Abstract
T-cell activation requires the influx of extracellular calcium, although mechanistic details regarding such activation are not fully defined. Here, we show that P2X7 receptors play a key role in calcium influx and downstream signaling events associated with the activation of T cells. By real-time PCR and immunohistochemistry, we find that Jurkat T cells and human CD4+ T cells express abundant P2X7 receptors. We show, using a novel fluorescent microscopy technique, that T-cell receptor (TCR) stimulation triggers the rapid release of ATP (<100 μM). This release of ATP is required for TCR-mediated calcium influx, NFAT activation, and interleukin-2 (IL-2) production. TCR activation up-regulates P2X7 receptor gene expression. Removal of extracellular ATP by apyrase or alkaline phosphatase treatment, inhibition of ATP release with the maxi-anion channel blocker gadolinium chloride, or siRNA silencing of P2X7 receptors blocks calcium entry and inhibits T-cell activation. Moreover, lymphocyte activation is impaired in C57BL/6 mice that express poorly functional P2X7 receptors, compared to control BALB/c mice, which express fully functional P2X7 receptors. We conclude that ATP release and autocrine, positive feedback through P2X7 receptors is required for the effective activation of T cells.—Yip, L., Woehrle, T., Corriden, R., Hirsh, M., Chen, Y., Inoue, Y., Ferrari, V., Insel, P. A., Junger, W. G. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors.
Keywords: interleukin-2, NFAT, calcium signaling, real-time PCR, siRNA silencing
T cells play a central role in controlling adaptive immune responses. Stimulation of T cells triggers a rapid rise in cytosolic Ca2+ that is required for activation of nuclear factor of activated T cells (NFAT), which leads to the expression of interleukin-2 (IL-2) and ultimately T-cell proliferation (1, 2). Previous work has demonstrated that osmotic stress and mechanical stimulation of T cells induce the release of cellular ATP, which regulates T-cell function (3,4,5). In addition, activated cytotoxic T cells can release ATP as a means of killing target cells (6,7,8).
The binding of extracellular ATP to P2X receptors, which are ATP-gated ion channels, induces the influx of extracellular Ca2+ (9, 10). Among the seven known P2X receptor subtypes (P2X1–7), P2X7 receptors are particularly highly expressed in immune tissues (9,10,11,12,13). In T cells, P2X7 receptors initiate Ca2+-dependent downstream signals that lead to T-cell activation and proliferation (4, 14). Activation of P2X7 receptors has also been implicated in the production of proinflammatory cytokines (e.g., IL-2, IL-1β, and IL-18) and in the regulation of cell proliferation and cell death (14,15,16,17,18). In human peripheral blood mononuclear cells (PBMCs) and T cells, P2X7 receptor stimulation induces proliferation and IL-2 synthesis (4, 14, 19), while pharmacological blockade of P2X7 receptors inhibits TCR-stimulated T-cell activation (18). Stimulation of P2X7 receptors also induces the shedding of L-selectin (CD62L), a process associated with T-cell activation (20,21,22).
While previous studies have shown that prolonged stimulation of P2X7 receptors with high concentrations of ATP induces cellular apoptosis (15, 23,24,25), transient stimulation of T cells with lower ATP concentrations, such as those secreted in an autocrine or paracrine fashion, can stimulate T-cell proliferation (23). Under such conditions, overexpression of P2X7 receptors can further promote T-cell proliferation (23, 26). Our previous work using Jurkat T cells has demonstrated that osmotic stress induces the release of endogenous ATP, which achieves extracellular concentrations of ∼10 μM (3). In contrast, apoptosis induced by P2X7 receptor in CD4+ and CD8+ T cells occurs only when extracellular ATP concentrations exceed 100 μM (15). Thus, we hypothesized that stimulation of P2X7 receptors by ATP concentrations that are released endogenously contribute to T-cell activation. The objective of the present study was to examine whether activation of T cells involves the release of endogenous ATP and if so, whether the released ATP acts via P2X7 receptors to regulate early T-cell activation processes, such as Ca2+ influx, NFAT activation, and IL-2 production.
MATERIALS AND METHODS
Cells and cell stimulation
Jurkat cells (E6–1 clone) from American Type Culture Collection (ATCC; Manassas, VA, USA) were maintained in complete RPMI 1640 medium (Irvine Scientific; Santa Ana, CA, USA) containing 10% heat-inactivated fetal calf serum (Omega Scientific; Tarzana, CA, USA), 50 μg/ml gentamicin (Life Technologies, Inc.; Grand Island, NY, USA), 10 mM HEPES (Fisher Scientific; Waltham, MA, USA), 1 mM sodium pyruvate (Fisher Scientific), 0.1 mM MEM nonessential amino acids (Fisher Scientific), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Invitrogen, Carlsbad, CA, USA). Human PBMCs and purified CD4+ T cells were prepared and maintained as described previously (3). Cells were stimulated with anti-CD3 (OKT-3; obtained from ATCC clone CRL8001) and anti-CD28 antibodies (BD Pharmingen; San Jose, CA, USA) as described previously (4). Mouse splenocytes (106) were isolated from the spleens of BALB/c or C57BL/6 mice (Charles River Labs, Wilmington, MA, USA), stimulated with phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO, USA) for 2 h, and IL-2 production of lymphocytes was assessed as described below. Unstimulated cells or cells stimulated with 50 ng/ml phorbol myristate acetate and 5 μM ionomycin served as negative or positive controls, respectively.
