Abstract
Background/Objective:
Bone density loss occurs rapidly after traumatic spinal cord injury (SCI) and is associated with low-energy fractures below the level of injury, commonly occurring around the knee. Bisphosphonates have been tested as potential agents to prevent bone loss after SCI, but no guidelines exist for clinical use of bisphosphonates in these patients. The objective of this study was to systematically review and evaluate evidence quality in studies of bisphosphonate use in patients with post-treatment follow-up of sublesional bone mineral density.
Methods:
Literature search in MEDLINE/PubMed and ISI database using key words bisphosphonates, spinal cord injury, quadriplegia, paraplegia, and tetraplegia.
Results:
The search identified 6 experimental studies and 1 quasi-experimental study of bisphosphonate therapy in patients with acute and chronic SCI. The studies were small and of fair or poor quality, and none included fracture outcomes. Mild attenuation of bone density loss with acute administration of bisphosphonates after SCI was found at some measurement sites but was not always maintained during follow-up.
Conclusions:
Data were insufficient to recommend routine use of bisphosphonates for fracture prevention in these patients. Current studies are limited by heterogeneity of patient populations and outcome measures. Uniform bone density measurement sites with rigorous quality control and compliance monitoring are needed to improve reliability of outcomes. Future studies should address specific populations (acute or chronic SCI) and should assess fracture outcomes.
Keywords: Spinal cord injuries; Bisphosphonates; Osteoporosis; Heterotopic ossification; Bone density, Tetraplegia; Paraplegia
INTRODUCTION
Osteoporosis is a common and important complication of spinal cord injury (SCI). Bone hyperresorption proceeds rapidly after the onset of injury and predominantly affects bones inferior to the level of the spinal cord lesion. In a study of 99 persons with motor complete SCI who had sustained a fragility fracture, bone mineral density (BMD) losses of 54% in the distal femur and 73% in the distal tibia occurred within the first 5 and 7 years after injury, respectively (1). Low BMD is associated with increased risk of fractures in persons with SCI (2). A study of 98 persons with paraplegia showed that the incidence of fracture increased with the duration of time since injury, from approximately 1% within the first year of injury to 4.6% in those with chronic SCI (20 to 29 years), with the median duration to first fragility fracture occurring at 8.5 years (3). A recent Canadian population-based study of individuals with SCI reported the prevalence of fractures among persons with traumatic SCI to be 7.3% (4). Complications of fractures include nonunion, infection, and skin ulcers, which can lead to further immobilization and decreased functional independence. Because more than one half of the 11,000 new cases of SCI reported in the United States each year occur in individuals younger than 30 years of age (5) and because the life expectancy after SCI has dramatically increased to greater than 30 years after injury (4), the complications in the decades after sustaining SCI constitute a significant disease burden in this patient population.
Despite an understanding of the compromised bone health after SCI, safe and effective diagnostic and treatment strategies are unproven and difficult to test. Standard osteoporosis diagnostic criteria (6), approaches to fracture risk assessment (7), and BMD measurement tools developed for postmenopausal women have not been adequately tested in people with SCI and may not be appropriate given the unique distribution of osteoporosis and associated fractures, risk factors, and pathophysiology of disease within the SCI population. World Health Organization (WHO) diagnostic criteria for osteoporosis were designed for postmenopausal women (6); the relationship between WHO classification and fracture risk for premenopausal women, young men, or persons with an SCI is unknown. The distal femur and proximal tibia have been proposed as sites for BMD measurements in patients with SCI because they are the most common sites of fracture after SCI (8–10). Hip BMD has been found to moderately correlate with distal femur BMD and is only marginally correlated with proximal tibia BMD (9). Furthermore, standardized DXA protocols for measurement of peripheral sites have not been available for use in interventional studies to date (9). Finally, according to the review by Giangregorio and McCartney (11), data regarding the effectiveness of strategies to build stronger bones around the knee through mechanical stress, such as static loading (weight bearing by tilt table), body weight–supported treadmill training, and muscle contraction using functional electrical stimulation, are inconclusive and require further study.
