Abstract
OBJECTIVE
We have previously described patterns of neonatal brain injury that correlate with global cognitive and motor outcomes. We now examine, in survivors of neonatal encephalopathy (presumed secondary to hypoxia-ischemia) without functional motor deficits, whether the severity and neuroanatomical involvement on neonatal magnetic resonance imaging (MRI) are associated with domain-specific cognitive outcomes, verbal (VIQ) and performance IQ (PIQ), at four years of age.
METHODS
In this prospective study, neonatal MRIs of 81 term infants with neonatal encephalopathy were scored for degree of injury in two common patterns: watershed-distribution (WS) and basal ganglia-distribution (BG). Follow-up evaluation at four years of age by examiners blinded to clinical history and MRIs included a five-point neuromotor score and the Wechsler Preschool and Primary Scale of Intelligence – Revised. In 64 subjects with no functional motor impairment, test of trend was used to examine the association of ordered WS and BG MRI scores with mean VIQ and PIQ.
RESULTS
Lower VIQs and PIQs were seen with increasing degree of injury on both WS and BG scales in univariate analyses (p≤0.05, all four analyses). When each MRI pattern score was adjusted for the other, only the association of decreasing VIQ with increasing WS injury remained significant (p=0.01; VIQ means across WS scores: 105–84). A suggestion of decreasing VIQ with increasing BG injury was also seen in the multivariate model (p=0.06; means across BG scores: 100–80), while no association was seen between PIQ and severity of injury in either MRI pattern.
CONCLUSIONS
In survivors of neonatal encephalopathy without functional motor deficits at 4 years of age, an increasing severity of watershed-distribution injury is associated with more impaired language-related abilities.
Keywords: Hypoxia-ischemia, brain, Magnetic resonance imaging, Cognition, Language, Intelligence tests, Infant, newborn, Child
INTRODUCTION
Hypoxic-ischemic encephalopathy, occurring in 1 to 6 of every 1000 live full-term births, accounts for a substantial proportion of neonatal brain injury representing a major cause of neurodevelopmental disability.1,2 Despite this, predicting outcomes after neonatal encephalopathy (a term used in this paper to refer to the condition of brain injury after presumed neonatal hypoxic-ischemic insult) remains a significant challenge.
Large variation in definitions of hypoxia-ischemia across studies has resulted in wide-ranging estimates of outcome (as reviewed by Dilenge et al.3 and van Handel et al.4). To improve accuracy and precision in identifying neonatal (hypoxic-ischemic) encephalopathy, MRI is increasingly applied to quantify the degree of brain injury and to identify the specific brain regions involved.5–8 Hypoxia-ischemia in newborns typically results in one of two characteristic patterns of brain injury: (1) a watershed-distribution (WS) pattern involving intervascular boundary-zone white matter, plus cortical gray matter when severe, and (2) a basal ganglia-distribution (BG) pattern involving deep grey nuclei, hippocampi, and perirolandic cortex, with further cortical involvement when severe.5,9 Barkovich et al.5 developed an MRI scoring system for these patterns of injury. Using such a system allows for a more direct examination of the association between brain structure and behavioral outcomes. In a previous study by our group,10 injury predominantly in the BG pattern was associated with the most impaired motor and general cognitive outcomes at 30 months of age, while injury predominantly in the WS pattern was often associated with cognitive deficits without motor deficits. Like the majority of studies of outcomes after neonatal encephalopathy,6,8,11,12 this study evaluated cognition in terms of the presence or absence of global cognitive impairment (i.e. “mental retardation”). While providing valuable information, this approach may overlook relatively isolated impairments in specific cognitive domains. The few studies that have examined specific cognitive domains have identified language and perceptual-motor skill deficits,11,13–17 even in the setting of normal overall cognition. To our knowledge, no studies to date have examined the association between domain-specific cognitive functions and the underlying neuroanatomical regions injured in perinatal hypoxic-ischemic brain injury.