ATP release
Jurkat cells were stimulated by ligation of CD3/CD28 using MACSibeads coated with anti-CD3 and anti-CD28 antibodies (1 bead/cell; Miltenyi Biotec; Auburn, CA, USA) and extracellular ATP levels near the cell surface were measured using fluorescence microscopy or HPLC analysis (3, 5, 17) or by use of an ATP Bioluminescence Assay Kit HSII from Roche (Palo Alto, CA, USA) and a Luminoskan chemiluminometer (Labsystems, Helsinki, Finland), according to the manufacturer’s instructions. The specificity of the fluorescence microscopy assay has previously been described (5). ADP, AMP, and adenosine levels in the bulk medium of anti-CD3- and anti-CD28-stimulated cells were also measured by HPLC (3, 5, 17).
Intracellular Ca2+ measurements
Cells were loaded with Fura-2-AM (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instructions, and changes in intracellular Ca2+ levels in response to cell stimulation were measured (4). To assess the role of P2 receptors and ATP in Ca2+ signaling, Fura-2 loaded cells were treated with suramin, NF023, or o-ATP (Sigma-Aldrich) for 1 h before stimulation or with apyrase (Sigma-Aldrich) or alkaline phosphatase (ALP; New England Biolabs; Ipswich, MA, USA) for 15 min prior to stimulation.
IL-2 measurements
IL-2 expression was measured in cell supernatants of CD3-stimulated PBMCs (2×105) or CD3/CD28-stimulated Jurkat cells (5×104) using an ELISA assay (3, 4). IL-2 expression in mouse lymphocytes was measured with an intracellular cytokine staining assay using flow cytometry (27). Mouse splenocytes were isolated and stimulated as described above, and then they were incubated in the presence of 2 μg/ml brefeldin A for 20 h, treated with 0.1% saponin in PBS containing 1% FCS and 2 mM EDTA, washed, and stained with PE-labeled anti-mouse IL-2 antibody (BD Pharmingen) or isotype controls. Cells were washed, fixed, and analyzed by flow cytometry. Lymphocytes were identified according to forward- and side-scatter properties, and data from 104 gated cells were acquired for each sample.
P2X7 receptor and IL-2 mRNA expression
We assayed P2X7 receptor mRNA transcript levels in Jurkat cells or human CD4+ T cells before or 1 h after stimulation with CD3/CD28-loaded beads using real-time RT-PCR (3, 17). The comparative Ct method for relative quantification of gene expression (ΔΔCt method) was used, and gene expression levels were normalized to gene expression of 18S rRNA (as a housekeeping gene control) using the following equation: normalized expression = 2[Ct(18S rRNA) − Ct(P2X7 receptor)]. P2X7 mRNA and 18S rRNA were assessed using the following primer sets: P2X7: 5′-ATC GGC TCA ACC CTC TCC TAC-3′, 5′-CTG GAG TAA GTG TCG ATG AGG AAG-3′; and 18S rRNA: 5′-CCG CAG CTA GGA ATA ATG GA-3′, 5′-CCC TCT TAA TCA TGG CCT CA-3′ (3). IL-2 mRNA expression was determined in anti-CD3/CD28-stimulated Jurkat cells in the presence or absence of 100 μM ATP using a primer set purchased from Qiagen (Germantown, MD, USA). This ATP concentration was chosen because it significantly enhances IL-2 secretion in stimulated Jurkat cells (Supplemental Fig. 1).
Plasmids, cell transfection, and NFAT-luciferase reporter gene assay
Plasmids containing wild-type or mutant (T1729A) human P2X7 receptor were kindly provided by Dr. J. Wiley (University of Sydney, Sydney, Australia). The NFAT-luciferase reporter plasmid was a gift from Dr. A. Altman (La Jolla Institute of Allergy and Immunology, San Diego, CA, USA), and the β-galactosidase control plasmid was purchased from Roche (Pleasanton, CA, USA). Jurkat cells were transiently transfected by electroporation using a Bio-Rad GenePulser X-cell (Bio-Rad, Hercules, CA, USA) set at 260 V and 950 μF with a 4-mm path length (28). After electroporation, cells were incubated for 24–72 h in complete culture medium. For reporter assays, Jurkat cells (107 in 0.4 ml RPMI) were transfected with 6 μg NFAT-luciferase reporter plasmid, 8 μg β-galactosidase reporter plasmid, and 5 μg wild-type P2X7 receptor plasmid or appropriate empty control. After incubation for 72 h, cells were stimulated with beads loaded with antibodies to CD3/CD28, as described above and incubated for another 8 h. Luciferase activity was measured using the Luciferase Substrate kit (BD Pharmingen); β-galactosidase activity was used to normalize for transfection efficiency (28).
P2X7 receptor silencing using siRNA
Three different siRNA constructs targeting the P2X7 receptor, and a Cy3-labeled nonsense siRNA construct were purchased from Ambion (Austin, TX, USA). siRNA transfection was performed by electroporation as described above. To determine the transfection efficiency, Jurkat cells were transfected with the Cy3-labeled nonsense siRNA construct. Cells were washed and visualized by microscopy 24 h posttransfection; >95% of cells contained Cy3-labeled siRNA (Fig. 6A). We selected the most effective of the three different siRNA constructs for further experiments. This siRNA silenced P2X7 mRNA levels in Jurkat cells by 90% (Fig. 6B). Ca2+ signaling, after silencing P2X7 receptors, was assessed using flow cytometry and Fluo-3-AM as a calcium probe (Molecular Probes). Briefly, cells were transfected with 3 μg siRNA and 3 μg empty DsRed-N1 plasmid to distinguish transfected from nontransfected cells. After 72 h, cells were loaded with Fluo-3-AM in the presence of Pluronic (Molecular Probes), according to the manufacturer’s instructions; changes in Fluo-3 fluorescence in response to cell stimulation were assessed by flow cytometry. Viable cells were selected based on their forward- and side-scatter properties, and changes in fluorescence were assessed in DsRed-positive cells. Cell responses were normalized using 5 μM ionomycin to account for differences in Fluo-3 loading between individual cell preparations. Results are expressed as percentage maximal response relative to stimulation of nonsilenced control cells.
