Abstract
Trauma-hemorrhage produces immunodepression in males but not in proestrus females and this difference is due to the presence of high estrogen in proestrus females. Although skin is the largest immunological organ of the body and is considered the first line of defense, no study to-date has examined whether trauma-hemorrhage has any effects on keratinocytes which are the major epidermal cell type (>90%) of skin. We therefore examined whether epidermal keratinocytes inflammatory response and the signal transduction pathways involved in the inflammatory response are altered following trauma-hemorrhage. C3H/HeN mice were subjected to trauma-hemorrhage and 2 hrs thereafter; keratinocytes were harvested and stimulated with LPS for 24 hrs (5μg/ml). Inflammatory mediators, Toll like receptor (TLR) and myeloid differentiation adaptor protein (MyD88) expression, and the activation of mitogen-activated protein kinase (MAPK) were determined. Trauma-hemorrhage increased the production of IL-6, IL-10, IL-12 and TNF-α, enhanced the expression of TLR4, MyD88 as well as the activation of MAPK proteins (p38, ERK and JNK) in epidermal keratinocytes. However, administration of a single dose of 17β-estradiol following trauma-hemorrhage prevented the increase in these inflammatory parameters under those conditions. These findings suggest that 17β-estradiol normalizes epidermal keratinocytes inflammatory responses following trauma-hemorrhage by preventing the upregulation of TLR4-mediated MAPK activation.
Keywords: MyD88, cytokines, MAPK, cell signaling
INTRODUCTION
Traumatic injury is one of the leading causes of death among young people in the United States (Moore 1998;Noel, et al. 2005c;Shelley, et al. 2003). Several lines of evidence indicate that trauma-hemorrhage produces immunodepression in males but not in proestrus females. Additional studies indicate that these differences are due to the presence of high estrogen in females (Angele, et al. 2000c;Knoferl, et al. 2001b;Meldrum et al. 2006;Noel, et al. 2005) Studies have also shown that administration of 17β-estradiol (E2) following trauma-hemorrhage normalizes immune cell function (Angele, et al. 2000;Knoferl, et al. 2001;Meldrum et al. 2006;Noel, et al. 2005). Furthermore, high levels of circulatory cytokines are the principal mediators of inflammatory response to trauma and have been associated with poor prognosis following major injury and sepsis (Chaudry, et al. 2003;Choudhry, et al. 2005).
The skin is considered to be the largest immunological organ of the body and is the first line of defense (Kupper 1989) The skin is directly exposed to the environment and therefore is vulnerable to an injury such as trauma or excessive blood loss. However, despite the fact that the skin is the largest immunological organ, no study to-date has examined whether trauma-hemorrhage has any deleterious effects on keratinocytes. Keratinocytes are the major cells in epidermis (>90%) (Grone 2002;Eckert et al.1986;Barker, et al. 1991) and are known to be capable of producing a number of cytokines (Kupper 1989).
Studies have shown the involvement of Toll-like receptors (TLR) 4 and mitogen activated protein kinases (MAPK) in inflammatory response of cells such as Kupffer cells (Thobe, et al. 2006;Frink et al. 2007;Hsieh et al. 2007). However, whether TLR4 and/or MAPK play(s) a role in keratinocytes following trauma-hemorrhage remains to be determined. TLRs are the cell surface components that participate in innate immune recognition (Pivarcsi, et al. 2003;Muzio et al. 2001; O’Neill 2002). Upon binding of ligand, TLR initiates host responses through a series of signaling event. In general, the cascade of events involves the activation of a common set of adaptor protein and the protein kinase (Han, et al. 1994). MyD88 is an adaptor protein that is shared by the entire TLR pathway. Upon activation, MyD88 is recruited to the TLR and thus regulate the downstream intracellular signaling cascade including the activation of MAPK (Fitzgerald, et al. 2001). Three MAPK family members namely p38, extra cellular signal regulated kinase (ERK), and C-jun amino terminal kinase (JNK) are shown to be critical in the regulation of many cellular responses (Li, et al. 2006;Zhang et al. 2005). The aim of our study therefore was to determine the effect of trauma-hemorrhage on epidermal keratinocytes inflammatory response. Since E2 was shown to protect immune response following trauma-hemorrhage, we also examined whether treatment of animals following trauma-hemorrhage with E2 has any salutary effects on keratinocytes. Our findings suggest that TLR4, MyD88 and MAPK are rapidly activated in keratinocytes following trauma-hemorrhage. This was accompanied with an increased production of inflammatory mediators. However, treatment of animals with E2 normalized both the upregulation of TLR4, MyD88, MAPK and the release of inflammatory mediators following trauma-hemorrhage.
