Abstract
Increasing interest has focused on capturing the complexity of tissues and organs in vitro as models of human pathophysiological processes. In particular, a need exists for a model that can investigate the interactions in three dimensions (3D) between epithelial tissues and a microvascular network since vascularization is vital for reconstructing functional tissues in vitro. Here, we implement a microfluidic platform to analyze angiogenesis in 3D cultures of rat primary hepatocytes and rat/human microvascular endothelial cells (rMVECs/hMVECs). Liver and vascular cells were cultured on each sidewall of a collagen gel scaffold between two microfluidic channels under static or flow conditions. Morphogenesis of 3D hepatocyte cultures was found to depend on diffusion and convection across the nascent tissue. Furthermore, rMVECs formed 3D capillary-like structures that extended across an intervening gel to the hepatocyte tissues in hepatocyte-rMVEC coculture while they formed 2D sheet-like structures in rMVEC monoculture. In addition, diffusion of fluorescent dextran across the gel scaffold was analyzed, demonstrating that secreted proteins from the hepatocytes and MVECs can be exchanged across the gel scaffold by diffusional transport. The experimental approach described here is useful more generally for investigating microvascular networks within 3D engineered tissues with multiple cell types in vitro.—Sudo, R., Chung, S., Zervantonakis, I. K., Vickerman, V., Toshimitsu, Y., Griffith, L. G., Kamm, R. D. Transport-mediated angiogenesis in 3D epithelial coculture.
Keywords: microfluidics, vascularization, tissue engineering
Increasing interest has focused on capturing the complexity of tissues and organs in vitro as models of human physiological and pathophysiological processes (1,2,3). Indeed, the function of many epithelial tissues, including liver, pancreas, and adrenal gland, depends critically on the rapid exchange of molecules between the epithelial layer and the blood. In vivo, such tissues are highly vascularized, with little extracellular matrix (ECM) or stroma separating the functional and highly metabolically active epithelial cells from the endothelial cells lining capillaries or sinusoids permeating the tissue. To recapitulate a capillary bed of such tissues in vitro, the key challenges are in directing new microvessels into an environment defined prominently by cell–cell contacts and meeting high metabolic demands.
Liver is one of the most highly vascularized organs and an important target of in vitro tissue engineering (4). Various three-dimensional (3D) hepatocyte culture models have been developed, and many maintain relatively high degrees of hepatocellular function. Few, however, directly address the creation of a microvascular network within the tissue (5,6,7,8). Numerous pathophysiological responses of liver involve interactions with the sinusoidal endothelium; hence, it is of interest to define methods for inducing formation of microvascular networks in 3D liver cultures. 3D microvascular networks can be formed readily by microvascular endothelial cells (MVECs) in various natural or synthetic hydrogels, though formation of true capillaries (vessels with lumens <30 μm in diameter and with continuous anastomosing luminal structures) occurs only strictly controlled conditions (9, 10). Myriad angiogenic factors work in concert with gel matrix mechanical properties to influence capillary formation and vessel maturation (11, 12). Perivascular cells such as pericytes, smooth muscle cells, and fibroblasts also play an important role in the formation and stabilization of capillary networks (13). Still, little is known concerning the interaction between the resulting capillary structures and epithelial cell types such as hepatocytes, particularly under conditions where the growing capillaries encounter epithelial tissues joined by tight junctions, as is observed in liver regeneration. When liver is surgically resected, mature hepatocytes in the parenchyma are stimulated to proliferate and form avascular clusters. The clusters are then infiltrated by surrounding endothelia (14).
Here, we implement a microfluidic platform to analyze angiogenesis in 3D cultures of rat primary hepatocytes and rat/human MVECs (rMVECs/hMVECs). This study aims to establish a microfluidic coculture system and to investigate the heterotypic interaction between 3D structures of hepatocytes and MVECs during capillary morphogenesis. Morphogenesis of 3D hepatocyte cultures was found to depend on diffusion and convection across the nascent tissue. We also observed that rMVECs formed 3D capillary-like structures that extended across an intervening gel to the 3D hepatocyte tissues, whereas capillary morphogenesis of hMVECs was inhibited in hepatocyte-hMVEC coculture. Finally, the experimental approach described here is useful more generally for investigating microvascular networks within 3D engineered tissues with multiple cell types and enables real-time monitoring of cellular events such as migration and morphogenesis.
MATERIALS AND METHODS
Microfluidic device preparation
The microfluidic system incorporates a micropatterned polydimethyl siloxane (PDMS; Silgard 184, Dow Chemical, Midland, MI, USA) device with coverslip and includes two parallel microfluidic channels and an intervening 3D gel scaffold (e.g., type I collagen) (Fig. 1A) based on a previous design (15, 16). Briefly, the shape and dimension of the gel region were designed to produce capillary forces sufficient to confine and support the injected gel solution. The width of the gel scaffold (750 μm) was selected to withstand the pressures and shear stresses associated with interstitial flow and to promote interactions between cells cultured in the two separate channels. Channel width and height were selected to reduce shear stress on the cells during medium replacement and to provide sufficient area for cell adhesion to the sidewall of the gel scaffold.
