Abstract
Given the variable protective efficacy provided by Mycobacterium bovis bacillus Calmette-Guérin (BCG), there is an urgent need to develop new vaccines against tuberculosis. As dendritic cells (DC) play a critical role in initiating and regulating a protective T cell response against the pathogens, the comprehension of mycobacterium-induced modulation of DC functions is critical to pinpoint new, immunological strategies. To this end, a comparative analysis of the effect induced by BCG and Mycobacterium tuberculosis (Mtb) infection on the DC immunophenotype indicated that BCG is less efficient in inducing DC maturation than Mtb. In addition, BCG-infected DC poorly expressed IFN-β and displayed a reduced production of IL-12 as compared with Mtb-stimulated cells. The impaired expression of IL-12p35 and IFN-β is likely a result of the inability of BCG to induce the activation of the IFN regulatory factor-3. Taking into account these data, we sought to investigate whether the exogenous addition of IFN-β, a cytokine that exerts important effects on the immune system, could enhance the Th1-polarizing capacity of BCG-infected DC. Interestingly, when DC infected by BCG were pretreated in vitro with IFN-β, they displayed a fully mature phenotype and released a significant amount of bioactive IL-12p70, which resulted in an enhanced Th1 response. This study demonstrates that IFN-β potentiates DC immunological functions following BCG infection, thus suggesting IFN-β as a possible candidate as vaccine adjuvant.
Keywords: IRF, Mycobacterium tuberculosis, cytokines
INTRODUCTION
Tuberculosis (TB) is still a leading cause of death throughout the world, despite the availability of the bacillus Calmette-Guerin (BCG) vaccine and antibiotic treatments [1]. Meta-analyses of BCG vaccination clearly indicated that BCG confers some protection against pulmonary and disseminated TB in children, principally miliary disease and meningitis, but it is unreliable against the pulmonary TB in adults [2]. Attempts over the years to design a vaccine more protective than BCG have been unsuccessful so far [3]. Thus, investigating the impact of BCG infection on the immune response is instrumental for setting a new, effective vaccine.
Protective immunity against Mycobacterium tuberculosis (Mtb) is associated with antigen presentation by the APC to CD4 and CD8 T cells, which in turn initiate a specific cellular immunity against the intracellular pathogen [4]. Dendritic cells (DC) are the most efficient APC, which are highly represented on the sites of Mtb infection at the onset of the inflammatory response [5,6,7]. DC are a central component of the immune system for their extraordinary capacity to initiate and modulate the immune responses elicited upon recognition of infectious agents. Indeed, immature DC play a crucial role in the surveillance of peripheral sites by migrating through all of the tissues and actively taking up foreign antigen [8]. Once in contact with pathogens, immature DC use various pattern recognition receptors (PRRs) to specifically recognize pathogen-related molecules. TLR are the best-characterized class of PRRs in the mammalian species [9]. Immediately after contact with and recognition of the microbes, DC undergo a process, termed maturation, modifying their phenotypical features and leading to production of cytokines that regulate the immune responses, acting sequentially in different microenvironments and on different leukocyte populations [8]. Indeed, mature DC migrate from peripheral tissues into draining lymphnodes, where they specifically promote the differentiation of effector T cells and the expansion of memory T cells involved in the adaptive immune response to infection.
We have shown previously that Mtb-infected, monocyte-derived DC (MoDC) are involved primarily in inducing an antimycobacterial T cell immune response [10]. After interacting with the pathogen, DC mature and acquire the ability to stimulate T cells through surface expression of MHC and costimulatory molecules, as well as secretion of immunoregulatory cytokines, such as IL-12 and type I IFN [10]. The production of IL-12 and type I IFN by DC early in an immune response is considered critical for the polarization of a CD4+ T lymphocyte response toward a Th1 pattern, a key process for the clearance of intracellular pathogens [4]. Indeed, we reported about the ability of Mtb to induce a selective expression of type I IFN genes in human DC [11]. In addition, we found that type I IFN cooperates with IL-12 to stimulate the expression of IFN-γ by T cells [10] and induces the expression of CXCL10, a chemokine involved in the selective recruitment of activated/effector cells implicated in the granuloma formation [12]. Based on these observations, in the present study, we investigated the capacity of BCG to confer to MoDC the property to promote a Th1-oriented T cell response. Indeed, given the role played by DC in initiating and regulating a protective T cell response against Mtb, we sought to characterize and to compare the effect induced by the infection of human MoDC with BCG and Mtb with particular attention to T cell-stimulatory capacity. Having found that Mtb and BCG are taken up by DC and survive similarly, the comparative analysis was extended to DC maturation, cytokine expression, and stimulatory properties on IFN-γ production from naive T cells. Differences in the production of IL-12 and IFN-β as well as in the expression of maturation markers were observed, indicating that BCG and Mtb differentially promote DC maturation and their T cell-stimulatory capacity. However, the exogenous addition of IFN-β restored a fully mature phenotype and the capacity to release IL-12 by BCG-infected DC, thus improving BCG immunogenicity.