MATERIALS and METHODS
Animals
Inbred male C3H/HeN (Charles River Laboratories, Wilmington, MA), 6–8 weeks of age (24–27g body wt.) were used in this study. The mice were allowed to acclimatize in the animal facility for at least one week prior to experimentation. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham, and were performed in accordance with the guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Trauma-hemorrhage procedure
Mice were fasted overnight but allowed water ad lib. The animals were anesthetized with isoflurane (Minrad, Bethehem, PA) and restrained in a supine position. A midline laparotomy (2 cm) was performed, which was closed in two layers with sutures (Ethilon 6/0, Ethicon, Somervile, NJ). Both femoral arteries and the right femoral vein were then cannulated with polyethylene 10 tubing. The areas of the incisions were bathed with lidocaine to prevent any discomfort to the animals. Blood pressure was measured via one of the arterial lines using a blood pressure analyzer. Within 10 min after awakening, animal were bled through the arterial catheter to a mean arterial blood pressure of 35±5 mmHg, which was maintained for 90 min. The mice then received E2 (1 mg/kg body weight), or an equal volume and concentration of the vehicle (cyclodextrin) intravenously at the onset of resuscitation; the animals were resuscitated via the venous line with four times the shed blood volume using Ringers lactate. After removing the catheter, the incisions were closed. Sham operation animals underwent the same surgical procedures, but were neither hemorrhaged nor resuscitated (Chaudry et al, 2003).
Preparation of Keratinocytes
Epidermal sheet isolation
The mice were anesthetized at 2 hrs after resuscitation, the skin was shaved, collected as longitudinal strips (about 5 strips-0.5 cm wide) and the excess fat from the skin was removed. The skin strips were then digested in the Dispase II-RPMI solution: 0.5% (Roche Cat # 10165859; Indianapolis, IN) for 30 min at 37°C in a shaker maintained at the speed of 20 rpm. Following this, the strips were transferred to RPMI solution containing 10% fetal bovine serum (FBS) and kept on ice. The wet strips were then transferred to the Petri dish with dermis down; the end of dermis was secured with tweezers and using a second set of tweezers the epidermis was separated from dermis.
Keratinocytes isolation
Keratinocyets were isolated using the method described previously (Dlugosz, et al. 1995) with some modification. Epidermis sheets were floated, (dermis side down) in 20 ml of 0.15% trypsin solution incubated at 37°C and 20 rpm rotation with agitation every 5 min for 45–50 min. In the last 30 min, 2 μl/ml of DNAase was added to the trypsin solution and vigorously pipetted for a few min to help break up the cells. The cells were filtered through a 250 μm sieve into a tube containing equal volume of RPMI-FBS with antibiotics to remove large debris. Following this, the cells were centrifuged for 5 min at 1200 rpm 4°C, the supernatant was discarded and the pellet was resuspended in 20 ml of 0.002% DNAase and culture medium. The suspension was allowed to sit for a few min, and then the cells were filtered through 250 μm sieve into Petri dish. The cells were then passed through a 26 gauge needle and centrifuged for 5 min at 1200 rpm at 4°C. The supernatants was discarded and cells were resuspended in 10 ml of keratinocytes serum free growth medium (SFM; Gibco Cat # 10725-018; Carlsbad, CA). The isolated cells from different groups (i.e., sham, trauma-hemorrhage and E2 treated) showed the typical keratinocytes morphology with viability greater than 95% when stained with trypan blue. Furthermore, there were no significant changes in keratinocytes number in any group. The attached cells were allowed to grow for 24 hrs in keratinocytes growth medium. The keratinocytes thus obtained were stimulated with or without and cultured in the presence of 5 μg/ml LPS (E. coli,055:B5; Sigma Chemical Co. St. Louis, MO) for the measurement of various parameters as listed below. The dose of LPS at 5 μg/ml used in this study was selected after performing a dose response study in which LPS was used at 100 ng/ml, 1 μg/ml, 5 μg/ml and 10 μg/ml (data not shown). Our results showed that keratinocytes responded poorly when they were stimulated with LPS at the concentration of 100 ng/ml and 1 μg/ml. We found that the optimal cytokine production in keratinocytes was achieved when they were stimulated with 5 μg/ml for 24 hrs.
Cell extracts preparation
Keratinocytes cell extracts were prepared for the measurements of TLR, MyD88 and MAPK. In brief, the adherent keratinocytes cells were stimulated with 5 μg/ml LPS for 30 min and lysed with 60 μl/3×105 cells of ice-cold lyses buffer (150 mM NaCl, 1 mM MgCl2, 50 mM HEPES, 1 mM EDTA, 0.5% Triton x100, 10% glycerol, 200 μM sodium orthovanadate, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 200 μM phenylmethylsulfonyl fluoride, Glycerol, Triton X-100 and deionized water) for 60 min at 4°C. Cell lysates were centrifuged at 12,000 rpm for 10 min at 4°C and supernatants were stored at −80°C until used for western blot analysis.
Western blot analysis
Cell extracts (15 μg protein) were electrophoretically separated on 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE), as described previously (Li et al., 2006b). Proteins were electroblotted to Immobilon-P membranes (Healthcare, Waukesha, WI) using Semidry Transblot System (Bio-Rad, Hercules, CA) at 11v for 45 min. Blots were then blocked for 2 hrs at room temperature with 5% nonfat dry milk in 50 mM Tris-HCl (pH7.5), 200 Mm NaCl, 0.05% Tween 20 (TBST). After three 5 min washes with TBST, membrane were incubated overnight at 4°C with antibodies against TLR4, MyD88, phospho-p38, phospho-p44/42 (phospho-ERK1/2), phospho-JNK, non phospho-p38, non phospho-ERK, nonphospho-JNK (Cell Signaling Technology, Beverly, MA) diluted in 1:1000 in TBST containing 0.5% non fat dry milk. After subsequent washes with TBST, the membranes were incubated for 1 hr with goat anti rabbit or goat anti mouse secondary antibody (diluted to 1:2000). Membranes were developed with an enhanced chemiluminescence (ECL) reagent followed by exposure to Biomax Light Film. After the films were developed, the density of the bands was determined using Ambis Optic Imaging System (GE Health Care, U.K.).
