (as indicated on the pathology report) familial adenomatous polyposis, ulcerative colitis, or Crohn's disease.
First primary colon cancer (ICD-O 2nd edition codes 18.0, 18.2 to 18.9) diagnosed between October 1, 1991 and September 30, 1994 in Northern California and Utah were eligible for the study; tumor blocks were obtained and genetic analyses completed between 1995 and 1999. Of 1917 colon cancer cases eligible for the study from Northern California and Utah, we extracted DNA on 1637 or 85 percent 26. Cases with a first primary tumor in the recto-sigmoid junction or rectum were identified between May 1997 and May 2001; tumor block ascertainment and genetic analyses were completed in 2007. Of the 1265 cases who consented to having their tissue released, we obtained DNA from 1022 cases (81 percent of cases). Of the 234 cases that we did not make DNA, 27 did not have a block available; we did not receive the block for 132, and we were unable to make DNA from the block received for 75.
Block retrieval involved obtaining biopsy prior to treatment as well as paraffin embedded tissue from the resection. In some instances, because of radiation prior to resection for rectal cases, tissue was limited from the resection and therefore, biopsy specimens were used for making tumor DNA. In Utah, blocks were requested for all cases except those who refused release of blocks. For those who were not interviewed and had not signed a medical record release, the Utah Cancer Registry retrieved the blocks and released them to the study without key identifiers of name, address, and complete date of birth (year and month of birth were released). At the KPMCP, samples were retrieved from persons who signed a consent form or who had died. Detailed methods for collection of tissue have been described 26.
Genetic Analysis
Genetic analyses were conducted on colon blocks between 1995 and 1999; similar analyses were performed on the rectal blocks analyzed between 2001 and 2007. Tumor DNA was obtained from paraffin-embedded tissue as described previously. Tumors were characterized by their genetic profile that include sequence data for exons 5 through 8, or the hotspots of the p53 gene; sequence data for Ki-ras codons 12 and 13; five CpG Island markers MINT1, MINT2, MINT31, p16, and MLH1; the V600E BRAF mutation, and microsatellite instability (MSI) as determined by BAT26 and TGFβRII 17, 21, 27, 28. Although this MSI assessment for this study preceded the Bethesda consensus panel, BAT26, a mononucleotide repeat is by itself a very good measure of generalized instability. BAT26 and TGFβRII show a high correlation with the Bethesda consensus panel 29. Using a hierarchical approach, we have shown that 95 percent of tumors can be classified as either stable or unstable using BAT26 alone. At this time there is no “consensus” as to the appropriate CpG island panel or method of detection to determine CIMP. However, we have used our panel to demonstrate significant relationships between CIMP and numerous clinicopathologic variables, including cigarette smoking and the BRAF V600E mutation, which were independent of microsatellite instability 17, 20 This work has helped to support the legitimacy of the CIMP concept 30. Germline DNA obtained from normal tissue in the paraffin blocks or from blood samples provided by study participants was used to verify that mutations were acquired rather than inherited.
Statistical analysis
The distribution of genetic alterations in p53, Ki-ras, BRAF V600E mutations; CIMP and MSI status were compared between colon and rectal and by proximal and distal colon site using a Mantel-Haenszel chi-squared test statistic. Proximal tumors were defined as tumors in the cecum through the transverse colon; distal tumors were those from the splenic flexure through the sigmoid colon; rectal cancer was defined as tumors located in the rectosigmoid junction or rectum. In addition to p53 or Ki-ras mutation status, tumors were also assessed as to specific types and locations of p53 or Ki-ras mutations. Data were analyzed by age (30 to 64 years and 65 to 79 years), and gender. Because associations did not differ by either age or gender, data are presented for the entire study population.
To help visualize the carcinogenic pathways for proximal, distal and rectal tumors, we used un-timed oncogenetic trees as described by Desper et al. in 1999 31. An oncogenetic tree is a rooted directed tree with edges pointing away from the root 31, 32. The root presents the state of tissue with none of the measured somatic alterations. Each of the other nodes is associated with a particular somatic alteration. According to the oncogenetic tree model, for an alteration to occur in a particular tumor, all of the alterations corresponding to nodes that lie on the directed path from the root to the corresponding node must also occur in the tumor. As a consequence, each tumor corresponds to a “subtree” of the oncogenetic tree containing all the alterations in the tumor. Each edge is labeled by the “transition probability” - the conditional probability that a tumor will acquire the alteration corresponding to the next node away from the root, given that the alteration corresponding to the node proximal to the root is already present. When two edges emanate from the same node, neither of the alterations on each edge is required before the other can occur. The method used to fit the oncogenetic tree is described in greater detail elsewhere 33, 34.
