Abstract
This tutorial review examines the fundamental aspects of a new class of contrast media for MRI based upon the chemical shift saturation transfer (CEST) mechanism. Several paramagnetic versions called PARACEST agents have shown utility as responsive agents for reporting physiological or metabolic information by MRI. It is shown that basic NMR exchange theory can be used to predict how parameters such as chemical shift, bound water lifetimes, and relaxation rates can be optimized to maximize the sensitivity of PARACEST agents.
Magnetic resonance imaging (MRI) is arguably the most important diagnostic imaging tool in clinical medicine today offering exquisite anatomical images of soft tissues based upon detection of protons largely in water and fat. Image contrast is readily manipulated by choosing from a standard set of pulse sequences that weight signal intensities based upon differences in proton densities and T1 and T2 relaxation rates. For many clinical applications, however, it now common practice to administer an exogenous contrast agent to highlight specific tissue regions based upon flow or agent biodistribution. MRI contrast agents so far have been largely confined to small paramagnetic metal complexes, typically gadolinium(III) complexes, that alter signal intensity by shortening the relaxation times of the water protons.1 The mechanism of action of this class of agents is described in more detail in a separate review in this edition. Although such first generation agents are widely used in clinical medicine, the physical properties of such agents are limited when considering a new generation of MRI contrast agents that will provide functional as well as anatomical information.2 New agents that operate by a CEST mechanism may ultimately be able to provide important metabolic information with exquisite anatomical resolution.
What is CEST?
CEST is an acronym for Chemical Exchange Saturation Transfer, the basics of which are well established in NMR spectroscopy. In early experiments, it was also referred to as Saturation Transfer or Magnetization Transfer (MT). As its name suggests CEST involves chemical exchange of a nucleus in the NMR experiment from one site to a chemically different site. Before introducing CEST, it is necessary to consider briefly the origin of an NMR signal, more detailed descriptions are available elsewhere.3 When a nucleus having a net magnetic spin (the proton spin quantum number, I, equals ½) is placed in a magnetic field, the spins orient either in the same direction as the magnetic field (low energy-α) or against the field (high energy-β) (Fig. 1). The distribution of spins between these two energy states is determined by the Boltzmann equation (eqn 1).
| (1) |
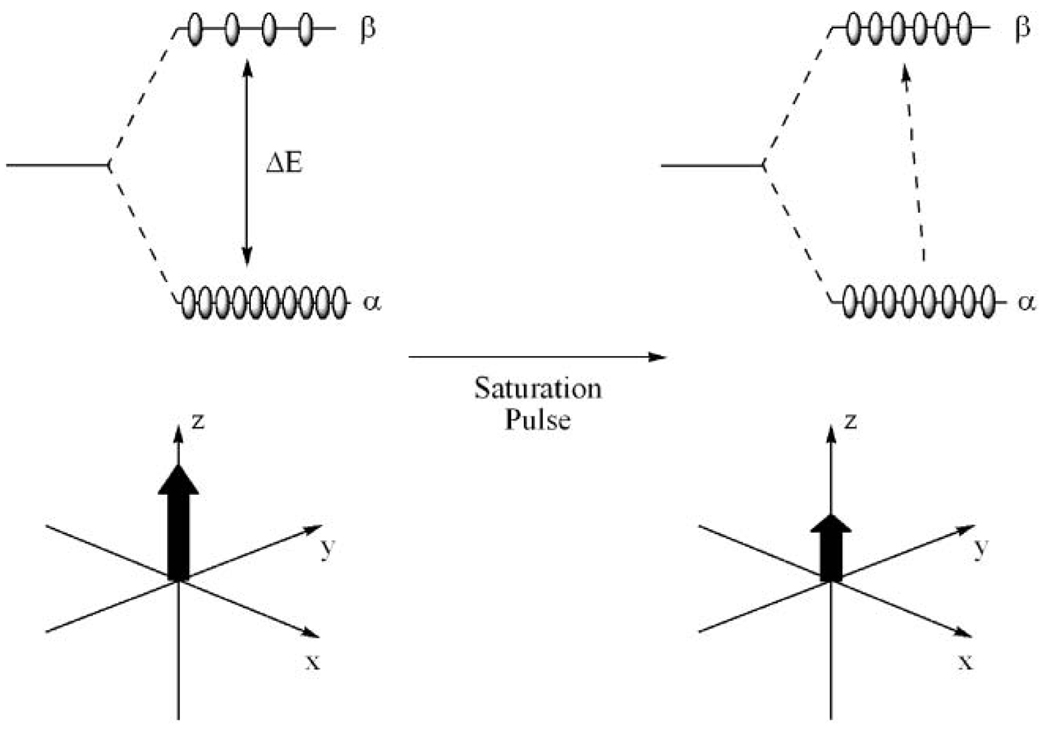
Fig. 1.
The Boltzmann distribution of spins in a magnetic field results in a bulk magnetization of the sample, represented by a vector along the z-axis (left). Application of a presaturation pulse increases the number of spins aligned against the magnetic field, reducing the magnitude of the bulk magnetization vector. This ultimately reduces the signal intensity of an NMR or MRI experiment.
At equilibrium the number of spins aligned with the magnetic field slightly exceeds the number aligned against it. The result is a bulk magnetization of the sample which may be thought of as a spin vector aligned with the magnetic field (along the z axis). Interrogation of this bulk magnetization with radiofrequency pulses is the origin of the signals observed in both NMR and MRI experiments. If a radiofrequency pulse of a suitable frequency is applied to this system prior to interrogation, then spins will be promoted from the low energy level to the high energy state. As fewer and fewer spins are aligned with the magnetic field, the magnitude of the bulk magnetization vector along the z-axis is reduced (Fig. 1). If enough energy is applied to the system, it is said to be saturated (Nα = Nβ) and the net magnetization is zero. The intensity of signals generated in both NMR and MRI experiments is proportional to the net magnetization along the z-axis so application of a presaturation pulse prior to interrogation reduces the observed signal intensity and if complete saturation is achieved then the signal will be completely eliminated.
As the name suggests, CEST considers the effects of presaturation in a system that is undergoing chemical exchange. In order for an effect to be observed, the exchange process must occur between two magnetically distinct environments and must be slow on the NMR timescale. “Slow” means that the rate of exchange (kex) must be no greater than the difference in frequency between the two chemical environments (Δω) (eqn 2).
| (2) |
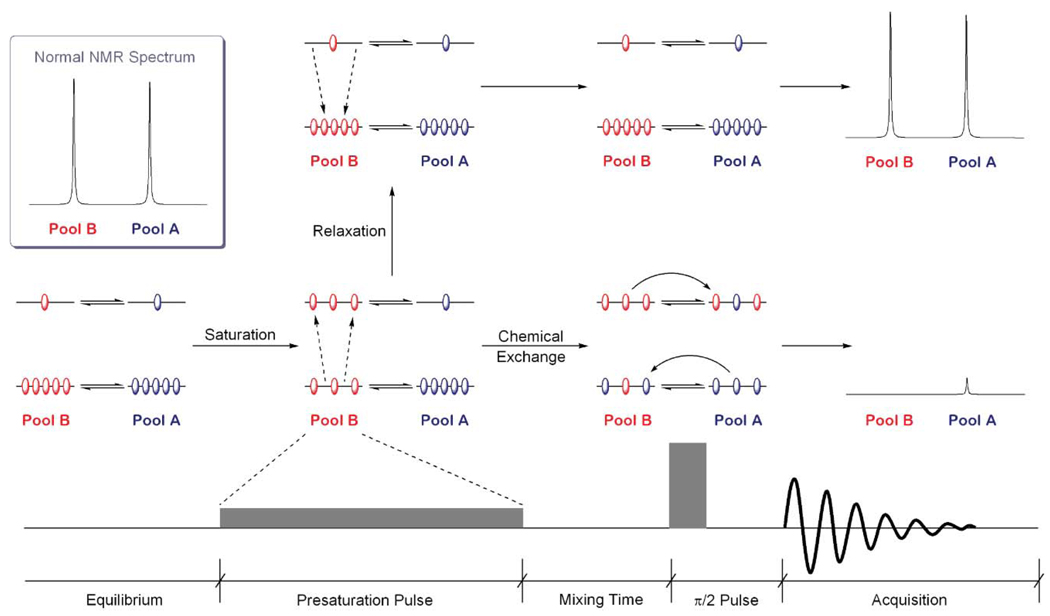
In a system that meets these conditions for two pools of exchanging protons, Pool A and Pool B, presaturation of the protons in Pool B also has an effect on the protons in Pool A. The presaturation pulse increases the number of spins aligned against the field in Pool B, thereby decreasing the bulk magnetization in this Pool. The spins in Pool A initially retain their normal Boltzmann equilibrium but, with time, chemical exchange perturbs this situation. The exchange process can be considered as two independent equilibria with identical forward and reverse rate constants (Fig. 2). The first equilibrium is that between the low energy states (α) of Pool A and Pool B and this will move to increase the number of spins in the low energy state of Pool B at the expense of those in Pool A. The second equilibrium is that between the two high energy states (β) and this will move such that the number of high energy spins in Pool A is increased at the expense of the number of high energy spins ion Pool B (Fig. 2). Thus, the result of chemical exchange is that the number of high energy spins in Pool A increases while the number of low energy spins in this pool decreases. In other words, the distribution of spins in Pool A moves closer to saturation, reducing the bulk magnetization along the z-axis. This means that when the system is interrogated by a 90° pulse to acquire an NMR spectrum, not only is the signal intensity of Pool B almost completely absent, but the intensity of Pool A is also decreased.