In the current study, we examined the relationship between neuroanatomic extent and severity of neonatal brain injury and future verbal and non-verbal cognitive abilities. To do so, we asked whether the severity and pattern of injury on neonatal MRI are associated with Wechsler Preschool and Primary Scale of Intelligence – Revised (WPPSI-R) VIQ and PIQ scores at four years of age. Recognizing that assessment of cognition by neuropsychological testing is influenced by neuromotor dysfunction, we evaluated only subjects with no functional motor impairment. As normal language function relies on anterior and posterior cortex and the underlying white matter connections, we hypothesized that VIQ scores would correlate negatively with degree of WS pattern injury. Alternatively, we hypothesized that PIQ scores would correlate negatively with degree of BG pattern injury, due to expected executive, working memory, and visuospatial impairment from striatal, hippocampal, and thalamic injury.
METHODS
Subjects
The present study included all eligible participants from a previously-described ongoing prospective cohort study of infants at risk for hypoxic-ischemic brain injury, including subjects enrolled from study initiation in 1994 through 2003.18 The protocol for the ongoing study was approved by the Committee on Human Research at the University of California – San Francisco, and subjects were studied only after voluntary informed consent was obtained from the parents. A cohort of 163 infants was derived from screening 5,562 consecutive term neonates born in or transferred to the UCSF Neonatal Intensive Care Unit (Figure 1). All enrolled participants had one or more of the following markers of hypoxia-ischemia: (a) 5-minute Apgar score of ≤ 5; (b) umbilical artery cord blood pH < 7.1; (c) umbilical artery base deficit ≤ −10; or (d) clinical brain dysfunction (defined as abnormal tone, feeding, alertness, respiratory status, or reflexes). An infant was excluded for any of the following: (1) evidence of in utero or perinatal infection; (2) major anomalies of the brain or other major organ systems; or (3) evidence of congenital metabolic disease. These broad criteria were chosen to include newborn infants with a wide range of injury and of neurodevelopmental outcomes, while including some marker of hypoxic-ischemic encephalopathy.19 Each cohort participant had a brain MRI obtained during the first two weeks of life. Of subjects enrolled in the cohort, 27 were excluded for lack of a neonatal-acquired MRI. Subjects were excluded from the current study if MRI showed evidence of focal infarction due to different etiology and specific motor and cognitive outcomes of neonatal stroke. All participants underwent a four-year follow-up evaluation including a standardized neurologic examination and neuropsychological evaluation. Participants were excluded from the current study if neurologic examination revealed functional motor impairment at 4-years-old (neuromotor score ≥ 3, see below).
Figure 1.
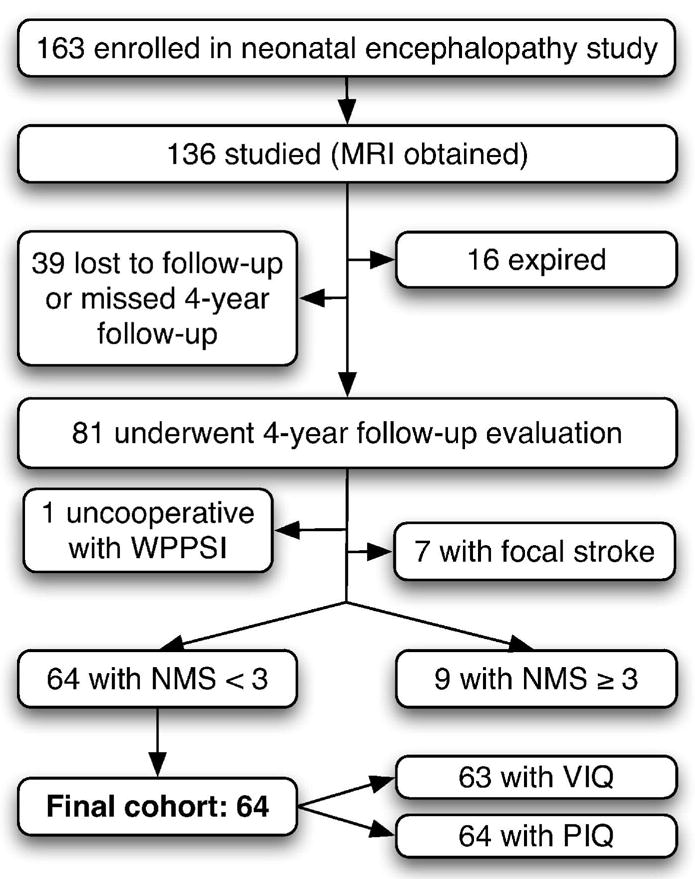
Flow diagram depicting subject selection for inclusion in final cohort.