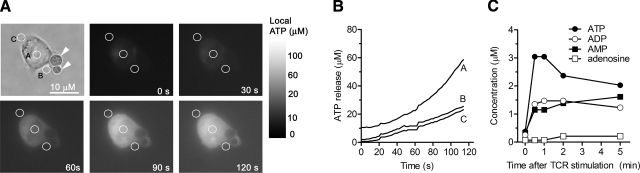
Figure 1.
Stimulation of Jurkat cells releases cellular ATP. A) Jurkat cells were stimulated by ligation of CD3/CD28 using MACSibeads coated with anti-CD3 and anti-CD28 antibodies (arrowheads), and extracellular ATP levels near the cell surface were visualized with a fluorescence microscope assay and estimated using ATP standards. B) Extracellular ATP concentrations determined from the indicated areas of interest shown in A. C) Changes in ATP, ADP, AMP, and adenosine concentrations as a function of time in the bulk cell supernatant of Jurkat cells (5×106) treated with anti-CD3 and anti-CD28 antibodies (1 μg/ml each) were measured with HPLC. Data shown in A and B are representative of 3 and 2 similar experiments, respectively, performed in separate experiments.
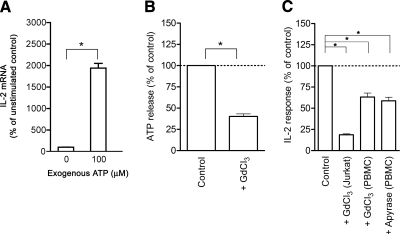
Figure 2.
Extracellular ATP is required for T-cell activation. A) IL-2 mRNA levels of Jurkat cells (107) stimulated for 1 h with anti-CD3/CD28 antibody-coated beads (1 bead/cell) in the presence or absence of 100 μM ATP. B) Inhibition of ATP release from Jurkat cells (107) treated with the maxi-anion channel blocker, GdCl3 (100 μM). Cells were treated in HBSS for 20 min at 37°C and stimulated with anti-CD3/CD28 antibodies (1 μg/ml each) for 1 min, and ATP release was determined using the ATP Bioluminescence Assay Kit HSII. C) GdCl3 (100 μM) and apyrase (20 U/ml) inhibit IL-2 mRNA expression of stimulated Jurkat cells and IL-2 production of stimulated human PBMCs. IL-2 mRNA expression was determined by real-time RT-PCR analysis, and IL-2 production of PBMCs was determined with ELISA. Data are means ± se. *P ≤ 0.05; n = 3; two-tailed unpaired Student’s t test.
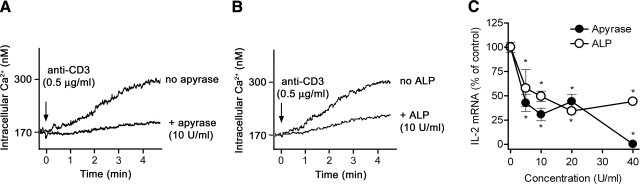
Figure 3.
Scavenging of released ATP inhibits calcium signaling and IL-2 expression. A, B) Scavenging extracellular ATP with 10 U/ml of apyrase (A) or alkaline phosphatase (ALP; B) inhibits intracellular Ca2+ signaling of Jurkat cells stimulated with anti-CD3 antibodies. C) Apyrase and ALP suppressed IL-2 mRNA transcription in response to stimulation of Jurkat cells by ligation of CD3 and CD28 for 4 h. IL-2 mRNA levels were determined by real-time RT-PCR, and intracellular Ca2+ mobilization was measured using Fura-2. Data shown in A and B are representative of 3 similar experiments performed separately. Data shown in C represent means ± se. *P ≤ 0.05; n = 3; two-tailed unpaired Student’s t test.
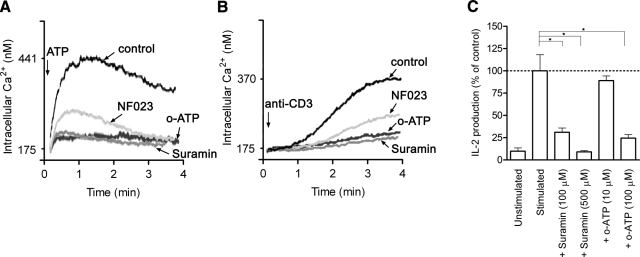
Figure 4.
P2X receptor antagonists inhibit Ca2+ signaling and IL-2 production. A, B) The nonselective P2X receptor antagonists suramin (100 μM) and NF023 (10 μM) and the P2X7-selective antagonist o-ATP (10 μM) blocked Ca2+ signaling in Jurkat cells stimulated with 2 mM ATP (A) or 1 μg/ml anti-CD3 antibodies (B). C) Suramin and o-ATP inhibited IL-2 production of stimulated PBMCs. Cells were treated with antagonists for 1 h prior to cell stimulation. Ca2+ mobilization was measured using Fura-2, and IL-2 production of PBMCs was determined by ELISA. Data in A and B are representative of 3 separate experiments; data in C represent means ± se. *P ≤ 0.05; n = 3; two-tailed unpaired Student’s t test.