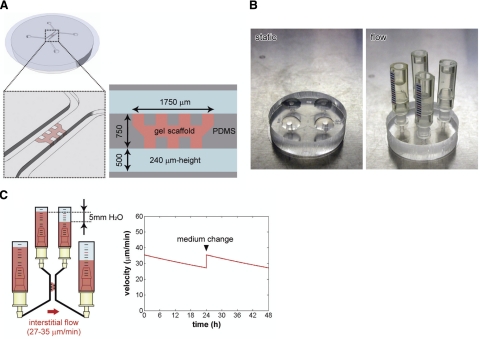
Figure 1.
The microfluidic coculture platform for the vascularization of tissue-engineered constructs. A) Schematic diagram and dimension of the microfluidic device made of PDMS. Two parallel microfluidic channels are formed between a micropatterned PDMS device and a coverslip. Gel scaffold (e.g., type I collagen) is located between the microfluidic channels with a mechanical support of PDMS posts. B) Pictures of the microfluidic device cultured under static and flow conditions. Droplets of culture medium are placed on each outlet of a microfluidic channel for static culture. Reservoirs are connected to microfluidic channels for flow culture. C) Interstitial flow across the gel scaffold was generated by a 5-mmH2O pressure difference between two microfluidic channels. The permeability of collagen gel with hepatocytes was determined by measuring displacement of the medium level in reservoirs and velocity was calculated based on the gel permeability and analytical solutions. The velocity decreases over time but is restored by changing the culture medium.
Soft lithography was used to produce the micropatterned PDMS device with SU-8 (MicroChem, Newton, MA, USA) patterned wafers (Supplemental Fig. 1A). Cured PDMS was removed from the wafer, trimmed, and punched to form inlets and outlets and then autoclaved. After plasma treatment (Harrick Plasma, Ithaca, NY, USA) in air to make the PDMS surface hydrophilic, prepolymerized collagen gel solution (2 mg/ml, pH 7.4; BD Biosciences, San Jose, CA, USA) was microinjected into the gel region, and the PDMS device was immediately bonded with a coverslip to form the microfluidic channels (Supplemental Fig. 1B). Next, the device was placed in the humidified 5% CO2 incubator at 37°C for 30 min for gelation, after which the microfluidic channels were filled with culture medium, and the device was left in the incubator until use.
Culture media
Hepatocytes were cultured in hepatocyte growth medium (HGM) (17) with the following modifications: 0.305 g/L niacinamide (Sigma-Aldrich, St. Louis, MO, USA); 2 g/L d-glucose; 1 mM l-glutamine; 54.4 μg/L ZnCl2; 75 μg/L ZnSO4 · 7H2O; 20 μg/L CuSO4 · 5H2O; 25 μg/L MnSO4; 20 ng/ml EGF; 0 ng/ml hepatocyte growth factor; 1 mM l-ascorbic acid 2-phosphate; and 90 μg/ml heparin. hMVECs were cultured in endothelial growth medium (EGM-2MV; Lonza, Walkerville, MD, USA). A 1:1 mixture of the modified HGM and EGM-2MV supplemented with 50 ng/ml vascular endothelial growth factor (VEGF), and 50 ng/ml basic fibroblast growth factor (bFGF) was used for hepatocyte-hMVEC coculture. rMVECs were cultured in MCDB-131 complete medium (VEC Technologies, Rensselaer, NY, USA), and a 1:1 mixture of the modified HGM and the MCDB-131 supplemented with 50 ng/ml VEGF and 50 ng/ml bFGF was used for hepatocyte-rMVEC coculture.
Hepatocyte culture
Hepatocytes were isolated from rats by a two-step collagenase perfusion (6). The number of viable cells was counted using the trypan blue-exclusion test, and the cell suspension was prepared at a density of 3 × 106 cells/ml. One of the two microfluidic channels was filled with this cell suspension, and the device was tipped on its side and maintained in the incubator for 30 min to allow cells to attach to the sidewall of the collagen gel scaffold. Returning the device to horizontal, cells were then cultured under either static or flow conditions. For static culture, a 70 μl droplet of the medium was placed on each channel outlet (Fig. 1B). For culture under flow, medium reservoirs were inserted into the channel outlets (Fig. 1B), and an interstitial flow across the gel scaffold was generated by a pressure difference of 5 mmH2O. Culture medium was changed daily (Fig. 1C). To calculate the gel scaffold permeability, the volume of medium passing through the gel was measured during day 1 of hepatocyte culture under forward flow. Using this volume and the cross-sectional area of the gel, average interstitial flow velocity was determined, from which hydraulic permeability was computed from Darcy’s law (for details, see Supplemental Data). Hepatocyte morphogenesis was quantified by measuring the area of hepatocytes in a 2D projection using ImageJ (http://rsb.info.nih.gov/ij/) (Supplemental Fig. 2A). More than 10 devices were cultured for each condition and experiments were repeated at least 3 times. Results were analyzed using a Student’s t test; differences were considered significant at P < 0.05.
MVEC culture
hMVECs were commercially obtained (human dermal microvascular endothelial cell, Lonza) and expanded for no more than 8 passages. The cell suspension was prepared at a density of 4 × 106 cells/ml and placed in one of the microfludic channels. The device was then held vertical in the incubator for 30 min to allow cell attachment to the sidewall of a gel scaffold, returned to horizontal, and cultured under static conditions. rMVECs were commercially obtained (rat lung microvessel endothelial cells, VEC Technologies) and expanded for no more than 10 population doublings. Filling followed the same procedure described for the hMVECs. Experiments were repeated at least 3 times, and representative results are shown.