Neonatal Neuroimaging
A neuroradiologist (AJB) blinded to the participants’ clinical condition scored each neonatal MRI on two scales that rate degree of injury in two common patterns (described in detail previously5): watershed-distribution (WS) and basal ganglia-distribution (BG). The severity of injury in the WS pattern was scored from 0 to 5, ranging from normal, to focal infarction, through increasing extent of white matter and cortical injury (Table 1); and the severity of injury in the BG pattern was scored from 0 to 4, ranging from normal deep grey matter through increasing extent of deep grey matter and maximally including cortical grey matter injury (Table 2). The inter-observer reliability of MRI scores in this cohort was previously reported with a kappa of 0.85,10 and the intra-observer reliability of MRI scores in this cohort was reported previously as ranging from a kappa of 0.85 to 1.0 (varying by MR sequence).5
Table 1.
Watershed-Distribution (WS) Pattern Injury Scale
| 0 | Normal |
| 1 | Single focal infarction |
| 2 | Abnormal signal in anterior or posterior watershed white matter |
| 3 | Abnormal signal in anterior or posterior watershed cortex and white matter |
| 4 | Abnormal signal in both anterior and posterior watershed zones |
| 5 | More extensive cortical involvement |
Table 2.
Basal Ganglia-Distribution (BG) Pattern Injury Scale
| 0 | Normal or isolated focal cortical infarct |
| 1 | Abnormal signal in thalamus |
| 2 | Abnormal signal in thalamus and lentiform nucleus |
| 3 | Abnormal signal in thalamus, lentiform nucleus, and perirolandic cortex |
| 4 | More extensive involvement |
Four-Year Clinical Evaluation
The neuropsychological evaluation consisted of the WPPSI-R, administered by a psychologist blinded to the child’s neonatal course, neurologist evaluation, and imaging. The WPPSI-R provides age-normed scaled VIQ and PIQ scores (mean ± SD of 100 ± 15 in typical children). The lowest possible formal scores are VIQ 46 and PIQ 45. VIQ and PIQ scores were obtained for each participant at four years of age. For the one subject in the final cohort whose language was too impaired to administer the VIQ, a “minimum score” of 1 less than the lowest possible formal score was assigned (i.e. VIQ 45).
The neurologic examination was performed by a pediatric neurologist blinded to the neonatal course, neuroimaging, and neuropsychological evaluation results. The neurologist scored neuromotor outcome using a validated neuromotor score (NMS) from 0 to 5 (Table 3).19
Table 3.
Neuromotor Score (NMS)
| 0 | Normal |
| 1 | Abnormal tone or reflexes or primitive reflexes |
| 2 | Abnormal tone and reflexes |
| 3 | Decreased power and tone or reflex abnormality |
| 4 | Cranial nerve involvement and any motor abnormality |
| 5 | Cranial nerve involvement and spastic quadriparesis |
Neuromotor Function
Performance on the WPPSI-R can be influenced by pure neuromotor dysfunction (pointing and oromotor-dependent verbal responses for VIQ subtests, manual dexterity and speed for PIQ subtests). To understand the relationship between injured brain structures and cognitive domain-specific function, it was important to control for functional motor impairments that may confound this relationship. Subjects were excluded from the current study if they had an NMS≥3, implying functional motor impairment (identifiable weakness and/or cranial nerve dysfunction).
Data Analysis/Statistical Methods
Unadjusted IQ-MRI Score Associations
Univariate linear regressions were used to evaluate the unadjusted association between each IQ score (VIQ and PIQ) and each MRI pattern scores (WS and BG scores). As WS and BG scores are based on ordinal scales, we were most interested in a trend in mean VIQ or PIQ scores across levels of WS or BG score. We therefore used the “test for trend” to assess for this type of departure from the null hypothesis of no association.