Fig. 2.
A presaturation pulse applied to Pool B alters the distribution of spins in this pool. If the R1 of Pool B is fast relative to the rate of chemical exchange with Pool A then the spins promoted to the high energy state will relax back to the equilibrium Boltzmann distribution and a normal NMR spectrum will be obtained. However, if chemical exchange is the faster process then the population densities of the high energy level will equilibrate altering the distribution of spins in Pool A, reducing the bulk magnetization of this pool. The result is that not only is the signal intensity of Pool B reduced but also that of Pool A.
This CEST process is in competition with relaxation. If the longitudinal relaxation rate (R1) of Pool B is faster than the forward rate constant of the exchange process (Pool B → Pool A) then the system will relax back to the Boltzmann distribution before exchange can transfer the altered spin state to Pool A. In this situation a normal NMR spectrum will be obtained (Fig. 2). Similarly, if the relaxation rate of Pool A is rapid, the system will have relaxed back to its Boltzmann distribution prior to interrogation of the system. So in addition to suitable exchange kinetics, a CEST effect will only be observed if the relaxation times of the two pools are long relative to the exchange rates.
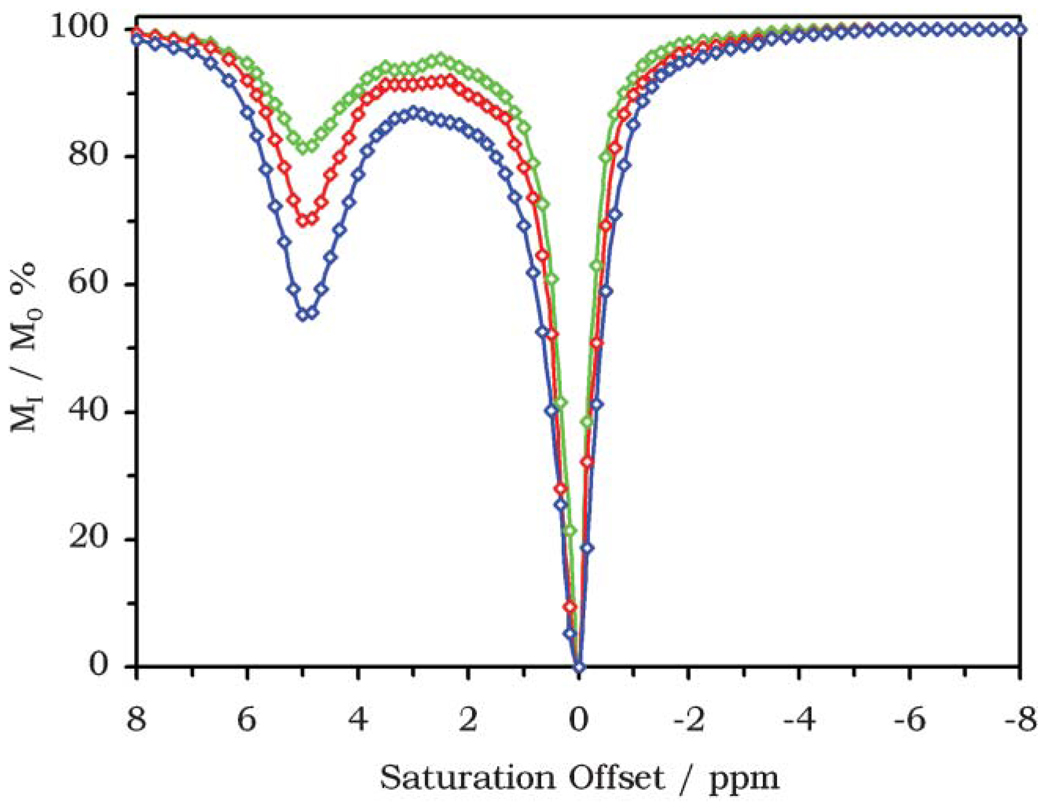
So one can see how the signal intensity of one resonance can be altered by applying a presaturation pulse to a second resonance in exchange with the first. This effect may be used to alter the signal intensity of tissue water to produce contrast in MR images. Although saturation transfer techniques have been used with endogenous systems for some years in MRI,4 it was not until 2000 that saturation transfer, and more specifically CEST, was proposed for generating image contrast using exogenous agents.5 Ward, et al., proposed that non-metal based contrast agents that operate by CEST would offer an advantage over conventional metal-based T1 or T2 shortening agents in that the effect of the contrast agent could be turned on and off by a presaturation pulse.5 Accordingly, a number of sugars, metabolites, amino acids and other small diamagnetic molecules possessing exchangeable protons were examined using a technique that has now become the standard means to assess the CEST capabilities of an agent. The intensity of the solvent water signal of a solution of the CEST agent was measured after application of a presaturation pulse. The frequency of this presaturation pulse is varied and the solvent water signal intensity plotted as a function of saturation frequency offset. When the presaturation frequency is off-resonance, no effect will be observed on the intensity of the solvent water peak. When the frequency of the presaturation pulse is at or close to the resonance frequency of the solvent water protons, direct saturation of water occurs and its signal intensity decreases. If, however, a presaturation pulse is applied at the resonance frequency of an exchangeable pool of protons, then the CEST effect will diminish the intensity of the solvent water peak and this is observed as a second negative peak in the CEST spectrum. The CEST spectrum of barbituric acid from Ward et al. is shown (Fig. 3), in which the effect of direct saturation can be observed at 0 ppm and a CEST peak arising from exchange of the –NH protons of barbituric acid is visible at +5 ppm.
Fig. 3.
CEST spectra of 125 mM (blue), 62.5 mM (red), and 31.25 mM (green) solutions of barbituric acid recorded at 300 MHz, pH 7.0 and 37 °C.
The spectrum shown in Fig. 3 demonstrates that this kind of diamagnetic compound can alter the bulk water signal and thereby act as contrast media for MRI. However, the main drawback of using this particular system of diamagnetic molecules can also be observed in this spectrum; the concentration of agent required to generate a significant change in the solvent water signal is quite high. Even a 15 mM solution of barbituric acid results in only a 5% decrease in solvent water signal intensity.5 This equates to approximately 100 times the concentration used for a conventional gadolinium agent in MRI. This is much higher than desirable for clinical use, so what is required is a more effective system by which exogenous CEST agents can be applied to generating image contrast in MRI.
PARACEST: its origins and advantages
Although the two exchanging pools leading to CEST must obey the slow exchange condition (eqn 2) so that one pool may be selectively saturated, exchange should also be as rapid as possible while maintaining this condition. Faster chemical exchange allows more spins to be transferred within a given time frame and faster exchange also reduces the detrimental effects of relaxation. From eqn 2 it can be seen that maximum exchange rate permissible within the intermediate-to-slow exchange condition is determined by the frequency difference between the two pools, Δω; the larger the value of Δω, the faster the permissible exchange. Hence, the effectiveness of a CEST agent may be improved by maximizing both Δω and the rate of chemical exchange. This goal is achieved by certain paramagnetic compounds having exchangeable proton sites (–NH, –OH or H2O) that are frequency shifted well away from the bulk water NMR frequency.
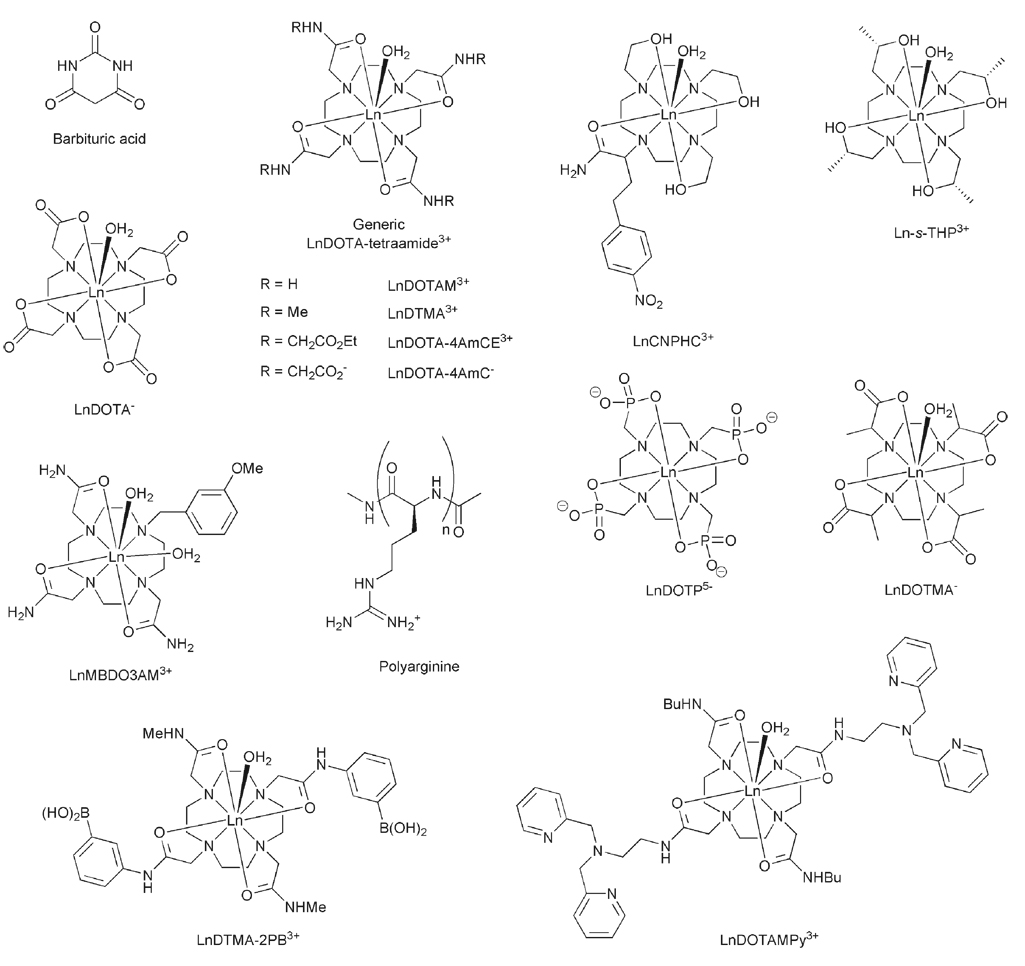
The paramagnetic complexes used as traditional T1-shortening agents have at least one water molecule coordinated to the metal center that exchanges relatively rapidly with bulk water. Typically, the rate of water exchange in these complexes exceeds that allowed by the slow exchange condition, thereby ruling out traditional T1 contrast agents as CEST agents. One class of paramagnetic lanthanide complexes that had been ruled out as T1-shortening agents because water exchange was too slow were complexes formed with tetramide derivatives of DOTA.6 Water exchange in some europium(III) DOTA tetraamide complexes has been observed to be so slow that a separate resonance for the Eu3+-coordinated water protons can be observed by high resolution NMR in dry acetonitrile7 and even in water as solvent.8 The importance of this for CEST was that the Eu3+-coordinated water molecule is shifted ~50 ppm away from the solvent water peak. Since Δω is approximately 10-fold larger in this system compared to the –NH resonance of barbituric acid, the maximum permitted exchange rate is also enhanced by a factor of 10. Using this example, it is possible to envision even more effective paramagnetic systems useful for generating CEST contrast, agents now referred to as PARACEST agents.
