Abstract
Background/Objective:
Patients with traumatic upper thoracic and cervical spinal cord injuries are at increased risk for the development of autonomic dysfunction, including thermodysregulation. Thermoregulation is identified as an autonomic function, although the exact mechanisms of thermodysregulation have not been completely recognized. Quad fever is a hyperthermic thermoregulatory disorder that occurs in people with acute cervical and upper thoracic spinal cord injuries. First described in 1982, it has not been widely discussed in the literature.
Methods:
Case reports of 5 patients with cervical spinal cord injury (SCI).
Results:
Five of 18 patients (28%) with acute cervical SCI who were admitted during a 1-year period had fatal complications caused by persistent hyperthermia of unknown origin.
Conclusions:
Patients with acute traumatic cervical and upper thoracic SCI are at risk for thermoregulatory dysfunction. Changes in the hypothalamic axis may be implicated, especially in the light of modification in hypothalamic afferent nerves, but this hypothesis has not yet been explored. Thermodysregulation may be an early sign of autonomic dysfunction. A comprehensive guideline is needed for the management of elevated body temperature in critically ill patients with cervical SCI, because this condition may be fatal.
Keywords: Spinal cord injuries, Fever, Hyperthermia, Hyperpyrexia, Quad fever, Thermoregulation, Autonomic dysfunction, Tetraplegia
INTRODUCTION
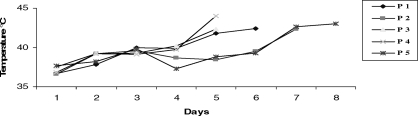
Many factors may cause fever in patients admitted to intensive care units (ICUs), both infectious and noninfectious. Discrimination between infectious and noninfectious causes of the fever may be challenging and requires careful clinical assessment. Potential exceptions to this condition are those patients in whom fever is detrimental to their outcome, especially those with central neurologic injury. Quad fever may be defined as an idiopathic extreme elevation in body core temperature beyond 40°C (101.5°F) in patients with spinal cord injury (SCI) (1). When the cervical spinal cord is injured, particularly at a high level, thermoregulation is impaired. Cervical SCI frequently occurs during the summer months, often after near drowning or a fall. We report 5 fatal cases of hyperthermia in patients with cervical SCI and tetraplegia (Figure 1). Our objective was to discuss the aspects of hyperthermia of unknown origin and outline a diagnostic approach to persistent fever in patients with cervical SCI.
Figure 1. Axillary temperature values of 5 patients during intensive care unit stay.
CASE REPORTS
Case 1
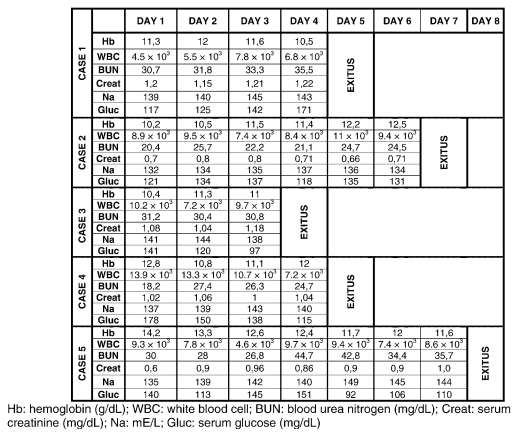
A previously healthy 39-year-old man, American Society of Anesthesiologists (ASA) I, Glasgow Coma Scale (GSC) 15, was brought to our university hospital emergency room after falling from a tree. Physical examination showed a C5 to C6 dislocation and 2/5 strength of left-right proximal and 1/5 left-right distal extremities, but flaccid total paralysis below T1. He had lack of sensation below the C5 level, and American Spinal Injury Association (ASIA) Impairment Scale grade was A. The patient underwent internal fixation under general anesthesia on the second day of the trauma. Anesthesia was induced with fentanyl, vecuronium, and propofol and maintained with sevoflurane. Mechanical ventilation was started on the fourth postoperative day. Electrolytes were regulated, and deep venous thrombosis (DVT) prophylaxis was initiated with low-molecular-weight heparin (LMWH). After trauma, the patient's axillary temperatures were as follows: day 1, 36.7°C; day 2, 37.8°C; day 3, 40.0°C; and day 4, 39.9°C. Fluid regimens contained a combination of colloids or crystalloids to maintain mean arterial pressure at 70 mmHg and urine output level at 1 mL/kg/h.