Figure 5.
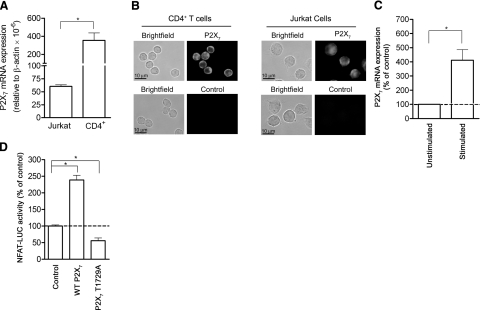
P2X7 receptors are involved in T-cell activation. A) P2X7 receptor mRNA expression in Jurkat cells and human peripheral CD4+ T cells was quantified using real-time RT-PCR analysis. B) Immuncytochemical assessment of P2X7 receptor expression of Jurkat cells and human CD4+ T cells was evaluated using a fluorescence microscope. Controls were probed with goat anti-rabbit Alexa 555 secondary antibody only. C) P2X7 receptor mRNA expression of Jurkat cells was determined with real-time RT-PCR 4 h after stimulation with or without anti-CD3/CD28 antibody-coated beads. D) NFAT activation of Jurkat cells overexpressing wild-type (WT) or mutated (T1729A) P2X7 receptor or empty expression vector (control) was assessed using a NFAT-luciferase reporter assay. Cells were stimulated with anti-CD3/CD28 antibody-coated beads. NFAT-luciferase activity was normalized using a β-galactosidase control reporter coexpressed in the cells. Transfected cells were cultured for 72 h, stimulated with anti-CD3/CD28 antibody-coated beads for 8 h, and luciferase activity was measured. Data represent means ± se of triplicates. *P ≤ 0.05; n ≥ 3; two-tailed unpaired Student’s t test.
Figure 6.
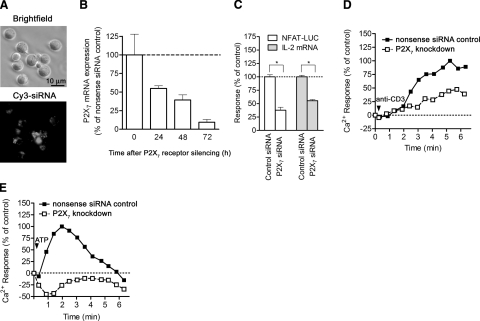
P2X7 receptors are involved in Ca2+ signaling and IL-2 expression. A) Jurkat cells electroporated with a Cy3-labeled nonsense siRNA construct were examined using a microscope to verify siRNA uptake. B) Jurkat cells were transfected with 3 μg siRNA targeting P2X7 or nonsense siRNA, and P2X7 gene expression was examined at the indicated time points by assessing mRNA levels using real-time RT-PCR. C) Jurkat cells were treated with nonsense control siRNA or with siRNA targeting P2X7 receptors for 72 h, then stimulated with anti-CD3/CD28 antibody-loaded beads, and NFAT-luciferase activation,and IL-2 transcription were determined. Data represent means ± se. *P ≤ 0.05; n = 3; two-tailed unpaired Student’s t test. D, E) Jurkat cells were treated with siRNA to silence P2X7 receptors or with nonsense control siRNA, and Ca2+ signaling in response to stimulation with 0.5 μg/ml anti-CD3 antibody (D) or 2 mM ATP (E) was determined by flow cytometry using Fluo-3 as a Ca2+ indicator. Data are representative of similar results obtained in 3 separate experiments.
Immunocytochemistry
Jurkat cells and human CD4+ T cells were fixed in 4% paraformaldehyde in PBS for 10 min on ice, blocked with 1% BSA in PBS, and stained with rabbit anti-P2X7 receptor antibody overnight at 4°C (1:100 in blocking buffer; Alomone Labs, Jerusalem, Israel). Washed cells were then incubated with goat anti-rabbit Alexa 555 antibody (Molecular Probes) for 1 h at room temperature (diluted 1:2000 in blocking buffer), washed again, and placed on cover slips in mounting medium (Vectashield, Vector Laboratories, Burlingame, CA, USA). As a control, cells were stained in the absence of primary antibody. Immunofluorescence and bright field images were captured using a Leica DMIRB fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Hamamatsu ORCA II camera (Hamamatsu Photonics, Hamamatsu, Japan).
Statistical analyses
Unless otherwise stated, data are expressed as means ± se of experiments performed in triplicate. For comparisons between groups, two-tailed unpaired Student’s t-tests were used. A value of P ≤ 0.05 was considered significant.
RESULTS
TCR stimulation induces ATP release
Released cellular ATP can serve as a paracrine signaling molecule for intercellular communication, or it can act in an autocrine manner to regulate cellular functions, including those of leukocytes (17, 29). We found that the ligation of the TCR/CD3 complex and CD28 coreceptor induces the release of ATP from Jurkat T cells (Fig. 1). ATP concentrations in the bulk medium reached levels of 3 μM in 30 s and remained elevated for >5 min (Fig. 1C), but extracellular ATP concentrations in close proximity to the cell surface achieved concentrations of >60 μM within 2 min after cell stimulation (Fig. 1A, B). These ATP concentrations are sufficient to stimulate P2X receptors (9, 30, 31) but are lower than the concentrations of ATP that induce apoptosis in CD4+ and CD8+ T cells (15). Low levels of ADP, AMP, and adenosine, which are likely generated from the breakdown of ATP, were also detected in the bulk medium during the 5-min stimulation period (Fig. 1C).