Hepatocyte-MVEC coculture
Hepatocytes were first seeded in the device as described above. Interstitial flow was applied to promote attachment of the cells to the gel scaffold. On day 1, the flow direction was reversed to remove debris. This pattern of flow application was designed based on our previous study where hepatocytes were cultured in a microreactor and formed 3D tissue-like structures under flow conditions (6). MVECs were added to the opposite side of the gel scaffold on day 2. In the case of rMVECs, the channel was filled with medium containing 50 μg/ml fibronectin for 45 min at 37°C prior to seeding. For all coculture experiments, interstitial flow was stopped at the time of MVEC seeding, and the subsequent coculture was performed under static conditions to promote two-way heterotypic interactions between the hepatocytes and MVECs by diffusional transport across the gel scaffold. Migration of rMVECs was quantified by measuring the perimeter and area of rMVECs in a 2D projection (Supplemental Fig. 2B). Experiments were repeated at least 3 times; 22 devices were cultured for the hepatocyte-rMVEC coculture and 21 devices were cultured for the rMVEC monoculture.
Immunocytochemistry
Cells were fixed with 2% paraformaldehyde for 60 min at room temperature and permeabilized with 0.1% Triton X-100 for 45 min. Actin filaments and nuclei were then stained with rhodamine-phalloidin (Sigma-Aldrich)/Alexa Fluor 488-phalloidin (Invitrogen, Carlsbad, CA, USA) and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich)/propidium iodide (Invitrogen), respectively. hMVECs were stained with rabbit anti-von Willebrand factor antibody and Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen) after the cells were incubated with Block Ace (Dainippon Pharmaceutical, Osaka, Japan). The cells were rinsed with PBS, and a z-axis series of fluorescence images was obtained using a confocal laser-scanning microscope (Olympus, Tokyo, Japan). Z stacks of the confocal images were reconstructed in 3D using ImageJ.
Assessment of ethoexyresorufin O-dealkylase (EROD) activity
EROD activity, a cytochrome P450-dependent reaction in hepatocytes, was probed by a fluorescence method based on a previous study with some modifications (18). Ethoxyresorufin is converted to fluorescent resorufin by P450 activity. To evaluate EROD activity, P450 activity was induced in hepatocytes by incubating the cells with 2 μM 3-methylcholantlene (Sigma-Aldrich) for 24 h. The EROD reaction was initiated by the addition of 20 μM ethoxyresorufin (Sigma-Aldrich).
Visualization of bile canaliculi (BC) formed in the 3D hepatocyte structures
To investigate whether hepatocytes formed BC in 3D tissue-like structures in the microfluidic device, the cells were treated for 15 min with fluorescein diacetate (FD; Sigma-Aldrich) at a final concentration of 2.5 μg/ml. Thereafter, the cells were rinsed three times with medium and were photographed using a phase-contrast microscope equipped for fluorescence imaging (Nikon, Tokyo, Japan). BC can be detected as green fluorescent networks because FD is metabolized within hepatocytes and the fluorescent metabolites are excreted into the BC (5).
Diffusion analysis
For characterizing the transport of growth factors secreted by cells, we performed diffusion experiments using a 40-kDa Texas Red-conjugated dextran (Invitrogen) mixed with culture medium at a final concentration of 1 ng/ml. The solution was added to one side of the collagen gel scaffold, and using fluorescent microscopy, the concentration fields were imaged at different time points; and the intensity profiles were analyzed using MATLAB (Mathworks, Boston, MA, USA). Finite element simulations were also performed to analyze the diffusion across the gel scaffold using COMSOL (Burlington, MA, USA) (15). Fluorescence intensity was normalized to compare the experimental results with simulations.
RESULTS
Microfluidic platform and interstitial flow application
To create a model for liver angiogenesis, we used a microfluidic platform including two parallel microfluidic channels and a 3D collagen gel scaffold located between the channels (Fig. 1A). Cells were seeded into the microfluidic platform via a microfluidic channel and cultured under static or flow conditions. Cells were cultured under static conditions by placing droplets of culture medium on each port of the channels or under flow by connecting reservoirs to the channels (Fig. 1B). Interstitial flow across the gel scaffold was generated by a small pressure difference (5 mmH2O) between the two microfluidic channels (Fig. 1C). Interstitial flow velocity was 27–35 μm/min, calculated by combining analytical solutions and measurements of displacement of the medium levels during the initial 24 h culture after hepatocyte seeding (for details, see Supplemental Data). The velocity decreased over time but was restored by changing the culture medium (Fig. 1C).
3D culture of hepatocytes in the microfluidic platform
We first established conditions for 3D cultures of hepatocytes in our microfluidic system. Hepatocytes were seeded on the sidewall of a collagen gel scaffold via one of the microfluidic channels, and interstitial flow was applied to promote 3D structure formation. To investigate the effect of flow direction on hepatocyte morphogenesis, we applied interstitial flow in two ways: 1) forward (i.e., from the hepatocyte-containing channel into the gel scaffold) flow followed by reverse flow; and 2) reverse flow from the beginning.
