Abstract
Kidney stone patients often have a decrease in BMD. It is unclear if reduced BMD is caused by a primary disorder of bone or dietary factors. To study the independent effects of hypercalciuria on bone, we used genetic hypercalciuric stone-forming (GHS) rats. GHS and control (Ctl) rats were fed a low Ca (0.02% Ca, LCD) or a high Ca (1.2% Ca, HCD) diet for 6 wk in metabolic cages. All comparisons are to Ctl rats. Urine Ca was greater in the GHS rats on both diets. GHS fed HCD had reduced cortical (humerus) and trabecular (L1–L5 vertebrae) BMD, whereas GHS rats fed LCD had a reduction in BMD similar to Ctl. GHS rats fed HCD had a decrease in trabecular volume and thickness, whereas LCD led to a ∼20-fold increase in both osteoid surface and volume. GHS rats fed HCD had no change in vertebral strength (failure stress), ductibility (failure strain), stiffness (modulus), or toughness, whereas in the humerus, there was reduced ductibility and toughness and an increase in modulus, indicating that the defect in mechanical properties is mainly manifested in cortical, rather than trabecular, bone. GHS rat cortical bone is more mineralized than trabecular bone and LCD led to a decrease in the mineralization profile. Thus, the GHS rats, fed an ample Ca diet, have reduced BMD with reduced trabecular volume, mineralized volume, and thickness, and their bones are more brittle and fracture prone, indicating that GHS rats have an intrinsic disorder of bone that is not secondary to diet.
Key words: BMD, bone strength, hypercalciuria, nephrolithiasis
INTRODUCTION
Hypercalciuria is the most common metabolic abnormality found in patients with calcium (Ca)-containing kidney stones.(1–5) Hypercalciuria raises urine supersaturation with respect to the solid phases of Ca hydrogen phosphate (CaHPO4, brushite) and Ca oxalate (CaOx), enhancing the probability of nucleation and growth of crystals into clinically significant stones.(4) Patients with hypercalciuria often excrete more Ca than they absorb, indicating a net loss of total body Ca.(1–13) The source of this additional urine Ca is almost certainly the skeleton, which is by far the largest repository of body Ca.(13–15)
A number of studies have shown a decrease, which is generally mild, in BMD in hypercalciuric stone formers compared with controls.(1,6,13,16–22) However, in human stone formers, it is difficult to determine whether the reduction in BMD is caused by a primary decrease in bone formation and/or an increase in bone resorption or an alteration in any of number of dietary factors that may be altered in stone formers over their lifetime. For example, a reduction in dietary Ca(23) or an increase in dietary sodium(24) and/or protein(25) would all be expected to alter urine Ca excretion and potentially decrease BMD.
To study the independent effect of hypercalciuria on bone, we used genetic hypercalciuric stone forming rats.(26–40) These rats were established by successively inbreeding the most hypercalciuric progeny of hypercalciuric Sprague-Dawley rats. Each rat excretes significantly more urinary Ca than do similarly fed controls.(26–40) The hypercalciuria is caused by increased intestinal Ca absorption,(39) coupled to a defect in renal tubular Ca reabsorption(33,37) and enhanced bone mineral resorption,(34) suggesting a systemic dysregulation of Ca homeostasis.(38) Virtually all of these hypercalciuric rats form kidney stones, whereas there was no evidence of stone formation in controls.(35) We have termed the rats genetic hypercalciuric stone forming (GHS) rats.(26–30,32,35,40) The stones formed by the GHS rats fed standard rat chow contain only Ca and phosphate.(28,30,32,35) The addition of hydroxyproline, a common amino acid and an oxalate precursor,(41) results in formation of Ca oxalate kidney stones.(26,40)
We used these GHS rats to test the hypothesis that, in this genetic model of hypercalciuria, there is a primary decrease in BMD and strength even when the rats were fed ample dietary Ca. We compared the BMD of the GHS rats to their parental strain (Sprague-Dawley) when fed an equal amount of a high and a low Ca diet and also compared the static histomorphometry and mechanical properties of the bone from these two strains of rats.
MATERIALS AND METHODS
Establishment of hypercalciuric rats
Adult Sprague-Dawley rats (Charles River Laboratories, Kingston, NY, USA) were initially screened for hypercalciuria by placing the rats in individual metabolic cages, feeding them a constant amount of a standard (1.2% Ca) Ca diet, and measuring urine Ca excretion. The most hypercalciuric male and female rats were used to breed the next generation. A similar protocol was used for screening and inbreeding of subsequent generations as described previously.(26–40,42,43)
Study protocol
Sixteen 46th generation female GHS rats, initially weighing on average 140 g, and 16 Sprague-Dawley rats (the parental strain of the GHS rats) were each placed in metabolic cages for 6 wk. Each rat was provided with 15 g/d of food and deionized distilled water ad libitum. The GHS and the Sprague-Dawley rats were each divided equally, by random allocation, into two groups. Some were fed a high Ca diet containing 1.2% Ca (HCD), and others were fed a matched low Ca diet containing 0.02% Ca (LCD). Every 2 wk, a 24-h urine collection was collected in concentrated HCl for urine Ca. The urine was refrigerated at 4°C and measured within 2 wk. Urine Ca was measured by reaction with arsenazo III and determined photometrically at 650 nm.(44) At the conclusion of the experiment (6 wk), each rat was killed. Their femurs, tibias, humeri, and lumbar spines were dissected out, cleaned, and stored at –80°C until analysis. Any rat that ate <14 g of food/d or drank <15 ml of water on any day would have been excluded from the study; however, all rats met these prospective criteria throughout the study. The rats were studied with strict adherence to the NIH Guide for the Care and Use of Laboratory Animals in the AALAC accredited vivarium at the University of Rochester.