First- and second-generation bisphosphonates (BPs) have been tested in small trials of persons with acute and chronic SCI in an effort to attenuate bone hyper-resorption by inhibiting osteoclastic activity (12). In postmenopausal women, BPs confer a protective effect against spine fractures that is clinically important (13) and greater than expected for the small post-treatment increases in BMD (14). Although BMD measures have been examined in patients with SCI, the antifracture efficacy with BPs in either the prevention or treatment of sublesional osteoporosis after SCI is not yet characterized (10).
This systematic review will summarize small randomized trials of BP treatment in persons with SCI with post-treatment follow-up of sublesional BMD. The purpose is to characterize patient populations tested, identify possible trends in post-treatment BMD measures, and use this information to provide direction for future research.
METHODS
The initial search strategy in the PubMed/MEDLINE database (1966 through December 2008) made use of key words bisphosphonates AND spinal cord injury OR quadriplegia OR paraplegia and limited results to human studies only, for a total yield of 134 articles. An additional query using key words bisphosphonates AND paraplegic OR tetraplegic identified 2 additional articles, but no new relevant studies. To supplement the PubMed search, a citation search was performed in the ISI database (1955 through December 2008) using key articles from the bibliography of frequently cited studies; again, no new relevant articles were found. References cited in review articles were studied to assure adequacy of systematic review.
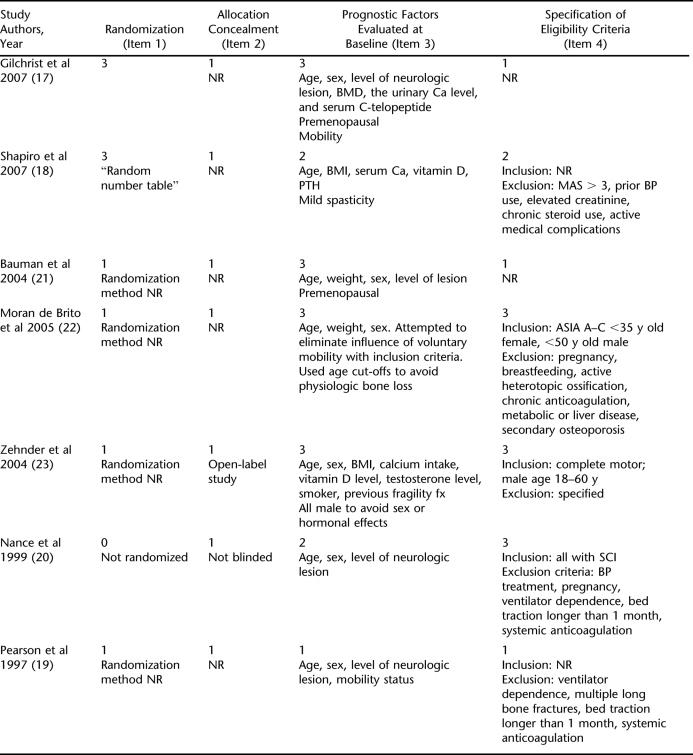
On the basis of an exploratory search of the literature, inclusion and exclusion criteria were established (Table 1). The study population was limited to persons with SCI with specified or presumed traumatic etiology. The primary outcome was BMD measured by dual-energy x-ray absorptiometry (DXA) because clinically this is the gold standard measure to assess fracture risk. The body of literature on BP therapy in persons with SCI includes few studies with rigorous study designs; therefore, broad inclusion criteria for study design were set.
Table 1.
Inclusion and Exclusion Criteria for Original Research Reports of BP Therapy in Persons With SCI
After a review of the title plus abstract or full text of the article, articles that met all eligibility criteria were selected. Three articles published before 1997 only reported histomorphometric outcomes; these articles were reviewed but were not assigned evidence ratings. Data expressed as numbers and/or means and SDs or 95% confidence intervals (CIs) were retrieved for baseline characteristics and BMD results. Intervention details were also assessed, including type of drug and route, timing, and frequency of administration. Main outcomes from the studies using DXA measurements were BMD at sublesional sites, considered clinically relevant because they are common fracture sites in patients with SCI.