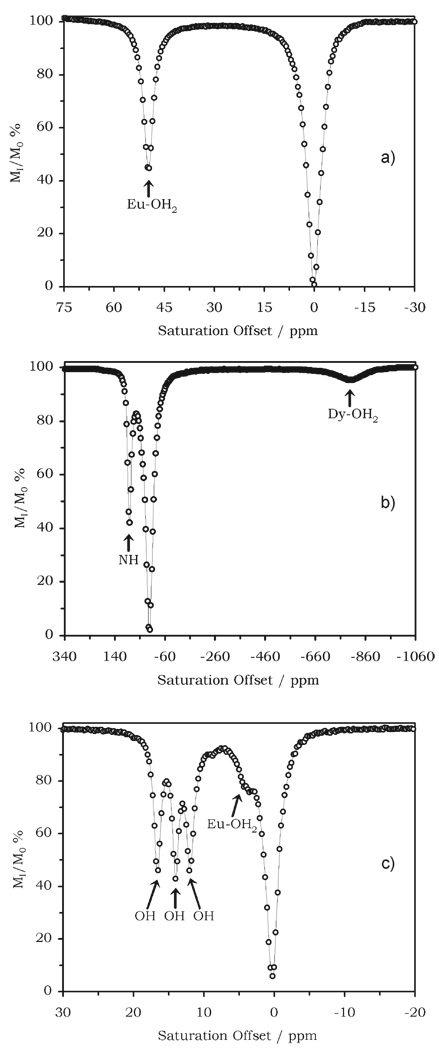
The CEST spectrum of EuDOTA-4AmCE3+ (Fig. 4a) shows that a substantial reduction in the solvent water intensity can be achieved by pre-irradiation at 50 ppm.9 Similar spectra have been recorded for many structurally related DOTA-tetraamide complexes10 and it has been shown that the Eu3+-coordinated water position does not vary much from this example. It should be pointed out, however, that the protons of the coordinated water molecule are not the only exchangeable protons of this complex. The amide protons are not shifted so strongly as those of the coordinated water molecule and, although it is difficult to see in the spectrum of the europium complex, they are easily detected in the spectra of other lanthanide complexes, such as those formed with ytterbium or dysprosium.11 The CEST spectrum of DyDOTAM3+ for example (Fig. 4b) shows a strong CEST effect arising from amide proton exchange at +80 ppm that is considerably larger than the CEST peak from the Dy3+-coordinated water molecule near −720 ppm. There are two principal reasons why the amide protons give rise to a larger CEST effect. First, the coordinated water protons of DyDOTAM3+ are more effectively relaxed by the dysprosium ion than the amide protons and we have already seen how competing relaxation is detrimental in CEST effectiveness. Secondly, there are eight exchanging amide protons in this complex compared to only two water protons. Hence, more exchanging protons translates to a larger CEST effect. Another strategy for increasing the number of exchangeable protons available for CEST is to replace amide coordinating groups with hydroxyethyl side-chains. Hydroxyl ethyl groups are known to retain their protons upon coordination to a lanthanide ion and this then provides another type of chemical exchange site for CEST activation. Interestingly, the rate of proton exchange in Eu-S-THP3+ was found to be too fast, relative to Δω, to allow a CEST effect to be observed.12 However, when one hydroxyethyl group was replaced by an amide pendant arm, as in EuCNPHC3+, then not only was CEST from the pendant hydroxyethyl groups observed (with the complex dissolved in acetonitrile) but each of the three magnetically non-equivalent exchange sites were clearly resolved (Fig. 4c).12 Although interesting from a structural point of view, the utility of such hydroxyethyl-based systems appears to be limited by the magnitude of Δω in these systems. As weak donors, the hydroxyl groups do not impart a strong ligand field on the europium ion and, since ligand field is one of the major factors in governing the magnitude of the hyperfine shifts in such systems, a weak ligand field gives rise to small Δω values and this ultimately limits the magnitude of the CEST effect produced by such systems. Furthermore, the rate of proton exchange between the coordinated hydroxyethyl groups and bulk water increases dramatically as water is added to this system and eventually exceeds that permitted by the CEST requirements and consequently no CEST effect is observed for this complex dissolved in pure water. Thus, it appears from the limited number of hydroxyethyl-based ligand systems examined so far that these are not a viable alternative as PARACEST compounds.
Fig. 4.
The CEST spectra of a) a 30 mM solution EuDOTA-4AmCE3+ recorded at 270 MHz and 25 °C; the peak at +50 ppm reflects exchange with the coordinated water molecule, b) DyDOTAM3+ recorded at 400 MHz and 25 °C; the peak at +80 ppm reflects exchange with the amide protons while the peak at −720 ppm reflects the coordinated water molecule, c) a 35 mM solution of EuCNPHC3+ recorded at 270 MHz and 25 °C; the three peaks at 16, 14 and 12 ppm reflect exchange with each of the hydroxyl protons.
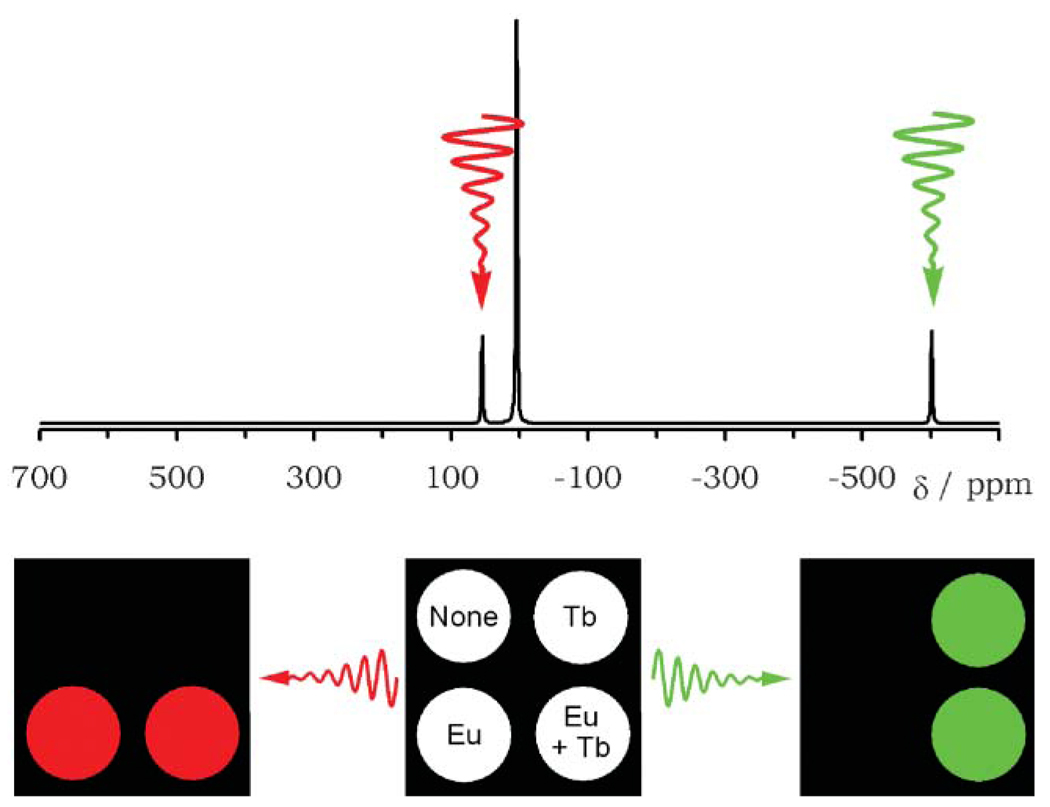
These systems do highlight one further advantage of using lanthanide-based agents for CEST. Because the coordination chemistry of the lanthanides are virtually identical along the series, isostructural complexes can be prepared with any lanthanide ion and any given ligand system. This means that the hyperfine shift characteristics and relaxation properties of a PARACEST agent can be tuned according to the choice of lanthanide ion. Indeed the resonance chosen for CEST activation can be shifted either upfield or downfield of solvent water with varying magnitude depending upon the lanthanide ion chosen. This means that more than one PARACEST agent could be administered simultaneously and each could be activated separately by an appropriate choice of CEST frequency. This concept was nicely demonstrated both in phantoms and in living cells by Aime and co-workers.13 A schematic of this experiment is illustrated in Fig. 5; four samples, one containing EuDOTA-4AmC− (Δω = 50 ppm downfield), one containing TbDOTA-4AmC− (Δω = 600 ppm upfield), one containing a mixture of both complexes and a control containing only water. When a presaturation pulse is applied at +50 ppm prior to image acquisition, a change in total water signal intensity is observed only in those samples containing the europium complex. No change in water intensity is observed in samples that do not contain the europium complex. Conversely, a presaturation pulse applied at −600 ppm activates only the terbium complex and a decrease in water intensity is observed only in those samples containing the terbium complex. As illustrated for the sample containing both complexes, the presence of a second complex does not affect the performance of the first complex as long as the saturation frequencies are well separated from one another. There are many imaging applications where the use of multiple PARACEST agents could be envisioned; for example, cell tracking experiments might allow more than one type of cell to be tracked simultaneously or two types of PARACEST agents could be given at equal concentrations, one for reporting a physiological measure such as pH and another to act simply as a concentration marker. Further ideas of using responsive PARACEST agents are developed below.