MATERIALS AND METHODS
Antibodies and other reagents
mAb specific for CD1a, CD14, CD38, CD86, HLA-DR, CD83, IgG1, and IgG2a (BD Bioscience PharMingen, San Diego, CA, USA) were used as direct conjugates to FITC or PE. Where indicated, 200 pM IFN-β (Avonex®, Biogen Inc., Cambridge, MA, USA) was used to treat DC cultures. Sendai virus (a kind gift of Dr. Ilkka Julkunen, National Public Health Institute, Helsinki, Finland) was used at a concentration of 60 hemagglutination U/ml and LPS from Escherichia coli 0111:B4 (Sigma-Aldrich, St. Louis, MO, USA), at 1 μg/ml.
MoDC preparation
MoDC were prepared as described previously [13]. Briefly, PBMCs were isolated from freshly collected buffy coats obtained from healthy voluntary blood donors (Blood Bank of University “La Sapienza”, Rome, Italy) by density gradient centrifugation using Lympholyte-H (Cedarlane, Hornby, Ontario, Canada). Monocytes were purified by positive sorting using anti-CD14-conjugated magnetic microbeads (Miltenyi, Bergisch Gladbech, Germany). The recovered cells were >99% CD14+, as determined by flow cytometry with anti-CD14 antibody. MoDC were generated by culturing monocytes in six-well tissue-culture plates (Costar Corp., Cambridge, MA, USA) with 50 ng/ml GM-CSF and 1000 U/ml IL-4 (R&D Systems, Abingdom, UK) for 5 days at 0.5 × 106 cells/ml in RPMI 1640 (BioWhittaker, Verviers, Belgium), supplemented with 2 mM L-glutamine and 15% FCS (BioWhittaker). No antibiotics were added to the cultures. At day 5, the cells were 90% CD1a+ and 95% CD14–.
Bacteria preparation and infection of DC
Mtb H37Rv (ATCC 27294, American Type Culture Collection, Manassas, VA, USA) and Mycobacterium bovis BCG (ATCC 27291) were grown with gentle agitation (80 rpm) in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI, USA), supplemented with 0.05% Tween 80 (Sigma-Aldrich) and 10% Middlebrook oleic acid albumin dextrose catalase enrichment (Becton Dickinson, Sparks, MD, USA). Logarithmically growing cultures were centrifuged at 800 rpm for 10 min to eliminate clumped mycobacteria and then washed three times in RPMI 1640. Mycobacteria were resuspended in RPMI 1640 containing 10% FCS and then stored at −80°C. Vials were thawed, and bacterial viability was determined by counting the number of CFU on Middlebrook 7H10 agar plates. All bacteria preparations were analyzed for LPS contamination by the Limulus lysate assay (BioWhittaker) and contained less than 1 EU/ml. DC cultures were infected with a multiplicity of infection (MOI) of one bacterium/cell.
CFU assay
Two hours after infection, the cell cultures were washed gently (three times) with RPMI 1640. MoDC were centrifuged at 150 g for 10 min to selectively spin down cells, while extracellular bacteria remain in the supernatants. Cells were resuspended in complete medium and cultured for the times indicated in each experiment. T0 referees to cell cultures washed after 2 h of infection and used to count the intracellular bacteria. Samples were assayed for CFU in triplicate. At each time-point, cells were lysed with water containing 0.05% saponin. Serial dilutions of the bacterial suspensions were plated (in duplicate) on Middlebrook 7H10 agar plates, and viable bacteria were evaluated after 3 weeks of incubation at 37°C.
Flow cytometry analysis
Approximately 1–2 × 105 cells were aliquoted into tubes and washed once in PBS containing 2% FCS. The cells were incubated with mAb at 4°C for 30 min. The DC were then washed and fixed overnight with 4% paraformaldehyde before analysis on a FACSCan using CellQuest software (Becton Dickinson).
Cytokine determination
Supernatants from Mtb- or BCG-infected MoDC- or IFN-β-pretreated cultures were harvested at 24 h after infection, filtered (0.2 μm), and stored at −80°C. IL-12p70 was measured with the human inflammation cytometric bead array (CBA; BD Bioscience PharMingen). The production of cytokines in MLR experiments was measured with human Th1/Th2 CBA (BD Bioscience PharMingen).
RNA isolation and real-time PCR quantifications
RNA was extracted from DC with a RNeasy kit (Qiagen Inc., Valencia, CA, USA), according to the manufacturer’s instructions. A phenol/chloroform extraction was performed to inactivate residual mycobacterial particles.
Reverse transcriptions were performed as described previously [14]. Quantitative PCR assays were performed at least in triplicates using the Platinum Taq DNA polymerase (Invitrogen Life Technologies, Frederick, MD, USA) and the SYBR Green I (BioWhittaker Molecular Applications, Rockland, ME, USA) on a LightCycler (Roche Diagnostics, Basel, CH, Switzerland). Primer pairs have been described previously [14]. Quantification data are presented as a ratio to the GAPDH mRNA level. Only ratios with a standard error (SE) 0.2 log (95% confidence limits) were considered for the determination of induction levels.