Adjustment for the Degree of Brain Injury in Other MRI Pattern
Correlation was expected between degree of WS injury and degree of BG injury (as both are thought to correspond to degree of hypoxia-ischemia experienced) and was confirmed to exist in this study. This correlation suggests the potential for confounding effect of each MR-pattern (WS or BG) injury score on the association between an IQ score and the other MR-pattern injury score (i.e. an apparent causal relationship between one MR-pattern score and an IQ score may, in fact, be due to injury in the other MR pattern). To control for this, we used multiple linear regression models that included both BG score and WS score as predictors of the IQ score. These models yielded estimated mean VIQ and PIQ scores at each level of WS score or BG score, adjusted for the complementary MR-pattern score. Test for statistical significance was performed using the test for trend across levels of WS score and BG score.
RESULTS
From the originally enrolled cohort, 136 subjects were studied with MRI (Figure 1). 16 of these subjects died before the age of four and 39 missed their four-year follow-up evaluation or have been lost to study follow-up. Four-year clinical follow-up evaluation had been completed by 81 subjects at the time of this study. No difference was found between distribution of WS and BG scores in subjects who missed the follow-up appointment compared to those who completed the four-year follow-up (Kruskal-Wallis test; WS: p=0.9; BG: p=0.6). Of the 81 subjects, one was uncooperative with WPPSI testing. Seven of 81 subjects were excluded from this study due to focal strokes identified on neonatal MRI. Of the remaining eligible participants, 9 had NMS scores ≥ 3, leaving 64 participants for inclusion in the current study. PIQ was measured in all 64, and VIQ was measured in 63 (VIQ not valid for one participant for whom appropriate interpreter was not available) (see Figure 1).
Of the 9 participants excluded due to NMS score representing “functional motor impairment,” all had the maximum NMS score of 5, indicating spastic quadriparesis plus cranial nerve abnormality. These subjects had the highest MRI pattern scores, such that the highest BG score (4) and the highest WS score (5) were comprised only of these subjects. Of these 9 subjects, 7 were too impaired (cognitively and/or motorically) for administration of the VIQ; one had VIQ 73 and one had VIQ 91. Eight subjects were too impaired for administration of the PIQ; one had PIQ 76.
The final cohort was comprised of 64 participants, 35 (55%) of which were male. Mean (±SD) for VIQ was 96 (±21) and for PIQ was 101 (±17). One subject (1.6%) received the VIQ “minimum score” of 45. Six (9%) had VIQ<70, 14 (22%) had VIQ 70–84, and 43 (67%) had VIQ≥85, while one had no VIQ measured. Clinical characteristics of the cohort, divided into three groups by VIQ score, are provided in Table 4. Statistically significant differences between the groups were only seen for median PIQ score (p<0.0001): middle and lowest VIQ groups had median PIQ 21 and 22 points lower than the highest VIQ group. In this cohort with no functional motor impairment, WS scores ranged from 0 to 4 (none with 5; subjects with WS score of 1 excluded). BG scores ranged from 0 to 3 (none with 4) (Figure 2).
Table 4.