DXA
The right femurs and lumbar vertebrae (L1–L5) were scanned using DXA (PIXImus; Lunar Corp/GE). An aluminum/lucite phantom (Lunar Corp/GE) was used to calibrate the machine. The cleaned excised bones were placed on a polystyrene tray to mimic soft tissues, and the BMD of the bones was determined.(45)
Mechanical tests
The mechanical properties of the humeri and vertebra were conducted using an Instron 1011 material testing system (Instron Canada). In both experiments, force and deformation data were collected a rate of 25 Hz using a 12-bit data acquisition card (National Instruments), LabVIEW 5.0 data acquisition software (National Instruments), and a Pentium II computer (Compaq Canada).
The diaphysis of the right humeri was tested in three-point bending according to the procedure described previously.(46) Briefly, samples were subjected to a preload of 2 N and deformed at a rate of 2 mm/min until failure. The point of failure was defined as a successive drop in load >10%. The body of the fifth lumbar vertebra was tested to failure in unconfined compression using a similar procedure described previously.(47) Briefly, a preload of 2 N was applied to the sample and deformed at a rate of 1 mm/min until failure occurred. In these experiments, the point of failure was defined as a successive drop in load >5%. In both tests, sample stress-strain curves were generated from the collected load-deformation data and the specimen geometry. From the resulting stress-strain curves, the following bone material properties were determined: (1) elastic modulus, (2) failure stress and strain, and (3) energy to failure (toughness).
Specimen processing for image analysis and histomorphometry
The distal femur of the rats was cleaned of surrounding tissues. The distal femurs were first dehydrated through graded alcohols to acetone (70%, 90%, 100%, and 100%), infiltrated in increasing strengths of spurr/acetone solutions (50%, 80%, 100%, and 100%), and embedded undecalcified in polymerized plastic Spurr blocks. On completion, thin sections were cut, on a rotary microtome (Reichert-Jung 2050) using a tungsten carbide knife, from the blocks and stained for use in image analysis and histomorphometry.
Histomorphometry
Static histomorphometry was performed on undecalcified Goldner's trichrome-stained section (5 μm) of distal femur. Measurements of the bone were taken from a 3-mm2 area in the central region beginning at 1 mm distal to the growth plate. The trabecular bone parameters were measured at a magnification of ×125. A semiautomated image analysis system (Bioquant; R&M Biometrics) was used to quantify the structure of the imaged bone. From these measurements, we determined the trabecular bone volume (BV), mineralized trabecular bone volume (Md.V), osteoid volume (OV), osteoid surface (OS), osteoid thickness (O.Th), and eroded surface (ES); trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were also calculated. The osteoid thickness was calculated as osteoid volume/osteoid surface. All the measurements and calculations were done following the American Society for Bone Mineral (ASBMR) nomenclature and guidelines.(48)
Backscattering electron microscope
After some sections were taken for histomorphometry, the plastic blocks from the distal femurs were polished to a 1-μm diamond finish on the Minimet Polisher (Buehler). Specimens were mounted on a plexi-glass plate and carbon-coated, the plate was placed on the stage of a scanning electron microscope (Philips XL300ESEM), and beam conditions were set at 20 kV accelerating voltage, a load current ranging from 58 to 87μA, and a spot size of 7. A backscattering electron (BSE) detector (Centaurus Detector) was used to detect the reflected electrons from the bone. Calibration of the machine was performed with silicon dioxide (SiO2, Z = 30) before and after taking images from each sample. Six to nine images at ×200 magnification were taken from each sample at a working distance of 15 mm. Regions of both cortical and trabecular bone in each sample were imaged; the nonmineralized tissues and the plastic matrix appeared black. The mineralized bone exhibited a range of grayscale values, and the area at each gray level was measured from the Backscattering images using Quantimet image analysis system (Leica). The percentage area (% area) of pixels excluding the plastic region was calculated. The gray level intensity distribution was described using the logit function, or cumulative log ratio,(49) and is defined as follows: logit = ln(proportion > X)/(proportion < X), where X is a given gray level. From the histogram, the gray level of the histogram peak (peak gray level) was determined and used to represent the degree of mineralization. Increasing gray levels represent a higher degree of mineralization. The full width at one half the maximum height (FWHMH) of the histogram represents the heterogeneity of the mineralization distribution and gives an approximation of the distribution of less-mineralized (younger) and more mineralized (older) bone.
Statistical analysis
All the data were analyzed using a one-way ANOVA with SPSS statistical software (SPSS, Chicago, IL, USA). Pairwise comparisons between groups were performed using the Fisher's PLSD posthoc test. Significance was considered at p < 0.05. All data are presented as mean ± SE.