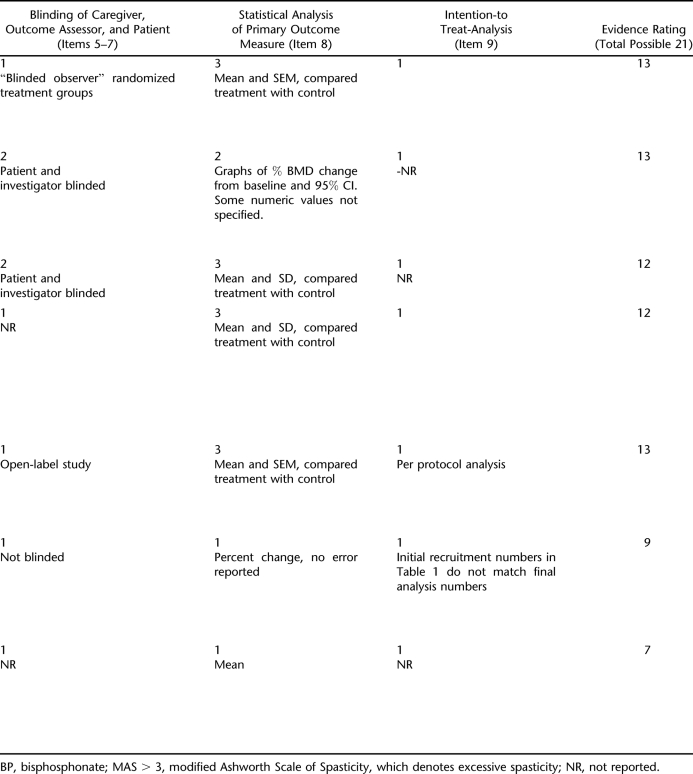
To establish minimum reference standards for the quality of selected studies, the internal validity of each of the included studies was assessed based on modified quality assessment criteria for experimental trials (trials that are randomized and include control/comparison group) from the Centre for Reviews and Dissemination (CRD 2001) (15). These criteria were developed by consensus rounds by international experts who determined that 9 items comprised the key quality components of experimental trials. The 9 items are referred to as the Delphi list and consist of (a) randomization; (b) adequate allocation concealment; (c) groups similar at baseline; (d) specification of eligibility criteria; (e) blinding of outcome assessor; (f) blinding of care provider; (g) blinding of patient; (h) presentation of point estimates and measures of variability for the primary outcome; and (i) intention-to-treat analysis (16).
A subscore of 1 (inadequate or not reported), 2 (fair), or 3 (good) was assigned for each category. All categories had a minimum subscore of 1 except for the randomization category, in which a study received a 0 if it was not randomized. Regarding item 3 on the Delphi list, because similarity of baseline factors often cannot be achieved with small sample sizes, and because the relative importance of each prognostic factor for fracture risk is not well established in persons with SCI, grades were not significantly decreased if some baseline prognostic factors were dissimilar in the treated and control groups. For item 4, 1 point was subtracted from the subscore if either inclusion or exclusion criteria were not specified, or an additional point was subtracted for specified criteria that were less rigorous. Separate CRD categories for blinding (items 5–7) were not possible because no studies reported blinding of the outcome assessor, care provider and patient, only 2 of 7 reported blinding of the patient and investigator, and the blinding methods were not described in detail. Therefore, these categories were combined into single subscore with a point given for each type of blinding. Subscores were added to determine overall evidence rating, with equal weights applied to all items: poor (7–11), fair (12–16), and good (17–21).