Subject Characteristics by Four-Year VIQ Groups and Total
| Characteristic, median (range) or n (%) | VIQ < 70 (n=6) | VIQ 70–84 (n=14) | VIQ ≥ 85 (n=43) | p-value* | Total (n=64**) |
|---|---|---|---|---|---|
| Male gender | 4 (67%) | 9 (64%) | 21 (49%) | 0.5 | 35 (55%) |
|
| |||||
| Gestational age, weeks | 40 (35–42) | 39 (36–42) | 40 (36–42) | 0.8 | 40 (35–42) |
|
| |||||
| Cesearean section | 4 (67%) | 5 (36%) | 16 (37%) | 0.4 | 26 (41%) |
|
| |||||
| Birth weight, grams | 3605 (2350–5120) | 3152.5 (2020–4490) | 3354 (2040–4575) | 0.4 | 3330 (2020–5120) |
|
| |||||
| Birth head circumference, cm | 35.5 (31–36) | 34 (32.5–36) | 35 (31–38) | 0.3 | 35 (31–38) |
|
| |||||
| 5-minute Apgar | 4.5 (4–6) | 5 (3–8) | 5 (1–9) | 0.6 | 5 (1–9) |
|
| |||||
| Encephalopathy score (0–6)† | 3 (2–6) | 4 (1–6) | 3 (0–6) | 0.2 | 3 (0–6) |
|
| |||||
| Resuscitation score (1–6)‡ | 4.5 (4–6) | 4.5 (3–5) | 4 (2–6) | 0.2 | 4 (2–6) |
|
| |||||
| Neonatal seizures | 1 (17%) | 4 (29%) | 5 (12%) | 0.2 | 10 (16%) |
|
| |||||
| PIQ | 87 (71–102) | 88 (70–101) | 109 (76–141) | 0.0001 | 100.5 (70–141) |
|
| |||||
| Ethnicity | |||||
| American Indian/Native Alaskan | 1 (2%) | ||||
| Asian/Pacific Islander | 14 (22%) | ||||
| Black, non-Hispanic | 2 (3%) | ||||
| Hispanic | 15 (23%) | ||||
| White, non-Hispanic | 32 (50%) | ||||
p-values were obtained using Fisher’s exact test (n) or Kruskal-Wallis test (median), and refer to comparison across the 3 groups
1 subject not in included in VIQ groups because no VIQ was measured
The severity of clinical brain dysfunction was graded from 0 (no clinical encephalopathy) to 6 (severe clinical encephalopathy) using a validated score on the basis of alertness, feeding, tone, respiratory status, reflexes, and seizure activity.21
The amount of resuscitation at birth was graded from 1 (no intervention) to 6 (endotracheal intubation with ventilation and sodium bicarbonate with or without epinephrine) using a previously validated score.10
Figure 2.
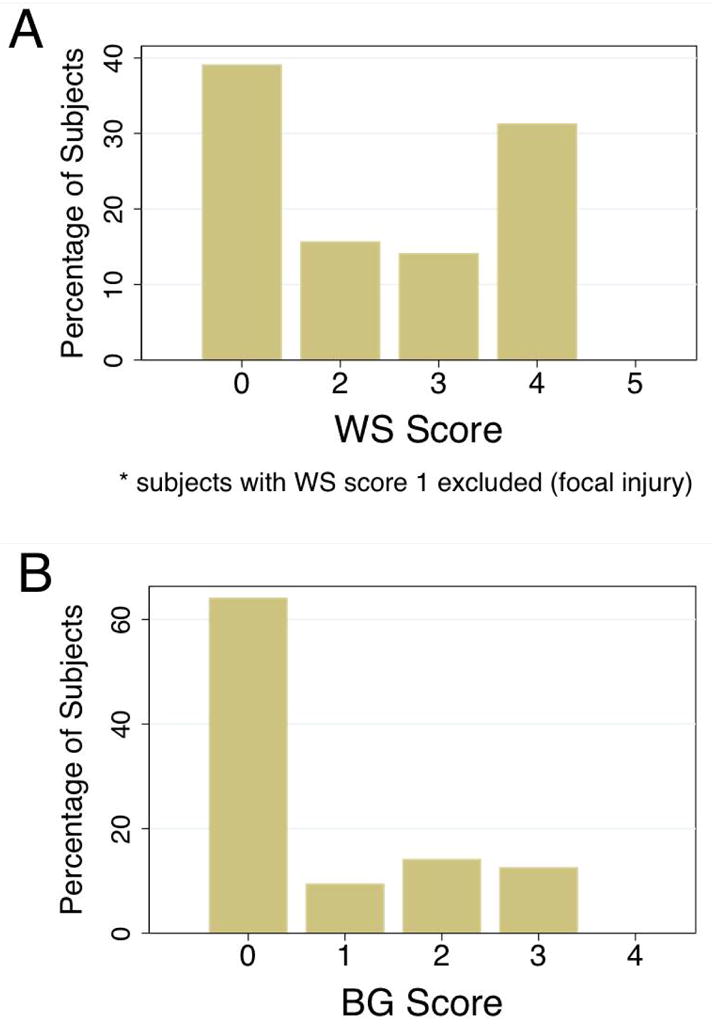
A. Distribution of WS scores for MRIs of 64 subjects. Subjects with WS score of 1 (focal stroke) were excluded from the analysis. B. Distribution of BG score for MRIs of 64 subjects.