Western blot analysis
Western blot was performed as described previously [11]. Briefly, 30 μg total cell extracts were separated by 10% SDS-PAGE gel and blotted onto nitrocellulose membranes.
Blots were incubated with rabbit polyclonal antibodies against IFN regulatory factor 3 (IRF-3; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and phosphorylated IRF-3 (Cell Signaling, Danvers, MA, USA) and reacted with anti-rabbit HRP-coupled secondary antibody (Amersham Pharmacia Biotech, Little Chalfont, UK) using an ECL system. Blots, after stripping, were incubated with IRF-3 antibody to verify the total content of the transcription factor.
MLR
MLR was performed as described previously [15]. Briefly, cord blood CD4+ T cells were purified by indirect magnetic sorting with a CD4+ T cell isolation kit (Miltenyi). Immature DC or BCG-infected or IFN-β-pretreated DC were resuspended in 5% human AB serum complete medium after 18 h of culture.
The proliferative response was assessed at various T cell:DC ratios using a fixed number of T cells (3×104) and evaluated after 5 days by measuring thymidine incorporation (0.5 μCi/well [3H] thymidine; Amersham Pharmacia Biotech).
Supernatants from T cells:DC coculture (ratio 1:10) were harvested at day 6 and analyzed for IFN-γ, TNF-α, IL-4, IL-2, IL-5, and IL-10 release by Th1/Th2 CBA (BD Bioscience PharMingen).
Statistical analysis
Statistical analysis was calculated using a two-tailed paired-data Student’s t-test. A P value <0.05 was considered statistically significant.
RESULTS
Maturation of human MoDC following BCG and Mtb infection
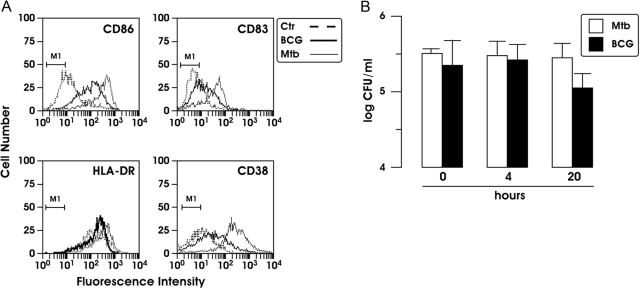
To compare the impact of BCG and Mtb infection on DC maturation, we evaluated by flow cytometry analysis the immunophenotype of MoDC focusing on the molecules involved in T cell activation. DC were infected with Mtb or BCG (MOI of 1) for 24 h, and cell-surface expression of CD86, CD83, HLA-DR, and CD38 was examined. Mtb- and BCG-infected DC showed an enhanced expression of the analyzed molecules compared with the uninfected counterpart. Notably, in BCG-infected cells, the induction of these markers was lower compared with that observed in Mtb-infected DC (Fig. 1A).
Fig. 1.
(A) Analysis of cell-surface phenotypes of Mtb- and BCG-infected MoDC. Cells were infected for 24 h at a MOI of 1, and the cell-surface expression of CD86, CD83, HLA-DR, and CD38 was analyzed by flow cytometry. A total of 5000 cells was analyzed per sample. Unstimulated and infected cells stained with a control (Ctr) Ab are represented by the M1 bar. The results shown are from one of three experiments that yielded similar results. (B) Analysis of Mtb and BCG infection and intracellular survival. DC (5×105) were lysed, and samples were plated to determine the number of bacterial CFU at various times after infection (0, 4, and 20 h). The results represent the means ± se of three independent experiments.
As BCG-infected DC displayed a less-mature phenotype compared with Mtb-infected cells, we assessed the capacity of the two mycobacteria to infect and survive in DC. The infection of DC with BCG and Mtb was studied over a 20-h period and evaluated by CFU (Fig. 1B). At various times after infection (0, 4, and 20 h), DC were lysed, and samples were plated to determine the number of intracellular bacteria. BCG and Mtb infected DC at a similar extent and survived intracellularly in a comparable way, as the number of CFU remained constant over a 20-h period of examination. In addition, the viability of infected DC was evaluated by phase-contrast light microscopic examination and trypan blue exclusion method (data not shown). Infection of DC with Mtb (H37Rv) or BCG at a MOI of 1 apparently did not affect cell viability during a 2-day follow-up period. Altogether, these results indicate that BCG is less efficient in the stimulation of DC maturation compared with Mtb, although both strains infected DC with the same efficiency.