RESULTS
Urine calcium excretion
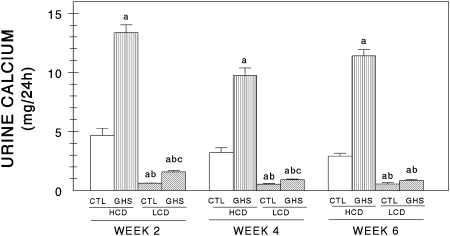
When fed the HCD, urine Ca excretion was significantly greater in the GHS rats than in the Ctl rats at weeks 2, 4, and 6 (Fig. 1). When fed the LCD, urine Ca excretion was again significantly greater in the GHS rats than in the Ctl rats on weeks 2 and 4 but not on week 6. Both strains of rats excreted more urine Ca when fed HCD compared with when fed LCD at all time periods studied.
FIG. 1.
Urine Ca excretion (mean ± SE) in the control (Ctl) and genetic hypercalciuric stone-forming (GHS) rats after 2, 4, and 6 wk of consuming 15 g/d of a high Ca diet (1.2% Ca, HCD) or a low Ca diet (0.02% Ca, LCD). The 24-h urines were collected in concentrated HCl and urine Ca was measured photometrically. aDifferent from Ctl eating HCD, p < 0.05; bdifferent from GHS eating HCD, p < 0.05; cdifferent from Ctl eating LCD, p < 0.05.
DXA
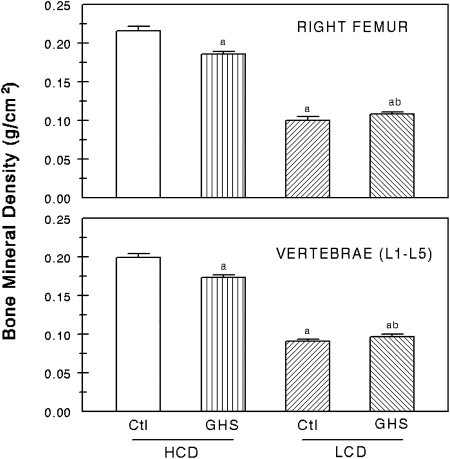
When fed HCD, BMD in both the right femur and in the vertebrae (L1–L5) was lower in the GHS rats than in the Ctl rats (Fig. 2). However, when fed LCD, there was no difference in BMD at either site between the GHS and Ctl rats. Both GHS and Ctl rats had substantially lower femoral and vertebral BMD when fed LCD compared with HCD.
FIG. 2.
BMD of the whole right femors, representing cortical bone, and the lumbar vertebrae (L1–L5) representing trabecular bone (mean ± SE). BMD was measured using DXA at the conclusion of the study. aDifferent from Ctl eating HCD, p < 0.05; bdifferent from GHS eating HCD, p < 0.05.
Histomorphometry
Compared with Ctl, trabecular bone volume (BV) and mineralized trabecular bone volume (Md.V) were both significantly lower in the GHS rats compared with the Ctl rats, when eating either diet (Table 1). Compared with HCD, BV and Md.V were significantly reduced with LCD in both rat strains. Compared with Ctl, neither osteoid volume (OV) nor osteoid surface (OS) was altered in GHS rats on either diet. However, compared with HCD, both OV and OS increased significantly with LCD in Ctl and in GHS rats. Compared with Ctl, with HCD, but not with LCD, trabecular thickness (Tb.Th) was significantly lower in the GHS rats. Compared with HCD, Tb.Th was significantly reduced with LCD in Ctl and in GHS rats. Compared with Ctl, trabecular number (Tb.N.) was not altered in GHS on HCD but was reduced on LCD. Compared with HCD, Tb.N was significantly reduced with LCD in both rat strains. Compared with Ctl, GHS rats had an increased trabecular separation (Tb.Sp) only while on LCD but not on HCD. Compared with HCD, Tb.Sp was significantly increased with LCD in both rat strains.
Table 1.
Histomorphometry
| Ctl HCD | GHS HCD | Ctl LCD | GHS LCD | |
| BV (%) | 18.5 ± 0.9 | 14.2 ± 0.4* | 9.3 ± 0.4* | 6.6 ± 0.4†‡ |
| Md.V (%) | 18.5 ± 0.9 | 14.2 ± 0.3* | 8.9 ± 0.4* | 6.4 ± 0.4†‡ |
| OV (%) | 0.16 ± 0.04 | 0.21 ± 0.14 | 3.6 ± 0.4* | 3.2 ± 0.4† |
| OS (%) | 1.3 ± 0.4 | 1.2 ± 0.6 | 23 ± 2* | 21 ± 2† |
| ES (%) | 0.51 ± 0.13 | 0.25 ± 0.04 | 0.80 ± 0.14 | 0.43 ± 0.15 |
| Tb.Th (μm) | 52 ± 1 | 40 ± 1* | 34 ± 2* | 32 ± 2† |
| O.Th (μm) | 3.4 ± 0.8 | 3.7 ± 1.2 | 2.8 ± 0.3 | 2.4 ± 0.1 |
| Tb.N (mm−1) | 3.56 ± 0.17 | 3.57 ± 0.08 | 2.79 ± 0.24* | 2.13 ± 0.16†‡ |
| Tb.Sp (μm) | 233 ± 13 | 241 ± 6 | 345 ± 34* | 464 ± 51†‡ |
Data are mean ± SE; n = 8 all groups.