The oncogenetic trees, together with edge labels, were constructed using a simple algorithm. The parent of each node was found by maximizing the weight function function . If the true oncogenetic tree is not skewed, a concept first described in Desper et al. 31, the algorithm is guaranteed to reconstruct the correct tree. A nonparametric bootstrap re-sampling method was used to estimate the amount of sampling variability in the fitted tree. The bootstrap method samples from the rows of the data with replacement N times, where N is the size of the original sample 35. An oncogenetic tree is fit to the resampled data. The procedure is repeated M times. We repeated the procedure M = 1000 times.
Results
The distribution of tumor mutations for proximal colon, distal colon, and rectal tumors is shown in Table 1. Significant differences between sub-sites of proximal colon, distal colon, and rectal cancer were identified. CIMP+, MSI+, and BRAF mutated tumors occurred with a much greater frequency in proximal colon tumors than in either distal colon or rectal tumors. There were more similarities for prevalence of tumor mutations or epigenetic changes between tumors in distal colon and rectum than between tumors of the proximal and distal colon. Similar associations were observed when the data were examined by age at diagnosis and gender of the study participants (data not shown in table).
Table 1.
Comparison of somatic mutations between proximal and distal colon and rectal tumors
| Proximal | Distal | Rectal | |||||
|---|---|---|---|---|---|---|---|
| Mutation or Alteration Statusa | N | (percent) | N | (percent) | N | (percent) | p-valueb |
| Cases with p53 status | 725 | (100.0) | 698 | (100.0) | 954 | (100.0) | |
| No p53 mutation | 437 | (60.3) | 342 | (49.0) | 509 | (53.4) | |
| p53 mutation | 288 | (39.7) | 356 | (51.0) | 445 | (46.6) | 0.01 |
| Cases with Ki-ras status | 702 | (100.0) | 674 | (100.0) | 1008 | (100.0) | |
| No Ki-ras mutation | 449 | (64.0) | 493 | (73.1) | 701 | (69.5) | |
| Ki-ras mutation | 253 | (36.0) | 181 | (26.9) | 307 | (30.5) | 0.03 |
| Cases with CIMP status | 607 | (100.0) | 595 | (100.0) | 889 | (100.0) | |
| CIMP- | 359 | (59.1) | 518 | (87.1) | 783 | (88.1) | |
| CIMP+ | 248 | (40.9) | 77 | (12.9) | 106 | (11.9) | <0.0001 |
| Cases with MSI status | 733 | (100.0) | 713 | (100.0) | 1007 | (100.0) | |
| MSI- | 559 | (76.3) | 686 | (96.2) | 985 | (97.8) | |
| MSI+ | 174 | (23.7) | 27 | (3.8) | 22 | (2.2) | <0.0001 |
| Cases with BRAF status | 572 | (100.0) | 576 | (100.0) | 986 | (100.0) | |
| BRAF- | 485 | (84.8) | 558 | (96.9) | 959 | (97.3) | |
| BRAF+ | 87 | (15.2) | 18 | (3.1) | 27 | (2.7) | <0.0001 |
| Total cases (non-missing)c | 501 | (100.0) | 502 | (100.0) | 858 | (100.0) | |
| No alterationsd | 62 | (12.4) | 119 | (23.7) | 236 | (27.5) | |
| One alteration | 176 | (35.1) | 279 | (55.6) | 440 | (51.3) | |
| Two or more alterations | 263 | (52.5) | 104 | (20.7) | 182 | (21.2) | <0.0001 |
p53: positive for one or more p53 mutations in exons 5-8; Ki-ras: positive for one or more Ki-ras mutations in codons 12 and 13; MSI+: BAT26 unstable; otherwise, TGFβRII unstable if BAT26 is ambiguous or missing; CIMP+: 2 of 5 markers methylated; BRAF+: BRAF V600E mutation.
Mantel-Haenszel chi-squared test, compares proximal, distal and rectal tumor site.