Stimulation of Jurkat cells in the presence of ATP at concentrations similar to those released endogenously in response to TCR/CD28 stimulation (100 μM) enhanced IL-2 transcription by >47-fold compared to controls stimulated in the absence of additional ATP (Fig. 2A). Treatment of Jurkat cells with 50 μM and 100 μM ATP also enhanced IL-2 release in stimulated Jurkat cells (Supplemental Fig. 1). Thus, ATP concentrations of ∼100 μM promote T-cell activation rather than apoptosis in Jurkat cells.
ATP release from Jurkat cells may occur through the stimulation of stretch-activated maxi-anion channels (32, 33). During T-cell activation, mechanical perturbations of the cell membrane by interactions of T cells with antigen-presenting cells may lead to ATP release through similar mechanisms. To examine this possibility, we treated Jurkat cells with the maxi-anion channel blocker GdCl3 (100 μM). We found that GdCl3 significantly inhibits ATP release and IL-2 expression of Jurkat cells and human PBMCs (Fig. 2B, C). Because GdCl3 does not completely abolish ATP release, other mechanisms besides Gd3+-sensitive channels may also contribute to the release of ATP from stimulated T cells. Endogenously released ATP plays an important role in mediating T-cell activation, as treatment of stimulated PBMCs with apyrase (20 U/ml) suppressed IL-2 production (Fig. 2C). This is consistent with our previous findings demonstrating that ATP, released endogenously in response to hypertonic saline treatment, enhances IL-2 release in human PBMCs (4).
TCR-induced ATP release and P2X receptors are required for Ca2+ mobilization and IL-2 synthesis
Treatment of peripheral T cells with exogenous ATP induces the influx of Ca2+ (14, 20). T-cell stimulation elicits the release of endogenous ATP (Fig. 1), but it is unclear whether released ATP contributes to the influx of Ca2+ that occurs in response to TCR stimulation (34). To test this possibility, we stimulated Jurkat cells in the presence or absence of the nucleotide-hydrolyzing enzymes apyrase or alkaline phosphatase and found that both enzymes inhibited TCR-stimulated Ca2+ influx and IL-2 expression (Fig. 3). Higher concentrations of apyrase and alkaline phosphatase (20 to 40 U/ml) did not result in a further inhibition of Ca2+ influx (data not shown). The doses of apyrase and alkaline phosphatase (>10 U/ml) required to inhibit Ca2+ influx and IL-2 expression are consistent with previous observations demonstrating the presence of an apyrase-resistant pericellular ATP pool that surrounds T cells (35). Our results imply that ATP released into the extracellular milieu is essential for Ca2+ influx and subsequent T-cell activation processes that result in IL-2 expression.
To evaluate the role of P2X receptors in the influx of Ca2+ and activation of IL-2 expression, we used pharmacological inhibitors to block P2X receptors. The P2X receptor antagonists suramin and NF023 block a number of P2X receptor subtypes, while o-ATP is a more selective inhibitor of P2X7 receptors (11, 36, 37). We found that suramin (100 μM), NF023 (10 μM), and o-ATP (10 μM) inhibited Ca2+ mobilization of Jurkat cells stimulated with anti-CD3 antibodies (1 μg/ml) or exogenous ATP (2 mM; Fig. 4A, B). Treatment of human PBMCs with suramin (100 and 500 μM) or o-ATP (10 and 100 μM) inhibited IL-2 production (Fig. 4C). These results are akin to findings that have demonstrated the ability of o-ATP to reduce IL-2 production and proliferation of CD3/CD28-stimulated naive CD4+ T cells (38). Taken together, the results support the conclusion that ATP acts on P2 receptors to mediate Ca2+ influx and IL-2 expression in response to T-cell stimulation. To examine the role of P2X7 receptors in T-cell activation, we performed experiments using immunohistochemistry and assessment of cells with decreased P2X7 receptor expression.
P2X7 receptor expression and function in T lymphocytes
Real-time RT-PCR analysis and immunocytochemical examination revealed that Jurkat cells and peripheral human CD4+ T cells express P2X7 receptors (Fig. 5A, B) and that stimulation of Jurkat cells by ligation of CD3/CD28 up-regulates P2X7 receptor expression (Fig. 5C). Together with the findings above, these data imply that changes in P2X7 receptor expression levels may serve to regulate T-cell activation processes. To test whether increases in P2X7 receptor expression promote T-cell activation, we utilized a NFAT-luciferase reporter assay (28). NFAT, which is activated in response to increases in intracellular calcium concentrations, is required for the induction of IL-2 gene transcription (2). We found that overexpression of human wild-type P2X7 receptors more than doubled NFAT activation in TCR/CD28-stimulated Jurkat cells compared to control cells that were transfected with an empty expression vector (Fig. 5D). A T1729A mutation occurs as a single nucleotide polymorphism in the human P2X7 gene, conferring a loss of receptor function due to the failure of the mutant receptors to traffic to the cell surface (39). Overexpression of the nonfunctional T1729A mutant P2X7 receptor resulted in suppressed NFAT activation in response to TCR/CD28 stimulation (Fig. 5D).