Cytokine analysis
For the cytokines measurements, keratinocytes were cultured with or without 5 μg/ml LPS for 24 hrs. The supernatants were then collected and the cytokine levels in the supernatants were measured using Cytometric bead array (CBA) according to the manufacturer’s instructions (BD Biosciences) and as described previously (Kawasaki et al., 2006). Briefly, 50 μl of mixed capture beads were incubated with 50 μl of supernatants and 50 μl PE detection reagents for 2 hrs at room temperature. The immunocomplexes were then washed and analyzed using LSRII flow cytometer (BD Biosciences, San Diego, CA). Data processing was carried by using FACS Diva and BD CBA software.
Statistics
Statistical analysis was performed using Sigma-Stat computer software (SPSS, Chicago, IL). Comparison between groups was performed using one way ANOVA. A p value of less than 0.05 was considered to be statistically significant for all analyses.
RESULTS
Upregulation of cytokines in epidermal keratinocytes following trauma-hemorrhage
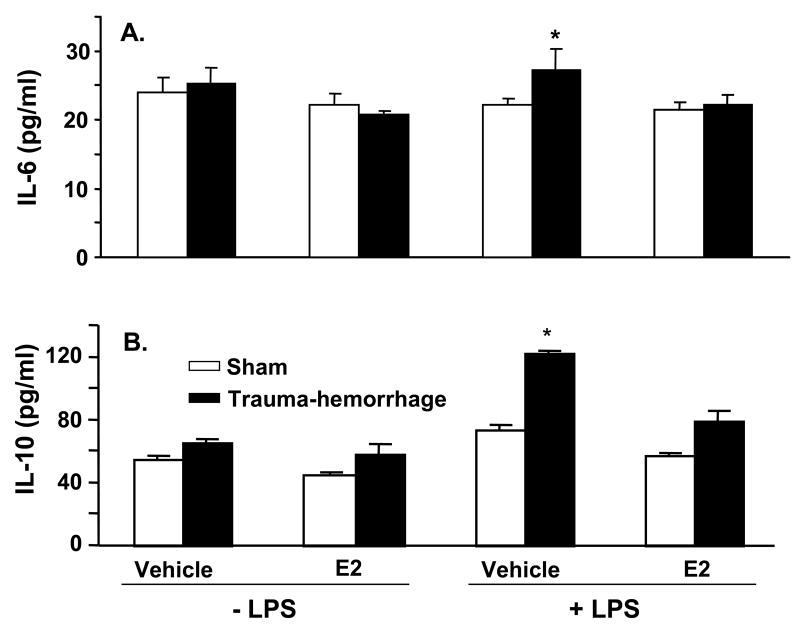
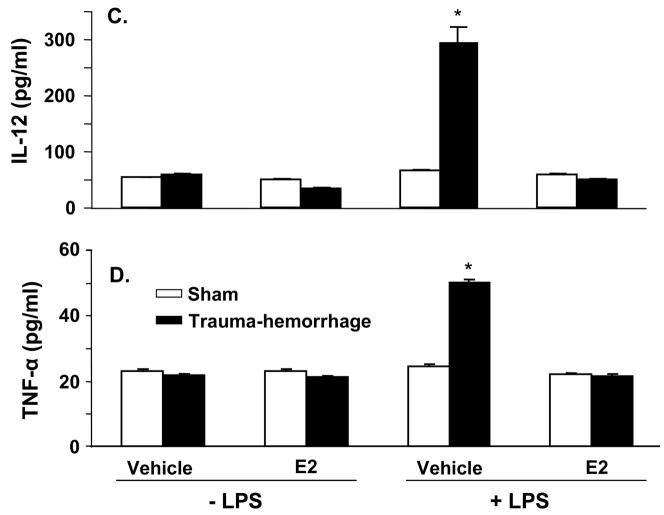
The cytokine (IL-6, IL-10, IL-12 and TNF-α) production by keratinocytes from male mice following trauma-hemorrhage is shown in Fig. 1. As shown in panels A–D of Fig. 1, there was no significant difference between cytokines released from unstimulated keratinocytes derived from trauma-hemorrhage or sham animals. However, upon stimulation with LPS, the levels of all four cytokines were markedly increased in keratinocytes cultures from trauma-hemorrhage animals as compared to the sham animals (p<0.05). Treatment of animals with E2 following trauma-hemorrhage normalized the cytokine release to those observed in sham animals (Fig. 1A–D). Treatment of sham animals with E2 did not influence cytokines release by their keratinocytes.
Figure 1.