Case subjects with status available for all alterations shown.
p53 and Ki-ras negative, MSI-, and CIMP- (0 or 1 markers methylated).
The specific types of p53 (Table 2) and Ki-ras (Table 3) mutations observed were similar for all three sites when compared among those with a p53 or Ki-ras mutation. Only when examining the p53 hot-spots did we observe a significant difference, where missense mutations in codon 175 resulting in an amino acid change from arginine (R) occurred more frequently in rectal tumors than in colon tumors. With respect to p53 hot-spots, proximal and distal colon tumors were more similar to each other than distal colon and rectal tumors (Table 2). Examination of Ki-ras mutations (Table 3) showed that rectal tumors were more likely to have mutations in codons 12 and 13 than either proximal or distal colon tumors.
Table 2.
Comparison of p53 mutations in somatic mutation with proximal and distal colon and rectal tumors
| Proximal | Distal | Rectal | |||||
|---|---|---|---|---|---|---|---|
| N | (percent) | N | (percent) | N | (percent) | p-valuea | |
| Cases with p53 statusb | 725 | (100.0) | 698 | (100.0) | 954 | (100.0) | |
| p53 negative | 437 | (60.3) | 342 | (49.0) | 509 | (53.4) | |
| p53 positive | 288 | (39.7) | 356 | (51.0) | 445 | (46.7) | 0.01 |
| Total p53 mutationsc | 303 | (100.0) | 378 | (100.0) | 472 | (100.0) | |
| Missense | 232 | (76.6) | 283 | (74.9) | 368 | (78.0) | |
| Stop codon | 24 | (7.9) | 41 | (10.9) | 51 | (10.8) | |
| Frameshift | 32 | (10.6) | 29 | (7.7) | 33 | (7.0) | |
| In frame ins./del. | 8 | (2.6) | 12 | (3.2) | 10 | (2.1) | |
| Splice site | 7 | (2.3) | 13 | (3.4) | 10 | (2.1) | 0.48 |
| Transitions | 214 | (70.6) | 279 | (73.8) | 339 | (71.8) | |
| Transversions | 48 | (15.8) | 57 | (15.1) | 88 | (18.7) | |
| Inactivating | 41 | (13.5) | 42 | (11.1) | 45 | (9.5) | 0.07 |
| Non-CpG Island | 158 | (52.2) | 192 | (50.8) | 247 | (52.3) | |
| CpG Island | 145 | (47.9) | 186 | (49.2) | 225 | (47.7) | 0.91 |
| Non-hot spot | 192 | (63.4) | 224 | (59.3) | 314 | (66.5) | |
| R175 | 19 | (6.3) | 28 | (7.4) | 43 | (9.1) | |
| G245 | 8 | (2.6) | 16 | (4.2) | 21 | (4.5) | |
| R248 | 30 | (9.9) | 44 | (11.6) | 45 | (9.5) | |
| R273 | 36 | (11.9) | 51 | (13.5) | 35 | (7.4) | |
| R282 | 18 | (5.9) | 15 | (4.0) | 14 | (3.0) | 0.10 |
| p53 Location | |||||||
| Helix | 72 | (23.8) | 91 | (24.1) | 73 | (15.5) | |
| L3 loop | 60 | (19.8) | 84 | (22.2) | 113 | (23.9) | |
| L2 loop | 42 | (13.9) | 63 | (16.7) | 102 | (21.6) | |
| β sandwich | 47 | (15.5) | 39 | (10.3) | 65 | (13.8) | |
| Loop-sheet | 20 | (6.6) | 27 | (7.1) | 27 | (5.7) | |
| Other | 62 | (20.5) | 74 | (19.6) | 92 | (19.5) | 0.24 |
Mantel-Haenszel chi-squared test, compares differences for proximal and distal colon and rectal.
Negative (no mutations) or positive for one or more p53 mutations in exons 5-8.
Table 3.