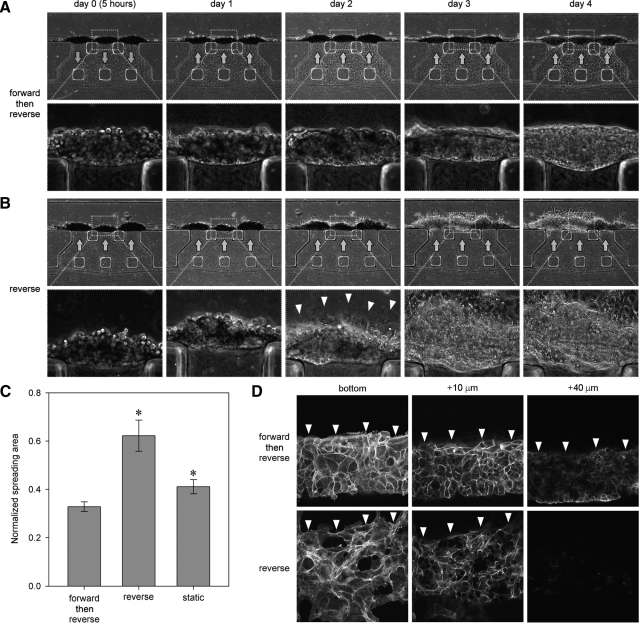
Hepatocytes adhered to the sidewall of the gel scaffold and gradually organized into 3D tissue-like structures when cultured under forward flow followed by reverse flow (Fig. 2A). In contrast, the cells tended to spread on the surface of the microfluidic channel when cultured under reverse flow from the beginning (Fig. 2B). Although cells behaved similarly until day 1 under both culture conditions, the cells under consistent reverse flow started to spread on the bottom surface of the microfluidic channel on day 2 (arrowheads, Fig. 2B), then gradually covered the entire bottom surface. This migration and spreading were predominantly observed under reverse flow. This difference in hepatocyte morphogenesis was confirmed by quantitative analysis (Fig. 2C). Forward flow during the initial seeding period produced a more uniform distribution of hepatocytes over the gel surface and inhibited 2D migration over the coverslip. Confocal laser-scanning microscopy confirmed that the hepatocytes formed thicker structures under forward flow than under reverse flow (Fig. 2D). Note that the 3D structures did not extend across the entire face of the gel region; instead, cells organized into compact clusters and tended to produce a thicker layer adjacent to the bottom surface of the channel.
Figure 2.
Effect of interstitial flow direction on the formation of 3D tissue-like structures by hepatocytes. A) Corresponding phase-contrast images of hepatocytes cultured in forward flow (arrows, day 0) followed by reverse flow (arrows, >day 1). Note that hepatocytes gradually organized into 3D tissue-like structures. B) Corresponding phase-contrast images of hepatocytes cultured in reverse flow. Cells started to spread on the microfluidic channel on day 2 (arrowheads, day 2). As cells migrated on the microfluidic channel, cell structures became thin. C) Quantification of hepatocyte morphogenesis on day 3. Area of hepatocytes spreading on the microfluidic channel was measured and normalized by the area of the microfluidic channel. Error bars = sem (n=10, N=3). *P < 0.05 vs. forward then reverse. D) Actin filaments were stained, and z-stack images were taken by a confocal laser-scanning microscope at the z-plane of bottom (near coverslip), 10 μm, and 40 μm elevations. Arrowheads indicate edge of hepatocyte tissue-like structures. Cells formed thicker structures in forward flow followed by reverse flow than those in reverse flow alone.
3D angiogenesis model created in the microfluidic platform
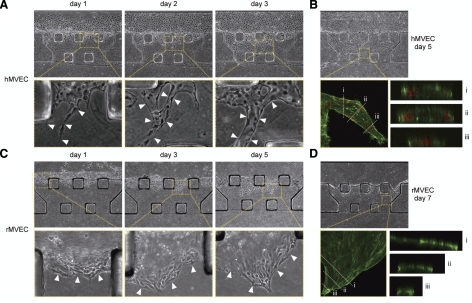
Next, we explored conditions that might facilitate 3D angiogenesis by either hMVECs or rMVECs. hMVECs were used for our culture system, since these cells have been used widely for in vitro angiogenesis models (10, 15, 16, 18). hMVECs were seeded on the sidewall of the collagen gel scaffold via the microfluidic channel and cultured under static conditions. They initially attached to the sidewall of the gel but also spread onto the walls of the microfluidic channel. Some cells formed vascular sprouts on day 1 (arrowheads, day 1, Fig. 3A). These spouts developed into capillary-like structures, which gradually extended across the gel scaffold (arrowheads, days 2 and 3, Fig. 3A). As the cells penetrated into the gel, they formed structures with identifiable lumens (Fig. 3B).
Figure 3.