To examine the role of P2X7 receptors in Ca2+ influx, NFAT activation, and IL-2 expression in more detail, we silenced the expression of P2X7 receptors using siRNA. Using Cy3-labeled nonsense siRNA, we found that >95% of treated Jurkat cells incorporated the siRNA constructs (Fig. 6A). Treatment with a P2X7 receptor siRNA construct reduced P2X7 receptor mRNA levels in a time-dependent manner, with a ∼90% decrease in receptor transcripts after 72 h of siRNA treatment (Fig. 6B). The decrease in P2X7 receptor expression resulted in the inhibition of NFAT activation and IL-2 mRNA synthesis in response to TCR/CD28 stimulation (Fig. 6C). NFAT activation and IL-2 mRNA synthesis require Ca2+ signaling (2). Consistent with evidence that P2X7 receptor stimulation involves Ca2+ influx, we found that silencing of P2X7 receptors inhibits Ca2+ signaling in response to cell stimulation by TCR or exogenous ATP (Fig. 6D, E). Intracellular Ca2+ concentrations decreased below baseline levels when cells with silenced P2X7 receptors were stimulated with ATP, suggesting that the lack of P2X7 receptors impairs the ability of the cells to replenish cytosolic Ca2+ sequestered in response to cell stimulation. Thus, P2X7 receptors may contribute more generally to Ca2+ homeostasis in T cells (40, 41).
Lack of functional P2X7 receptors impairs T-cell function
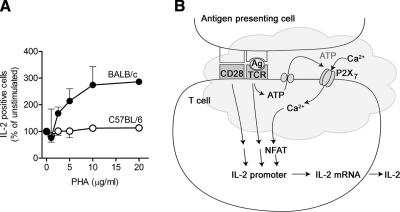
C57BL/6 mice have a mutation in the P2X7 receptor gene that impairs the function of the receptor; by contrast, BALB/c mice express fully functional P2X7 receptors (42). Consequently, T cells of BALB/c mice are more sensitive to ATP and other P2X7 receptor agonists compared to T cells of C57BL/6 mice (15, 42). We thus used splenocytes from these two mouse strains as a further means to examine the role of P2X7 receptors in T-cell activation. We found that lymphocytes of BALB/c mice showed robust IL-2 production in response to stimulation with PHA while lymphocytes of C57BL/6 mice failed to induce IL-2 production, even at the highest PHA concentration tested (Fig. 7A). Studies comparing ATP-induced responses in T-cell subsets of C57BL/6, BALB/c, and P2X7 receptor knockout mice have previously been reported (15). Of particular interest are data demonstrating increased proliferation of CD4+ T cells from BALB/c, but not C57BL/6 and P2X7 knockout mice in response to stimulation with 100 μM ATP. The differences in the sensitivity of CD4+ T cells to ATP was found to correlate with different levels of P2X7 receptor that are expressed by the T cells of these strains (15). Our findings are consistent with these data and, together with the siRNA silencing data above, provide further support for the notion that P2X7 receptors play an essential role in the activation of T cells.
Figure 7.
P2X7 receptors regulate IL-2 production in mouse splenocytes and a model for the role of ATP release and P2X7 receptors in T-cell activation. A) IL-2 expression of splenocytes from C57BL/6 mice lacking functional P2X7 receptors or BALB/c mice with functional P2X7 receptors was assessed using intracellular cytokine staining and flow cytometry. Splenocytes were stimulated overnight with the indicated concentrations of phytohemagglutinin (PHA), and IL-2 expression of lymphocytes was assessed by intracellular staining. Data are representative of 3 experiments; values are means ± se of triplicate measurements. B) Schematic depiction of the role of ATP release and P2X7 receptors in T-cell activation. TCR activation by antigen presenting cells (APC) induces ATP release, resulting in the activation of P2X7 receptors that facilitate Ca2+ entry, which, in turn, induces T-cell activation events, through the activation of NFAT and IL-2 transcription.
DISCUSSION
The current findings provide evidence that release of cellular ATP by T cells and stimulation of P2X7 receptors regulate T-cell activation. We found that T-cell stimulation up-regulates P2X7 receptor expression, while silencing of P2X7 receptors suppresses T-cell activation events that include Ca2+ influx, NFAT activation, and IL-2 expression. We and others have shown that the release of cellular ATP from leukocytes and other cells occurs in a controlled manner and that released ATP serves as a local autocrine or paracrine signaling molecule that regulates the functions of numerous cell types (17, 29, 43, 44).
We found that Jurkat T cells release ATP within seconds after TCR stimulation and that the extracellular ATP concentrations generated near the cell surface are sufficient to activate P2X7 receptors of the cells (45). Released ATP can be converted to ADP, AMP, and adenosine by Jurkat cells (3), but ATP levels measured in the bulk medium of stimulated cells are highest, with adenosine levels remaining relatively low (∼0.2 μM) throughout the 5-min stimulation period (Fig. 1C). Adenosine can inhibit IL-2 release of stimulated Jurkat cells; however, this requires treatment with adenosine concentrations of ≥1 μM (3).
We found that Jurkat cells release more cellular ATP in response to TCR stimulation than with other stimuli, such as osmotic stress and local mechanical stimulation (3,4,5). We speculate that this greater release of ATP may be a consequence of sustained local perturbations of the plasma membrane caused by interaction of cells with the beads used for activation, thus perhaps modeling the interaction of T cells with antigen-presenting cells.
These close intercellular interactions may induce greater concentrations of extracellular ATP within the immune synapse that forms between T cells and antigen-presenting cells (APC), thereby facilitating the initiation of events that are critical for T-cell activation (29). This suggestion is consistent with the slightly higher level of ATP measured near the beads than at the rear of the cell. However, ATP was detected throughout the cell surface on cell activation and thus may mediate signal transduction events outside the T-cell:APC contact site. This idea is consistent with previous findings, indicating that signal transduction in activated T cells can occur outside of the immunological synapse (46).