Comparison of Ki-ras mutation between proximal and distal colon and rectal tumors
| Proximal | Distal | Rectal | |||||
|---|---|---|---|---|---|---|---|
| N | (percent) | N | (percent) | N | (percent) | p-valuea | |
| Cases with Ki-ras Statusb | 702 | (100.0) | 674 | (100.0) | 1008 | (100.0) | |
| Ki-ras negative | 449 | (64.0) | 493 | (73.2) | 701 | (69.5) | |
| Ki-ras positive | 253 | (36.0) | 181 | (26.9) | 307 | (30.5) | 0.03 |
| Total Ki-ras mutationsc | 255 | (100.0) | 183 | (100.0) | 307 | (100.0) | |
| Codon 12 | 203 | (79.6) | 137 | (74.9) | 241 | (78.5) | |
| Codon 13 | 52 | (20.4) | 46 | (25.1) | 66 | (21.5) | 0.79 |
| Transitions | 165 | (64.7) | 109 | (59.6) | 193 | (62.9) | |
| Transversions | 90 | (35.3) | 74 | (40.4) | 114 | (37.1) | 0.69 |
| Codon 12 G>A | 115 | (45.1) | 63 | (34.4) | 133 | (43.3) | |
| Codon 12 G>T | 76 | (29.8) | 64 | (35.0) | 84 | (27.4) | |
| Codon 13 G>A | 50 | (19.6) | 46 | (25.1) | 60 | (19.5) | |
| Non-hot spot | 14 | (5.5) | 10 | (5.5) | 30 | (9.8) | 0.30 |
Mantel-Haenszel chi-squared test, compares differences between, proximal, distal, and rectal tumors
Negative (no mutations) or positive for one or more Ki-ras mutations in codons 12 and 13.
Total of mutations by type may be greater than total Ki-ras+ cases because of individuals with multiple mutations.
Examination of the prevalence of CIMP, MSI, and BRAF alterations showed differences for CIMP overall as well as for specific CIMP markers, the number of CIMP markers methylated, and CIMP+ tumors with and without MSI and BRAF mutations (Table 4). Distal and rectal tumors were similar in all categories. CIMP+ proximal tumors contained a much higher proportion of MSI+ tumors (43.2 percent) than CIMP+ distal colon or rectal tumors (9.5 percent and 3.8 percent respectively).
Table 4.
Comparison of CIMP, MSI, and BRAF in proximal and distal colon and rectal cases
| Proximal | Distal | Rectal | |||||
|---|---|---|---|---|---|---|---|
| N | (percent) | N | (percent) | N | (percent) | p-valuea | |
| Cases with CIMP statusb | 607 | (100.0) | 595 | (100.0) | 889 | (100.0) | |
| CIMP- | 359 | (59.1) | 518 | (87.1) | 783 | (88.1) | |
| CIMP+ | 248 | (40.9) | 77 | (12.9) | 106 | (11.9) | <0.0001 |
| Cases with MLH1 status | 587 | (100.0) | 577 | (100.0) | 909 | (100.0) | |
| MLH1- | 485 | (82.6) | 560 | (97.1) | 899 | (98.9) | |
| MLH1 + | 102 | (17.4) | 17 | (3.0) | 10 | (1.1) | <0.0001 |
| Cases with p16 status | 618 | (100.0) | 606 | (100.0) | 946 | (100.0) | |
| p16- | 471 | (76.2) | 558 | (92.1) | 868 | (91.8) | |
| p16+ | 147 | (23.8) | 48 | (7.9) | 78 | (8.3) | <0.0001 |
| Cases with MINT1 status | 608 | (100.0) | 601 | (100.0) | 920 | (100.0) | |
| MINT1- | 442 | (72.7) | 561 | (93.3) | 884 | (96.1) | |
| MINT1 + | 166 | (27.3) | 40 | (6.7) | 36 | (3.9) | <0.0001 |
| Cases with MINT2 status | 613 | (100.0) | 602 | (100.0) | 906 | (100.0) | |
| MINT2- | 380 | (62.0) | 482 | (80.1) | 727 | (80.2) | |
| MINT2+ | 233 | (38.0) | 120 | (19.9) | 179 | (19.8) | <0.0001 |
| Cases with MINT31 status | 590 | (100.0) | 599 | (100.0) | 913 | (100.0) | |