Axillary temperature increased to 41.8°C on the fifth day, and he remained persistently unresponsive to antipyretic and inotropic agents. Vigorous cooling efforts with ice packs, gastric and rectal lavage, and antipyretic agents were ineffective. Laboratory values were unremarkable; daily changes are shown in Table 1. Although the chest radiograph was normal on the fifth hospital day, arterial blood gases indicated hypoxia (pH: 7.41, PaO2: 76.2, PaCO2: 43.3, HCO3: 26.7, SaO2: 95% with FiO2 1.0). Thyroid function tests were normal [thyroid-stimulating hormone < 5 μIU/mL]. A cranial computed tomography (CT) scan showed no signs of meningeal inflammation or other changes. Blood, sputum, stool, urine, and cerebrospinal fluid cultures were negative. On the sixth hospital day, the axillary temperature increased to 42.4°C, systolic pressure was 80 mmHg, and pulse rate was irregular at 120 to 135 beats/min. Despite dopamine administration, cardiac arrest occurred, and he did not respond to resuscitation.
Table 1.
Daily Laboratory Values for 5 Patients
Case 2
A 50-year-old man, ASA I, GCS 15, was brought to the hospital emergency room after near drowning. CT scan of cervical spine showed dislocation at the C5 to C6 level, linear fracture of the C6 spinous process, and spinal cord contusion. He showed 1/5 strength in the proximal and distal upper extremities bilaterally, but flaccid paralysis below injury level. His injury was categorized as ASIA grade A. On day 1, he underwent mechanical ventilation. Body temperature remained elevated after the injury; maximal daily axillary temperatures were as follows: day 1, 36.6°C; day 2, 39.2°C; day 3, 39.6°C; day 4, 38.7°C; day 5, 38.4°C; and day 6, 39.5°C.
On the seventh ICU day, temperature rose to 42.3°C. The patient's mental status was altered, and he became hemodynamically unstable, with a systolic pressure of 70 mmHg and an irregular pulse rate of 115 to 145 beats/min. Aggressive fluid administration of colloids and crystalloids was needed to maintain the mean arterial pressure at 70 mmHg and urine output at 1 mL/kg/h. Electrolytes were monitored, and DVT prophylaxis was initiated with LMWH after he was admitted to the ICU.
Despite treatment with dopamine, he remained persistently unresponsive to antipyretic and inotropic agents. Blood, tracheal aspirate, and urinary cultures were negative for bacterial growth. No identifiable source for the fever could be identified. Hyperthermia did not resolve with aggressive cooling. Endocrine function (including cortisol and TSH < 5 μIU/mL) were normal. Daily changes are given in Table 1. Although the chest radiograph was normal, on the sixth hospital day, arterial blood gases indicated hypoxia (pH: 7.48, PaO2: 74.3, PaCO2: 38.4, HCO3: 26.7, SaO2: 94% with FiO2 1.0). On the seventh hospital day, his axillary temperature increased to 42.3°C and cardiac arrest occurred. No source for the fever was identified.
Case 3
A previously healthy 52-year-old man, ASA I, GCS 15, was admitted after a fall. Physical examination showed a C6 corpus fracture with flaccid paralysis of upper and lower extremities. Physical examination showed 2/5 strength of proximal and 1/5 distal extremities, and he had flaccid paralysis below the T4 level (ASIA grade A). He underwent surgery 2 days after the accident. On the second postoperative day (4 days after the accident), his axillary temperature progressively increased to 40.2°C. Physical examination showed no obvious source for the elevated temperature. Blood, sputum, stool, urine, and cerebrospinal fluid cultures were taken. A CT scan of the head was negative. Thyroid function tests were normal (TSH < 5 μIU/mL).
On the fifth postoperative day, temperature rose to 42.3°C; systolic arterial pressure was 90 mmHg, and pulse rate was irregular at 95 to 120 beats/min. An electrocardiogram showed ventricular tachycardia and ventricular arrhythmia, which returned to normal sinus rhythm with repeated direct current (DC) cardioversion.