3D angiogenesis model created in the microfluidic platform. A) Corresponding phase-contrast images of hMVECs cultured under static conditions. Cells penetrated into the collagen gel scaffold and formed vascular sprouts (arrowheads, day 1). Spouts extended and formed capillary-like structures (arrowheads, days 2 and 3). B) Corresponding phase-contrast and fluorescent images. hMVECs were fixed on day 5 and stained for actin filaments (green) and nuclei (red). Cross-section images showed that hMVECs formed capillary-like structures with lumens (i, ii), whereas the tip cells formed no lumen (iii). C) Corresponding phase-contrast images of rMVECs cultured under static conditions. Cells migrated into the gel scaffold as a sheet-like structure (arrowheads). D) Corresponding phase-contrast and fluorescent images. rMVECs were fixed on day 7 and stained for actin filaments (green). Cross-section images showed that the cells formed a sheet-like structure (i–iii).
rMVECs were also used for our culture system, which allowed us to create a coculture model of hepatocytes and MVECs derived from the same species. In contrast to the results with hMVECs, rMVECs failed to form capillary-like structures. Instead, they tended to migrate into the gel as a sheet, although the cells transiently formed sprout-like structures at the leading edge (Fig. 3C). This behavior was due to the tendency for cells to attach to the sidewall of the gel scaffold and migrate along the interface between the gel and the coverslip. Confocal images of the migrating cells confirmed the 2D sheet-like geometry of the formed structures (Fig. 3D).
3D hepatocyte-MVEC coculture in the microfluidic platform
Finally, the hepatocyte and 3D angiogenesis models were integrated and tested for their ability to form vascularized tissue-engineered constructs. Since the microfluidic platform offers considerable flexibility, several coculture procedures were tried with different spatiotemporal manipulation of hepatocytes, MVECs, and interstitial flow. Cells were seeded either on the sidewall of or inside the gel scaffold using different sequences of cell seeding, with and without interstitial flow (Supplemental Fig. 3). On the one hand, rMVECs/hMVECs formed capillary-like structures when embedded within a gel scaffold, the capillary-like structures were not extensive. On the other hand, hepatocytes tended to remain isolated when embedded within the gel scaffold, although some cells migrated and connected to each other to form clusters (data not shown). After trying various combinations, we found that seeding hepatocytes on the sidewall of the gel scaffold and MVECs on the other sidewall (Supplemental Fig. 3A) showed the greatest promise in terms of the morphogenesis of each cell type.
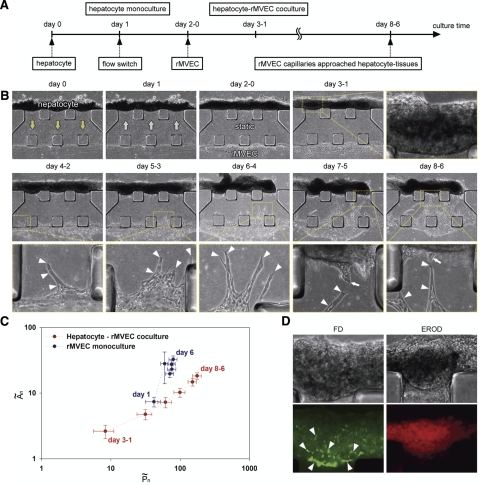
In hepatocyte-rMVEC coculture, cell seeding and culture conditions were dynamically changed to promote heterotypic cell interaction (Fig. 4A). First, hepatocytes were seeded on the gel scaffold sidewall and interstitial flow was applied as above to promote cell–cell adhesions (arrows, day 0, Fig. 4B). On day 1, the flow direction was reversed primarily to flush debris and nonadherent cells from the system (arrows, day 1, Fig. 4B). Coculture was started by seeding rMVECs on the other side of the gel scaffold on day 2 (day 2-0, Fig. 4B). Interstitial flow was stopped for the rMVEC seeding, and the cells were subsequently cultured under static conditions to promote mutual heterotypic interactions across the gel scaffold by diffusion.
Figure 4.
Hepatocyte-rMVEC coculture in the microfluidic platform. A) Experimental protocol for coculture. B) Corresponding phase-contrast images. Hepatocytes were seeded on the sidewall of a collagen gel scaffold, and interstitial flow was applied (arrows, day 0). Flow direction was reversed on day 1 (arrows, day 1). Interstitial flow was stopped and rMVECs were added to the other side of the gel scaffold on day 2 (day 2-0). Hepatocytes formed 3D tissue-like structures (day 3-1). Some rMVECs started to form vascular sprouts on day 4-2 (arrowheads, day 4-2). Vascular sprouts extended across the gel scaffold and approached the hepatocyte tissue-like structures (arrowheads). Some hepatocytes also migrated toward the capillary-like structures of rMVECs (arrows, days 7-5 and 8-6). C) Quantification of rMVEC morphogenesis. graph shows the relation between the normalized area increase Ãn and perimeter increase P̃n of the migrating rMVECs on day n. In coculture, the data represent larger P̃n and smaller Ãn than those of a control culture. Each plot represents the values on each day. “Day 3-1” represents day 3 of hepatocytes and day 1 of rMVECs. Error bars = sem (n=16, N=4 for hepatocyte-rMVEC coculture; n=15, N=3 for rMVEC culture). D) Corresponding phase-contrast and fluorescent images of hepatocyte tissue-like structures. Left panels: metabolite of FD secreted into BC (arrowheads) in hepatocytes on day 13-11 of coculture. Right panels: EROD activity of hepatocytes on day 10-8 of coculture.