Fig. 5.
A simulated 1H NMR spectrum showing the coordinated water proton resonances of EuDOTA-4AmC− (+50 ppm) and TbDOTA-4AmC− (−600 ppm) in water (resonances arising from the ligand were omitted for clarity). Below this is a schematic representation of the result of selectively irradiating these resonances on samples containing EuDOTA-4AmC−, TbDOTA-4AmC− and a mixture of the two. It is seen that each complex may be activated selectively regardless of the presence or absence of the other complex.
PARACEST agents as pH sensors
Two emerging areas of MRI contrast agent development are responsive agents and targeted agents. Contrast agents that respond to changes in the concentration of biologically important species, sometimes known as “smart” agents, are of particular interest since these agents would render MRI a true molecular imaging technique in addition to anatomical imaging. Many gadolinium-based agents that exhibit a change in relaxivity with changes in pH, metal ion concentration, endogenous anions and enzyme activity have been reported.2 One of the main difficulties in using gadolinium-based agents as responsive contrast agent is that the T1 effects of gadolinium are never completely silent due to the outer-sphere relaxation effect. Thus, for quantitative measures of pH, metal ion concentration, or enzyme activity for example, one must account for all forms of Gd3+ in solution, not an intractable problem but certainly a difficult one. Consider the example of a pH responsive agent that exhibits a doubling of its relaxivity in response to a change in pH from 7.4 to 6. If the pH is 7.4 in one tissue volume element and pH 6 in a second, nearby volume element and the concentration of the agent is the same in both elements, then the voxel at pH 6 will appear brighter than that at pH 7.4. However, if the pH is 7.4 in both voxels but the concentration of the agent is twice as high in one voxel than the other, then a similar difference in image contrast would be observed. In other words the two situations are indistinguishable. So, in order to generate an accurate pH map using this traditional gadolinium agent, it is necessary to account for the local concentration of the agent in each image voxel. Although this has been accomplished in pH mapping studies,14,15 the experimental protocol is more cumbersome than one would like for a clinical application.
We have seen that CEST originates from chemical exchange of protons and/or water molecules the magnitude of the CEST effect is related to the rate of this exchange. Since proton exchange in these systems is often directly influenced by pH, so is the magnitude of the CEST effect. This feature, recognized from the very beginning of the development of exogenous CEST agents,16 allows a direct measure of pH if the concentration of the CEST agent is known, just as is the case for traditional Gd3+-based contrast agents. But, unlike traditional Gd3+-based agents, it is possible to administer and independently visualize two CEST agents in the same experiment (Fig. 5) and this feature could be used to develop a concentration independent method of determining pH. For example, the exchange of the amide protons of YbDOTA-4AmC−, shifted 20 ppm upfield, respond differently to changes in pH than does the exchange of protons of the coordinated water molecule of EuDOTA-4AmC−, shifted 50 ppm downfield. By selectively saturating first one pool of protons followed by the other, it is possible to acquire two different CEST pH profiles for a solution containing both complexes.17 Since these complexes are isostructural, their distribution in tissue should be identical. The ratio of the two CEST profiles is therefore concentration independent and can be used to determine pH directly without knowing the analytical concentration of either agent.
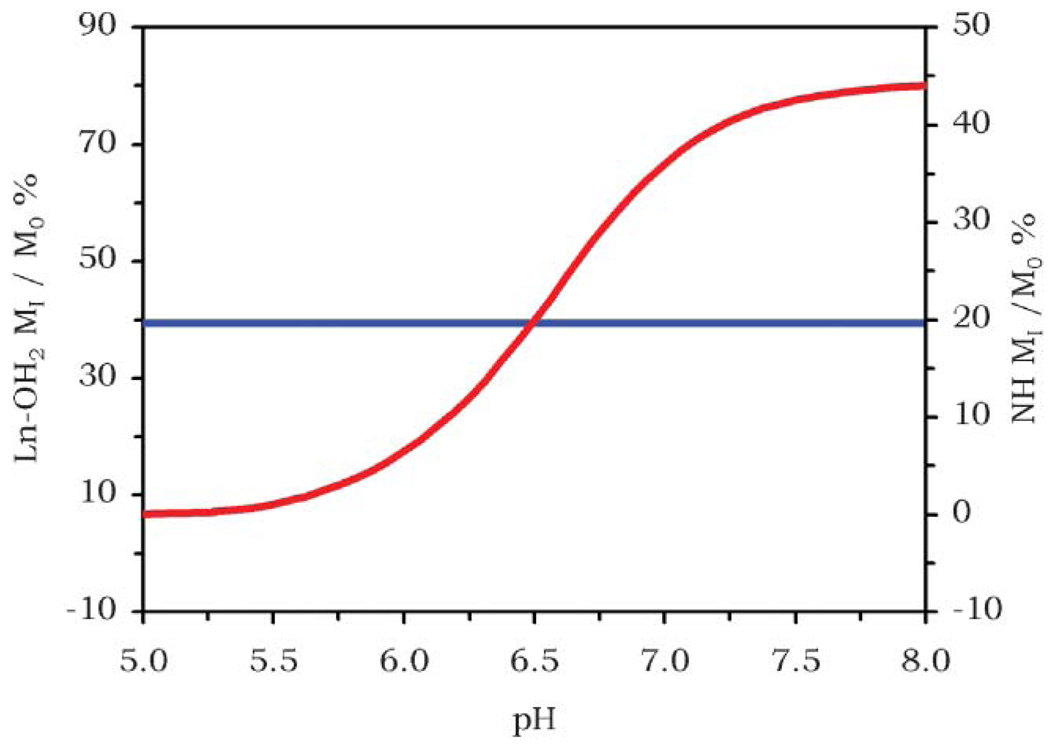
Of course it would be preferable to administer a single compound rather than a cocktail of compounds, so single agent ratiometric approaches have been explored.18 It can be seen from the CEST spectrum of DyDOTAM3+ (Fig. 4) that, if the conditions are right, a CEST effect can be observed from both the coordinated water molecule and amide protons of a single complex. If the pH response of the CEST effect arising from the amide protons is different from that arising from the coordinated water molecule then the same ratiometric approach could be employed using just this one complex. Although the CEST effect arising from the coordinated water molecule of EuDOTA-4AmC− is reasonably high, the effect arising from the amide protons in this same complex is relatively small.18 Δω for the –NH protons is also typically small so the CEST peak arising from this exchange is often poorly resolved from that of the solvent water peak. Conversely, CEST from the –NH groups of YbDOTA-4AmC− is favorably resolved but unfortunately CEST from the exchanging water molecule in this complex cannot be detected, probably because water exchange is too fast in this case.17,18 Lanthanide complexes of DOTA-4AmC from the early part of the lanthanide series, and in particular praseodymium, were found to have CEST effects of similar magnitude for the two pools of protons.18,19 The CEST effect arising from the coordinated water molecule of PrDOTA-4AmC− was found to independent of pH between 5 and 8 while the effect arising from the amide protons, on the other hand, exhibits a marked pH dependence (Fig. 6).18,19 Thus, absolute pH can be determined using this single complex by using the CEST effect from the coordinated water molecule protons as a concentration marker and using the ratio of the two CEST effects as a direct measure of pH.
Fig. 6.
The ratio between the CEST effects arising from the coordinated water molecule (blue line) and the amide protons (red line) of PrDOTA-4AmC− may be used to determine pH without knowing the concentration of the complex in solution. Data taken from reference 18.