RESULTS
BMD Outcomes
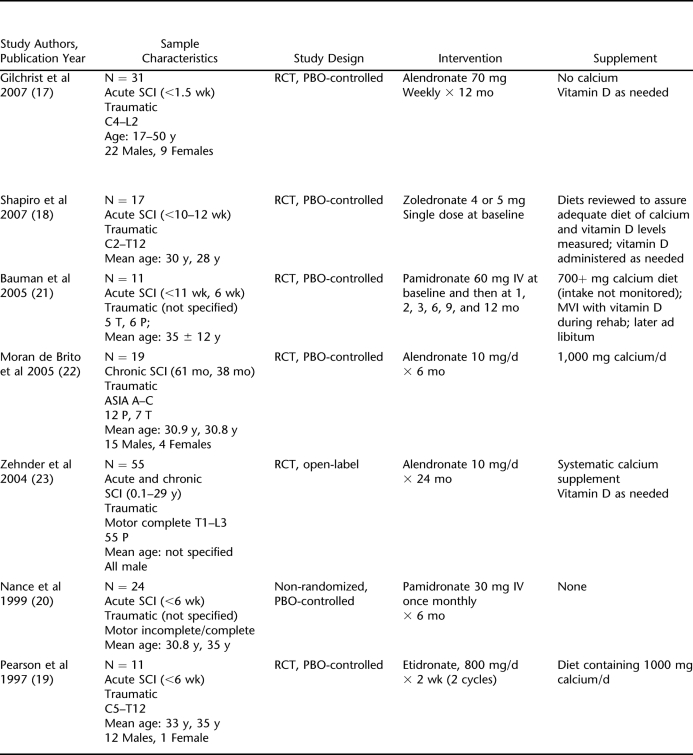
Six randomized controlled trials (RCTs) and 1 quasi-experimental study were identified as having BMD measured by DXA as the primary outcome and were published in 1997 and after. DXA was the only imaging modality used in the studies. Although some studies reported quality monitoring with DXA precision studies (Table 2), the studies by Nance et al (20) and Gilchrist et al measured DXA machine precision error on ambulatory persons rather than persons with SCI.
Table 2.
Summary of Prospective Studies With DXA as the Measurement Tool
Of the selected studies, 5 administered BPs in the acute phase of SCI, 1 in the chronic phase, and 1 in the acute and chronic phases (Table 2). When alendronate was administered within 10 days of acute SCI and a weekly 70-mg dose was continued for 12 months, BMD declined at a slower rate in the treatment group, with a resulting 7% difference (−5.6 ± 1% vs −12.7 ± 1.4%; P < 0.001) in the total body leg, and 17.6% difference (−3.3 ± 0.9% vs −20.9 ± 1.9%; P < 0.001) in the total hip BMD, in the treatment group compared with placebo controls (17). When a single dose of 4 or 5 mg of zoledronic acid was administered within 10 to 12 weeks after injury in a different study, bone loss was attenuated at proximal femur sites for 6 months and at the femur shaft for 12 months to a greater extent in the treatment group compared with the placebo group (18). The treatment effect did not persist at the proximal femur site, however, because BMD values in both groups were comparable at 12 months. BMD values at the femur shaft were maintained near baseline up to 12 months in the treatment group while decreasing in the placebo group. In an older study with administration of a first-generation BP, etidronate 800 mg daily × 2 weeks (2 cycles 13 weeks apart) starting within 6 weeks of SCI, BMD was significantly decreased at 12 months (percentage loss at 12 months: 26% distal femur, 22% proximal tibia). A treatment effect, with BMD being maintained near baseline, was found only in the 2 patients who received treatment and were ambulatory (19).
Two trials tested sequential administration of intravenous pamidronate over a 12-month period. One of these trials tested pamidronate 30 mg administered every 4 weeks for 6 treatments. Results showed amelioration of BMD loss at 12 months after injury in the treatment group compared with the placebo group (6.8% vs 13.5% mean overall BMD loss, respectively, P = 0.11; 4.7% vs 10.8% distal femur, P = 0.033) (20). However, the other trial of pamidronate 60 mg administered 7 times over 12 months showed that a significant treatment effect on BMD did not persist relative to placebo in continued follow-up at 24 months (−19 ± 17% vs −28 ± 10% distal femur, −36 ± 29% vs −42 ± 14% proximal tibia) (21).