Keratinocytes inflammatory response following trauma-hemorrhage. Male mice were subjected to trauma-hemorrhage and E2 treatment. Keratinocytes from sham and trauma-hemorrhage animals were isolated 2 hrs thereafter and treated with 5μg/ml LPS for 24 hrs and supernatants were harvested. The levels of IL-6 (A), IL-10 (B), IL-12 (C) and TNF-α (D) in the supernatants were analyzed using Cytokine Bead Array. Data are means ± SEM from six animals in each group. *p<0.05 compared to all other groups.
Expression of TLR4 in epidermal keratinocytes
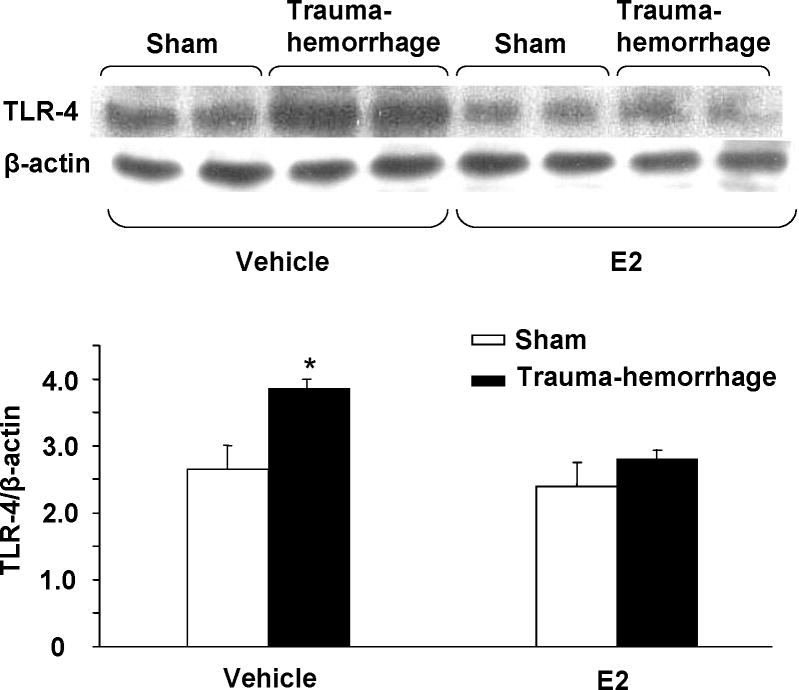
The expression of TLR4 in keratinocytes was assessed using Western-blot technique. In unstimulated keratinocytes, there was no significant difference in TLR4 expression in mouse following trauma-hemorrhage compared to sham operation (data not shown). However, stimulation of keratinocytes with LPS for 30 min resulted in a significant increase in TLR4 expression in cells from both sham and trauma-hemorrhage animals. Nonetheless, the increase in TLR4 expression was significantly higher in keratinocytes harvested from trauma-hemorrhage animals as compared to shams (Fig. 2). Administration of E2 normalized the TLR4 expression in keratinocytes following trauma-hemorrhage (Fig. 2). There was no significant difference in TLR4 expression in keratinocytes from sham mouse with or without E2 treatment.
Figure 2.
TLR-4 expression in keratinocytes following trauma-hemorrhage. Keratinocytes were isolated (1×106 cells/ml), and stimulated with LPS (5μg/ml) for 30 min and lysed. The lysates were analyzed for TLR-4 expression by Western blot, which was stripped and reprobed for β-actin protein content in various lanes. Blots obtained from six animals in each group were analyzed using densitometry. Densitometric values were normalized to the β-actin and are shown in bar graph as mean ± SEM. *p<0.05 compared to all other groups.
Expression of MyD88 in keratinocytes
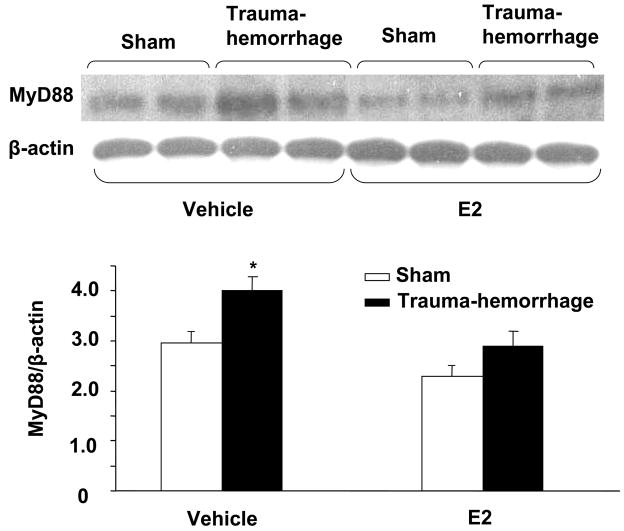
Since MyD88 connects TLR4 with its downstream signaling cascade, we examined the effect of trauma-hemorrhage on MyD88 expression in keratinocytes. Similar to TLR4 expression, there was no significant difference in MyD88 expression in unstimulated keratinocytes harvested from sham or trauma-hemorrhage animals (data not shown). The results obtained after stimulation of keratinocytes with LPS for 30 min are shown in Fig. 3. These results suggest that the expression of MyD88 in keratinocytes was significantly increased following trauma-hemorrhage (p<0.05). However, treatment of animals with E2 decreased the increase in MyD88 expression following trauma-hemorrhage and the values were similar to shams (Fig. 3).