* Different from control rats eating the high Ca diet, p < 0.001.
† Different from GHS rats eating the high Ca diet, p < 0.001.
‡ Different from control rats eating the low Ca diet, p < 0.001.
Ctl, control rats; GHS, genetic hypercalciuric stone forming rats; HCD, high Ca (1.2%) diet; LCD, low Ca (0.02%) diet; BV, bone volume; Md.V, mineralized trabecular bone volume; OV, osteoid volume; OS, osteoid surface; ES, eroded surface; Tb.Th, trabecular thickness; O.Th, osteoid thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
Degree of mineralization (BSE)
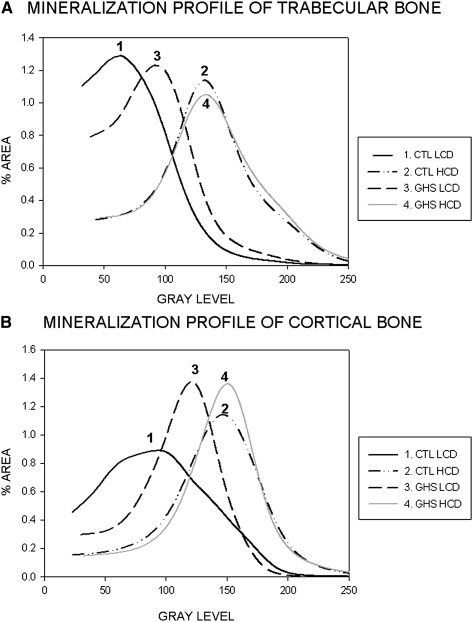
In general, the cortical bone is more mineralized than the trabecular bone as shown by the shift of the curves toward higher gray levels in Fig. 3B compared with Fig. 3A. In both trabecular and cortical bone, the mineralization profile was significantly lower (p < 0.05) in LCD compared with HCD in both the GSH and Ctl rats as indicated by the lower gray level in both LCD groups using the logit function.
FIG. 3.
Histograms of mineralization profile for trabecular bone (A) and cortical bone (B). Higher gray level (shift of curve to right) indicated an increased level of mineralization. Increased width of curve indicates increased degree of heterogenicity.
When fed HCD, both GSH and Ctl rats had similar trabecular and cortical bone mineralization profile (curves 2 and 4 in Figs. 3A and 3B). However, when both the GHS and Ctl rats were fed LCD, there is a decrease in their degree of mineralization. This shift toward lower degree of mineralization is greater in the Ctl rats (27% decrease in the peak of the gray level in trabecular and 21% decrease in cortical bone) than in the GHS rats (17% decrease in trabecular and 11% decrease in cortical bone). Thus, the GHS rats retain a higher degree of mineralization when fed LCD compared with Ctl rats.
The Ctl rats fed LCD have an increase in the heterogeneity of the mineralization distribution as indicated by wider curves in Figs. 3A and 3B and a significant increase in mineralization as described by the parameter FWHMH for both trabecular and cortical bone, which is not observed in the GHS rats.
Mechanical tests
The failure properties of the humeri were significantly different between the GHS and Ctl rats and were also significantly affected by diet (Table 2).
Table 2.
Mechanical Properties
| Ctl HCD | GHS HCD | Ctl LCD | GHS LCD | |
| Humeri (cortical bone) | ||||
| Failure stress (MPa) | 202 ± 5 | 193 ± 9 | 118 ± 10* | 148 ± 8†‡ |
| Failure strain | 0.064 ± 0.003 | 0.044 ± 0.002* | 0.099 ± 0.004* | 0.065 ± 0.004†‡ |
| Modulus (MPa) | 3253 ± 165 | 4633 ± 461* | 1164 ± 96* | 2278 ± 215†‡ |
| Toughness (J/mm2) | 7.8 ± 0.4 | 4.6 ± 0.3* | 8.5 ± 0.9 | 6.2 ± 0.3†‡ |
| Fifth lumbar vertebrae (trabecular bone) | ||||
| Failure stress (MPa) | 19.8 ± 1.7 | 16.3 ± 1.5 | 7.5 ± 0.7* | 5.8 ± 0.7† |
| Failure strain | 0.078 ± 0.007 | 0.070 ± 0.006 | 0.093 ± 0.018 | 0.059 ± 0.009‡ |
| Modulus (MPa) | 251 ± 6 | 238 ± 24 | 88 ± 11* | 89 ± 6† |
| Toughness (J/mm2) | 0.87 ± 0.12 | 0.62 ± 0.9 | 0.37 ± 0.7* | 0.23 ± 0.6† |
Data are mean ± SE; n = 8 all groups.
* Different from Ctl eating HCD, p < 0.001.
† Different from GHS eating HCD, p < 0.001.
‡ Different from Ctl eating LCD, p < 0.001.
Ctl, control rats; GHS, genetic hypercalciuric stone forming rats; HCD, high Ca diet,1.2% Ca; LCD, low Ca diet, 0.02% Ca.