In the four unadjusted (univariate) analyses, lower VIQs and lower PIQs were associated with increasing degree of injury on both WS and BG scales (p≤0.05, all four analyses). Range of estimated mean VIQs was 107–82 across WS scores and 102–79 across BG scores. Range of estimated mean PIQs was 109–96 across WS scores and 105–86 across BG scores.
When the association between the MRI pattern score and the VIQ or PIQ score was adjusted for the complementary MRI pattern score (Figure 3), only the association of decreasing VIQ with increasing WS injury remained statistically significant (p=0.01; range of estimated mean VIQs across WS scores: 105–84). The test for trend suggested that VIQs decrease with increasing BG injury as well, though this was not statistically significant (p=0.06; range of estimated mean VIQs across BG scores: 100–81). No association was seen between PIQ and severity of injury in either MRI pattern (WS: p=0.24, range of estimated mean PIQs: 108–97; BG: p=0.15, range of estimated mean PIQs: 105–86).
Figure 3.
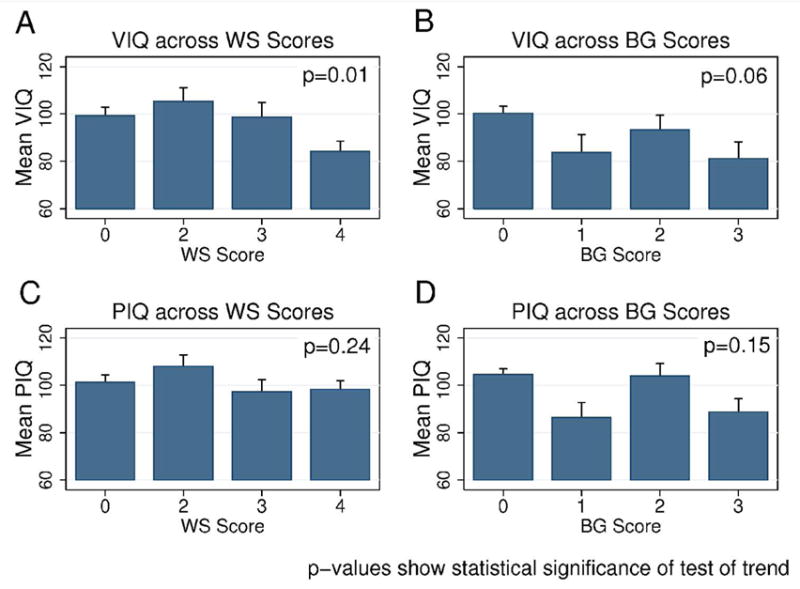
A. Estimated mean VIQ (±SE) across levels of WS score. B. Estimated mean VIQ (±SE) across levels of BG score. C. Estimated mean PIQ (±SE) across levels of WS score. D. Estimated mean PIQ (±SE) across levels of BG score. Each p-value represents statistical significance for test of trend across WS or BG levels
DISCUSSION
In this prospective study, we examined the association of MRI patterns of brain injury in neonatal encephalopathy presumed secondary to hypoxia-ischemia with verbal (VIQ) and non-verbal (PIQ) cognitive outcomes at four years old in children with no functional motor impairment. Our results demonstrated an independent association between degree of WS injury in the neonatal period and future verbal abilities measured by the WPPSI-R. The association identified between degree of BG injury and verbal abilities was not statistically significant, suggesting a less robust, if truly existent, association.
Studies of neonatal hypoxic-ischemic encephalopathy using the traditional biochemical and/or clinical markers have yielded mixed results with a broad range of possible sequelae in the setting of moderate encephalopathy,3,4 suggesting significant heterogeneity of this group. Studies incorporating prospective neuroimaging10,12 have begun to show that pattern of brain injury, and not just severity, is important in determining future domain (motor versus cognitive) impairment. Viewing cognition in mostly general and dichotomous terms (e.g. “mental retardation” vs “no mental retardation”)6,8,11,12 has likely contributed to the often ambiguous results obtained about those with a history of moderate encephalopathy. The current study is, to our knowledge, the first prospective study of neonatal encephalopathy due to presumed hypoxic-ischemia to show that the distribution of brain injury is also important in determining the specific pattern of future cognitive dysfunction.