Our results obtained with GdCl3 suggest that T cells release ATP via stretch-activated anion channels. However, because GdCl3 did not completely block ATP release or IL-2 production in response to T-cell stimulation, other mechanisms of ATP release, such as vesicular release or release from pannexin-1 or connexin hemichannels, may also be involved in the release of ATP from stimulated T cells (38, 47, 48). A recent study has shown that ATP release from naive CD4+ T cells can occur through pannexin-1 hemichannels (38). De novo synthesis of extracellular ATP through sequential phosphor-transfer reactions by ecto-adenylate kinase and NDP kinase expressed on the T-cell surface may also contribute to the accumulation of extracellular ATP (43, 49). In addition to ATP that is released by stimulation of T cells, Yegutkin et al. (35) have shown that a pericellular “ATP halo” envelopes resting T cells, which may mediate various autocrine and paracrine signaling events.
Although ATP concentrations of >300 μM can induce apoptosis in mouse peripheral T cells, Jurkat cells are comparatively insensitive to ATP-induced apoptosis; instead, they proliferate in the presence of ATP at concentrations as high as 3 mM (14, 15). We find that exogenous ATP concentrations similar to those released in response to the stimulation of Jurkat cells enhance IL-2 mRNA expression, consistent with evidence that exogenous ATP in this concentration range augments IL-2 production of activated Jurkat cells (Supplemental Fig. 1) (4).
Differences in the abundance of P2X7 receptors in Jurkat cells vs. CD4+ cells may explain why Jurkat cells are less sensitive to ATP-induced apoptosis than are CD4+ cells. Human CD4+ T cells have a ∼6-fold greater copy number of P2X7 mRNA than do Jurkat cells (Fig. 5A). By comparing the responses of T cells isolated from P2X7 receptor knockout mice with T cells from strains of wild-type mice, Aswad et al. (15) showed that the abundance of functional P2X7 receptors expressed by T cells correlates with the degree of apoptosis that is induced in response to ATP stimulation. Thus, the lower P2X7 receptor expression in Jurkat cells, a cell line originally derived from a patient with acute T-cell leukemia, may protect the cells from apoptosis and thereby contribute to their growth. P2X7 receptor expression correlates with cell proliferation (23, 26), implying that the apoptotic vs. proliferative roles of P2X7 receptors differ among different types of T cells.
Influx of extracellular Ca2+ is an early signaling event that is critical for T-cell activation (50), including, as our data suggest, in response to stimulation by P2X7 receptors. We found that stimulation of Jurkat cells with exogenous ATP induces rapid Ca2+ influx, while the removal of endogenous ATP that is released in response to cell stimulation or elimination of P2X7 receptors with antagonists or siRNA inhibits Ca2+ signaling and downstream events that lead to IL-2 production (Figs. 3 and 4). Inhibition of P2X7 receptors in other cell types, (i.e., human and mouse peripheral T cells) also suppresses cell activation (18, 38).
A defect in P2X7 receptor signaling or polymorphisms in the P2X7 receptor gene that result in a loss of receptor function (T1729A, A1513C, and G496A) can lead to decreased immune cell function (39, 51, 52). Here, we show that C57BL/6 mice, which carry an allelic mutation that impairs P2X7 receptor function (42), are unable to produce IL-2 in response to cell stimulation. Experiments using P2X7 receptor knockout mice or leukocytes isolated from patients with genetic defects in P2X7 receptors support their importance in T-cell function (53). In addition, single nucleotide polymorphisms that lead to the gain of function of P2X7 receptors increase Ca2+ influx in lymphocytes of patients with chronic lymphocytic leukemia (CLL) (54). Lymphocytes of patients with the aggressive variant of CLL can have up-regulated P2X7 receptor expression that enhances Ca2+ influx and cell proliferation (23, 55).
Overall, the current findings imply an essential role for ATP release and autocrine or paracrine feedback through P2X7 receptors in T-cell activation (Fig. 7B). Such localized feedback processes may be important for the induction of T-cell proliferation and for the amplification and modulation of T-cell responses to cues such as those provided by antigen-presenting cells. Future studies that explore the role of P2X7 receptor splice variants and other P2X receptor subtypes should aid in defining the precise role of ATP and P2X receptors in T-cell activation.
Supplementary Material
Acknowledgments
We thank Dr. Amnon Altman for his advice and Dr. Marion Schneider for the helpful discussions of our results. This study was supported in part by National Institute of General Medical Sciences grants R01 GM-51477, GM-60475 (W.G.J.), and GM-66232; a grant from the Lymphoma and Leukemia Society (P.A.I.); DOD/CDMRP grant PR043034 (W.G.J.); and NIH GCRC grant 5MO1-RR-00827-25.