When fed both HCD and LCD, the humeral (cortical) bone of the GHS rats was more brittle, characterized by an increase in stiffness (modulus) with an associated reduction in both toughness and ductility (failure strain). Interestingly, the opposite effect was observed in both rat strains when fed LCD compared with HCD: a decrease in strength (failure stress) and stiffness and an associated increase in ductility.
In the vertebral bone, there was only a significant effect of diet on the failure properties of trabecular bone (Table 2). Both GHS and Ctl rats fed LCD had a general loss of the vertebral trabecular bone mechanical properties (strength, stiffness, and toughness, by 57–65%).
DISCUSSION
Patients with idiopathic hypercalciuria often have a reduction in BMD.(1,6,13,16–22) Whether this reduction is caused by a primary disorder of bone formation and/or resorption related to the hypercalciuria or caused by differences in dietary intake, perhaps over a lifetime, between stone formers compared with normal controls is not known and virtually impossible to determine in humans. Using a genetic strain of rats that has been bred for hypercalciuria and spontaneously produce kidney stones, the GHS rats, we tested the hypothesis that there is a primary abnormality in bone mineral not related to differences in diet. We found that even when the GHS rats were fed HCD, there were significant differences in bone mineral compared with identically fed control rats. When compared with controls, the bone of the GHS rats not only had a reduction in density but had reduced trabecular volume and thickness and was more brittle and fracture prone.
Compared with the control rats, the cortical bone (humeri) in GHS rats differed more than trabecular (vertebral) bone. In GHS rats consuming HCD, the cortical bone was stiffer and less ductile, leading to a decrease in toughness that may induce an increased risk of fracture. These differences were not seen in the trabecular bone. However, the cortical bone of the GHS rats on LCD was stronger and stiffer than the control rats fed the same diet. This may be explained by the smaller decrease in mineralization found in the GHS rats compared with the controls when fed LCD. This effect concurs with data showing that rats fed a high fluoride diet were more resistant to bone loss during a period of Ca deficiency than control rats.(50)
The defects in mechanical properties in the GHS rats fed both the HCD and LCD are mainly manifested in cortical rather than trabecular bone. Histomorphometric analysis showed that the GHS rats fed the HCD had a significant decrease in trabecular volume and trabecular thickness (Table 1), yet the mechanical properties of their vertebrae (trabecular bone), whereas numerically lower, did not differ significantly. In both control and GHS rats, the LCD induced a significant increase in osteoid surface and volume, indicating an increase in the rate of bone formation or a decrease in the rate of mineralization. Further studies, perhaps using tetracycline labeling, will be necessary to determine the dynamics of mineralization when the rats are fed LCD.
Compared with non–stone formers, hypercalciuric stone formers often have a mild decrease in BMD.(1,6,13,16–22) Pietschmann et al.(16) found lower spinal BMD in hypercalciuric compared with normocalciuric patients, and Jaeger et al.(17) found that stone formers were slightly shorter and had a significantly lower BMD at specific sites compared with controls. Giannini et al.(18) found that recurrent hypercalciuric stone formers had reduced BMD compared with normal controls, whereas Misael da Silva et al.(19) examined bone formation and resorption parameters in 40 stone formers and classified 10 as osteopenic. Tasca et al.(20) found a more negative Z-score in hypercalciuric patients than in controls. Analysis of the Third National Health and Nutrition Examination Survey (NHANES III) showed that men with a history of kidney stones have a lower femoral neck BMD than those without a history of stones.(51) Analysis of almost 6000 older men again showed an association of kidney stones with decreased BMD.(52) Calcium stone formers seem to have an increased risk of fractures.(51,53) Data from NHANES III showed an increased risk of wrist and spine fractures in stone formers,(51) and in a retrospective analysis, stone formers had an increased incidence of vertebral fractures but not fractures at other sites.(53) BMD is correlated inversely with urine Ca excretion in men(54) and in women.(55)
Although these studies in humans showed, in general, a reduction in BMD in stone formers, there are many dietary influences on BMD over a lifetime. Stone formers often consume a diet poor in Ca, with ample sodium and protein.(56) Each of these dietary constituents influences urine Ca excretion,(23–25) which potentially can alter the bone mineral. For example, Lauderdale et al.,(51) in analyzing NHANES III, has shown that there is a reduction in BMD in stone formers if they habitually consume virtually no milk. Walser(24) showed that Ca clearance is directly correlated with sodium clearance. Asplin et al.(15) has shown that, in stone formers, there is an increase in urine ammonia excretion, indicating increased net acid intake that is generally from dietary protein, which is correlated inversely with femoral neck and vertebral BMD.
In a previous study, PTH was found to be lower in the GHS rats compared with control Sprague-Dawley rats fed a high calcium diet.(57) This observation suggests that, in the GHS, the principle mechanism of the hypercalciuria is not an inability of the renal tubule to conserve calcium but either an increase in bone resorption or intestinal calcium absorption. Compared with controls, levels of 1,25(OH)2D were normal to elevated,(31,37,38,58,59) and levels of the vitamin D receptor were markedly elevated in the bone, intestine and kidney of the GHS rats.(31,34,38,59,60) The elevated levels of the vitamin D receptor suggest that the normal to elevated levels of 1,25(OH)2D are more effective and result in an enhanced effect of 1,25(OH)2D, which could be acting on the bone to increase resorption and/or the intestine to increase absorption.