Other responsive PARACEST agents
Temperature
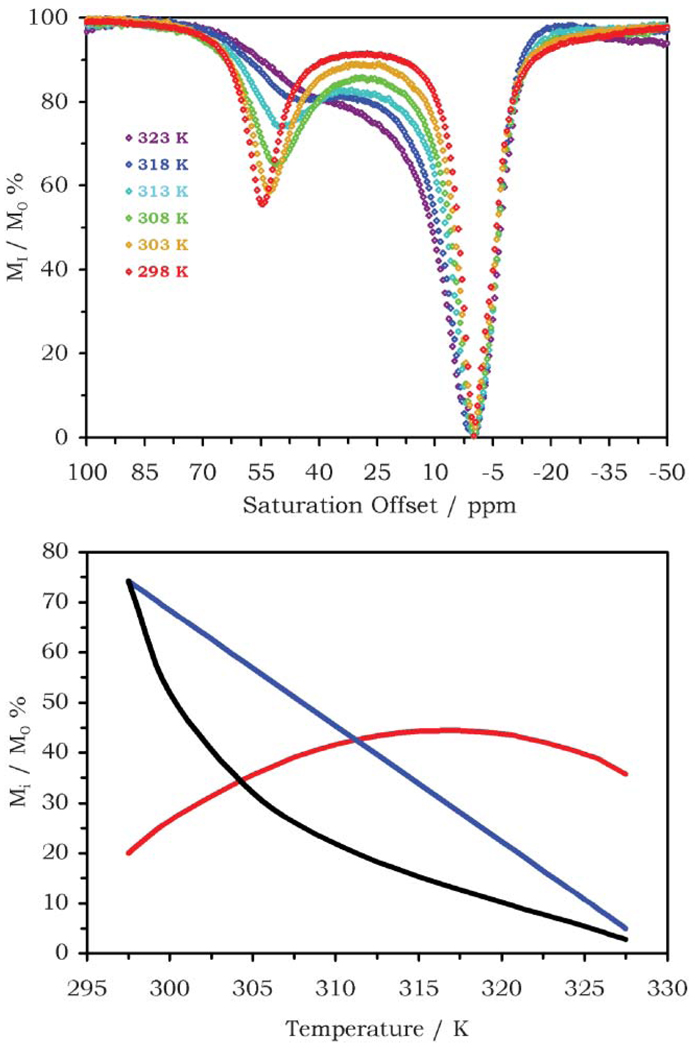
In addition to changes in pH, the rate of proton exchange is also affected by temperature. The rate of exchange accelerates with increasing temperature according to the Arrhenius equation, and this has a profound effect upon the magnitude and shape of the peaks in the CEST spectrum (Fig. 7). A peak in the CEST spectrum arising from protons in very slow exchange is relatively sharp but low in intensity. As the exchange rate increases, the peak shape remains the same but the intensity increases until it reaches a maximum. After the optimal exchange rate is exceeded the peak broadens, shifts towards the solvent peak and decreases in intensity. Furthermore, the hyperfine shift induced by the paramagnetic lanthanide ion is also temperature dependent, decreasing rather sharply with increasing temperature. These changes in CEST may be used to measure sample temperatures. Aime and co-workers used the ratiometric approach developed to measure pH to the measurement of temperature with PrDOTA-4AmC−.18 Acceleration of the coordinated water exchange rate with increasing temperature resulted in the linear decrease in the CEST effect. The CEST effect arising from the amide protons exhibits a more complex behaviour with increasing temperature, arising from the interplay of changing Δω, kex and T1. Initially the CEST effect arising from the amide protons increases, maximises and then falls at higher temperatures. Although the CEST effect of both pools exhibits a pH response, so neither pool can be viewed as a simple concentration marker, taking the ratio of the two pools still affords a concentration independent method of measuring temperature (Fig. 7).
Fig. 7.
The CEST spectra of 10 mM solution of EuDOTA-4AmCE3+ recorded at 400 MHz, pH 7.3, B1 = 714 Hz and different temperatures (top), from reference 20; and the ratiometric determination of temperature using PrDOTA-4AmC− comparing the water (blue) and amide protons (red), the ratio ST(H2O)/ST(NH) is shown in black (bottom).
Zhang et al. recently took a different approach to measure temperature with PARACEST agents.20 Rather than using the magnitude of the CEST effect, they monitored the frequency of the CEST peak with changing temperature. In an imaging context this means acquiring a collection of images in which the frequency of the presaturation pulse has been varied over the range of interest. In phantom systems using EuDOTA-4AmC−, it was possible to determine the temperature of the sample using this method with sufficient accuracy that even slight temperature gradients in the sample could be observed. Since it is the frequency of the presaturation pulse that is being monitored in this method, this technique is also independent of the concentration of the CEST agent.
Metabolites
Changes in Δω have also been utilized as a method for detecting the presence of lactate by CEST imaging.21 Many endogenous anions, including lactate, are known to displace the two water molecules coordinated to the metal ion in heptadentate DO3A-triamide lanthanide complexes such as YbMBDO3AM3+.21,22 The displacement of water by lactate induces a substantial change in the chemical shift of the amide proton resonances in the Yb complex, from −29.1 ppm to −15.5 ppm. In the absence of lactate, a 60% reduction in the solvent water signal intensity was observed for a 9.3 mM solution of the complex upon presaturation at −29.1 ppm. As lactate was added to the solution, the intensity of the solvent water signal increased after presaturation at −29.1 ppm until the complex is completely saturated with lactate. The effect of lactate on the water signal intensity after presaturation at −15.5 ppm was somewhat different because even in the absence of lactate there is still a reduction in the solvent water signal after presaturation. Nonetheless, the magnitude of the CEST effect observed after presaturation at this frequency was still observed to increase with increasing lactate concentration. Although it was not proposed by the authors at the time, the ratio of the CEST effects obtained by irradiating at these two frequencies could also be used to give a concentration independent ratiometric method of determining the concentration of lactate in solution. It is not clear, however, to what extent other endogenous anions would interfere with the operation of this agent in vivo.
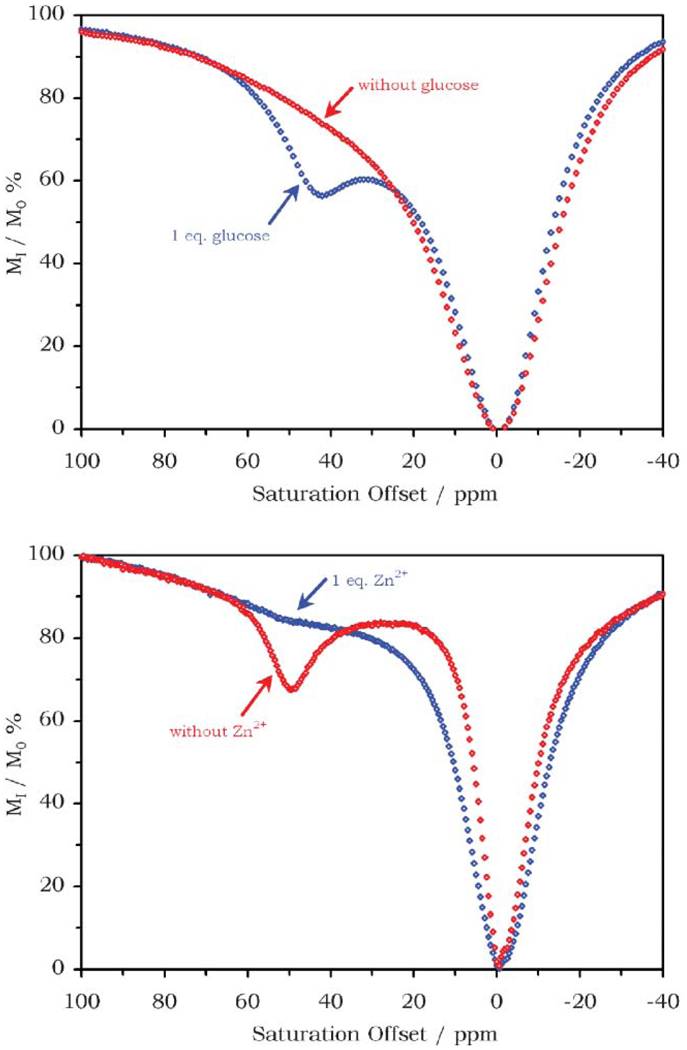
Groups that can induce changes in factors that affect CEST, such as the chemical shift or exchange rate of exchangeable protons, may also be introduced into the design of a PARACEST agent to render it responsive. For instance, phenyl boronate groups are known to reversibly bind cis-diols, and as such have been proposed as glucose sensors. Two meta-phenyl boronates have been incorporated into a simple tetraamide complex as amide substituents in a trans-orientation. The CEST properties of the europium complex, EuDTMA-2PB3+, were measured in the absence and presence of glucose at pH 7.4 (Fig. 8a).23 The spectrum obtained in the absence of glucose does not exhibit a single well defined peak corresponding to the coordinated water molecule. Instead the bulk water signal intensity is altered by a presaturation over a broad range of frequencies between 20 and 60 ppm, characteristic of a system close to breaching the slow exchange threshold. In contrast the spectrum obtained in the presence of equimolar glucose shows a clear CEST peak arising from the coordinated water molecule near 40 ppm, indicating that water exchange is now much slower. This deceleration of water exchange is thought to be the result of the phenyl boronate groups binding glucose directly above the site of the coordinated water molecule, hindering the process of water exchange.24 The difference in the CEST spectrum observed upon addition of glucose can be used to measure the concentration of glucose in solution. Even though a CEST effect from only one pool of exchanging protons is observed it is still possible to use a ratiometric method that does not require knowledge of the complex concentration to calculate the response to glucose binding. Simply comparing the magnitude of the CEST effect at two points on these profiles at which the response to glucose is different will provide enough information to determine the glucose concentration. For example, the magnitude of the CEST effect when irradiated at 25 ppm will change only slightly compared to that obtained when irradiating at 40 ppm. This single pool ratiometric approach has shown that it is possible to determine glucose concentration in the physiologically relevant range (~5 mM) for imaging systems.24 Of course glucose is not the only sugar that contains a cis-diol; however, experiments with other sugars, such as fructose and galactose, showed that EuDTMA-2PB3+ has a strong preference for glucose over these other sugars, and since the physiological concentrations of these other sugars are normally much lower than that of glucose, they are not anticipated to prevent the use of this complex in vivo.