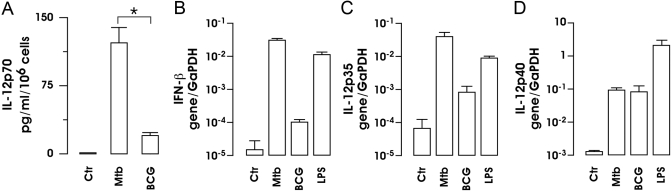
Next, we investigated the possibility that BCG might have had a different ability to stimulate the production of cytokines involved in the polarization of Th1 lymphocytes. To this aim, the accumulation of IL-12p70 in the supernatants of infected DC cultures and the expression of IFN-β RNA were analyzed following BCG and Mtb infection (Fig. 2, A and B). Cell-culture supernatants were collected at 24 h after infection with Mtb or BCG, and the production of IL-12 was analyzed by CBA (Fig. 2A). As shown previously [10], Mtb infection induced a robust production of IL-12 by DC, whereas BCG-infected DC produced a lower amount of this cytokine. A more striking result was obtained from the analysis of IFN-β expression performed by real-time RT-PCR. Indeed, a poor induction of IFN-β mRNA was observed in BCG-infected DC, and the expression of the IFN-β mRNA was induced rapidly 8 h after exposure of the DC to Mtb or to LPS (Fig. 2B), as demonstrated previously [11].
Fig. 2.
IL-12 and IFN-β expression in MoDC following Mtb and BCG infection. (A) Supernatants from Mtb- and BCG-infected DC cultures were collected after 24 h and analyzed by CBA for IL-12p70 production. The results represent the means ± se of four separate experiments. *, P = 0.042 (BCG vs. Mtb). (B–D) Total RNA was extracted 8 h after treatment with LPS or infection with Mtb or BCG. IFN-β gene (B), IL-12 p35 (C), and IL-12 p40 (D) expression was analyzed by real-time RT-PCR. All quantification data are presented as a ratio to the GAPDH level. The results shown are from one of three experiments performed with RNA extracted from different DC cultures that yielded similar results.
To investigate whether the reduced secretion of IL-12 by BCG-infected cells was caused by a reduced transcription of IL-12p35 and p40 genes, the expression of these two subunits was analyzed by real-time PCR (Fig. 2, C and D). Total RNA was extracted 8 h after stimulation from Mtb-, BCG-, and LPS-treated DC. As shown in Figure 2, C and D, LPS and Mtb induced the expression of IL-12p35 and IL-12p40 subunits, and BCG induced only the expression of IL-12p40. This result confirms the CBA data shown in Figure 2A and also indicates that the impaired expression of bioactive IL-12p70 upon BCG infection was mainly the result of a reduced IL-12p35 gene expression.
As it has been demonstrated that the activation of IRF-3 regulates the expression of IFN-β [16] and IL-12p35 [17], we investigated the possibility that a differential activation of this transcription factor could be involved in the regulation of these cytokines following BCG or Mtb infection. Immunoblots were performed with whole cell extracts prepared at 6 h following infection of DC with BCG or Mtb (Fig. 3). As expected, Mtb infection resulted in an evident increase of IRF-3 activation, and IRF-3 phosphorylation was undetected in BCG-infected cells. A strong activation of IRF-3 was also found following 3 h of Sendai virus infection or LPS treatment (Fig. 3), stimuli used as positive control for IRF-3 phosphorylation. No differences in the total IRF-3 content were observed in all tested samples (Fig. 3, lower panel). Thus, the lack of IRF-3 activation is likely correlated with a weak expression of IFN-β and a reduced release of IL-12p70 in BCG-infected DC.
Fig. 3.
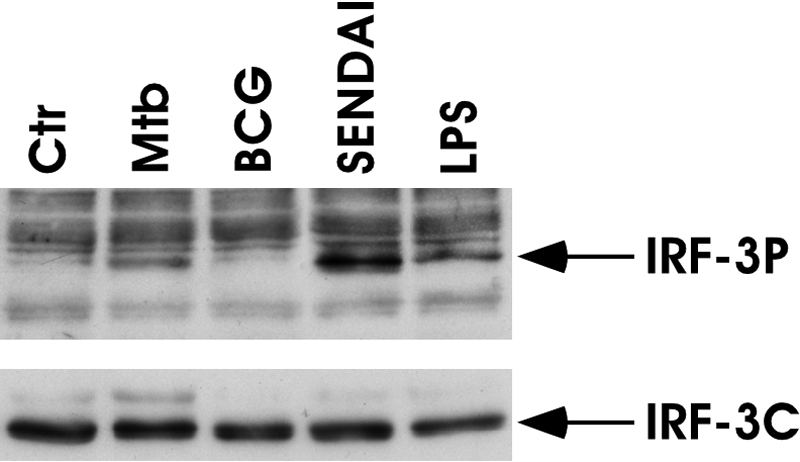
Analysis of IRF-3 phosphorylation in stimulated DC. BCG and Mtb infection. Total cell extracts were prepared 6 h following infection with BCG and Mtb, 3 h after Sendai virus infection or LPS treatment. Whole cell extracts (30 μg) were analyzed on a SDS-7% PAGE gel and subjected to immunoblot analysis with anti-IRF-3 antibody to detect the phosphorylated IRF-3 isoforms (IRF-3P; upper panel). The total content of IRF-3 was evaluated as an internal loading control (IRF-3C; lower panel). The results shown are from one of three experiments performed with cell extracts from different DC cultures that yielded similar results.