Administration of BPs to persons with chronic SCI improved sublesional BMD loss relative to pretreatment values, although statistical significance was only achieved in 2 of 9 BMD parameters, and there was virtually no variation in the lower extremity BMD values in the treatment group relative to placebo (0.01 ± 0.02% vs −0.01 ± 0.05% lower extremity) (22). Administration of 10 mg daily oral alendronate plus elemental calcium to a population of people with acute and chronic SCI for a sustained period of 24 months showed a statistically significant treatment effect (2.0 ± 2.9% vs −10.8 ± 2.7% distal tibia, P < 0.017) compared with calcium alone, but not, on average, a reparation of bone loss relative to BMD before SCI (23).
Selected Prognostic Factors
As noted above, factors independently associated with osteoporosis in persons with SCI are not clearly established, although conventional factors associated with bone health in postmenopausal women may be considered (Tables 2 and 3). Age, weight, and sex are the most important determinants of BMD (24,25), and these factors were reported in all of the studies. Calcium and vitamin D, which may also have independent effects on BMD (17), were not consistently measured and were coadministered with BPs in nearly all studies (Table 2). Past BP use was an exclusion criterion in only one study (20). An association between weight bearing and BMD has been hypothesized, but these studies overall did not show clear evidence regarding this prognostic factor. In one study of administration of alendronate, BMD loss in the proximal knee (distal femur) for ASIA A compared with ASIA D was 12.5% vs 3% (20). In the study of Pearson et al (19), which administered etidronate, a significant interaction was seen between ambulation and treatment status, and ambulation was found to have a protective effect in treated individuals. The number of ambulatory treated individuals was small (n = 2), however, and ambulation did not affect the BMD of the control group. Spasticity, like ambulation, is a potential source of mechanical stress on bone and therefore has been postulated to have a protective effect on bone, yet only one study took into account the degree of spasticity in the patient population (18).
Table 3.
Evidence Grading (Items From 2001 CRD Criteria)
Evidence Ratings
Of the 7 studies with DXA BMD outcomes, 5 were rated fair and 2 were rated poor in evidence quality. Grading for each category is highlighted in the text and in Table 3:
Randomization method was not reported in any studies except those by Shapiro et al (18) and Gilchrist et al (17).
Allocation concealment was not reported in any of the randomized studies, and the study by Zehnder et al (23) was open label.
The more recent studies tended to be more rigorous about reporting prognostic factors at baseline, including age, sex, BMI, and a measure of calcium and/or vitamin D levels.
Regarding the eligibility criteria, the studies by Gilchrist et al (17) and Zehnder et al (23) did not specify eligibility criteria, and the study by Pearson et al (19) did not specify inclusion criteria. Although the study by Pearson et al (19) did specify exclusion criteria, it failed to include pertinent criteria mentioned in other studies such as excessive spasticity (Modified Ashworth Score >3), prior BP use, elevated creatinine, chronic steroid use, active medical complications, active heterotopic ossification, and pregnancy.
Three criteria describing blinding of the patient, observer and outcome assessor were combined into 1 category.
All studies received a full or nearly full grade for point estimates and measure of variability presented for the primary outcome measure except for the 2 oldest studies.
No study performed or reported intention-to-treat analysis.
Bone Histomorphometry Studies
Bone histomorphometry was the outcome measure cited in 3 studies published before 1997 (26–28). One of these studies reported no further decrease in bone mineral content, and a second study reported a slight increase in bone volume in samples from treated patients compared with placebo controls.
DISCUSSION
In this review of RCTs and quasi-experimental studies, treatment regimens of second-generation BPs administered in the acute stages of SCI attenuated bone hyper-resorption in treated patients compared with placebo during the treatment period in most studies. However, acute treatment failed to restore sublesional BMD to preinjury levels and may not result in a long-term therapeutic effect. A study of intravenous pamidronate administered for 12 months showed no difference in bone loss from baseline in treated patients vs controls by 24 months (12 months after treatment ended). The effect size does not seem to be as significant when administered in persons with chronic compared with acute SCI.