Hepatocytes gradually organized into 3D tissue-like structures in coculture similar to those formed in monoculture (Figs. 2A and 4B). We investigated the differentiation of hepatocytes in the 3D tissue-like structures from days 8 to 13, because rMVECs formed capillary-like structures extending toward the hepatocyte tissues during this period. Hepatocytes in the 3D tissues formed BC, which are highly differentiated structures of hepatocytes and exhibited cytochrome P450 activity (Fig. 4D) in coculture. These results suggest that hepatocytes maintained their differentiated function in the microfluidic coculture system. rMVECs attached to the other sidewall of the gel scaffold and some cells penetrated and formed vascular sprouts within 2–3 days. These sprouts extended across the gel scaffold and approached the hepatocyte tissue-like structures (arrowheads, days 4-2–8-6, Fig. 4B). Note that rMVEC capillary morphogenesis was observed only in coculture (17/22 devices) since the cells formed 2D sheet-like structures in monoculture (21/21 devices). Quantitative analysis clearly delineated the different types of structures between coculture and monoculture (Fig. 4C). In coculture, the ratio of normalized area increase (Ãn) to normalized perimeter increase (P̃n) of migrating rMVECs was smaller than that in rMVEC monoculture due to the perimeter increase when the cells formed complex capillary-like structures. Furthermore, confocal laser-scanning microscopy revealed that some rMVECs formed capillary-like structures with continuous lumina (Fig. 5).
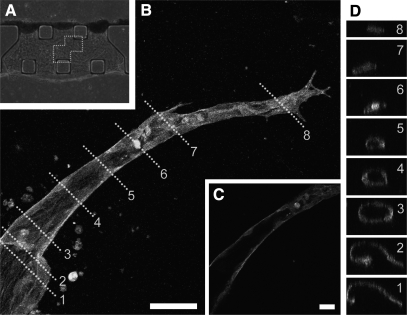
Figure 5.
Tube formation process of rMVECs in hepatocyte-rMVEC coculture. A) Phase-contrast image of rMVECs in coculture. B) A z-projection image of a capillary-like structure stained with rhodamine-phalloidin. Cells were cultured under static conditions and fixed on day 12-10 of coculture. Image field corresponds to dotted frame in A. C) Cross-sectional image of capillary-like structures along the direction of the structure. Note that rMVECs formed a luminal structure, although no lumen was found at the tip region. D) Perpendicular cross-sectional images of capillary-like structures in B. Numbers correspond to dotted lines in B. Scale bars = 50 μm (B); 20 μm (C).
In direct contrast to the behavior observed with rMVECs, capillary formation of hMVECs was inhibited in the hepatocyte-hMVEC coculture compared to that in hMVEC monoculture (Supplemental Fig. 4).
Diffusion analysis
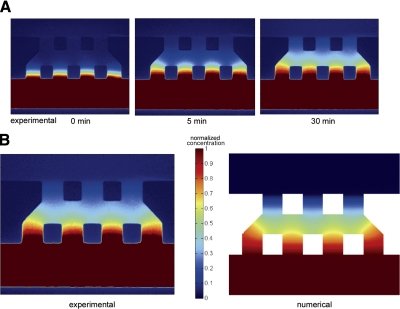
To demonstrate diffusive transport across the gel, we injected fluorescent dextran (MW 40kD) into one channel and imaged the evolution of the concentration profiles across the gel using fluorescent microscopy (Fig. 6). We also simulated soluble factor transport numerically and, by fitting these predictions to the experimental data, calculated the diffusion coefficient of dextran in the collagen gel to be 6.6 × 10−11 m2/s.
Figure 6.
Diffusion analysis in a gel scaffold. A) Distribution of 40-kDa fluorescent dextran across the gel scaffold in the microfluidic platform at 0, 5, and 30 min. B) Experimental results in steady state (left) and corresponding numerical simulation (right). Intensity was normalized to maximum value to compare experimental results with simulations. Colorimetric images are shown for normalized concentration.
DISCUSSION
Interstitial flow promotes the formation of 3D tissue-like structures of hepatocytes
Conditions for forming viable, functional, 3D cultures of hepatocytes in our microfluidic system were established through a series of experiments in which interstitial flow could be controlled. The computed velocity of the initial forward flow was 27–35 μm/min, comparable to physiological values previously reported (∼36 μm/min) (19). Hepatocytes formed 3D tissue-like structures when cultured under forward flow followed by reverse flow, whereas they started to spread on the surface of the microfluidic channel on day 2 when cultured under consistent reverse flow. These results demonstrate that the initial forward flow regulates hepatocyte migration after day 2 and suggest that cells cultured under forward flow followed by reverse flow preferentially form 3D structures. Forward flow appears to help the cells attach to the sidewall of the gel scaffold as well as to each other, potentially due to a different mechanical balance between cell–substratum adhesion and cell–cell cohesion. The adhesion-to-cohesion ratio plays an important role in cell organization (20). The initial forward flow application might enhance cell–cell cohesion, causing it to dominate over cell–substratum adhesion. Consequently, the cells maintained 3D structures even after the flow direction was switched from forward to reverse, although these structures were thicker adjacent to the bottom surface of the channel likely due to the effects of gravity. In contrast, cells spread on the surface of the microfluidic channel and formed 2D structures when cultured without the initial forward flow application. This could be a consequence of cell–substratum adhesion dominating over cell–cell cohesion. These results demonstrate that interstitial flow application can be used to promote cell organization into 3D structures via the enhancement of cell–cell cohesion.