Effect of exogenous IFN-β on DC maturation stimulated by BCG
Given the fact that BCG-infected MoDC poorly express IFN-β, we asked whether the exogenous addition of this cytokine could modify the response of DC to BCG infection. To this aim, the immunophenotype of DC and IL-12p70 expression was evaluated in DC pretreated for 4 h with 200 pM IFN-β before BCG infection. Having controlled that IFN-β pretreatment did not modify the capacity of DC to internalize and to kill BCG (data not shown), the expression of CD38, CD83, and CD86 was compared in BCG-infected DC, with or without the IFN-β pretreatment (Table 1). As shown previously, IFN-β-treated MoDC displayed a selective, increased expression of CD38 and CD86 but not that of CD83 [18]. Interestingly, a strong effect of IFN-β in inducing CD83, CD38, and CD86 expression was observed in IFN-β pretreated, BCG-infected MoDC compared with cells stimulated with BCG alone.
TABLE 1.
IFN-β Effect on the Expression of Maturation Markers in DC Infected with BCG
| CD38 | CD83 | CD86 | |
|---|---|---|---|
| Ctr | 10 ± 2 | 6 ± 1 | 18 ± 7 |
| IFN-β | 59 ± 17 | 8 ± 2 | 50 ± 15 |
| BCG | 14 ± 3 | 10 ± 1.4 | 33 ± 8 |
| BCG+ IFN-β | 85 ± 22 | 17 ± 1 | 73 ± 18 |
The expression of the cell-surface molecules was evaluated using the mean fluorescence intensity (MFI) after subtraction of the values of the isotype controls. Value are reported as MFI ± SE measured in five independent experiments. CD86: P = 0.045 BCG versus BCG + IFNβ; CD83: P = 0.018 BCG versus BCG + IFNβ; CD38: P = 0.025 BCG versus BCG + IFN-β.
The effect of IFN-β was also investigated on the release of IL-12p70 from BCG-infected MoDC cultures. Supernatants were harvested from MoDC stimulated for 24 h with BCG, with or without 4-h IFN-β pretreatment, and analyzed by CBA for IL-12p70 production (Fig. 4). The presence of IFN-β strongly increased the amount of IL-12p70 secreted by BCG-infected DC, and IFN-β per se did not modify the steady-state level of IL-12 production. Taken together, these data suggest that IFN-β strengthens the maturation of BCG-infected DC by inducing the expression of maturative markers and IL-12p70 production.
Fig. 4.
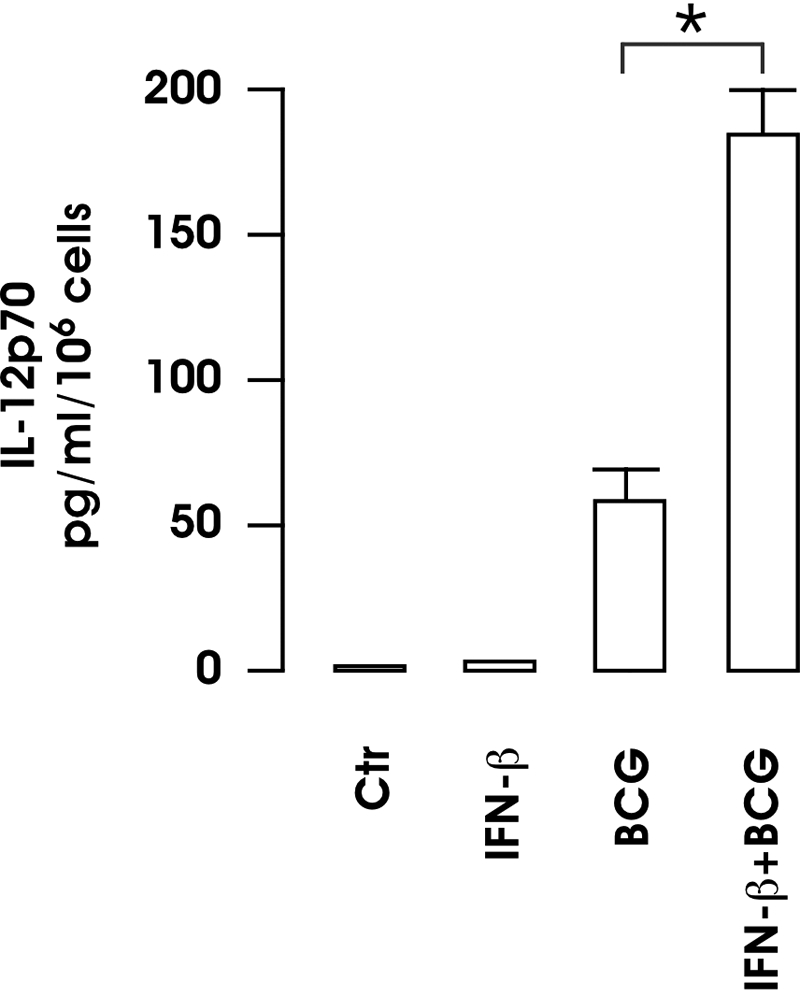
Cytokine production by BCG-infected DC following treatment with IFN-β. DC were infected with BCG, with or without a 4-h pretreatment with 200 pM IFN-β. After 24 h, supernatants were collected and analyzed by CBA for IL-12p70. The results represent the means ± se of four separate experiments. *, P = 0.0123 (BCG vs. BCG+IFN-β).