Methodological Considerations in the Studies
The overall quality assessment scores using the CRD criteria showed poor or fair quality of the clinical studies. Although well-done RCTs can provide the best information regarding the effectiveness of a medical intervention, poorly designed trials may yield misleading results and inconsistent findings in a systematic review. Therefore, interpretation of findings from these studies must be guarded.
Although 6 of the 7 studies used an experimental design, the studies were too small to show the usual strengths of large, randomized trials. Small sample sizes yielded power sufficient for only marked changes in BMD to be statistically significant; therefore, underestimation of effect cannot be ruled out. For example, the power calculations cited in the studies of Gilchrist et al (17) and Zehnder et al (23) reported sufficient statistical power (80%) to show a difference in bone loss of 7% per year or more and 15% over 24 months at the 0.05 significance level, respectively. Many studies failed to provide adequate specification of the method of randomization (lack of description regarding sequence generation for randomization) and/or allocation concealment and failed to perform intention-to-treat analysis. Additionally, equivalence of baseline risk factors for bone loss in the treatment and control groups was not possible to assess in many studies because few patient characteristics were reported. Small sample sizes likely precluded equal distribution of risk factors in the 2 groups at baseline.
More detailed quality assessment showed several potential confounders including varying administration of calcium and vitamin D and variations in spasticity and mobility in participants, all of which may have influenced outcomes. An example of potential effects of mobility on BMD was the greater BMD loss for patients with ASIA A compared with patients with ASIA D in the study of Nance et al (20), which suggested that nonambulatory subjects receive less protective effect from treatment at common fracture sites. Additionally, different dosing regimens could have led to different outcomes in the studies. Timing of the first dose of BP in acute SCI ranged from 1.5 to 12 weeks after injury and differed by as much as 5 weeks in the control and treatment groups within a study. If weight-bearing activity is independently associated with BMD, changes in weight-bearing activity after discharge from rehabilitation compared with during rehabilitation or differential engagement in activity between the treatment or placebo groups may have affected outcomes. A recent study examining the level of activity in persons with SCI after discharge from acute inpatient rehabilitation showed that dynamic activities decreased by one third (P < .001) and that this change was independently associated with level and completeness of lesion (29). Whether these potential confounders and other sources of bias influenced findings toward or away from the null hypothesis is difficult to ascertain.
Quality control measures such as multiple measurements to determine precision of DXA machine and/or having a single technician performing scans to avoid interoperator variability were also not routinely incorporated, and compliance monitoring was not frequently reported. Quality control of DXA measurements could have affected the accuracy of results and are noted in Table 2; however, this was not included in the evidence ratings because it is not part of the CRD criteria.
In addition to assessing the quality of study methods, outcome and quality improvement measures must be judged for their clinical relevance to the specific treatment population. The main outcome of most of the studies reviewed is the percentage change in sublesional BMD. Because the association between sublesional BMD and fracture outcomes has not been tested, this measure is of unknown clinical significance in SCI. Generalizability of the reported outcomes is limited by heterogeneity in the study performed on a mixed population of persons with acute and chronic SCI and heterogeneity of DXA measurement sites. Although most studies using DXA measured BMD at the common fracture sites (distal femur or proximal tibia) in the SCI population, the studies by Gilchrist et al (17) and Shapiro et al (18) failed to do so. This is likely not appropriate given that performing DXA studies at the knee in the SCI population can be challenging because of poor resolution and difficulty with positioning caused by spasticity (9). Additionally, imprecision is likely in longitudinal studies that report a percentage change in BMD over time, because there may be measurement error at each study visit.
Limitations
Several limitations should be considered in the interpretation of these findings. Results of a systematic review are influenced by publication bias and bias caused by trial design characteristics. Our evidence rating system gave equal weight to the major Delphi criteria; this weighting would not capture differential effects of individual components of trial quality.