Figure 3.
MyD88 expression in keratinocytes following trauma-hemorrhage. Keratinocytes were isolated (1×106 cells/ml), stimulated with LPS (5μg/ml) for 30 min and lysed. Lysates were analyzed for MyD88 expression by Western blot, which was stripped and reprobed for β-actin protein content in various lanes. Blots obtained from six animals in each group were analyzed using densitometry. Densitometric values were normalized to the β-actin and are shown in bar graph as mean ± SEM. *p<0.05 compared to all other groups.
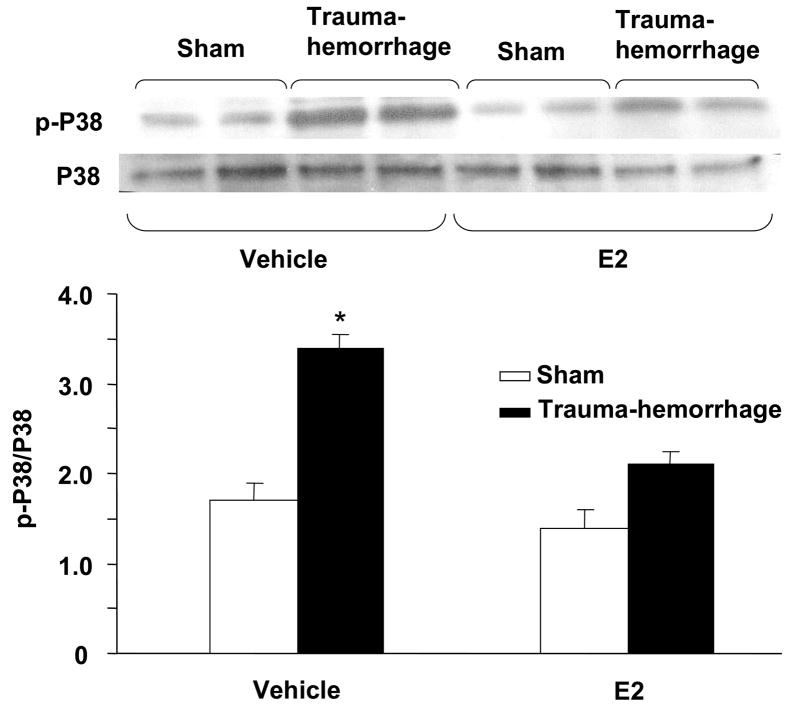
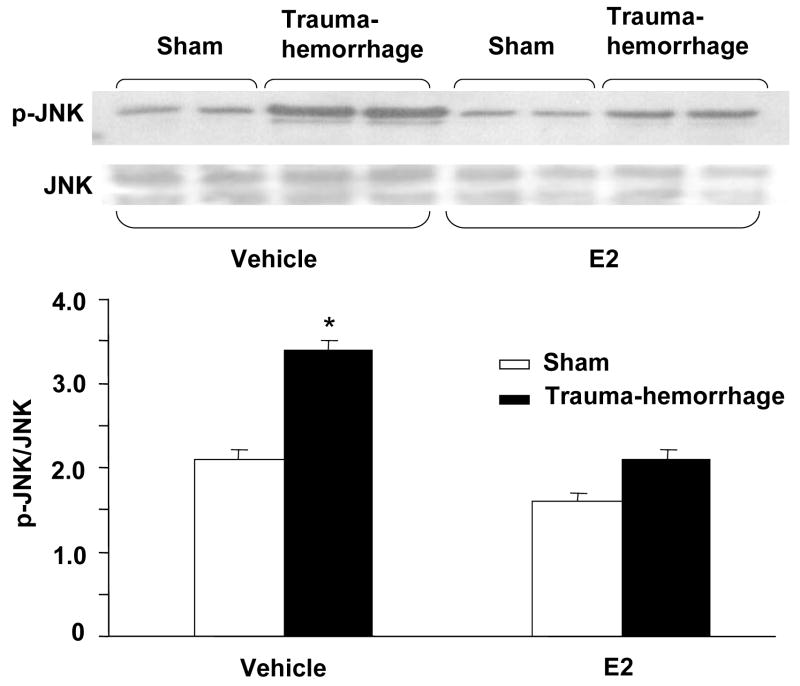
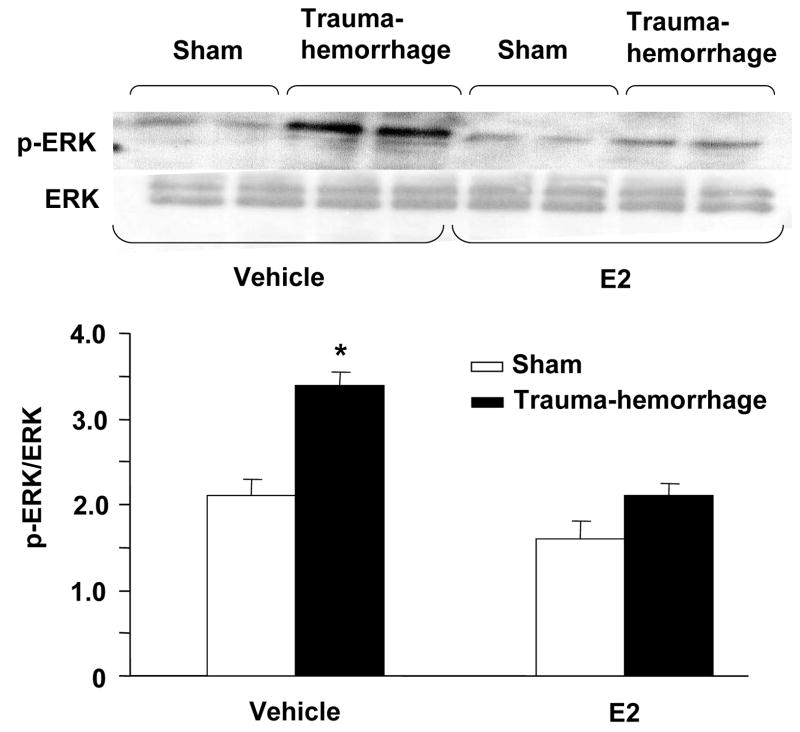
Phosphorylation of p38, ERK and JNK in keratinocytes
We further characterized the downstream signaling cascade by evaluating the effect of trauma-hemorrhage on MAPK signaling pathway. The activation of MAPK members, p38, ERK and JNK is crucial for the production of inflammatory cytokines that we have examined in this study. There was no significant difference in MAPK activation in unstimulated keratinocytes cells (data not shown). The results as shown in Figs. 4–6 suggest that the phosphorylation of p38, ERK and JNK was significantly increased keratinocytes following trauma-hemorrhage as compared to sham animals. The upregulation of p38 (Fig. 4), ERK (Fig. 5) and JNK (Fig. 6) following trauma-hemorrhage was, however, normalized if the animals were treated with E2 following trauma-hemorrhage. No significant difference in the phosphorylation of p38, ERK and JNK was observed in sham operated animals with or without E2 treatment.
Figure 4.
p38 phosphorylation and protein expression in epidermal keratinocytes following trauma-hemorrhage. Keratinocytes were isolated (1×106 cells/ml) and stimulated with LPS (5μg/ml) for 30 min and lysed, lysates were analyzed for p38 phosphorylation by Western blot. The blots were stripped and reprobed for p38 total protein contents in various lanes. Blots from 6 animals in each group were analyzed using densitometry. Densitometric values for phosphorylation were normalized to p38 protein and are shown in bar graph as mean ± SEM. *p<0.05 compared to all other groups.
Figure 6.