Analysis of T cell priming and polarization after IFN-β pretreatment of BCG-infected DC
To study whether the effect induced by IFN-β on BCG-stimulated DC maturation resulted in an enhanced capacity to promote the expansion of Th1-oriented CD4 T cells, we studied T cell proliferation and polarization by MLR.
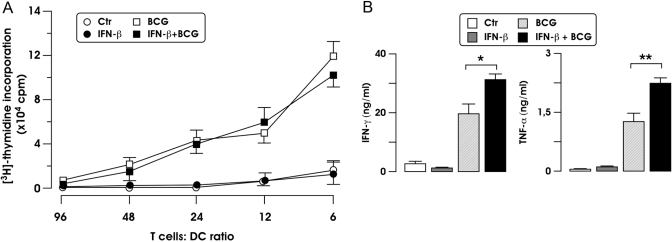
As shown in Figure 5A, the IFN-β pretreatment did not affect the ability of BCG-infected DC to induce proliferation of naïve, allogeneic cord blood CD4+ T cells. However, the production of IFN-γ from T cells stimulated with BCG-infected DC was increased further when T cells were cocultured with BCG-infected DC that had been pretreated with IFN-β (Fig. 5B). Similarly, the pretreatment of BCG-stimulated DC with IFN-β enhanced the expression of TNF-α from T cells (Fig. 5B). Conversely, no induction of IL-4, IL-5, IL-2, or IL-10 was observed when T cells were cocultured with BCG-stimulated DC in the presence or absence of IFN-β for 6 days (data not shown).
Fig. 5.
Stimulation of naïve CD4+ T cells by BCG-infected DC following treatment with IFN-β. (A) MoDC were left untreated or infected for 24 h with BCG, with or without IFN-β. A MLR assay was set up with DC cultured at various cell numbers with 3 × 104-purified, allogeneic, naïve CD4+ T cells isolated from cord blood. The proliferative response was measured after 6 days and is expressed as mean cpm of triplicate cultures ± sd. One representative experiments out of three performed is shown. (B) MoDC stimulated as in A were cultured at a 1:10 ratio (stimulator:effector cell) with allogeneic, naïve T cells. After 6 days, supernatants were collected to measure IFN-γ (B) and TNF-α, and IFN-γ and TNF-α production means ± se of three independent experiments are shown. *, P = 0.043, for IFN-γ; **, P = 0.040, for TNF-α (BCG vs. BCG+IFN-β).
DISCUSSION
Mtb is a facultative, intracellular pathogen, which is controlled principally by cell-mediated immunity. Indeed, the elimination of Mtb is dependent mainly on the release of IFN-γ, which synergizes with TNF-α in activating macrophages and in isolating Mtb inside the granuloma, composed primarly of CD4+ and CD8+ T cells, but a complex array of T cells, including γδ- and CD1-restricted αβ T cells, is likely involved in orchestrating immune responses and in containing the infection [4]. In particular, although CD4+ T cells, restricted by gene products of the MHC class II, play a major role, MHC class I-restricted CD8+ T cells contribute to protection, notably in later phases of the infectious process. In addition, γδ- and CD1-restricted T cells with specificity for nonproteinaceous antigen are stimulated and shape the subsequent development of Th1-dominant immunity [19]. Accordingly, the induction of IFN-γ-expressing T cells is a central, recurring theme in the search for new vaccines or suitable adjuvants to specifically enhance a protective immune response against Mtb. In particular, the desirable immunological consequence of vaccination with BCG is the priming of a Th1-oriented CD4 T cell response that strengthens, through the release of IFN-γ, the antimicrobial property of macrophages [4, 20].