Clinical Implications and Future Studies
Certainly this patient population is at high risk of accelerated bone loss often at an early age, and a better understanding of effective interventions in the acute setting before bone hyper-resorption would be beneficial both to reduce the low-energy fracture incidence in chronic SCI and to prevent fracture associated with weight-bearing activities during physical rehabilitation to optimize recovery after injury. However, given the generally low internal validity of studies, poor statistical power, lack of clinically relevant outcomes, and uncertain generalizability, interpretation of findings is guarded. The minimal evidence currently available does not provide adequate justification for routine use of BP therapy for prevention or treatment of BMD loss after SCI.
A major limitation of current studies in the SCI population is that most BMD measurements were at atypical anatomical sites with an unknown relationship to fracture. Precision studies of DXA in patients with SCI will need to be performed to determine the least significant change at the unique anatomical sites (distal femur and proximal tibia) studied in SCI, and therefore, the percent change that is outside the limits of machine precision. Adopting a standardized DXA protocol for measurement of common fracture sites, such as that proposed by Shields et al, will facilitate direct comparison of outcomes among studies and enhance generalizability, as well as improve reliability of knee BMD measurements.
Current research in osteoporosis reinforces the view that treatment should be directed on the basis of fracture probability rather than on a single BMD threshold. Research in persons with SCI must clarify the relative importance of risk factors that might contribute or protect against fracture risk, including level of injury, sex, spasticity, and ambulatory status. A positive correlation of BP therapy with ambulation was evident in some studies. Because degree of ambulation could potentially have an independent effect on BMD measures, larger randomized studies with equivalent baseline ambulation status in treatment and control groups should be considered. Also, static loading or ambulation combined with acute administration of BPs with a different dosing schedule could be tested in patients with SCI.
In designing future studies, consideration should be given to special factors in patients with SCI that warrant concern about potential risks of BP use, especially in pregnant women. BPs are FDA-approved for the treatment of osteoporosis in postmenopausal women and men and for treatment of glucocorticoid-induced osteoporosis. Caution must be exercised when using BPs in women with SCI who are of reproductive age, because BPs are a Category C drug and have a very long half-life (eg, estimated half-life of alendronate is up to 12 years) (30). Because BPs accumulate in the maternal skeleton, cross the placenta, and accumulate in the fetal skeleton, and because of reports of toxic effects in pregnant rats, these agents are rarely used in women of reproductive age (31). Two of the 7 studies reviewed cited pregnancy or breastfeeding as an exclusion criterion (20,22). Additionally, a case series of patients who sustained unusual fractures after prolonged use of alendronate (32) may have implications in young patients with traumatic SCI, who could potentially take BPs for many years.
If warranted, prospective studies with long-term follow-up and fracture incidence outcomes will be necessary to better characterize the therapeutic effectiveness of BPs and therefore facilitate evaluation of the cost-benefit ratio of implementing therapy.
CONCLUSION
This systematic review identified 6 experimental studies and 1 quasi-experimental study of BP therapy in acute and chronic SCI. The studies were small and of fair or poor quality, and none included fracture outcomes. Data were insufficient to recommend routine use of BPs for fracture prevention in these patients. Future research should define risk factors associated with low-energy fractures in the SCI population and should evaluate whether the modest effect of bisphosphonates on BMD in SCI is associated with fracture risk reduction. We strongly recommend conducting future research within a specific population (acute or chronic SCI) with consistent timing of treatment administration to minimize heterogeneity in the study population. We also encourage adoption of uniform outcome measures with rigorous quality control and compliance monitoring to improve reliability of outcomes.
Footnotes
Dr Gourlay was supported by Grant K23RR024685 from the National Center for Research Resources.
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the funding agency.
Table 2.
Extended
Table 3.