JNK phosphorylation and protein expression in epidermal keratinocytes following trauma-hemorrhage. Keratinocytes were isolated (1×106 cells/ml) and stimulated with LPS (5μg/ml) for 30 min and lysed; lysates were analyzed for JNK phosphorylation by Western blot. The blots were stripped and reprobed for JNK total protein contents in various lanes. Blots from 6 animals in each group were analyzed using densitometry. Densitometric values for phosphorylation were normalized to JNK protein and are shown in bar graph as mean ± SEM. *p<0.05 compared to all other groups.
Figure 5.
ERK phosphorylation and protein expression in epidermal keratinocytes following trauma-hemorrhage. Keratinocytes were isolated (1×106 cells/ml) and stimulated with LPS (5μg/ml) for 30 min and Lysates of keratinocytes were analyzed for ERK phosphorylation by Western blot. The blots were stripped and reprobed for ERK total protein contents in various lanes. Blots from 6 animals in each group were analyzed using densitometry. Densitometric values for phosphorylation were normalized to ERK protein and are shown in bar graph as mean ± SEM. *p<0.05 compared to all other groups.
DISCUSSION
The epidermis is an important site of host interactions with the keratinocytes, the main cell type in the epidermis (>90%) (Grone et al. 2002;Eckert et al.1986), and participates in the host defense. Epidermal keratinocytes are different from other immune cells because not only do they form a physical barrier, but also because they initiate and regulate the cutaneous inflammation and immune response by producing a number of cytokines. Previous studies have shown that trauma-hemorrhage induces an inflammatory response by Kupffer cells (resident macrophage population of liver) that is characterized by an increase in systemic levels of IL-6, IL-10 and TNF-α shortly after injury (Ayala, et al. 1992). The increase in Kupffer cell cytokine production is accompanied with a decrease in immune cell functions including the antigen presentation function of macrophages (Ayala, et al. 1992;Chaudry, et al. 2003). Studies have also shown that although Kupffer cells produces more cytokines, the macrophages harvested from spleen have decreased ability to produces cytokines following trauma-hemorrhage (Ayala, et al. 1991).
Although the skin is the largest immunological organ of the body and is the first line of defense, no-study to date has examined whether trauma-hemorrhage influences the ability of keratinocytes to produce the inflammatory mediators. The present study was there conducted to determine the inflammatory response of keratinocytes following trauma-hemorrhage and the mechanism involved in that response.