The hypercalciuria in the GHS rats is caused by a systemic dysregulation of Ca transport.(38) We showed increased intestinal Ca absorption,(39) a defect in renal tubular Ca reabsorption,(33,37) and enhanced bone mineral resorption(34) in these rats. To study bone homeostasis in GHS rats in vivo, we fed GHS and control rats a diet with adequate Ca and switched them to a low Ca diet.(37) The low Ca diet led to the renal excretion of more Ca than was present in the diet; thus, the GHS rats were in negative Ca balance. To confirm that bone was the source of the additional urine Ca, we continued both groups of rats on the low Ca diet and injected some with the bone resorption blocker alendronate.(29) Alendronate caused a significant decrease in urine Ca in the GHS but not in control and brought the urine Ca in the GHS rats to below the dietary Ca intake, indicating that, on a low Ca diet, there is a significant contribution of bone Ca to the increased urine Ca excretion. To support that there was a primary abnormality in the bone homeostasis in the GHS rats, we cultured neonatal bone (calvariae) from GHS and control rats. We found that cultured calvariae exhibited greater sensitivity to 1,25(OH)2D3 than did bone from controls.(34) In contrast, PTH induced similar bone resorption in control and GHS calvariae. Immunoblot analysis showed a 4-fold increase in the level of 1,25(OH)2D3 receptors (VDR) in GHS calvariae compared with control calvariae, similar to the increased intestinal receptors described previously.(31,34,38) There was no comparable change in VDR RNA levels as measured by slot blot analysis, suggesting the altered regulation of the VDR occurs post-transcriptionally. The current data, the first studying bone from the GHS rats in vivo, support that there is a primary defect in bone homeostasis in this animal model of hypercalciuria induced stone formation.
Thus, using the GHS rats, an excellent model of hypercalciuria and stone formation, we showed reduced BMD in both cortical (femoral) and trabecular (vertebral) bone. We showed that the cortical bone is more brittle, whereas the trabecular bone is less affected. Whether therapy to reduce the hypercalciuria will correct these abnormalities of bone has yet to be determined.
ACKNOWLEDGMENTS
This work was supported in part by NIH Grants RO1 AR 46289 and RO1 DK 75462 (both to D.A.B.) from the National Institutes of Health.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Monk RD, Bushinsky DA. Kidney stones. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA, USA: W.B. Saunders; 2008. pp. 1311–1326. [Google Scholar]
- 2.Monk RD, Bushinsky DA. Nephrolithiasis and nephrocalcinosis. In: Frehally J, Floege J, Johnson RJ, editors. Comprehensive Clinical Nephrology. 3rd ed. London, UK: Mosby; 2007. pp. 641–655. [Google Scholar]
- 3.Bushinsky DA. Nephrolithiasis. J Am Soc Nephrol. 1998;9:917–924. doi: 10.1681/ASN.V95917. [DOI] [PubMed] [Google Scholar]
- 4.Bushinsky DA, Coe FL, Moe OW. Nephrolithiasis. In: Brenner BM, editor. The Kidney. 8th ed. Philadelphia, PA, USA: W.B. Saunders; 2008. pp. 1299–1349. [Google Scholar]
- 5.Bushinsky DA. Renal lithiasis. In: Humes HD, editor. Kelly's Textbook of Medicine. New York, NY, USA: Lippincott Williams & Wilkens; 2000. pp. 1243–1248. [Google Scholar]
- 6.Bushinsky DA. Recurrent hypercalciuric nephrolithiasis—does diet help? N Engl J Med. 2002;346:124–125. doi: 10.1056/NEJM200201103460210. [DOI] [PubMed] [Google Scholar]
- 7.Bushinsky DA. Calcium nephrolithiasis. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th ed. Washington, DC, USA: American Society of Bone and Mineral Research; 2008. pp. 460–464. [Google Scholar]
- 8.Consensus Conference. Prevention and treatment of kidney stones. JAMA. 1988;260:977–981. [PubMed] [Google Scholar]
- 9.Pak CYC. Pathophysiology of calcium nephrolithiasis. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. 2nd ed. New York, NY, USA: Raven Press; 1992. pp. 2461–2480. [Google Scholar]
- 10.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coe FL, Bushinsky DA. Pathophysiology of hypercalciuria. Am J Physiol. 1984;247:F1–F13. doi: 10.1152/ajprenal.1984.247.1.F1. [DOI] [PubMed] [Google Scholar]
- 12.Edwards NA, Hodgkinson A. Metabolic studies in patients with idiopathic hypercalciuria. Clin Sci. 1965;29:143–157. [PubMed] [Google Scholar]
- 13.Coe FL, Favus MJ, Crockett T, Strauss AL, Parks JH, Porat A, Gantt C, Sherwood LM. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. Am J Med. 1982;72:25–32. doi: 10.1016/0002-9343(82)90567-8. [DOI] [PubMed] [Google Scholar]
- 14.Monk RD, Bushinsky DA. Pathogenesis of idiopathic hypercalciuria. In: Coe F, Favus M, Pak C, Parks J, Preminger G, editors. Kidney Stones: Medical and Surgical Management. Philadelphia, PA, USA: Lippincott-Raven; 1996. pp. 759–772. [Google Scholar]
