Abstract
We undertook this study to estimate the event-free survival (EFS) of patients with newly diagnosed supratentorial primitive neuroectodermal tumor (SPNET) treated with risk-adapted craniospinal irradiation (CSI) with additional radiation to the primary tumor site and subsequent high-dose chemotherapy supported by stem cell rescue. Between 1996 and 2003, 16 patients with SPNET were enrolled. High-risk (HR) disease was differentiated from average-risk (AR) disease by the presence of residual tumor (M0 and tumor size > 1.5 cm2) or disseminated disease in the neuraxis (M1–M3). Patients received risk-adapted CSI: those with AR disease received 23.4 Gy; those with HR disease, 36–39.6 Gy. The tumor bed received a total of 55.8 Gy. Subsequently, all patients received four cycles of high-dose cyclophosphamide, cisplatin, and vincristine with stem cell support. The median age at diagnosis was 7.9 years; eight patients were female. Seven patients had pineal PNET. Twelve patients are alive at a median follow-up of 5.4 years. The 5-year EFS and overall survival (OS) estimates for all patients were 68% ± 14% and 73% ± 13%. The 5-year EFS and OS estimates were 75% ± 17% and 88% ± 13%, respectively, for the eight patients with AR disease and 60% ± 19% and 58% ± 19%, respectively, for the eight with HR disease. No deaths were due to toxicity. High-dose cyclophosphamide-based chemotherapy with stem cell support after risk-adapted CSI results in excellent EFS estimates for patients with newly diagnosed AR SPNET. Further, this chemotherapy allows for a reduction in the dose of CSI used to treat AR SPNET without compromising EFS.
Keywords: autologous stem cell rescue, craniospinal radiotherapy, dose-intensive chemotherapy, event-free survival, risk-adapted therapy, supratentorial PNET
Supratentorial primitive neuroectodermal tumors (SPNETs) in children are rare and represent less than 2.5% of childhood brain tumors.1,2 Histologically, SPNETs are very similar to medulloblastomas that are called primitive neuroectodermal tumors (PNETs) of the cerebellum.3 However, SPNETs are thought to be less curable than medulloblastoma.4,5 Therefore, higher doses of radiation therapy are used routinely to treat SPNET, regardless of the stage of the disease. Emerging evidence that SPNETs and medulloblastomas contain distinct sets of molecular alterations suggests that biological differences between these two tumor types might account for their differential responses to therapy.6–9 Nevertheless, SPNETs are responsive to conventional treatments. For example, high-dose chemotherapy with autologous bone marrow or stem cell rescue has produced durable responses and prolonged event-free survival (EFS) in young patients with SPNET who did not receive craniospinal irradiation (CSI) and in patients with recurrent SPNET.10–12
Here we report the results of a pilot study that evaluated reduced-dose CSI (23.4 Gy) followed by high-dose chemotherapy supported by stem cell rescue in the treatment of newly diagnosed patients with SPNET who were ≥ 3 years of age and were classified as having average-risk (AR) disease. We also studied the effectiveness of this chemotherapy in association with 36–39.6 Gy CSI to treat patients ≥ 3 years of age with high-risk (HR) SPNET.
Patients and Methods
Patients
Between October 1996 and August 2003, 16 patients with newly diagnosed SPNET received treatment at one of the following participating institutions: Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA (n = 6); Royal Children’s Hospital, Melbourne, Australia (n = 2); Royal Children’s Hospital, Brisbane, Australia (n = 2); and St. Jude Children’s Research Hospital, Memphis, Tennessee, USA (n = 6). All patients who were eligible were entered on the study sequentially at the respective centers. There was no selection bias.
Patients ≥ 3 and ≤ 21 years of age at the time of diagnosis who had not previously received chemotherapy or radiation therapy were eligible for enrollment on the protocol. Previous corticosteroid therapy was not a criterion for exclusion. For most patients, treatment began within 28 days of definitive surgery. The extent of surgical resection was defined as gross total resection (GTR) if there was no evidence of residual disease after resection, near-total resection (NTR) if postoperative MRI revealed residual disease ≤ 1.5 cm2, and subtotal resection (STR) if 25% or more of the original tumor remained as residual disease.