| MINT31- | 319 | (54.1) | 489 | (81.6) | 740 | (81.1) | |
| MINT31+ | 271 | (45.9) | 110 | (18.4) | 173 | (19.0) | <0.0001 |
| Number of markers methylated | |||||||
| 0 | 243 | (40.0) | 383 | (64.4) | 594 | (66.8) | |
| 1 | 116 | (19.1) | 135 | (22.7) | 189 | (21.3) | |
| 2 | 87 | (14.3) | 48 | (8.1) | 67 | (7.5) | |
| 3 | 61 | (10.1) | 17 | (2.9) | 28 | (3.2) | |
| 4 | 62 | (10.2) | 10 | (1.7) | 10 | (1.1) | |
| 5 | 38 | (6.3) | 2 | (0.3) | 1 | (0.1) | <0.0001 |
| CIMP- cases with MSI status | 345 | (100.0) | 501 | (100.0) | 779 | (100.0) | |
| MSI- | 319 | (92.5) | 485 | (96.8) | 766 | (98.3) | |
| MSI+ | 26 | (7.5) | 16 | (3.2) | 13 | (1.7) | <0.0001 |
| CIMP+ cases with MSI status | 243 | (100.0) | 74 | (100.0) | 105 | (100.0) | |
| MSI- | 138 | (56.8) | 67 | (90.5) | 101 | (96.2) | |
| MSI+ | 105 | (43.2) | 7 | (9.5) | 4 | (3.8) | <0.0001 |
| CIMP- cases with BRAF status | 328 | (100.0) | 485 | (100.0) | 768 | (100.0) | |
| BRAF- | 319 | (97.3) | 482 | (99.4) | 760 | (99.0) | |
| BRAF+ | 9 | (2.7) | 3 | (0.6) | 8 | (1.0) | 0.06 |
| CIMP+ cases with BRAF status | 231 | (100.0) | 73 | (100.0) | 102 | (100.0) | |
| BRAF- | 155 | (67.1) | 58 | (79.5) | 84 | (82.4) | |
| BRAF+ | 76 | (32.9) | 15 | (20.5) | 18 | (17.7) | <0.01 |
Mantel-Haenszel chi-squared test, compares differences by proximal, distal, and rectal tumors.
CIMP-: 0 or 1 of 5 markers methylated; CIMP+: 2 or more of 5 markers methylated.
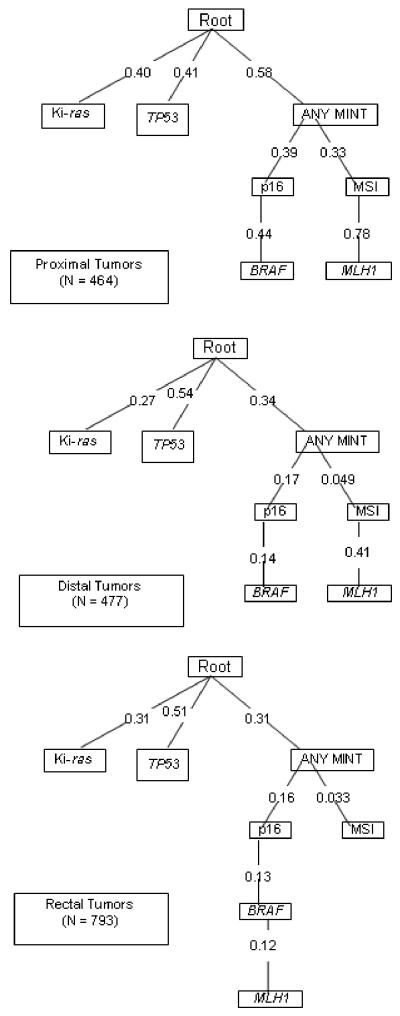
The oncogenetic trees describe the probability of developing various mutations given the underlying system or root and any previously acquired mutations. Trees for proximal, distal, and rectal tumors were created separately. As shown in Figure 1, the estimated proportion of tumors that will develop Ki-ras mutations increases as one goes from proximal colon to rectal tumors. The estimated proportion of tumors that will develop a p53 mutation is the same for rectal and distal colon tumors and almost twice that of proximal tumors. On the other hand, proximal tumors were more likely to have one or more MINT1, MINT2, or MINT31 (labeled any MINT) marker methylated with subsequent paths that go the MSI and MLH1 route and another that is more likely to move towards p16 and BRAF mutations. Rectal tumors were least likely to have a MSI positive pathway if any MINT markers were methylated; methylated MINT markers were more likely to subsequently acquire a p16, BRAF and MLH1 methylated marker. Bootstrap resampling of the oncogenetic trees consistently showed the same three major pathways for all trees generated. However, for proximal colon tumor, there was variation in the location of BRAF, where in some trees it followed the p16 methylation and in others it followed the MSI and MLH1 methylation path. Because of the rarity of BRAF mutations and MSI for both distal colon and rectal tumor sites, that arm of the tree was less stable following the ANY MINT pathway, where MSI and the methylation markers fell in different orders generating many unique trees.