On the fifth postoperative day, the chest radiograph was negative, but physical examination showed dyspnea, and mechanical ventilation was started. Blood gases showed the following—pH: 7.51, PaO2: 68.1, PaCO2: 26.8.4, HCO3: 23.6, and SaO2: 95% with FiO2 0.6. A combination of colloids and crystalloids was administered to maintain a mean arterial pressure at 70 mmHg and urine output at 1 mL/kg/h. DVT prophylaxis was initiated with LMWH, and dopamine was given to maintain blood pressure. Core temperature was 42.3°C, and he remained persistently unresponsive to antipyretic and inotropic agents. Laboratory values are given in Table 1. Sequential cultures were negative. Applying ice packs for cooling, gastric lavage with cold water, and antipyretic agents were not effective. On the sixth hospital day, axillary temperature was still 42.3°C and the patient died.
Case 4
A 40-year-old man, ASA I, GCS 15, who fell into a swimming pool, was admitted to the university hospital emergency service. Cervical spine CT examination confirmed a C5 corpus fracture and flaccid paralysis of the upper and lower extremities. Extremity motor examination showed 2/5 strength proximally and plegia distally. Sensory examination showed hypoesthesia below the T5 level and anesthesia below the T8 level. He had paraplegia with ASIA grade A. After emergency diskectomy with fusion, he developed respiratory distress and was admitted to the ICU, where mechanical ventilation was started. Maximal axillary temperatures on each postoperative day were as follows: day 1, 36.8°C; day 2, 39.2°C; day 3, 39.1°C; and day 4, 39.7°C. Physical examination showed no obvious source for the elevated temperature in the early postoperative period. After resuscitation and cooling measures, a CT scan showed no signs of meningitis or other pathologies. Thyroid function tests were normal (TSH < 5 μIU/ml).
On the fifth postoperative day, his temperature rose to 44.0°C; systolic pressure was 90 mmHg, and pulse rate was irregular at 95 to 120 beats/min. An electrocardiogram showed sinus tachycardia and ventricular arrhythmia. Arterial blood gases showed the following—pH: 7.53, PaO2: 159 mmHg, PaCO2: 30 mmHg, HCO3: 26.9, SaO2: 99% with FiO2 0.5. The patient remained persistently unresponsive to antipyretics and inotropic agents. Aggressive management of fluids and electrolytes was maintained, and DVT prophylaxis was initiated with LMWH. Laboratory values are shown in Table 1. Cooling with ice packs, gastric lavage, and antipyretic agents were ineffective. Blood, sputum, stool, urine, and cerebrospinal fluid cultures were without any growth, excluding an obvious bacterial cause. On the fifth hospital day, his axillary temperature increased to 44.0°C. Cardiac arrest occurred, and resuscitation efforts failed; no source of fever was determined.
Case 5
A 29-year-old man, ASA I, GCS 15, was admitted to the university hospital emergency service after an accident. Cervical spine CT examination confirmed a C1 anterior and posterior corpus linear fracture and a C4 to C5 linear corpus fracture with flaccid paralysis of upper and lower extremities. Sensory examination showed anesthesia below the T4 level. Motor assessment revealed paraplegia (ASIA grade A), and mechanical ventilation was required. Postoperative maximal skin temperatures on each postoperative day were as follows: day 1, 37.7°C; day 2, 38.2°C; day 3, 39.8°C; day 4, 37.3°C; day 5, 38.8°C; day 6, 39.3°C; day 7, 42.6°C. On the seventh day, despite supportive interventions, systolic pressure was 85 mmHg with an irregular pulse rate of 90 to 115 beats/min. An electrocardiogram showed sinus tachycardia, ventricular arrhythmia, and ST-T wave changes. Arterial blood gases showed the following—pH: 7.44, PaO2: 100 mmHg, PaCO2: 46 mmHg, HCO3: 30.4, SaO2: 99% with FiO2 1.0. After administration of dopamine 5 μg/kg/h, axillary temperature was 43.0°C; the patient remained persistently unresponsive to antipyretic agents, and urinary output decreased. Table 1 shows the daily laboratory values. Thyroid function tests were normal. Cooling with ice packs, gastric lavage, and antipyretic agents were ineffective. Blood, sputum, stool, urine, and cerebrospinal fluid cultures showed no growth, excluding an obvious bacterial cause. No source of fever was determined. On the eighth hospital day, axillary temperature increased to 43.0°C, cardiac arrest occurred, and attempts at resuscitation failed.