Eligibility criteria based on organ function were described previously.13,14 The protocol was approved prior to patient enrollment and then annually by the institutional review board of each participating institution, and informed consent and assent for treatment were obtained from all patients, parents, or legal guardians as appropriate. This study is registered with ClinicalTrials. gov (no. NCT00003211).
AR disease was defined as (1) the absence of meta-static disease confirmed by gadolinium-enhanced MRI of the head and spine, cytological analysis of lumbar cerebrospinal fluid (CSF) at least 10 days after surgical resection and bone scintigraphy (M0); (2) GTR or residual disease ≤ 1.5 cm2 as recorded in the operative notes and confirmed by gadolinium-enhanced MRI of the head performed no more than 48 h after resection. HR disease was defined as (1) the presence of metastatic disease in the brain (M2) or spine (M3) or the existence of malignant cells in the lumbar spinal fluid (M1) or (2) the presence of residual disease >1.5 cm2. We excluded patients with bony metastasis or extraneural metastasis from this study since the study protocol involved stem cell or bone marrow rescue.
Treatment Regimen
After resection of the primary tumor, patients with HR disease underwent a 6-week phase II window of topotecan therapy, after which the tumor’s response was evaluated by imaging studies.15 Patients who declined the topotecan therapy option or were enrolled after the accrual target for this therapy received radiation therapy following surgery. Patients with HR disease underwent CSI (36 Gy for M0 with residual tumor >1.5 cm2 and for M1; 36–39.6 Gy for M2; and 39.6 Gy for M3) with three-dimensional conformal boost to the tumor bed (total dose, 55.8 Gy) and, where appropriate, to local sites of metastasis (total dose, 50.4 Gy). Patients with AR disease received 23.4 Gy CSI and 55.8 Gy to the primary tumor bed. The median duration of radiation therapy was 1.4 months. After a 6-week rest, patients began four cycles of nonmyeloablative high-dose chemotherapy, each followed by autologous stem cell or bone marrow rescue13,14 (Table 1). The planned total duration of chemotherapy was 16 weeks.
Table 1.
Schedule for each cycle of chemotherapy
| Day | Chemotherapy |
|---|---|
| − 4 | Cisplatin 75 mg/m2 i.v.; vincristine 1.5 mg/m2 (maximum dose, 2 mg) i.v. |
| − 3 | Cyclophosphamide 2 g/m2 i.v.; mesna given by continuous infusion |
| − 2 | Cyclophosphamide 2 g/m2 i.v.; mesna given by continuous infusion |
| − 1 | Postchemotherapy hydration |
| 0 | Infusion of PBSC or bone marrow cells |
| +1 | G-CSF 5 μg/kg daily s.c. or i.v. until ANC ≥ 2,000 on 2 consecutive days after the expected ANC nadir |
| +6 | Vincristine 1.5 mg/m2 (maximum dose, 2 mg) i.v. |
Abbreviations: PBSC, peripheral blood stem cells; G-CSF, granulocyte colony–stimulating factor; ANC, absolute neutrophil count.