Figure 1.
Oncogenetic Tree Analysis of probability of various tumor mutations in proximal, distal, and rectal tumors.
Discussion
Our study corroborates previous reports of differences in tumor mutations observed for proximal and distal colon and rectal cancers 18, 36, 37. Of note in our large population-based study, is the similarity in tumor marker distribution observed for rectal and distal colon tumors. While proximal tumors were more likely to have MSI or CIMP+, distal colon and rectal tumors were more likely to p53 and Ki-ras mutations.
Defining disease pathways that lead to colorectal cancer is an ongoing concern. While early work by Vogelstein 38 suggested a sequence of mutational events that lead to colon cancer, evidence is accumulating that several mutational pathways are involved in the carcinogenic process 39. Data presented here further support previous observations that multiple disease pathways of acquired DNA alterations are involved in developing colon and rectal tumors. Over 50 percent of distal and rectal tumors had a single somatic mutation and 34.6 percent of proximal tumors had a single mutation. Of those with only one mutation, the majority were p53 mutations followed by Ki-ras mutations. While common multiple mutations for proximal tumors included MSI and CIMP or BRAF, a subset of CIMP+ tumors with p53 and Ki-ras were observed, suggesting unique disease pathways. Studies have also shown inverse relationships between MSI and p53 and Ki-ras mutations 29 Previous studies have reported that some risk factors may be associated with specific mutations. Of interest are the observations that cigarette smoking and alcohol intake are associated more strongly with MSI tumors 19, 24 than either Ki-ras or p53- mutated colon tumors. Examination of specific exposures and rectal tumor mutations will further our understanding of risk factors as well as the carcinogenic process.
Oncogenetic tree analysis allowed us to visually display probabilities of unique mutational pathways. These trees, although statistically generated, are extremely close to the actual proportion of tumors with specific mutations. Resampling and bootstrap methods were used to validate the tree structure and the three main branches were reproduced in all trees generated, suggesting stability to the three main colorectal mutation pathways. The shapes of the trees; i.e., the relationships among the alterations, were similar for all three sites; in all three trees, Ki-ras and p53 mutations were not dependent on any other alteration, whereas the DNA methylation, MSI, and BRAF mutation consistently fell in the same pathway. It was the probabilities of the pathways that differed among the three site-specific trees. These oncogenetic trees reinforce the unique mutational pathways to colon and rectal cancer that exist and show the higher degree of similarity to distal and rectal tumors than to proximal and distal tumors.
Our findings have implications for future studies. Some epidemiologic and molecular studies examine associations with colon cancer which include both proximal and distal colon tumors and sometimes rectal tumors. Our data suggest that these tumor sites have different mutational characteristics. This is illustrated most vividly when looking at CIMP data, where nearly 41 percent of proximal tumors are CIMP+ while only 13 percent of distal and 12 percent of rectal tumors are CIMP+. Among the methylation markers proximal tumors are more likely to have MLH1 methylation (over 17 percent) whereas this marker is seldom methylated in either distal colon or rectal tumors (3 percent and 1 percent, respectively). The differences between proximal and distal colon tumors and similarities between distal colon and rectal tumors may have potential implications for epidemiologic and molecular association studies. Distinguishing site-specific associations between proximal and distal colon tumors may be important as are distinguishing between colon and rectal cancer.
Our study has limitations. The genetic markers evaluated, although identical for colon and rectal tumors were based on markers available when the colon samples were analyzed in the mid 1990s. While new markers might provide additional information, the existing set of markers is still believed to be relevant to colorectal cancer. Because of limitations in cost-efficient manner to analyze the APC gene, we have not included it in our set of tumor markers. Likewise, we have sequenced the hot spots for the p53 and Ki-ras genes, which could result in missed mutations. However, we believe that our sequencing information provides additional data beyond that obtained from immunohistochemistry alone 40. Although we believe that our study adds to the literature on pathogenesis of colorectal cancer, we also believe that more work is needed to better understand these pathways.