The induction of a functional Th1 response is crucially dependent on the profile of the cytokines released following the exposure of different APC, such as DC, to Mtb. After interacting with Mtb, DC mature and acquire the ability to stimulate T cells through the surface expression of MHC and costimulatory molecules as well as the secretion of immunoregulatory cytokines, such as IL-12 and type I IFN, which play a key role in driving Th1 polarization [10]. Keeping with these observations, here, we compared the ability of BCG and Mtb to endow DC with a Th1-polarizing capacity through the analysis of the phenotype and the expression of the immunoregulatory cytokines IL-12 and IFN-β. Notably, we observed that BCG-infected DC displayed a less-mature phenotype compared with Mtb-infected cells (Fig. 1). In addition, an impaired transcription of IFN-β and IL-12p35 genes was found in BCG-infected MoDC, leading to a poor or missing production of these two cytokines (Fig. 2). Accordingly, BCG-infected MoDC primed naive T cells for an allogeneic response, inducing a weak Th1 polarization (Fig. 5). Noteworthy, the exogenous addition of IFN-β endowed BCG-infected MoDC with a stronger Th1-polarizing capacity (Fig. 5). Indeed, IFN-β reinforces the maturation of DC infected with BCG by enhancing the expression of maturative markers, promoting IL-12p70 production (Table 1 and Fig. 4) and consequently, the capacity to expand IFN-γ- and TNF-α-expressing T cells (Fig. 5). The combined production of these cytokines in CD4 T cells might represent a good immune correlate of protection as a result of their capacity to enhance the killing of intracellular bacteria by activated macrophages [21, 22]. However, although our data suggest that IFN-β may promote a protective immune response against Mtb, the significance of type I IFN in the pathogenesis and control of mycobacterial infection is still controversial. Indeed, Mtb was shown to have a slight growth advantage in the lung of type I IFNR knockout mice following aerosol infection [23], and other reports showed that type I IFN promote mycobacterial growth [24,25,26]. Despite these evidences, the therapeutic use of recombinant IFN-α and -β is not associated with the reactivation of mycobacterial infection. Furthermore, a more rapid decrease in the number of bacilli identified in sputum and an improvement of the course of pulmonary TB were observed when aerolized IFN-α was administered to patients receiving antimicrobial treatment [27, 28]. Therefore, owing to their immunomodulatory properties and to a long record of clinical use, type I IFNs are good candidates to be used also as adjuvants for vaccination [29, 30].
In the attempt to identify the molecular mechanisms underlying the impaired expression of IFN-β and IL-12 in BCG-infected DC, we investigated the activation of IRF-3, a factor activated in Mtb-infected DC [11], which regulates IFN-β gene transcription [31] and the expression of the IL-12p35 subunit [17]. Having found that BCG does not induce IRF-3 phosphorylation, which in turn, occurs upon Mtb infection [11, 32], we suppose that the absence of IRF-3 activation could account for the impaired expression of IL-12 and IFN-β in BCG-infected MoDC (Fig. 3). On the other hand, it is conceivable that in the absence of IRF-3 phosphorylation, the expression of IL-12p70 in BCG-infected DC can be induced by IFN-β, likely through the activation of IRF-1 [18], which replaces IRF-3 binding to the IRF-E element, present within the IL-12p35 gene promoter [33]. A similar mechanism probably occurs in IFN-γ-primed DC stimulated with heat-inactivated Mtb, where the restored expression of IL-12p35 leads to IL-12p70 accumulation [34].
Interestingly, our data about the impaired expression of IFN-β and IL-12 in BCG-infected MoDC are consistent with a recent paper by Stanley and colleagues [25], which describes the triggering of type I IFN induction by the ESX-1 secretion system in murine macrophages infected with Mtb. ESX-1 is the major secretion system of Mtb required for the secretion of early secreted antigenic target 6 (ESAT6) and culture filtrate protein 10 (CFP10) virulence factors [35, 36]. In Mtb-infected cells, CFP10 and ESAT6 might gain access to the cytosol for the interaction with intracellular signaling pathways, including the TNFR-associated NF-κB kinase-binding kinase 1 pathway, which leads to IRF-3 activation and to IFN-β production [11, 32]. Therefore, in this scenario, it is likely that the lack of IRF-3 phosphorylation in BCG-infected MoDC might be a result of the absence of ESAT6 and CFP10 expression in this vaccinal strain [37]. This hypothesis correlates well with a recent paper by van der Wel et al. [38], showing that the cytosolic entry of Mtb in human myeloid cells requires the secretion of ESAT6 and CFP10. Therefore, the vaccinal strategies based on BCG should take in consideration the impaired expression of IL-12 and IFN-β from BCG-infected DC. These findings can open new perspectives for the TB vaccine and suggest that IFN-β could be considered as an adjuvant able to improve BCG immunogenicity by influencing critical steps of innate and adaptive immunity.
Acknowledgments
This work was supported by the ISS-NIH program (#5303) and a grant of the Istituto Superiore di Sanità (6ACF). We are grateful to Sandra Pellegrini (Pasteur Institute, Paris, France) and Martina Severa (Istituto Superiore di Sanità, Rome, Italy) for helpful discussion and critical reading of the manuscript. We also thank Eugenio Morassi for preparing drawings.