Capillary morphogenesis in the 3D hepatocyte-MVEC coculture
In the present experiments we created a microfluidic coculture model by integrating a 3D hepatocyte culture and an in vitro angiogenesis model. The morphogenesis of hepatocytes was induced by the application of interstitial flow, and MVECs were then added to start coculture. This coculture model recapitulates the process that occurs during liver regeneration where hepatocytes form clusters, which are subsequently penetrated by capillaries (21).
rMVECs formed 3D capillary-like structures that extended across an intervening gel to the 3D hepatocyte tissues in hepatocyte-rMVEC coculture, whereas rMVECs formed 2D sheet-like structures without hepatocytes. These results suggest that heterotypic interactions promote capillary morphogenesis of rMVECs in coculture. In our coculture system, hepatocytes appeared to secrete soluble factors to promote capillary morphogenesis of rMVECs. Hepatocytes produce a wide array of angiogenic factors such as VEGF, transforming growth factor α (TGFα), acidic FGF (aFGF), bFGF, EGF, and angiopoietin 1 during liver regeneration (22). Since the culture medium already contained VEGF, bFGF, and EGF, possible soluble factors that promoted the capillary morphogenesis may be TGFα, aFGF, and angiopoietin 1. Hepatocytes secrete not only angiogenic growth factors but also ECM proteins such as fibronectin (21), which can be transported by diffusion and convection across a collagen gel scaffold in our microfluidic platform. Modification of collagen gel by the ECM proteins may also affect rMVEC morphogenesis. Thus, the angiogenic growth factors and ECM proteins secreted by hepatocytes may play an important role in promoting capillary morphogenesis of rMVECs in coculture. Heterotypic interactions between parenchymal cells and nonparenchymal neighbors have also been investigated in previous studies. By controlling temporal cell–cell interactions in a 2D microdevice, it was found that the interaction was essential for maintaining hepatocyte differentiation (23, 24).
Capillary-like structures formed by rMVECs extended toward the hepatocyte tissue-like structures. When the rMVECs neared the hepatocyte layer, some hepatocytes migrated toward the capillary-like structures (arrows, days 7-5 and 8-6, Fig. 4B). Similar hepatocyte migration toward MVECs was also observed in a coculture system composed of hepatocytes and 2D networks of MVECs on Matrigel where hepatocytes were attracted by hepatocyte growth factor secreted by MVECs (18). In some devices, the extending capillary-like structures reached and contacted the hepatocytes. When the capillary-like structures became attached to the hepatocytes, the tip cells either remained attached (Supplemental Fig. 5A) or they veered away (Supplemental Fig. 5B). Despite these interactions rMVEC capillary-like structures failed to penetrate hepatocyte tissue-like structures, nor did we observe a robust outgrowth of hepatic parenchymal or nonparenchymal cells (stellate cells or macrophages) toward the MVECs. In vivo, angiogenesis in avascular, matrix-poor hepatocyte islands following partial hepatectomy is initiated by hepatic stellate cells, which extend projections in between hepatocytes and secrete laminin, providing a beachhead for subsequent invasion by sinusoidal endothelial cells (14, 21). These observations, therefore, suggest that stellate cells, present only in minute fractions in the present experiments, may be essential in promoting endothelial integration into hepatocyte structure.
In our coculture system rMVECs and hMVECs exhibited different behavior. In particular, hMVECs fail to form capillary-like structures in coculture whereas they form 3D capillary-like structures without hepatocytes. Since hepatocytes were isolated from rats, it is possible that hMVECs fail to sense some key signals from rat hepatocytes due to the different species. Further investigation will be needed to elucidate the detailed mechanism of the heterotypic interaction. When rMVECs/hMVECs were added to the other side of the hepatocytes, the cells attached and spread on the hepatocyte-free portion of the gel scaffold surface (#2, Supplemental Fig. 6). However, fewer MVECs attached to the gel that was exposed to hepatocytes (#1, Supplemental Fig. 6). This pattern of behavior suggests that soluble factors produced by hepatocytes were convected by the initial interstitial flow, accumulated within the gel scaffold regions where flow occurred, and exerted a long-term influence on attachment of rMVECs/hMVECs and capillary formation.
Interstitial flow was found to promote 3D tissue-like structure formation by hepatocytes during the first day following cell seeding but appeared not to be necessary afterward. While the effect of forward flow on the hepatocytes can be explained by the effect of flow to facilitate cell–cell cohesion, the reason for the prolonged effect on MVEC sprouting and capillary formation is less obvious. Two factors, however, can help to explain this. First, as discussed above, factors secreted by the hepatocytes and convected through the gel during this initial phase apparently adhere to the collagen matrix and have lasting effects. Second, even in the absence of flow, transport can occur by diffusion. The results of diffusion analysis suggest that secreted proteins from the hepatocytes and MVECs can be exchanged across the gel scaffold, over a distance of 750 μm, by diffusive transport within tens of minutes.