Of the relatively small number of studies of specific cognitive outcome patterns in this condition, one domain in which isolated cognitive dysfunction has been identified is language.14,16,20 D’Souza and colleagues found speech and language deficits in approximately 1/3 of survivors of severe perinatal asphyxia in the setting of normal general intellect.14 In subjects with a history of moderate encephalopathy, Marlow et al. identified lower scores in language and verbal memory tests compared to controls.16 Our findings provide evidence that such domain-specific cognitive function may reflect underlying brain structures injured, specifically the white matter and cortical injury seen in the WS pattern of injury.
Prior studies examining specific cognitive domains after presumed hypoxic-ischemic neonatal brain injury have also identified deficits in non-language domains.13,16,17 In the current study, no association was identified between PIQ (which includes subtests involving visuospatial knowledge, visual memory, and executive function) and either BG or WS injury. This leaves the question of what type of brain injury leads to deficits in the non-verbal cognitive abilities measured by PIQ? The presence of associations in the unadjusted analyses with BG and WS, but lack of an independent association with either pattern of injury suggests that it is the interaction of these injury patterns that results in the non-verbal cognitive deficits. In other words, PIQ is mediated by the degree of injury in multiple areas, including areas involved in both patterns of injury. This could provide a neuroanatomic explanation of the finding of Marlow et al.16 of visuospatial impairment in subjects with more severe, but not moderate, encephalopathy, compared to controls.
One limitation to the current study is the relatively imprecise nature of the VIQ and PIQ scores, each comprised of multiple subtests requiring inter-related cognitive abilities. More detailed neuropsychological testing of specific cognitive domains (e.g. verbal memory, vocabulary production, and sentence comprehension) could provide even greater information about the spectrum of cognitive deficits associated with different neuroanatomic patterns of perinatal brain injury.
The aim of the current study was to understand the relationship between injured brain structures and cognitive domain-specific function, independent of neuromotor function. The small number of subjects with functional motor impairment, their severity of impairment (mostly precluding neuropsychological testing), and their limited variability of MRI pattern scores, statistical analysis incorporating this group was felt to provide inaccurate results. To address the question of association between specific brain regions injured and domain-specific cognitive outcomes, we therefore limited our study to subjects with no functional motor impairment (NMS<3). Given this, our findings must be interpreted with caution in children with functional motor deficits.
Interestingly, in children with no functional motor impairment we found no clear association between degree of BG injury and verbal or non-verbal cognitive outcomes. When examining the entire cohort (i.e. those with and without motor impairment) at 30 months, we have previously shown10 that injury predominating in the BG pattern was associated with the worst cognitive and motor outcomes at 30 months. In contrast, in the whole cohort, those with mostly WS pattern of injury had cognitive impairments less severe than those with the BG pattern, and did not have motor impairments. These previous findings are still in keeping with what we are describing here in those without motor impairment. Poor cognitive outcomes with BG injury seen in the previous study may not be attributable to basal ganglia, thalamic, or perirolandic injury given the occurrence of more diffuse cortical injury in the most extreme BG pattern (Table 2), present only in those with functional motor impairment at four years old. Further, given the high co-occurrence of WS injury with BG injury, the presence of WS injury in those with predominantly BG pattern injury in the prior study may have contributed importantly to the severe cognitive impairments in these children. Therefore, when we examined only subjects without motor deficits and adjusted for severity of WS injury in this study, the severity of BG injury was not significantly associated with the severity of cognitive deficits. Additionally, some of the apparent “severe cognitive impairment” seen when BG injury predominates may reflect inability to assess cognition accurately in the face of severe neuromotor disability.
Our current findings of an independent association between degree of WS injury and future language-related deficits demonstrate the use of neuroimaging to identify neuroanatomic distribution of brain injury in neonatal hypoxic-ischemic encephalopathy and their relation to domain-specific cognitive outcomes. Future studies that examine the association between perinatal brain injury distribution and cognitive outcome patterns are needed, including examination of these associations in children with motor impairment and examination with more detailed measures of specific cognitive domains. Such studies will hopefully lead to better ability to predict cognitive outcomes after neonatal encephalopathy, a worthy goal as we enter an era of experimental treatments.