Fig. 8.
The behaviour of PARACEST agents designed to sense the presence of glucose and zinc. Top: The effect of glucose on the CEST effect of a 10 mM solution of EuDTMA-2PB3+, in the absence (red) and presence (blue) of 10 mM glucose (25 °C, pH 7.4, B1 = 1000 Hz). Bottom: The CEST spectra of a 20 mM solution of EuDOTAMPy3+ in the absence (red) and presence (blue) of 20 mM Zn2+ (25 °C, pH 8, B1 = 1000 Hz).
Although the experiment has not yet been performed, it is easy to see from the spectra shown (Fig. 7) how this single-pool ratiometric approach could also be applied to the measurement of temperature and, in principle, any of the other responsive PARACEST agents discussed here, such as a recently reported sensor for the biologically important Zn2+ ion.25 The approach taken in this case was similar to that used in the glucose sensor. Two bis(pyridylmethyl)amino groups were incorporated into a DOTA-tetraamide complex (EuDOTAMPy3+) with the expectation that when the four pyridyl groups came together to bind a zinc ion above the water coordination site of the complex the rate of water exchange would again be modulated, resulting in a measurable difference in CEST efficiency. The CEST spectrum of EuDOTAMPy3+ recorded at pH 8 in the absence of zinc is typical of tetraamide complexes of europium, exhibiting a CEST peak at 50 ppm that reflects the coordinated water molecule. Addition of an equimolar amount of zinc chloride to the solution resulted in broadening and a reduction in the intensity of this CEST peak (Fig. 8b). This behavior, also observed when temperature is increased (Fig. 7), is indicative of an acceleration in water or water proton exchange upon binding of Zn2+. This is in contrast to the effect observed for the glucose binding system, which appears to arise from a slowing of the water exchange rate with the binding event. This observation suggests that the mode of operation of these systems may be much more complex than initially predicted. Despite uncertainty in mechanism of action, this system can clearly be applied to the measurement of zinc ions by CEST imaging and by using the single-pool ratiometric approach described above this can be done without knowing the concentration of the agent in solution.
Using exchange theory to optimize PARACEST agents
One of the limitations of conventional contrast media is the high doses required to generate image contrast; typical doses can be 5 g or more for a 70 kg individual. If PARACEST agents are to be used in a clinical setting it is important that they are at least as sensitive as the existing range of contrast agents. From the experience of researchers engaged in functional MRI (fMRI) it is generally accepted that a change in the water signal intensity of 5% is sufficient to generate contrast in an MR image. But this change must be achieved within strict power limits. The question of power limits is more acute when considering CEST imaging techniques because a long presaturation pulse must be applied to the sample prior to data acquisition. The development of CEST agents for clinical application must take place within the specific absorption rate (SAR) power limitations of clinical scanners and should therefore be as sensitive as possible while operating within clinically acceptable SAR limits.
The factors that govern the CEST effect are described by the Bloch model26 and so it should be possible to calculate the parameters required to maximize CEST, reducing the required dose and the required presaturation power. Let’s consider an idealized system in which some of the protons on a PARACEST agent (Pool B) exchange with those of the solvent water (Pool A). In this prototype CEST experiment, Δω is sufficiently large that irradiation of the protons of Pool B does not result in any direct saturation of the protons in Pool A. After the saturation of Pool B, exchange causes partial saturation of Pool A and the intensity of the A NMR signal is somewhat lower. The extent of this change in signal intensity depends on several different NMR parameters, some determined by the CEST agent, some by the solvent water, and others controlled by operator guided instrument settings.
In tissue, the concentration of water protons (Pool A) is on the order of ~80 M whereas the concentration of the contrast agent must be no more than millimolar, preferably even lower. However, an NMR experiment detects nuclear magnetization, not the nuclei themselves but since the magnetization of each pool at equilibrium is given by the Boltzmann distribution (eqn 1) then the magnetization, M, of each pool will be proportional to their concentrations, [H]a = M0 a and [H]b = M0 b. This allows us to relate the exchange of nuclear magnetization between the two pools to the actual exchange of protons. When exchange occurs, protons move from Pool A to Pool B at a rate ka (ka = 1/τa) where τa is the residence lifetime of bulk water protons (Pool A). As long as the exchangeable protons on the CEST agent are completely saturated, and some early publications make this assumption, then the protons that leave Pool A that have a z-magnetization, Mz a, will be replaced by protons from Pool B with no magnetization. This will cause the bulk magnetization of Pool A to decrease at the rate kaMz a. Of course spin–lattice relaxation will simultaneously work to return the z-magnetization of Pool A to its equilibrium value at a rate [M0 a − Mz a]/T1a, where T1a is the longitudinal relaxation time of bulk water protons. Once the system has reached steady-state, these rates will be equal, such that:
| (3) |
This can be rearranged to give the normalized steady-state value of Mz a, often referred to as the Z-value. Note that smaller Z-values indicate larger CEST effects.
| (4) |
From eqn 4, one can see that the observed CEST effect will be larger when the residence lifetime of a proton in Pool A, τa, is short and the longitudinal relaxation time of this pool, T1a, is long. T1a is an intrinsic property of bulk water and is typically longer than 1 s unless a relaxation agent is present. It should be noted that the T1a of bulk water tends to be longer at higher fields so this implies, everything else being equal, that the CEST effect will magnify at higher imaging fields. τa, on the other hand, is a feature of interaction between the solvent water and the PARACEST agent and this can be manipulated by chemical design so as to position it in an optimal range. Mass balance dictates that kbM0 b = kaM0 a and τa = τb (M0 b/M0 a) so eqn 4 can be written in terms of agent concentration and lanthanide bound water lifetime (τb).
| (5) |
Eqn 5 shows that a maximum CEST effect is obtained when the residence lifetime of a proton on the agent, τb, is as short as possible and the concentration of the agent (M0 b) is as high as possible. In addition to these factors, the observed CEST effect also depends upon the extent of saturation of Pool B, less than complete saturation will result in lower CEST contrast. The extent of saturation of Pool B is determined by the power of the applied presaturation pulse, B1 (ω1 = 2πB1), as well as other physical characteristics of the nuclear spin system including relaxation times, chemical shifts and exchange rates.27 So, the dichotomy in this experiment is that CEST will nearly always increase with larger applied B1, yet this (power) is the very parameter that we need to minimize for in vivo work. To do this, there are essentially three parameters that can be modified, the residence lifetime of the protons on the agent, τb, the chemical shift of the exchangeable protons, Δω, and the relaxation properties of the agent. Clearly, Δω should be as large as possible so that one does not indirectly saturate bulk water protons while saturating the exchanging Pool B protons. Eqn 5 shows that shorter values of τb result in larger CEST effects if the protons of Pool B are satisfactorily saturated. However, if τb is too short, then the protons of Pool B will be poorly saturated and this limits the observed CEST effect. Since these two effects run counter to one another, an optimal value of τb must be calculated. A numerical analysis that involves solution of the Bloch equations formulated to include exchange between two pools of protons shows that this optimal value is dependent upon the saturation power, ω1.27
| (6) |
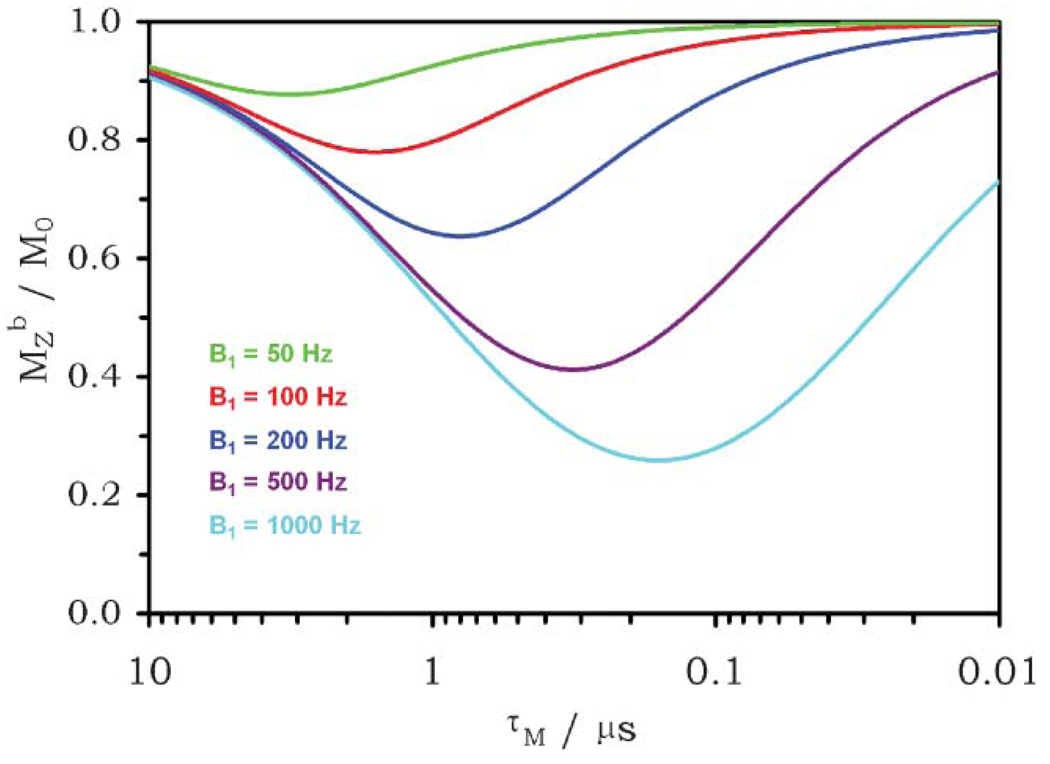
This fundamental concept is graphically illustrated by Fig. 9. It shows that for a relatively small B1 of 50 Hz, an optimal CEST effect will be observed for an agent with a bound water lifetime, τb, of 3 ms, a value that is much longer than any PARACEST agent reported to date. For B1 values of 100, 200 or 500 Hz, the optimal bound water lifetimes decrease to 1.5 ms, 735 µs, and 296 µs. respectively. Thus, a higher presaturation power allows one to choose an agent undergoing faster water exchange. This is a very useful principle to keep in mind for PARACEST design.