In summary, our data suggest mutational pathways that are different for proximal vs distal and rectal cancers. Given similarities in mutational status of distal colon and rectal cancer, it is possible that distal colon and rectal cancer share more molecular and potentially etiologic similarities than do proximal and distal colon tumors. Additional work to capture the associations between colorectal cancer tumor site and specific mutational spectra is currently being undertaken and will provide further insight into the etiology of colorectal cancer.
Acknowledgments
Acknowledgements: We would like to acknowledge the contributions of Leslie Palmer, and Judy Morse to the data collection and management efforts of this study and to Erica Wolff and Michael Hoffman for genotyping, and the core facility of the University of Utah Health Sciences Center for sequencing. We also acknowledge the support of the Biostatistics Core, NCI Cancer Center Support Grant P30CA042014, Huntsman Cancer Institute/Huntsman Cancer Foundation and support of the Data Survey and Management Core .of the Huntsman Cancer Foundation. The Institutional Review Board of all collaborating parties have reviewed and approved this project.
Grant support: This study was funded by CA48998 and CA61757 to Dr. Slattery. This research was supported by the Utah Cancer Registry, which is funded by Contract #N01-PC-67000 from the National Cancer Institute, with additional support from the State of Utah Department of Health and the University of Utah, the Northern California Cancer Registry, and the Sacramento Tumor Registry. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Cancer Institute.
Footnotes
No Conflict of Interest exists for any of the authors
No reprints are available
References
- 1.Slattery ML, Levin TR, Ma K, Goldgar D, Holubkov R, Edwards S. Family history and colorectal cancer: predictors of risk. Cancer Causes Control. 2003;14:879–87. doi: 10.1023/b:caco.0000003840.94591.76. [DOI] [PubMed] [Google Scholar]
- 2.Arbman G, Axelson O, Fredriksson M, Nilsson E, Sjodahl R. Do occupational factors influence the risk of colon and rectal cancer in different ways? Cancer. 1993;72:2543–9. doi: 10.1002/1097-0142(19931101)72:9<2543::aid-cncr2820720906>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Slattery M, Mineau G, Kerber R. Reproductive factors and colon cancer: the influences of age, tumor site, and family history on risk (Utah, United States) Cancer Causes Control. 1995;6:332–8. doi: 10.1007/BF00051408. [DOI] [PubMed] [Google Scholar]
- 4.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73:1134–40. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slattery ML, Caan BJ, Benson J, Murtaugh M. Energy balance and rectal cancer: an evaluation of energy intake, energy expenditure, and body mass index. Nutr Cancer. 2003;46:166–71. doi: 10.1207/S15327914NC4602_09. [DOI] [PubMed] [Google Scholar]
- 6.slattery ML, Ballard-Barbash R, Potter JD, et al. Sex-specific differences in colon cancer associated with p53 mutations. Nutr Cancer. 2004;49:41–8. doi: 10.1207/s15327914nc4901_6. [DOI] [PubMed] [Google Scholar]
- 7.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–52. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Colbert LH, Hartman TJ, Malila N, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–8. [PubMed] [Google Scholar]
- 9.Caan BJ, Coates AO, Slattery ML, Potter JD, Quesenberry CP, Jr., Edwards SM. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord. 1998;22:178–84. doi: 10.1038/sj.ijo.0800561. [DOI] [PubMed] [Google Scholar]
- 10.Slattery ML, Neuhausen SL, Hoffman M, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111:750–6. doi: 10.1002/ijc.20330. [DOI] [PubMed] [Google Scholar]
- 11.Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369–75. doi: 10.1097/00008469-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Burt RW, Leppert MF, Slattery ML, et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology. 2004;127:444–51. doi: 10.1053/j.gastro.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–6. doi: 10.1126/science.8484121. see comments. [DOI] [PubMed] [Google Scholar]
- 14.Kuwada SK, Burt RW. The clinical features of the hereditary and nonhereditary polyposis syndromes. Surgical oncology clinics of North America. 1996;5:553–67. [PubMed] [Google Scholar]