Our findings suggest that keratinocytes harvested from trauma-hemorrhage mice produce more IL-6, IL-10, IL-12 and TNF-α compared to sham animals. Although keratinocytes are the main producers of cytokines in skin, the mechanisms involved in the secretion of these cytokines following trauma-hemorrhage remained unclear. Therefore our studies further investigated the involvement of TLR4 and MyD88, and MAPK activation in keratinocytes following trauma-hemorrhage. The findings from these studies indicated that trauma-hemorrhage results in increased TLR4 and MyD88 expression in keratinocytes. This finding corroborates our previous studies in which we have shown that the increase in Kupffer cells IL-6 production is accompanied with an in increase in TLR4 and MyD88 expression following hypoxia (Zheng, et al. 2006). TLR4 expression has been found in heart, lung, brain and many other tissues. A large number of studies have shown that LPS utilizes TLR4 to stimulate the cells (Maung, et al. 2005;Muzio et al. 2001), and thus over-expression of TLR4 is shown to enhance the sensitivity of human keratinocytes to the LPS (Pivarcsi, et al. 2003;Muzio et al. 2001). The signaling induced by TLR4 is complex and involves both MyD88-depedent and -independent mechanisms (Zheng, et al. 2006). It has been shown that the adaptor protein MyD88 following activation is recruited to the cytoplasmic domain of TLR4 and activates a downstream kinase cascade involving p38, ERK, and JNK kinase. Our results demonstrate that trauma-hemorrhage up-regulates kertinocytes p38, ERK and JNK phosphorylation compared to sham. The activation of these proteins is likely to contribute to the elevated levels of inflammatory cytokine release by epidermal keratinocytes.
A number of studies from our and other laboratories have shown that female sex hormone E2 protects immune response following trauma-hemorrhage (Choudhry, et al. 2005). To determine whether E2 also has similar protective effects in keratinocytes, we treated a group of animals with E2 at the onset of resuscitation. The effect of this treatment was then determined on keratinocytes responses. The findings suggest that treatment of animals with E2 after trauma-hemorrhage normalizes keratinocytes TLR4 and MyD88 expression, as well as the activation of p38, ERK and JNK under those conditions. In addition, keratinocytes cytokine production was also restored to the levels observed in shams. These results indicate that E2 also produces salutary effects in keratinocytes following trauma-hemorrhage.
Previous studies have indicated that the gender dimorphic difference in the inflammatory response and survival following trauma-hemorrhage is regulated by sex steroids (Angele, et al. 2000;Choudhry, et al. 2005). Studies have also shown that macrophage antigen presentation, T cell proliferation, and Th-1 cytokines production (IL-2 and IFN-γ) are decreased in male animals following trauma-hemorrhage; while the production of Th-2 cytokines (IL-4 and IL-10) is augmented under those conditions (Ayala, et al. 1994;Choudhry, et al. 2005). On the other hand, females in the proestrus stage of estrus cycle have normal/maintained immune responses following trauma-hemorrhage. Moreover, depletion of E2 by ovariectomy prior to trauma-hemorrhage resulted in depressed immune responses following trauma-hemorrhage similar to that observed in male mice under those conditions (Choudhry, et al. 2005;Knoferl, et al. 2001). In contrast, treatment of male or ovariectomized female mice with female sex steroid, E2, prevented the depression in splenic, peritoneal macrophage and Th1 cytokine production following trauma-hemorrhage (Knoferl, 2001). Although we have not determined keratinocyte responses in proestrus mice following trauma-hemorrhage in this study, based upon the studies performed in our laboratory we would anticipate that keratinocyte responses in proestrus females and males treated with 17β-estradiol would be similar following trauma-hemorrhage.
Studies have also shown that administration of E2 following trauma-hemorrhage prevented the increase in the phosphorylation of Kupffer cells p38, ERK and JNK and restored the production of inflammatory cytokines. These findings indicate that E2 protects immune functions following trauma-hemorrhage. Furthermore the findings that E2 also protects keratinocyte functions following trauma-hemorrhage suggest that the effect of E2 is not organ specific, and that it produces a global protective following trauma-hemorrhage.
The precise mechanism of the salutary effects of E2 on keratinocytes after trauma-hemorrhage remains to be established. There are two major subtypes of estrogen receptors (ER), ER-α and ER-β, however, several isoforms of ER-α and ER-β have been reported to-date. The distribution of ER may vary from organ to organ. In a recent study, we found that heart is primarily rich in ER-β, intestine in both ER-α and -β, lung has more ER-βthan -α and liver was found to be rich in ER-α (Yu, et al. 2006). Studies have also shown that ER distribution may be further affected by trauma-hemorrhage (Yu, et al. 2006). Furthermore, studies have also suggested that ERs are localized in the cytoplasm and nucleus of the cell. Thus, it remains to be established which of the two receptors is more critical to E2-mediated effects on keratinocytes following trauma-hemorrhage and whether the salutary effects of E2 are mediated via receptors located on cell or nuclear membrane.
We recognize that LPS at a dose of 5 μg/ml may activate other TLRs such as TLR2. However, a recent study showed that although both TLR4 and TLR2 are expressed in keratinocytes; the expression of TLR2 was more pronounced in the suprabasal layer of keratinocytes and not at the basal layer (Pivarcsi, et al. 2003). Since we have used basal layer which predominantly expresses TLR4, the observed LPS-mediated effects are likely due to TLR4 and not TLR2. However more studies are needed to confirm this. Furthermore, we also recognize that we stimulated cells for 30 minutes for the measurement of signaling molecules (i.e., TLR4 and MAPK). In contrast, cells were cultured with LPS for 24 hours for the measurement of their capacity to release inflammatory mediators. These timings were established in our preliminary studies (unpublished) and we found that stimulation of cells with 5μg/ml LPS for 30 min is the optimal time for activation of TLR4, MyD88 and MAPK activation.