Fig. 9.
The magnitude of the CEST effect calculated for typical parameters for a 20 mM solution of a PARACEST agent (T1bulk = 2.5 s, T1bound = 0.2 s, Δω = 10 kHz) as a function of the water exchange rate (1/τM) for different presaturation powers (B1). The advantage of selecting an appropriate water exchange rate is evident.
The theoretical description outlined above applies to conventional CEST experiments that involve presaturation at an exchanging site but CEST effects may be generated by other methods. The effect of applying a 360° pulse to a resonance in an NMR experiment is to rotate the bulk magnetization by 360° and return it to its equilibrium position along the z axis. In the absence of exchange, a single 360° pulse, or a train of pulses equivalent to a 360° pulse, applied on resonance prior to an observe pulse should have no net effect upon the intensity of the measured bulk water signal. If, however, a 360° pulse is applied to a system in rapid chemical exchange, then the effect will be quite different. Consider a bulk water spin that exchanges with a spin on a PARACEST agent that resonates at a different remote frequency, then for the duration of its time on the PARACEST agent this spin will not be subjected to the effects of the 360° pulse. When this spin returns to bulk water, its magnetization will lag behind those spins that did not exchange so once the 360° pulse is complete, its magnetization will not lie along the z-axis. The result is that the bulk water signal intensity is reduced. This is also an effect due to CEST but, in this case, a high power presaturation pulse at the remote bound water site is not required, a significant advantage for in vivo applications. This technique has been demonstrated by applying a WALTZ-16* pulse train on the water resonance.28 In the absence of a PARACEST agent, the majority of the bulk water signal remained unchanged after application of the WALTZ-16* pulse train. However, in the presence of 41 µM TmDOTA-4AmC−, a 32% reduction in solvent water intensity was observed. This demonstrated that novel methods can be developed to “activate” PARACEST agents and that the lower detection limit of these agents may be in the low µM range. The great advantage of this method is that lower B1 fields can be employed to create a detectable CEST effect than is possible with normal CEST activation by saturation of an exchanging pool of protons. Of course, since this CEST activation method is quite different from the more usual presaturation CEST activation method, the criteria for a PARACEST agent in this type of experiment are very different from that of a traditional PARACEST agent. First, it is beneficial for the frequency of the exchangeable pool on the PARACEST agent to lie as far as possible from that of the solvent water. This will minimize the effect of the pulse train of the protons in this pool improving the amount of contrast generated. Importantly, from this point of view is the fact that relaxation is not in competition with this CEST mechanism, in fact, a certain amount of relaxation may even enhance the contrast generated. This is significant because most of the lanthanides that induce the largest chemical shifts, such as dysprosium and thulium, also have powerful relaxation effects and this can be a problem in traditional CEST agents.
Highly sensitive supramolecular PARACEST agents
In addition to the methods described above for improving the sensitivity of PARACEST agents, it has been shown that it is possible to design highly sensitive CEST agents by moving beyond the simple complex toward supramolecular structures. Supramolecular assemblies may be envisioned that could entrap a much larger number of exchangeable protons than can be included in a simple paramagnetic complex. In such a system, the rate of proton exchange between the solvent and the supramolecular assembly must be slow enough to meet the slow exchange condition (eqn 2). The exchangeable protons entrapped in the supramolecular assembly are still required to have a substantial shift difference (Δω) from the solvent water signal and this is where another property of the lanthanide ions may be used. Lanthanide ions have formed the basis of shift reagents for a wide range of NMR applications,29 so including a suitable lanthanide complex in the supramolecular assembly will have the effect of increasing the Δω of these protons. However, the requirements for this lanthanide complex are different from those of a discrete PARACEST agent; the rate of exchange of protons between the complex and the solvent water need not meet the slow exchange condition. Indeed, exchange itself is not necessary as can be seen from the first example of this type of supramolecular PARACEST agent.30 TmDOTP5− is a shift reagent that has been used in 23Na NMR experiments but does not possess a coordinated water molecule and has no exchangeable protons. When this anionic complex is added to the polyarginine polycation, the two species interact via normal ion-pair attractions. The result is that the TmDOTP5− complex induces a shift in the exchangeable protons of polyarginine and exchange of these protons with those of the solvent is now able to produce a CEST effect. An optimal ratio of 18 : 1, TmDOTP5− : poly-Arg, when irradiated between 20 and 30 ppm, gives rise to a 5% reduction in solvent signal intensity at 30 µM TmDOTP5− and 1.7 µM polyarginine (B1 = 86.7 µT).
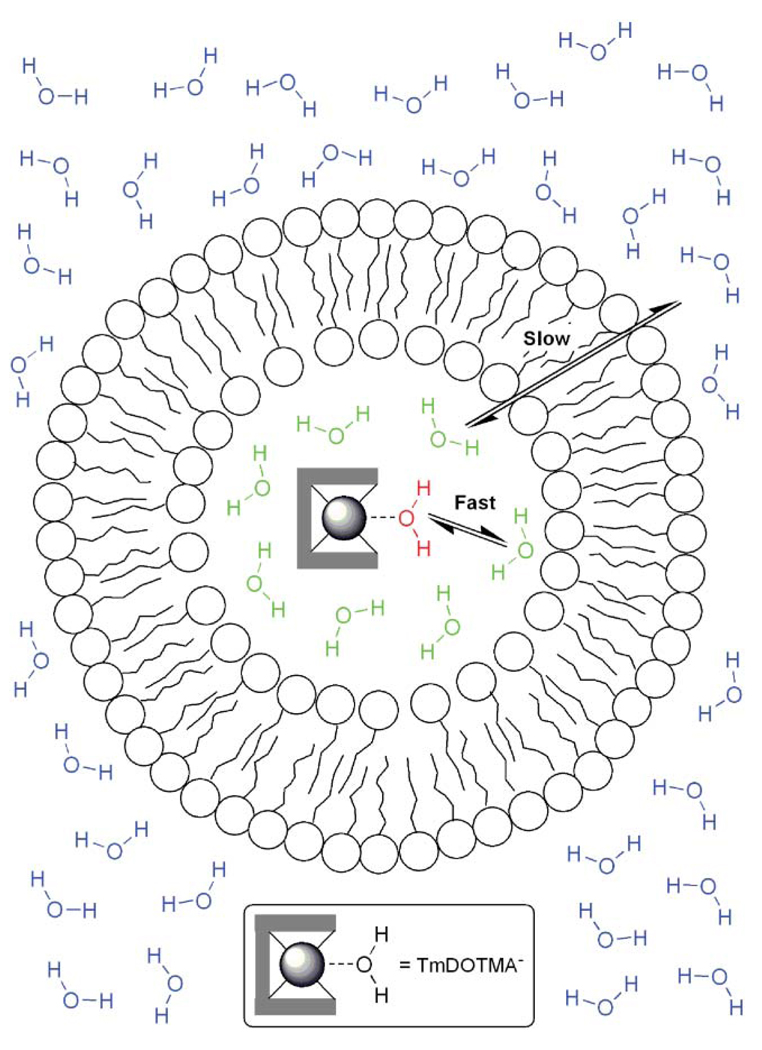
Even greater advances in sensitivity could be achieved by encapsulating the shift reagent inside a liposome. Because the rate of water exchange across the lipid bilayer of the liposome is slow, the presence of a shift reagent entrapped within the liposome gives rise to two signals in the NMR spectrum—one from bulk water and another from water entrapped within the liposome (Fig. 10). So this type of assembly, christened a LIPOCEST agent,31 could be used to generate CEST contrast. Liposomes made up from phospholipids and cholesterol could be prepared that entrapped, on average, 9.2 × 10− 18 L of a 0.1 M solution of TmDOTMA−. 31 The shift of the entrapped water molecules is 3.1 ppm downfield of the solvent water protons and presaturation at this frequency leads to a reduction in the intensity of the solvent water signal. The solvent water signal was reduced by 5% in the presence of just 90 pM LIPOCEST agent (B1 = 12 µT) which, although this still equates to 42 µM in TmDOTMA−, is still a remarkably low level of detection for any MRI contrast agent.