Exchange of soluble factors between different cell types by either diffusive or convective transport is essential to the development of a complex tissue such as liver. Local delivery of soluble factors is one of the advantages of using a microfluidic device since cells cultured in a microfluidic channel are in an environment with small dimensions and a high surface area-to-volume ratio compared to macroscale culture conditions (25,26,27). Moreover, conditions can be changed over time by the sequenced delivery of exogenous factors via the medium channels. Secreted molecules from the cells accumulate in their immediate vicinity and facilitate both feedback regulation and paracrine signaling. Localization of soluble factors was shown to be important by Helm et al. (28); directional biasing of the VEGF distribution around endothelia in fibrin gel by interstitial flow significantly promoted capillary morphogenesis.
The microfluidic coculture system reported here was created using rat hepatocytes and rMVECs. This coculture system may also be applied, however, to human hepatocytes and hMVECs in a similar fashion, which would have obvious advantages as an in vitro coculture model for human liver regeneration. Furthermore, the microfluidic platform can also be applied to the other epithelial cell types since the formation of 3D structures is controlled by a mechanical effect on the balance between cell–cell cohesion and cell–substrate adhesion. In the longer term, the experimental approach described here is useful more generally for investigating microvascular networks within 3D engineered tissues with multiple cell types in vitro. This may ultimately lead to successful clinical use of tissue-engineered constructs in organ replacement as well as a physiological experimental model spanning from 2D culture models to experimental animal models.
Supplementary Material
Acknowledgments
We thank Jose Antonio Sanz-Hererrea for analyzing the diffusion experiments; Chen-rei Wan and Nathan Hammond for critical reading of the manuscript; and Laura Vineyard, Romie Littrell, and Ta-Chun Hang for technical assistance. We also appreciate helpful discussions with Carlos Semino at the Massachusetts Institute of Technology (MIT) and Donna Stolz at the University of Pittsburgh. This research is supported by the National Institute of Biomedical Imaging and Bioengineering (EB003805), Draper Laboratories Inc. (UR&D project DL-H-550151), Singapore-MIT Alliance for Research and Technology, and Japan Society for the Promotion of Science postdoctoral fellowships for research abroad (R.S.).
References
- Griffith L G, Swartz M A. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Yamada K M, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Nerem R M. Tissue engineering: the hope, the hype, and the future. Tissue Eng. 2006;12:1143–1150. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- Griffith L G, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- Sudo R, Mitaka T, Ikeda M, Tanishita K. Reconstruction of 3D stacked-up structures by rat small hepatocytes on microporous membranes. FASEB J. 2005;19:1695–1717. doi: 10.1096/fj.04-3269fje. [DOI] [PubMed] [Google Scholar]
- Hwa A J, Fry R C, Sivaraman A, So P T, Samson L D, Stolz D B, Griffith L G. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564–2579. doi: 10.1096/fj.06-7473com. [DOI] [PubMed] [Google Scholar]
- Toh Y C, Zhang C, Zhang J, Khong Y M, Chang S, Samper V D, van Noort D, Hutmacher D W, Yu H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- Nahmias Y, Berthiaume F, Yarmush M L. Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol. 2007;103:309–329. doi: 10.1007/10_029. [DOI] [PubMed] [Google Scholar]
- Davis G E, Bayless K J, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252–275. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- Vailhé B, Vittet D, Feige J J. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81:439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- Yamamura N, Sudo R, Ikeda M, Tanishita K. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007;13:1443–14453. doi: 10.1089/ten.2006.0333. [DOI] [PubMed] [Google Scholar]
- Jain R K, Au P, Tam J, Duda D G, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Rouwkema J, Macdonald M, Garfein E S, Kohane D S, Darland D C, Marini R, van Blitterswijk C A, Mulligan R C, D'Amore P A, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Ross M A, Sander C M, Kleeb T B, Watkins S C, Stolz D B. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135–1148. doi: 10.1053/jhep.2001.29624. [DOI] [PubMed] [Google Scholar]
- Vickerman V, Blundo J, Chung S, Kamm R D. Design, fabrication and implementation of a novel multi parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8:1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Sudo R, Mack P J, Wan C, Vickerman V, Kamm R D. A new microfluidic platform to study cell migration. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- Block G D, Locker J, Bowen W C, Petersen B E, Katyal S, Strom S C, Riley T, Howard T A, Michalopoulos G K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias Y, Schwartz R E, Hu W S, Verfaillie C M, Odde D J. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. 2006;12:1627–1638. doi: 10.1089/ten.2006.12.1627. [DOI] [PubMed] [Google Scholar]
- Chary S R, Jain R K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D A, Griffith L G. Who’s got pull around here? Cell organization in development and tissue engineering. Proc Natl Acad Sci U S A. 2001;98:4282–4284. doi: 10.1073/pnas.081083698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta P S. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Bhatia S N, Balis U J, Yarmush M L, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Hui E E, Bhatia S N. Micromechanical control of cell–cell interactions. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G M, Zeringue H C, Beebe D J. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4:91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti J P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Toh Y C, Voldman J, Yu H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip. 2007;7:681–694. doi: 10.1039/b704602b. [DOI] [PubMed] [Google Scholar]
- Helm C L, Fleury M E, Zisch A H, Boschetti F, Swartz M A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci U S A. 2005;102:15779–15784. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.