- 15.Samowitz WS, Curtin K, Lin HH, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–8. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 16.Samowitz WS, Slattery ML. Microsatellite instability in colorectal adenomas. Gastroenterology. 1997;112:1515–9. doi: 10.1016/s0016-5085(97)70032-5. [DOI] [PubMed] [Google Scholar]
- 17.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Russo A, Bazan V, Iacopetta B, Kerr D, Soussi T, Gebbia N. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518–28. doi: 10.1200/JCO.2005.00.471. [DOI] [PubMed] [Google Scholar]
- 19.Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92:1831–6. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 20.Samowitz WS, Albertsen H, Sweeney C, et al. Association of Smoking, CpG Island Methylator Phenotype, and V600E BRAF Mutations in Colon Cancer. Journal of the National Cancer Institute. 2006;98:1731–8. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 21.Slattery ML, Curtin K, Ma K, et al. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol Biomarkers Prev. 2002;vol. 11:541–8. [PubMed] [Google Scholar]
- 22.Slattery ML, Anderson K, Curtin K, et al. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res. 2001;483:73–81. doi: 10.1016/s0027-5107(01)00228-7. [DOI] [PubMed] [Google Scholar]
- 23.Slattery ML, Curtin K, Anderson K, et al. Associations between dietary intake and Kiras mutations in colon tumors: a population-based study. Cancer Res. 2000;60:6935–41. [PubMed] [Google Scholar]
- 24.Slattery ML, Anderson K, Curtin K, KN Ma, Schaffer D, Samowitz W. Dietary intake and microsatellite instability in colon tumors. Int J Cancer. 2001;93:601–7. doi: 10.1002/ijc.1370. [DOI] [PubMed] [Google Scholar]
- 25.Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer--beyond physical activity. Cancer Res. 1997;57:75–80. [PubMed] [Google Scholar]
- 26.Slattery ML, Edwards SL, Palmer L, et al. Use of archival tissue in epidemiologic studies: collection procedures and assessment of potential sources of bias. Mutat Res. 2000;432:7–14. doi: 10.1016/s1383-5726(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 27.Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–7. [PubMed] [Google Scholar]
- 28.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 29.Samowitz WS, Holden JA, Curtin K, et al. Inverse relationship between microsatellite instability and K-ras and p53 gene alterations in colon cancer. Am J Pathol. 2001;158:1517–24. doi: 10.1016/S0002-9440(10)64102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issa JP, Shen L, Toyota M. CIMP, at last. Gastroenterology. 2005;129:1121–4. doi: 10.1053/j.gastro.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Desper R, Jiang F, Kallioniemi OP, Moch H, Papadimitriou CH, Schaffer AA. Inferring tree models for oncogenesis from comparative genome hybridization data. J Comput Biol. 1999;6:37–51. doi: 10.1089/cmb.1999.6.37. [DOI] [PubMed] [Google Scholar]
- 32.Desper R, Jiang F, Kallioniemi OP, Moch H, Papadimitriou CH, Schaffer AA. Distance-based reconstruction of tree models for oncogenesis. J Comput Biol. 2000;7:789–803. doi: 10.1089/10665270050514936. [DOI] [PubMed] [Google Scholar]
- 33.Szabo A, Boucher K. Estimating an oncogenetic tree when false negatives and positives are present. Math Biosci. 2002;176:219–36. doi: 10.1016/s0025-5564(02)00086-x. [DOI] [PubMed] [Google Scholar]
- 34.Szabo A, Boucher K. Oncogenetic Trees. In: Hanin LG, Tan W-T, editors. Handbook of Cancer Models with Applications to Cancer Screening, Cancer Treatment and Risk Assessment. World Scientific; Singapore and River Edge, NJ: to appear. [Google Scholar]
- 35.Efron BE, Efron TR. Monographs on Statistics and Applied Probability. vol. 57. Chapman and Hall; New York and London: 1993. An introduction to the bootstrap. [Google Scholar]
- 36.Kim JC, Cho YK, Roh SA, et al. Individual tumorigenesis pathways of sporadic colorectal adenocarcinomas are associated with the biological behavior of tumors. Cancer science. 2008;99:1348–54. doi: 10.1111/j.1349-7006.2008.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–23. [PubMed] [Google Scholar]
- 38.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 39.Samowitz WS, Slattery ML, Sweeney C, Herrick J, Wolff RK, Albertsen H. APC mutations and other genetic and epigenetic changes in colon cancer. Mol Cancer Res. 2007;5:165–70. doi: 10.1158/1541-7786.MCR-06-0398. [DOI] [PubMed] [Google Scholar]
- 40.Curtin K, Slattery ML, Edwards S, Holden JA, Samowitz Ws. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2004;12:380–6. doi: 10.1097/00129039-200412000-00017. [DOI] [PubMed] [Google Scholar]