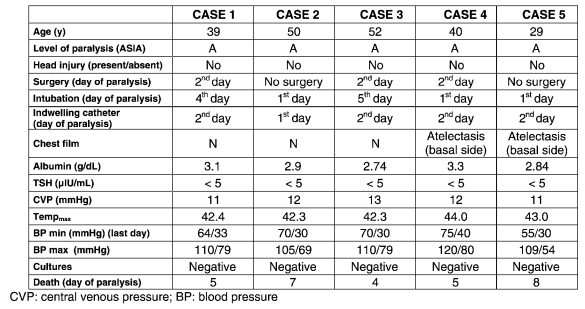
Demographic, clinical data, and hemodynamic variables of the patients are summarized in Table 2.
Table 2.
Demographic, Clinical Data, and Hemodynamic Variables
DISCUSSION
Body temperature changes during the course of the day, controlled by the thermoregulatory center of the anterior hypothalamus (2). Thermoregulation is a complex system involving several different factors. The human body is able to preserve a stable temperature because the hypothalamic thermoregulatory center balances the excess heat production by cooling mechanisms such as vasodilatation (3,4). After high cervical SCI, the resultant autonomic dysfunction may be manifested as thermodysregulation.
There are 3 categories of thermodysregulation (5). Poikilothermia is the best known in the first category, which is related to hypothermia resulting from prolonged cold exposure. The second category includes “quad fever,” which refers to fever without an infectious basis that appears in the first several weeks to months after cervical SCI. The third is exercise-induced fever. Along with total loss of temperature control, such as in the cases reported here, these conditions may have devastating and lethal sequelae (4,6).
Uncontrolled environmental conditions can affect body temperature; therefore, special hospital areas should maintain a constant temperature. The recommendations of the Intensive Care Society specify that ICUs and their patient rooms should provide air-conditioning with ambient adjustable temperature in the range of 16°C to 27°C (7). In our ICU, the temperature is maintained at 23°C to 24°C. Therefore, extremes of body temperature among patients in the ICU cannot be attributed to temperature changes caused by environmental conditions.
Sugarman (1) described quad fever in individuals with tetraplegia and occasionally in those with high paraplegia. These patients had fever, often exceeding 40°C (101.5°F), and fever was encountered later in their courses. Quad fever is considered only after all infectious or inflammatory causes have been ruled out. Quad fever is defined as an early phenomenon in patients with incomplete cervical SCI. These 5 patients had cervical SCI and hyperthermia consistent with quad fever related to autonomic dysfunction after acute SCI.
Fever is associated with poor outcome in up to 70% of ICU patients with or without bacterial infections. The most important noninfectious causes of severe hyperthermia are heat stroke, malignant hyperthermia, neuroleptic malignant syndrome, thromboembolism, thermoregulatory dysfunction, and medication-related fever, which are caused by failure of thermoregulation. Each of these situations can be associated with severe systemic complications and death (8,9). Malignant hyperthermia is a rare fatal genetic disorder that manifests itself in susceptible patients after induction with certain anesthetic agents. These patients develop elevated body temperature, increased muscle metabolism, muscle rigidity, rhabdomyolysis, acidosis, and cardiovascular instability (10). Three of our patients suffered fatal hyperthermia episodes after the postoperative period, and all of them died within 1 week. The same anesthetic agents were administered to these patients (induction with fentanyl, vecuronium, and propofol and maintenance with sevoflurane and remifentanil infusion). Their clinical findings were similar to those of malignant hyperthermia, but some of the clinical signs, such as increased muscle metabolism, muscle rigidity, and rhabdomyolysis, were absent. The onset of malignant hyperthermia occurs usually within 1 hour of administration of general anesthesia; rarely, it may be delayed up to 10 hours after induction (11). In this series, however, body temperatures increased a few days after the operation in the 3 patients who had surgery. In addition, fatal stress-induced malignant hyperthermia in patients with head injury has been reported (12). The clinical findings and histories of the patients presented were not consistent with these causes of malignant hyperthermia.