Acknowledgments
Funding: This publication was made possible by Grant Number UL RR024131-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsible of the authors and do not necessarily represent the official view of the NCRR or the NIH. Information on NCRR is available at http://www.ncrr.nih.gov Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. This research is also supported by the National Institutes of Health (NS35902). KJS is supported by the NINDS Neurological Sciences Academic Development Award (NS01692). SPM is a Canadian Institutes of Health Research Clinician Scientist and Michael Smith Foundation for Health Research Scholar.
Thanks to Rita Jeremy, PhD, and Agnes Bartha, MD, for their work on this study and to Hannah Glass, MD CM, FRCP(C), MAS, and the UCSF Master’s Program in Clinical Research Seminar for helpful discussion.
Abbreviations
- MRI
magnetic resonance imaging
- VIQ
verbal IQ
- PIQ
performance IQ
- WS
watershed-distribution
- BG
basal ganglia-distribution
- WPPSI-R
Wechsler Preschool and Primary Scale of Intelligence – Revised
- NMS
neuromotor score
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
References
- 1.Volpe JJ. Neurology of the newborn. Philadelphia : 2001. [Google Scholar]
- 2.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 3.Dilenge ME, Majnemer A, Shevell MI. Long-Term developmental outcome of asphyxiated term neonates. J Child Neurol. 2001;16:781–792. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 4.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-Term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur J Pediatr. 2007;166:645–654. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: Evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuenzle C, Baenziger O, Martin E, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics. 1994;25:191–200. doi: 10.1055/s-2008-1073021. [DOI] [PubMed] [Google Scholar]
- 7.L’Abee C, de Vries LS, van der Grond J, Groenendaal F. Early diffusion-weighted MRI and 1h-magnetic resonance spectroscopy in asphyxiated full-term neonates. Biol Neonate. 2005;88:306–312. doi: 10.1159/000087628. [DOI] [PubMed] [Google Scholar]
- 8.Barnett A, Mercuri E, Rutherford M, et al. Neurological and perceptual-motor outcome at 5 – 6 years of age in children with neonatal encephalopathy: Relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–248. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- 9.Sie LT, van der Knaap MS, Oosting J, de Vries LS, Lafeber HN, Valk J. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31:128–136. doi: 10.1055/s-2000-7496. [DOI] [PubMed] [Google Scholar]
- 10.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Robertson CM, Finer NN. Educational readiness of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Dev Behav Pediatr. 1988;9:298–306. [PubMed] [Google Scholar]
- 12.Roland EH, Poskitt K, Rodriguez E, Lupton BA, Hill A. Perinatal hypoxic-ischemic thalamic injury: Clinical features and neuroimaging. Ann Neurol. 1998;44:161–166. doi: 10.1002/ana.410440205. [DOI] [PubMed] [Google Scholar]
- 13.Mañeru C, Junqué C, Botet F, Tallada M, Guardia J. Neuropsychological long-term sequelae of perinatal asphyxia. Brain Inj. 2001;15:1029–1039. doi: 10.1080/02699050110074178. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza SW, McCartney E, Nolan M, Taylor IG. Hearing, speech, and language in survivors of severe perinatal asphyxia. Arch Dis Child. 1981;56:245–252. doi: 10.1136/adc.56.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moster D, Lie RT, Markestad T. Joint association of apgar scores and early neonatal symptoms with minor disabilities at school age. Arch Dis Child Fetal Neonatal Ed. 2002;86:F16–F21. doi: 10.1136/fn.86.1.F16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90:F380–F387. doi: 10.1136/adc.2004.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114:753–760. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 18.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 19.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21:788–793. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 20.Robertson C, Finer N. Term infants with hypoxic-ischemic encephalopathy: Outcome at 3.5 years. Dev Med Child Neurol. 1985;27:473–484. doi: 10.1111/j.1469-8749.1985.tb04571.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–99. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]