Fig. 10.
The water exchange rate in TmDOTMA− is too fast for the complex to be used as a PARACEST agent. However, encapsulation of the complex in a liposome means TmDOTMA− can behave as a shift reagent that acts only on the water molecules trapped inside the liposome. Exchange of these shifted water molecules across the liposome membrane is slow, which means that the entire liposome can be used as a PARACEST agent.
Conclusions
We have seen that chemical exchange saturation transfer (CEST) is an effective method of generating negative image contrast in MRI. The application of paramagnetic metal complexes, and in particular those of lanthanide ions, as PARACEST agents offers a number of advantages over diamagnetic agents, primarily arising from their hyperfine shifting properties leading to increases in Δω. In addition to the potential improvement in sensitivity that can be gained from this increase in Δω, PARACEST agents offer the possibility of designing agents that can be applied, in a truly practical sense, to the problem of molecular imaging with MRI. The application of a ratiometric analysis of the CEST generated image contrast, whether from one or two exchanging pools, allows for the quantitative determination of a range of analytes. Systems that have been devised so far include pH, temperature, lactate, glucose and zinc sensors. Since any change in the CEST properties of an agent can be measured ratiometrically, it is relatively easy to consider using PARACEST agents for the detection and quantification of nearly any biologically important metabolite. As more agents are developed, MRI will become more than a tool for anatomical imaging but a true molecular imaging technique as well.
Chart 1.
Acknowledgements
The authors thank Dr Shanrong Zhang of the University of Washington for kindly supplying the spectra presented in Fig. 7, and Dr Jimin Ren of UT Southwestern Medical Center for the spectra presented in Fig. 8. The authors also thank the National Institutes of Health (EB-04285, MW), (CA-115531 and RR-02584, ADS) and the Robert A. Welch Foundation (AT-584) for financial assistance.
Biographies

Mark Woods received his BSc (Hons) degree from the University of York in 1995. He went on to gain his PhD from the University of Durham in 1998, studying under the supervision of Prof. David Parker. In 1999 he was a TMS fellow at the Université Claude Bernard Lyon 1 where he worked on palladium catalysed cascade reactions. In 2000 he returned to the field of lanthanide chemistry joining the group of Prof. Dean Sherry at the University of Texas at Dallas as a post-doctoral research associate. He took up his current position as Senior Research Scientist (Imaging) at Macrocyclics in 2004.

Donald E. Woessner received a PhD in Physical Chemistry from the University of Illinois in 1957, studying nuclear spin relaxation times under the supervision of Prof. Herbert S. Gutowsky. In these studies, he constructed the first pulsed NMR apparatus at a chemistry department of an American university. After a postdoctoral fellowship at Illinois, he joined the Mobil Field Research Laboratory in Dallas in 1958 to study NMR relaxation of molecules at solid surfaces in support of NMR oil well logging research. He retired from Mobil in 1992 and accepted an appointment as Adjunct Professor of Radiology at UT Southwestern Medical Center at Dallas where he studies theoretically the mechanism of imaging agents. He has been honored with the W. T. Doherty Award (1975) and the Southwest Regional Award of the American Chemical Society (1991).

A. Dean Sherry received a PhD in Inorganic Chemistry from Kansas State University in 1971 on Lewis acid–base theory and thermodynamics of hydrogen bonding. He was awarded a National Institutes of Health Postdoctoral Fellowship (1971–72) to study lanthanide cations as spectroscopic probes of protein structure. He joined Chemistry faculty at UT-Dallas in 1972 and was promoted to Professor in 1982. He served as Chairman of the department from 1979–1990. In 1990, he accepted an appointment as Professor of Radiology at UT-Southwestern Medical Center in Dallas where he studies intermediary metabolism in animal models and develops metabolic imaging agents. He was recently named Director of the Advanced Imaging Research Center on that campus. He has been honored with the W. T. Doherty Award (1990) and the Chancellor’s Outstanding Teaching Award (1994). Dr Sherry is the Scientific Founder of Macrocyclics, Inc.
References
- 1.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293. doi: 10.1021/cr980440x. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.Woods M, Zhang S, Sherry AD. Curr. Med. Chem. 2004;4:349. doi: 10.2174/1568013043357338. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders JKM, Hunter BK. Modern NMR Spectroscopy; A Guide for Chemists. Oxford, UK: Oxford University Press; 1997. and references cited therein. [Google Scholar]
- 4.Henkelman RM, Stanisz GJ, Graham SJ. NMR Biomed. 2001;14:57. doi: 10.1002/nbm.683. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 5.Ward KM, Aletras AH, Balaban RS. J. Magn. Reson. 2000;143:79. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 6.Aime S, Barge A, Botta M, De Sousa AS, Parker D. Angew. Chem., Int. Ed. 1998;37:2673. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673::AID-ANIE2673>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. J. Am. Chem. Soc. 1999;121:5762. [Google Scholar]
- 8.Zhang S, Wu K, Biewer MC, Sherry AD. Inorg. Chem. 2001;40:4284. doi: 10.1021/ic0003877. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Winter P, Wu K, Sherry AD. J. Am. Chem. Soc. 2001;123:1517. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. Acc. Chem. Res. 2003;36:783. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Michaudet L, Burgess S, Sherry AD. Angew. Chem., Int. Ed. 2002;41:1919. [PubMed] [Google Scholar]
- 12.Woods M, Woessner DE, Zhao P, Pasha A, Morrow JR, Vasalatiy O, Sherry AD. unpublished results. [Google Scholar]
- 13.Aime S, Carrera C, Delli Castelli D, Geninatti Crich S, Terreno E. Angew. Chem., Int. Ed. 2005;44:1813. doi: 10.1002/anie.200462566. [DOI] [PubMed] [Google Scholar]
- 14.Raghunand N, Howison C, Sherry AD, Zhang S, Gillies RJ. Magn. Reson. Med. 2003;49:249. doi: 10.1002/mrm.10347. [DOI] [PubMed] [Google Scholar]
- 15.Raghunand N, Howison C, Sherry AD, Zhang S, Gillies RJ. Magn. Reson. Med. 2006;55:309. doi: 10.1002/mrm.10347. [DOI] [PubMed] [Google Scholar]
- 16.Ward KM, Balaban RS. Magn. Reson. Med. 2000;44:799. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Aime S, Barge A, Delli Castelli D, Fedeli F, Mortillaro A, Nielsen FU, Terreno E. Magn. Reson. Med. 2002;47:639. doi: 10.1002/mrm.10106. [DOI] [PubMed] [Google Scholar]
- 18.Terreno E, Delli Castelli D, Cravotto G, Milone L, Aime S. Invest. Radiol. 2004;39:235. doi: 10.1097/01.rli.0000116607.26372.d0. [DOI] [PubMed] [Google Scholar]
- 19.Aime S, Delli Castelli D, Terreno E. Angew. Chem., Int. Ed. 2002;41:4334. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Malloy C, Sherry AD. J. Am. Chem. Soc. 2005;127:17572. doi: 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aime S, Delli Castelli D, Fedeli F, Terreno E. J. Am. Chem. Soc. 2002;124:9364. doi: 10.1021/ja0264044. [DOI] [PubMed] [Google Scholar]
- 22.Dickins RS, Gunnlaugsson T, Parker D, Peacock RD. Chem. Commun. 1998:1643. [Google Scholar]
- 23.Zhang S, Trokowski R, Sherry AD. J. Am. Chem. Soc. 2003;125:15288. doi: 10.1021/ja038345f. [DOI] [PubMed] [Google Scholar]
- 24.Trokowski R, Zhang S, Sherry AD. Bioconjugate Chem. 2004;15:1431. doi: 10.1021/bc0498976. [DOI] [PubMed] [Google Scholar]
- 25.Trokowski R, Ren JM, Kalman FK, Sherry AD. Angew.Chem., Int. Ed. 2005;44:6920. doi: 10.1002/anie.200502173. [DOI] [PubMed] [Google Scholar]
- 26.Bloch F. Phys. Rev. 1946;70:460. [Google Scholar]
- 27.Woessner DE, Zhang S, Merritt ME, Sherry AD. Magn. Reson. Med. 2005;53:790. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 28.Vinogradov E, Zhang SR, Lubag A, Balschi JA, Sherry AD, Lenkinski RE. J. Magn. Reson. 2005;176:54. doi: 10.1016/j.jmr.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Peters JA, Huskens J, Raber DJ. Prog. Nucl. Magn. Reson.Spectrosc. 1996;28:283. [Google Scholar]
- 30.Aime S, Delli Castelli D, Terreno E. Angew. Chem., Int. Ed. 2003;42:4527. doi: 10.1002/anie.200352132. [DOI] [PubMed] [Google Scholar]
- 31.Aime S, Delli Castelli D, Terreno E. Angew. Chem., Int. Ed. 2005;44:5513. doi: 10.1002/anie.200501473. [DOI] [PubMed] [Google Scholar]