Inappropriate use of psychotropic drugs and illicit drugs such as monoamine-oxidase inhibitors, tricyclic antidepressants, or amphetamines may cause drug-induced hyperthermia. Neuroleptic malignant syndrome is an idiosyncratic reaction to antipsychotic agents, which is also characterized by muscle rigidity, altered mental status, choreoathetosis, tremors, and evidence of autonomic dysfunction, such as sweating, unstable blood pressure, and dysrhythmias (13). These complications seem to be caused by the inhibition of central dopamine receptors in the hypothalamus, which results in increased heat generation and decreased heat delivery (14). None of these 5 patients received haloperidol or other antipsychotic drugs in the ICU period. However, 4 patients were sedated with midazolam or opioids during the ICU period. Therefore, we did not suspect neuroleptic malignant syndrome or drug-induced hyperthermia. Standard venous thromboembolism prophylaxis was initiated in all patients, and none showed evidence of venous thromboembolism. The etiologic spectrum of hyperthermia is extensive; it may be caused by infectious, endocrine, central nervous system, and toxic etiologies. Hence, its diagnosis may differ considerably from 1 patient to another (15). Thyrotoxicosis may also cause increased thermogenesis (16). Because TSH levels were within the normal range in all patients, thyrotoxicosis-induced fever was not considered.
It is important to recognize the difference between fever, which is caused by inflammatory or infectious conditions, and hyperthermia caused by thermoregulatory dysfunction, because hyperthermia can be progressive, fatal, and unresponsive to antipyretic agents and cooling interventions. The hyperthermia episodes in this series of patients occurred within 1 week of trauma. All of them had high cervical SCI with ASIA grade A and required intubation and mechanical ventilation.
Neurogenic shock is a form of distributive shock that involves loss of peripheral vasomotor control as a result of neurologic injury to the nervous system, especially noticeable in cervical injuries (17). A decrease in vascular resistance with associated vascular dilatation and pooling of blood in peripheral vascular beds and development of shunts and capillary leak after neurologic dysfunction results in hypotension. Also, loss of sympathetic vascular tone causes warm, dry skin and bradycardia after cervical SCI. Plasma volume expansion is the mainstay therapy in these patients. The amount of fluid administered is based on improvement of clinical signs, particularly blood pressure, pulse pressure, and heart rate. Central venous pressure and urinary output also provide indications of restoration of vital organ perfusion but only at the macrocirculatory level. In our patients, fluids (crystalloids and colloids) were given to maintain a central venous pressure of 10 to 15 mmHg, mean arterial pressure of 70 mmHg, and urine output of 1 mL/kg/h. Fluid resuscitation eventually restored global hemodynamic parameters; however, restoration of microcirculation might have been inadequate. On the other hand, inflammation has a significant pathophysiologic role in renal ischemia and reperfusion injury. Also, fever is an important manifestation of inflammation. All these changes caused prerenal azotemia, a well-known reason for high blood urea nitrogen/creatinine ratio as seen in our patients.
Distributive shock and fluid resuscitation may precipitate electrolyte imbalance (Na+, K+, and Cl−). Distributive shock may also decrease plasma albumin level. In this situation, distributive shock and inflammation can cause an increase in the BUN/creatinine ratio, a decrease in the albumin level, and an electrolyte imbalance.
In ICU patients, single fever spikes from 38.9°C to 41.1°C that terminate spontaneously within 24 hours are almost always noninfectious in nature and are most commonly caused by blood/blood products transfusion, insertion/removal of devices, or manipulation of a colonized/infected mucosal surface (18). Some clinical studies showed that hospital-acquired sinusitis is a frequent cause of fever with unknown origin in patients with orotracheal intubation and critically ill patients on mechanical ventilation (19). In addition, sputum production is increased in patients with cervical SCI (20). However, none of our patients had a history of infection; moreover, their sequential tracheal aspirate cultures were negative during the episodes. Their chest radiographs did not show any infiltration or consolidation caused by infections.
Patients with cervical SCI are prone to atelectasis, especially at the basal side of their lungs (21,22). Atelectasis may also cause fever in patients, especially in the early postoperative period; however, such extremely high temperatures in these patients could not be explained solely by atelectasis.
A total of 18 patients with acute cervical SCI were admitted to our ICU during a 1-year period, 5 of whom had complications caused by persistent high fever. Therefore, the approximate incidence of fever of unknown origin, or quad fever, was 28%.
CONCLUSION
This case series indicates that patients with acute traumatic cervical SCI are at risk for thermoregulatory dysfunction. Changes in the hypothalamic axis may be implicated, especially in the light of modification in hypothalamic afferent nerves, but this hypothesis has not yet been explored. Fever etiologies were not identified in this case series. Thermodysregulation may be an early sign of autonomic function after CSCI. There is a need for a comprehensive guideline for the management of elevated body temperature in critically ill patients with CSCI, because this condition may be fatal. Clinicians should include thermodysregulatory causes in the differential diagnosis of fever in individuals with acute high-level SCI.