Extended
REFERENCES
- Eser P, Frotzler A, Zehnder Y, et al. Relationship between the duration of paralysis and bone structure: A pQCT study of spinal cord injured individuals. Bone. 2004;34(5):869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(4):208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- Hitzig SL, Tonack M, Campbell KA, et al. Secondary health complications in an aging Canadian spinal cord injury sample. Am J Phys Med Rehabil. 2008;87(7):545–555. doi: 10.1097/PHM.0b013e31817c16d6. [DOI] [PubMed] [Google Scholar]
- CDC, National Center for Injury Prevention and Control. Spinal cord injury (SCI): fact sheet. http://www.cdc.gov/ncipc/factsheets/scifacts.htm. Accessed March 15, 2009.
- WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg. 1962;103(6):732–739. doi: 10.1016/0002-9610(62)90256-8. [DOI] [PubMed] [Google Scholar]
- Shields RK, Schlechte J, Dudley-Javoroski S, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005;86(10):1969–1973. doi: 10.1016/j.apmr.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol (Oxf). 2006;65(5):555–565. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29(5):489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanick JI, Liu K, Nierman DM, Stein A. Effect of a convenient single 90-mg pamidronate dose on biochemical markers of bone metabolism in patients with acute spinal cord injury. J Spinal Cord Med. 2006;29(4):406–412. doi: 10.1080/10790268.2006.11753890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;1:CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112(4):281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- Kahn KS, terRiet G, Popay J, Nixon J, Kleijnen J. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. Phase 5: study quality assessment. http://www.york.ac.uk/inst/crd/report4.htm. Accessed March 15, 2009.
- Verhagen AP, de Vet HC, de Bie RA, et al. The delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92(4):1385–1390. doi: 10.1210/jc.2006-2013. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Smith B, Beck T, et al. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int. 2007;80(5):316–322. doi: 10.1007/s00223-007-9012-6. [DOI] [PubMed] [Google Scholar]
- Pearson EG, Nance PW, Leslie WD, Ludwig S. Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil. 1997;78(3):269–272. doi: 10.1016/s0003-9993(97)90032-0. [DOI] [PubMed] [Google Scholar]
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999;80(3):243–251. doi: 10.1016/s0003-9993(99)90133-8. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Wecht JM, Kirshblum S, et al. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305–313. doi: 10.1682/jrrd.2004.05.0062. [DOI] [PubMed] [Google Scholar]
- Moran de Brito CM, Battistella LR, Saito ET, Sakamoto H. Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord. 2005;43(6):341–348. doi: 10.1038/sj.sc.3101725. [DOI] [PubMed] [Google Scholar]
- Zehnder Y, Risi S, Michel D, et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res. 2004;19(7):1067–1074. doi: 10.1359/JBMR.040313. [DOI] [PubMed] [Google Scholar]
- Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham osteoporosis study. J Bone Miner Res. 2000;15(4):710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Bauer DC, Vogt TM, Fox KM. Axial bone mass in older women. study of osteoporotic fractures research group. Ann Intern Med. 1996;124(2):187–196. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- Chappard D, Minaire P, Privat C, et al. Effects of tiludronate on bone loss in paraplegic patients. J Bone Miner Res. 1995;10(1):112–118. doi: 10.1002/jbmr.5650100116. [DOI] [PubMed] [Google Scholar]
- Minaire P, Berard E, Meunier PJ, Edouard C, Goedert G, Pilonchery G. Effects of disodium dichloroethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest. 1981;68(4):1086–1092. doi: 10.1172/JCI110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaire P, Depassio J, Berard E, et al. Effects of clodronate on immobilization bone loss. Bone. 1987;8(suppl 1):S63–S68. [PubMed] [Google Scholar]
- van den Berg-Emons RJ, Bussmann JB, Haisma JA, et al. A prospective study on physical activity levels after spinal cord injury during inpatient rehabilitation and the year after discharge. Arch Phys Med Rehabil. 2008;89(11):2094–2101. doi: 10.1016/j.apmr.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- Cohen A, Shane E. Treatment of premenopausal women with low bone mineral density. Curr Osteoporos Rep. 2008;6(1):39–46. doi: 10.1007/s11914-008-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]