- 15.Asplin JR, Bauer KA, Kinder J, Muller G, Coe BJ, Parks JH, Coe FL. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003;63:662–669. doi: 10.1046/j.1523-1755.2003.00763.x. [DOI] [PubMed] [Google Scholar]
- 16.Pietschmann F, Breslau NA, Pak CYC. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res. 1992;7:1383–1388. doi: 10.1002/jbmr.5650071205. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger P, Lippuner K, Casez JP, Hess B, Ackerman D, Hug C. Low bone mass in idiopathic renal stone formers: Magnitude and significance. J Bone Miner Res. 1994;9:1525–1532. doi: 10.1002/jbmr.5650091004. [DOI] [PubMed] [Google Scholar]
- 18.Giannini S, Nobile M, Sartori L, Calo L, Tasca A, Dalle Carbonare L, Ciuffreda M, D'Angelo A, Pagano F, Crepaldi G. Bone density and skeletal metabolism are altered in idiopathic hypercalciuria. Clin Nephrol. 1998;50:94–100. [PubMed] [Google Scholar]
- 19.Misael da Silva AM, dos Reis LM, Pereira RC, Futata E, Branco-Martins CT, Noronha IL, Wajchemberg BL, Jorgetti V. Bone involvement in idiopathic hypercalciuria. Clin Nephrol. 2002;57:183–191. doi: 10.5414/cnp57183. [DOI] [PubMed] [Google Scholar]
- 20.Tasca A, Cacciola A, Ferrarese P, Ioverno E, Visona E, Bernardi C, Nobile M, Giannini S. Bone alterations in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Urology. 2002;59:865–869. doi: 10.1016/s0090-4295(02)01626-6. [DOI] [PubMed] [Google Scholar]
- 21.Heilberg IP, Martini LA, Teixeira SH, Szejnfeld VL, Carvalho AB, Lobao R, Draibe SA. Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron. 1998;79:430–437. doi: 10.1159/000045089. [DOI] [PubMed] [Google Scholar]
- 22.Bataille P, Achard JM, Fournier A, Boudailliez B, Westell PF, Esper NE, Bergot C, Jans I, Lalau JD, Petit J, Henon G, Jeantet MAL, Bouillon R, Sebert JL. Diet, vitamin D and vertebral mineral density in hypercalciuric calcium stone formers. Kidney Int. 1991;39:1193–1205. doi: 10.1038/ki.1991.151. [DOI] [PubMed] [Google Scholar]
- 23.Hunt CD, Johnson LK. Calcium requirements: New estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies. Am J Clin Nutr. 2007;86:1054–1063. doi: 10.1093/ajcn/86.4.1054. [DOI] [PubMed] [Google Scholar]
- 24.Walser M. Calcium clearance as a function of sodium clearance in the dog. Am J Physiol. 1961;200:1099–1104. doi: 10.1152/ajplegacy.1961.200.5.1099. [DOI] [PubMed] [Google Scholar]
- 25.Lemann J, Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol. 2003;285:F811–F832. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 26.Evan AP, Bledsoe SB, Smith SB, Bushinsky DA. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int. 2004;65:154–161. doi: 10.1111/j.1523-1755.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 27.Bushinsky DA, Bashir MA, Riordon DR, Nakagawa Y, Coe FL, Grynpas MD. Increased dietary oxalate does not increase urinary calcium oxalate saturation in hypercalciuric rats. Kidney Int. 1999;55:602–612. doi: 10.1046/j.1523-1755.1999.00281.x. [DOI] [PubMed] [Google Scholar]
- 28.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone forming rats. Kidney Int. 2001;59:1415–1423. doi: 10.1046/j.1523-1755.2001.0590041415.x. [DOI] [PubMed] [Google Scholar]
- 29.Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int. 1999;55:234–243. doi: 10.1046/j.1523-1755.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 30.Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2000;57:550–560. doi: 10.1046/j.1523-1755.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 31.Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3: A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest. 1998;101:2223–2232. doi: 10.1172/JCI1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asplin JR, Bushinsky DA, Singharetnam W, Riordon D, Parks JH, Coe FL. Relationship between supersaturation and crystal inhibition in hypercalciuric rats. Kidney Int. 1997;51:640–645. doi: 10.1038/ki.1997.93. [DOI] [PubMed] [Google Scholar]
- 33.Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int. 1997;51:1540–1547. doi: 10.1038/ki.1997.212. [DOI] [PubMed] [Google Scholar]
- 34.Krieger NS, Stathopoulos VM, Bushinsky DA. Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol Cell Physiol. 1996;271:C130–C135. doi: 10.1152/ajpcell.1996.271.1.C130. [DOI] [PubMed] [Google Scholar]
- 35.Bushinsky DA, Grynpas MD, Nilsson EL, Nakagawa Y, Coe FL. Stone formation in genetic hypercalciuric rats. Kidney Int. 1995;48:1705–1713. doi: 10.1038/ki.1995.468. [DOI] [PubMed] [Google Scholar]
- 36.Bushinsky DA, Kim M, Sessler NE, Nakagawa Y, Coe FL. Increased urinary saturation and kidney calcium content in genetic hypercalciuric rats. Kidney Int. 1994;45:58–65. doi: 10.1038/ki.1994.7. [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Sessler NE, Tembe V, Favus MJ, Bushinsky DA. Response of genetic hypercalciuric rats to a low calcium diet. Kidney Int. 1993;43:189–196. doi: 10.1038/ki.1993.31. [DOI] [PubMed] [Google Scholar]