REFERENCES
- Sugarman B. Fever in recently injured quadriplegic persons. Arch Phys Med Rehabil. 1982;63(12):639–640. [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974;71:1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- Lee-Chiong TL, Stitt JT. Disorders of temperature regulation. Compr Ther. 1995;21(12):697–704. [PubMed] [Google Scholar]
- Downey JA, Huckaba CE, Myers SJ, Darling RC. Thermoregulation in the spinal man. J Appl Physiol. 1973;34(6):790–794. doi: 10.1152/jappl.1973.34.6.790. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Karlsson AK, Wecht JM, Wuermser LA, Mathias CJ, Marino RJ. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to international standards for neurological assessment. J Rehabil Res Dev. 2007;44(1):103–112. doi: 10.1682/jrrd.2005.10.0159. [DOI] [PubMed] [Google Scholar]
- Randall WC, Wurster RD, Lewin RJ. Responses of patients with high spinal transection to high ambient temperatures. J Appl Physiol. 1966;21(3):985–993. doi: 10.1152/jappl.1966.21.3.985. [DOI] [PubMed] [Google Scholar]
- The Intensive Care Society. Standards, Safety and Quality Committee page. Standards for Intensive Care Units. (Updated May 1997). Available at: http://www.ics.ac.uk/icmprof/standards.asp?menuid=7. Accessed November 7, 2008.
- Khosla R, Guntupalli KK. Heat-related illnesses. Crit Care Clin. 1999;15(2):251–263. doi: 10.1016/s0749-0704(05)70053-1. [DOI] [PubMed] [Google Scholar]
- Sugarman B, Brown D, Musher D. Fever and infection in spinal cord injury patients. JAMA. 1982;248(1):66–70. [PubMed] [Google Scholar]
- Denborough M. Malignant hyperthermia. Review. Lancet. 1998;352(9134):1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- Hadad E, Weinbroum AA, Ben-Abraham R. Drug-induced hyperthermia and muscle rigidity: a practical approach. Review. Eur J Emerg Med. 2003(2);10:149–154. [DOI] [PubMed]
- Feuerman T, Gade GF, Reynolds R. Stress-induced malignant hyperthermia in a head-injured patient. Case report. J Neurosurg. 1988;68(2):297–299. doi: 10.3171/jns.1988.68.2.0297. [DOI] [PubMed] [Google Scholar]
- Velamoor VR. Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Saf. 1998;19:73–82. doi: 10.2165/00002018-199819010-00006. [DOI] [PubMed] [Google Scholar]
- Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185–202. doi: 10.1016/s0025-7125(16)30278-4. [DOI] [PubMed] [Google Scholar]
- Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999;25(7):668–673. doi: 10.1007/s001340050928. [DOI] [PubMed] [Google Scholar]
- Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263–277. [PubMed] [Google Scholar]
- Papadakos PJ. Approach to shock. In: Apostolakos MJ, Papadakos PJ, editors. The Intensive Care Manual. New York: McGraw-Hill; 2001. pp. 55–70. [Google Scholar]
- Cunha BA. Fever in the intensive care unit. Intensive Care Med. 1999;25(7):648–651. doi: 10.1007/s001340050925. [DOI] [PubMed] [Google Scholar]
- van Zanten AR, Dixon JM, Nipshagen MD, de Bree R, Girbes AR, Polderman KH. Hospital-acquired sinusitis is a common cause of fever of unknown origin in orotracheally intubated critically ill patients. Crit Care. 2005;9(5):R583–R590. doi: 10.1186/cc3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar KR, Brown R, O'Sullivan DD, Melia S, Duggan M, Reid L. Bronchial mucus hypersecretion in acute quadriplegia. Macromolecular yields and glycoconjugate composition. Am Rev Respir Dis. 1991;143(3):640–648. doi: 10.1164/ajrccm/143.3.640. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82(10):803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Lanig IS, Peterson WP. The respiratory system in spinal cord injury. Phys Med Rehabil Clin North Am. 2000;11(1):29–43. vii. [PubMed] [Google Scholar]