References
- North R J, Jung Y J. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Maartens G, Wilkinson R J. Tuberculosis. Lancet. 2007;370:2030–2043. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124:683–687. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Flynn J L, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- Holt P G, Schon-Hegrad M A. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology. 1987;62:349–356. [PMC free article] [PubMed] [Google Scholar]
- Sertl K, Takemura T, Tschachler E, Ferrans V J, Kaliner M A, Shevach E M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986;163:436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haarst J M, Hoogsteden H C, de Wit H J, Verhoeven G T, Havenith C E, Drexhage H A. Dendritic cells and their precursors isolated from human bronchoalveolar lavage: immunocytologic and functional properties. Am J Respir Cell Mol Biol. 1994;11:344–350. doi: 10.1165/ajrcmb.11.3.8086170. [DOI] [PubMed] [Google Scholar]
- Steinman R M. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp. 2006;279:101–109. [PubMed] [Google Scholar]
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia E M. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–7041. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- Remoli M E, Giacomini E, Lutfalla G, Dondi E, Orefici G, Battistini A, Uze G, Pellegrini S, Coccia E M. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–374. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- Lande R, Giacomini E, Grassi T, Remoli M E, Iona E, Miettinen M, Julkunen I, Coccia E M. IFN-α β released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- Giacomini E, Sotolongo A, Iona E, Severa M, Remoli M E, Gafa V, Lande R, Fattorini L, Smith I, Manganelli R, Coccia E M. Infection of human dendritic cells with a Mycobacterium tuberculosis sigE mutant stimulates production of high levels of interleukin-10 but low levels of CXCL10: impact on the T-cell response. Infect Immun. 2006;74:3296–3304. doi: 10.1128/IAI.01687-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E M, Severa M, Giacomini E, Monneron D, Remoli M E, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and λ interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Gafa V, Lande R, Gagliardi M C, Severa M, Giacomini E, Remoli M E, Nisini R, Ramoni C, Di Francesco P, Aldebert D, Grillot R, Coccia E M. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect Immun. 2006;74:1480–1489. doi: 10.1128/IAI.74.3.1480-1489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. Convergence of the NF-κB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Goriely S, Molle C, Nguyen M, Albarani V, Haddou N O, Lin R, De Wit D, Flamand V, Willems F, Goldman M. Interferon regulatory factor 3 is involved in Toll-like receptor 4 (TLR4)- and TLR3-induced IL-12p35 gene activation. Blood. 2006;107:1078–1084. doi: 10.1182/blood-2005-06-2416. [DOI] [PubMed] [Google Scholar]
- Remoli M E, Gafa V, Giacomini E, Severa M, Lande R, Coccia E M. IFN-β modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y. Immunological protection against Mycobacterium tuberculosis infection. Crit Rev Immunol. 2006;26:515–526. doi: 10.1615/critrevimmunol.v26.i6.40. [DOI] [PubMed] [Google Scholar]
- Baumann S, Nasser Eddine A, Kaufmann S H. Progress in tuberculosis vaccine development. Curr Opin Immunol. 2006;18:438–448. doi: 10.1016/j.coi.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Seder R A, Darrah P, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Cooper A M, Pearl J E, Brooks J V, Ehlers S, Orme I M. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68:6879–6882. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser J M, Barry C E, III, Freedman V H, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc Natl Acad Sci USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S A, Johndrow J E, Manzanillo P, Cox J S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- Bouchonnet F, Boechat N, Bonay M, Hance A J. α/β Interferon impairs the ability of human macrophages to control growth of Mycobacterium bovis BCG. Infect Immun. 2002;70:3020–3025. doi: 10.1128/IAI.70.6.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giosue S, Casarini M, Alemanno L, Galluccio G, Mattia P, Pedicelli G, Rebek L, Bisetti A, Ameglio F. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 1998;158:1156–1162. doi: 10.1164/ajrccm.158.4.9803065. [DOI] [PubMed] [Google Scholar]
- Giosue S, Casarini M, Ameglio F, Zangrilli P, Palla M, Altieri A M, Bisetti A. Aerosolized interferon-α treatment in patients with multi-drug-resistant pulmonary tuberculosis. Eur Cytokine Netw. 2000;11:99–104. [PubMed] [Google Scholar]
- Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- Bracci L, La Sorsa V, Belardelli F, Proietti E. Type I interferons as vaccine adjuvants against infectious diseases and cancer. Expert Rev Vaccines. 2008;7:373–381. doi: 10.1586/14760584.7.3.373. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever B R, Grandvaux N, Zhou G P, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Liu J, Cao S, Herman L M, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-γ-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Lyakh L, Batoni G, Esin S, Winkler-Pickett R, Consolaro M, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein R, Carra G, Trinchieri G. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn K M, Hickey M J, Mathur S K, Zakel K L, Grotzke J E, Lewinsohn D M, Smith S, Sherman D R. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Rosenkrands I, Andersen P, Cole S T, Brosch R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 2004;12:500–508. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters P. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]