- 38.Li X-Q, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats: A cause of intestinal calcium hyperabsorption. J Clin Invest. 1993;91:661–667. doi: 10.1172/JCI116246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats: Inherited defect in intestinal calcium transport. J Clin Invest. 1988;82:1585–1591. doi: 10.1172/JCI113770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bushinsky DA, Asplin JR, Grynpas MD, Evan AP, Parker WR, Alexander KM, Coe FL. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2002;61:975–987. doi: 10.1046/j.1523-1755.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- 41.Hagler L, Herman RH. Oxalate metabolism. I. Am J Clin Nutr. 1973;26:758–765. doi: 10.1093/ajcn/26.6.758. [DOI] [PubMed] [Google Scholar]
- 42.Bushinsky DA. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 1999;8:479–488. doi: 10.1097/00041552-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Bushinsky DA. Genetic Hypercalciuric stone forming rats. Semin Nephrol. 1996;16:448–457. [PubMed] [Google Scholar]
- 44.Michalylova V, Ilkova P. Photometric determination of microamounts of calcium with arsenazo III. Anal Chim Acta. 1971;53:194–198. [Google Scholar]
- 45.Nagy TR, Clair AL. Precision and accuracy of dual-energy x-ray absorption for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 46.Kasra M, Vanin CM, MacLusky NJ, Casper RF, Grynpas MD. Effects of different estrogen and progestin regimens on the mechanical properties of rat femur. J Orthop Res. 1997;15:118–123. doi: 10.1002/jor.1100150117. [DOI] [PubMed] [Google Scholar]
- 47.Chachra D, Kasra M, Vanin CM, MacLusky NJ, Casper RF, Grynpas M. The effect of different hormone replacement therapy regimens on the mechanical properties of rat vertebrae. Calcif Tissue Int. 1995;56:130–143. doi: 10.1007/BF00296344. [DOI] [PubMed] [Google Scholar]
- 48.Parfitt AM, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Reker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 49.Bracci PM, Bull SB, Grynpas M. Analysis of compositional bone density data using log ration transformations. Biometrics. 1998;54:337–349. [PubMed] [Google Scholar]
- 50.Ericsson Y, Ekberg O. Dietetically provoked general and alveolar osteopenia in rats and its prevention or cure by calcium and fluoride. J Periodontal Res. 1975;10:56–69. doi: 10.1111/j.1600-0765.1975.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 51.Lauderdale DS, Thisted RA, Wen M, Favus M. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res. 2001;16:1893–1898. doi: 10.1359/jbmr.2001.16.10.1893. [DOI] [PubMed] [Google Scholar]
- 52.Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, Ensrud K, Lau EM, Orwoll ES for the MRoS Research Group. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporosis Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 53.Melton LJI, Crowson CS, Khosla S, Wilson DM, Fallon WM. Fracture rick among patients with urolithiasis: A population based cohort study. Kidney Int. 1998;53:459–464. doi: 10.1046/j.1523-1755.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 54.Vezzoli G, Soldati L, Arcidiacono T, Terranegra A, Biasion R, Russo CR, Lauretani F, Bandinelli S, Bartali B, Cherubini A, Cusi D, Ferrucci L. Urine calcium is a determinant of bone mineral density in elderly men participating in the InCHIANTI study. Kidney Int. 2005;67:2006–2014. doi: 10.1111/j.1523-1755.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 55.Giannini S, Nobile M, Dalle Carbonare L, Lodetti MG, Sella S, Vittadello G, Minicuci N, Crepaldi G. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol. 2003;149:209–213. doi: 10.1530/eje.0.1490209. [DOI] [PubMed] [Google Scholar]
- 56.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 57.Bushinsky DA, LaPlante K, Asplin JR. Effect of cinacalcet on urine calcium excretion and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int. 2006;69:1586–1592. doi: 10.1038/sj.ki.5000324. [DOI] [PubMed] [Google Scholar]
- 58.Yao J, Karnauskas AJ, Bushinsky DA, Favus MJ. Regulation of renal calcium-sensing receptor gene expression in response to 1,25(OH)2D3 in genetic hypercalciuric stone forming rats. J Am Soc Nephrol. 2005;16:1300–1308. doi: 10.1681/ASN.2004110991. [DOI] [PubMed] [Google Scholar]
- 59.Karnauskas AJ, van Leeuwen JP, van den Bemd GJ, Kathpalia PP, DeLuca HF, Bushinsky DA, Favus MJ. Mechanism and function of high vitamin D receptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res. 2005;20:447–454. doi: 10.1359/JBMR.041120. [DOI] [PubMed] [Google Scholar]
- 60.Favus MJ. Hypercalciuria: Lessons from studies of genetic hypercalciuric rats. J Am Soc Nephrol. 1994;5:S54–S58. doi: 10.1681/ASN.V55s54. [DOI] [PubMed] [Google Scholar]