We also recognize that this study was performed only at a single time point, i.e., at 2 hrs after treatment and thus it remains unclear whether the protective effects of E2 on keratinocytes signaling and cytokine production are sustained for a period longer than 2 hrs. However, our previous studies have shown that if the improvement in cell and organ function by any pharmacological agent is evident at 2, 5 or 24 hrs after treatment, the salutary effects are sustained for prolonged intervals (Mizushima, et al. 2000;Chaudry, et al. 2003). Thus, although the present study did not evaluate the effects of E2 at multiple time points, based on our previous work, it would appear that the salutary effects of E2 on keratinocytes cytokine release and other signaling molecules would be evident even if one measured those effects at another time point following trauma-hemorrhage and resuscitation.
In conclusion, our findings for the first time provide evidence that trauma-hemorrhage induces keratinocytes inflammatory response. We also found that the treatment of animals with estrogen normalizes the inflammatory responses of keratinocytes following trauma-hemorrhage. Furthermore, our studies suggest that the protective effect of E2 is mediated via down-regulation of MAPK proteins. Altogether these findings provide a novel insight into the molecular mechanisms responsible for the inflammatory response of keratinocytes following trauma-hemorrhage.
Acknowledgments
This work was supported by NIH grant GM37127. The authors wish to express their thanks to Dr. Sanjay Pradhan for his kind help in teaching the keratinocyte isolation procedure and Dr. Nochiketa Mohanty for his help with the cytokine analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ayala A, Ertel W, Chaudry IH. Trauma induced suppression of antigen presentation and expression of major histocompatibility class II antigen complex in leukocytes. Shock. 1996;5:79–90. doi: 10.1097/00024382-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ayala A, Perrin MM, Ertel W, Chaudry IH. Differential effects of haemorrhage on Kupffer cells: decreased antigen presentation despite increased inflammatory cytokine (IL- 1, IL-6 and TNF) release. Cytokine. 1992;4:66–75. doi: 10.1016/1043-4666(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Perrin MM, Wang P, Ertel W, Chaudry IH. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity. Involvement of cell-associated tumor necrosis factor and reactive nitrogen. J Immunol. 1991;147:4147–4154. [PubMed] [Google Scholar]
- 5.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 6.Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, III, Bland KI. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- 7.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24 Suppl 1:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 8.Dlugosz AA, Glick AB, Tennenbaum T, Weinberg WC, Yuspa SH. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 9.Eckert RL, Green H. Structure and evolution of the human involucrin gene. Cell. 1986;46:583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 11.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007 doi: 10.1016/j.molimm.2006.12.009. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Grone A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh YC, Frink M, Thobe BM, Hsu JT, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. 17beta-Estradiol downregulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Mol Immunol. 2007;44:2165–2172. doi: 10.1016/j.molimm.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoferl MW, Jarrar D, Angele MK, Ayala A, Schwacha MG, Bland KI, Chaudry IH. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2001;281:C1131–C1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 16.Kupper TS. Production of cytokines by epithelial tissues. A new model for cutaneous inflammation. Am J Dermatopathol. 1989;11:69–73. doi: 10.1097/00000372-198902000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;79:453–462. doi: 10.1189/jlb.0705369. [DOI] [PubMed] [Google Scholar]
- 18.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 19.Meldrum DR. Estrogen increases protective proteins following trauma and hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2006;290:R809–R811. doi: 10.1152/ajpregu.00802.2005. [DOI] [PubMed] [Google Scholar]
- 20.Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg. 2000;232:673–679. doi: 10.1097/00000658-200011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore EE. Mesenteric lymph: the critical bridge between dysfunctional gut and multiple organ failure. Shock. 1998;10:415–416. [PubMed] [Google Scholar]
- 22.Muzio M, Mantovani A. Toll-like receptors (TLRs) signalling and expression pattern. J Endotoxin Res. 2001;7:297–300. [PubMed] [Google Scholar]
- 23.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23:115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002;23:296–300. doi: 10.1016/s1471-4906(02)02222-6. [DOI] [PubMed] [Google Scholar]
- 25.Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, Beer Z, Bata-Csorgoo Z, Magocsi M, Rajnavolgyi E, Dobozy A, Kemeny L. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 26.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 27.Thobe BM, Hildebrand F, Frink M, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell Toll like receptor (TLR) 2-, 4- and 9-mediated signaling following trauma hemorrhage. J Cell Physiol. 2007;210:667–675. doi: 10.1002/jcp.20860. [DOI] [PubMed] [Google Scholar]
- 28.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2:20–27. [PubMed] [Google Scholar]
- 30.Zheng R, Pan G, Thobe BM, Choudhry MA, Matsutani T, Samy TSA, Kang SC, Bland KI, Chaudry IH. MyD88 and Src are differently regulated in Kupffer cells of males and proestrus females following hypoxia. Mol Med. 2006;1(2):65–73. doi: 10.2119/2006-00030.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]