References
- Zeyda M, Stulnig T M. Lipid Rafts & Co.: an integrated model of membrane organization in T cell activation. Prog Lipid Res. 2006;45:187–202. doi: 10.1016/j.plipres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Hogan P G, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Yip L, Cheung C W, Corriden R, Chen Y, Insel P A, Junger W G. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007;27:242–250. doi: 10.1097/01.shk.0000245014.96419.3a. [DOI] [PubMed] [Google Scholar]
- Loomis W H, Namiki S, Ostrom R S, Insel P A, Junger W G. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003;278:4590–4596. doi: 10.1074/jbc.M207868200. [DOI] [PubMed] [Google Scholar]
- Corriden R, Insel P A, Junger W G. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol. 2007;293:C1420–C1425. doi: 10.1152/ajpcell.00271.2007. [DOI] [PubMed] [Google Scholar]
- Filippini A, Taffs R E, Sitkovsky M V. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redegeld F, Filippini A, Sitkovsky M. Comparative studies of the cytotoxic T lymphocyte-mediated cytotoxicity and of extracellular ATP-induced cell lysis. Different requirements in extracellular Mg2+ and pH. J Immunol. 1991;147:3638–3645. [PubMed] [Google Scholar]
- Blanchard D K, Wei S, Duan C, Pericle F, Diaz J I, Djeu J Y. Role of extracellular adenosine triphosphate in the cytotoxic T-lymphocyte-mediated lysis of antigen presenting cells. Blood. 1995;85:3173–3182. [PubMed] [Google Scholar]
- North R A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North R A. Signaling at purinergic P2X receptors. 2009 doi: 10.1146/annurev.physiol.70.113006.100630. [E-pub ahead of print] Annu. Rev. Physiol. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz J M, Morelli A, Torboli M, Bolognesi G, Baricordi O R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Wang L, Jacobsen S E, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells [Online] BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R, Townsend-Nicholson A, Burnstock G. P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res. 2000;300:295–306. doi: 10.1007/s004410000206. [DOI] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, Bulfone-Paus S. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-κB. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V B, Hart J, Wewers M D. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel P A, Junger W G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 Receptor. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Baricordi O R, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274:33,206–33,208. doi: 10.1074/jbc.274.47.33206. [DOI] [PubMed] [Google Scholar]
- Gu B J, Wiley J S. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107:4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- Labasi J M, Petrushova N, Donovan C, McCurdy S, Lira P, Payette M M, Brissette W, Wicks J R, Audoly L, Gabel C A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Jamieson G P, Snook M B, Thurlow P J, Wiley J S. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gu B, Bendall L J, Wiley J S. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–951. [PubMed] [Google Scholar]
- Adinolfi E, Callegari M G, Ferrari D, Bolognesi C, Minelli M, Wieckowski M R, Pinton P, Rizzuto R, Di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M J, Lee H J, Lee Y S, Kim J H, Park J K, Chang W K, Shin H C, Kim D K. Extracellular ATP is involved in the induction of apoptosis in murine hematopoietic cells. Biol Pharm Bull. 2007;30:671–676. doi: 10.1248/bpb.30.671. [DOI] [PubMed] [Google Scholar]
- Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006;177:2842–2850. doi: 10.4049/jimmunol.177.5.2842. [DOI] [PubMed] [Google Scholar]
- Baricordi O R, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, Di Virgilio F. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- Hirsh M I, Hashiguchi N, Junger W G. Hypertonic saline increases gammadeltaT cell-mediated killing of activated neutrophils. Crit Care Med. 2008;36:3220–3225. doi: 10.1097/CCM.0b013e31818f238e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet C, Canonigo A J, Billadeau D D, Altman A. Membrane localization and function of Vav3 in T cells depend on its association with the adapter SLP-76. J Biol Chem. 2005;280:15289–15299. doi: 10.1074/jbc.M500275200. [DOI] [PubMed] [Google Scholar]
- Dubyak G R. Purinergic signaling at immunological synapses. J Auton Nerv Syst. 2000;81:64–68. doi: 10.1016/s0165-1838(00)00155-7. [DOI] [PubMed] [Google Scholar]
- Gever J R, Cockayne D A, Dillon M P, Burnstock G, Ford A P. Pharmacology of P2X channels. Pflügers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Klapperstuck M, Buttner C, Schmalzing G, Markwardt F. Functional evidence of distinct ATP activation sites at the human P2X(7) receptor. J Physiol. 2001;534:25–35. doi: 10.1111/j.1469-7793.2001.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- Liu H T, Toychiev A H, Takahashi N, Sabirov R Z, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- Cahalan M D, Zhang S L, Yeromin A V, Ohlsen K, Roos J, Stauderman K A. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin G G, Mikhailov A, Samburski S S, Jalkanen S. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17:3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch A E. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- Schenk U, Westendorf A M, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels [Online] Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- Wiley J S, Dao-Ung L P, Li C, Shemon A N, Gu B J, Smart M L, Fuller S J, Barden J A, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- Shivnan E, Alexander D R. Protein kinase C activation inhibits TCR-mediated calcium influx but not inositol trisphosphate production in HPB-ALL T cells. J Immunol. 1995;154:1146–1156. [PubMed] [Google Scholar]
- Balasubramanyam M, Gardner J P. Protein kinase C modulates cytosolic free calcium by stimulating calcium pump activity in Jurkat T cells. Cell Calcium. 1995;18:526–541. doi: 10.1016/0143-4160(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Yegutkin G G, Henttinen T, Samburski S S, Spychala J, Jalkanen S. The evidence for two opposite, ATP-generating and ATP-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem J. 2002;367:121–128. doi: 10.1042/BJ20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom R S, Gregorian C, Insel P A. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275:11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Costello P S, Gallagher M, Cantrell D A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- Leybaert L, Braet K, Vandamme W, Cabooter L, Martin P E, Evans W H. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10:251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Yegutkin G G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gardner P. Calcium and T lymphocyte activation. Cell. 1989;59:15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- Sluyter R, Shemon A N, Wiley J S. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol. 2004;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- Saunders B M, Fernando S L, Sluyter R, Britton W J, Wiley J S. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003;171:5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- Chen L, Brosnan C F. Regulation of immune response by P2X7 receptor. Crit Rev Immunol. 2006;26:499–513. doi: 10.1615/critrevimmunol.v26.i6.30. [DOI] [PubMed] [Google Scholar]
- Cabrini G, Falzoni S, Forchap S L, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi O R, Di Virgilio F. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, Cuneo A, Castoldi G, Di Virgilio F, Baricordi O R. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.v99.2.706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.