Patient Monitoring and Follow-up
During protocol-directed therapy, disease status and types of toxicity that the patients experienced were monitored. Version 2 of the NCI’s Common Toxicity Criteria (http://ctep.cancer.gov/reporting/ctc.html) was used to grade toxicity.16 Disease status was assessed by MRI of the head and spine and CSF cytology. Definitions of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) have been described previously.14
Evaluation of cognitive performance was completed with the age-appropriate Wechsler Intelligence scale17 administered in an abbreviated format at multiple time points. The abbreviated form administers three subtests from the standard administration (Information, Similarities, Block Design) and, utilizing a formula derived from the standard administration with persons from the general population, results in estimated full-scale IQ (EIQ).18 Patients who were ≥ 5 years of age at time of testing also completed a test of academic achievement to give measures of reading, mathematical reasoning, and spelling (n = 12).19 All quotients were adjusted for age using general population norms with a mean of 100 and a standard deviation of 15, and scores were expected to be maintained over time (slope of zero). Parent measures of child behavior were also collected by using the Child Behavior Checklist.20
Statistical Considerations
EFS was measured from the date of study enrollment to the date of occurrence of the first evidence of recurrent disease, second malignancy, or death from any cause after the start of radiation therapy. Overall survival (OS) was measured from the date of study enrollment to the date of death from any cause or last follow-up. EFS and OS were estimated using Kaplan-Meier methods.21 Standard error was calculated according to the method of Peto et al.22,23
Linear random coefficients models were used to estimate the mean change in intellect, academic achievement, and social competency per year.24 Each patient’s score and the time at which that score was obtained were used to create a regression line. The intercept of the line provided an estimate of the score at diagnosis, and the slope of the line indicated the rate of change. The intercept and slope of an individual patient’s regression line were considered random effects and were used to estimate the average change of the scores over time of the population. Computer implementation was via the MIXED procedure in SAS (version 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Sixteen children and adolescents 3.8–12.9 years of age (median, 7.9 years) with SPNET were treated on the protocol. Thirteen patients were Caucasian; the remaining three patients comprised one Hispanic, one African American, and one “other.” Eight patients were male. Seven patients had pineoblastoma. Metastatic staging showed that 11 patients had M0 disease; 1, M2; and 4, M3. GTR was achieved in six patients; two patients had STR, and one underwent NTR. Seven patients underwent biopsy only. Patients 1 and 2 (Table 2) had pineoblastoma and after biopsies were left with residual disease <1.5 cm2, so they were classified as having AR disease. Eight patients each were classified as having AR and HR disease (Table 2).
Table 2.
Clinical characteristics of patients with SPNET
| Patient No. | Age (Years) | Diagnosis | M Stage | Surgery | No. Cycles Topotecan (Response) | CSI Dose | Disease Status after RT | HD Chemotherapy (No. Cycles) | EFS (Years) | Failure Location | EIQ (Years from Diagnosis) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average-risk patients | |||||||||||
| 1 | 7.8 | Pineoblastoma | 0 | BX | — | 23.4 | SD | 4 | 4.3 | — | 122 (2.8) |
| 2 | 11.7 | Pineoblastoma | 0 | BX | — | 23.4 | NED | 2 | 0.41 | CSF | — |
| 3 | 3.6 | PNET | 0 | GT | — | 23.4 | NED | 4 | 7.0 | — | — |
| 4 | 4.0 | PNET | 0 | NT | — | 23.4 | SD | 4 | 7.8 | — | 80 (3.6) |
| 5 | 4.9 | PNET | 0 | GT | — | 23.4 | NED | 4 | 5.4 | — | 88 (3.5) |
| 6 | 7.0 | PNET | 0 | GT | — | 23.4 | NED | 4 | 1.2 | L | — |
| 7 | 7.3 | PNET | 0 | GT | — | 23.4 | NED | 4 | 5.3 | — | 102 (2.7) |
| 8 | 8.8 | PNET | 0 | GT | — | 23.4 | NED | 4 | 3.9 | — | — |
| High-risk patients | |||||||||||
| 9 | 6.0 | Pineoblastoma | 3 | GT | 2 (SD) | 39.6 | SD | 0 | 4.0 | — | — |
| 10 | 9.6 | Pineoblastoma | 0 | ST | 2 (PR) | 36.0 | SD | 4 | 3.1 | D | 88 (2.6) |
| 11 | 10.7 | Pineoblastoma | 3 | BX | 0 | 39.6 | PR | 4 | 4.7 | — | — |
| 12 | 4.0 | PNET | 3 | ST | 2 (PD) | 39.6 | PD | 0 | 0.36 | L + D | — |
| 13 | 8.0 | PNET | 3 | BX | 0 | 39.6 | PR | 1 | 6.3 | — | — |
| 14 | 8.1 | Pineoblastoma | 0 | BX | 0 | 36.0 | SD | 4 | 3.7 | — | — |
| 15 | 10.1 | Pineoblastoma | 0 | BX | 0 | 36.0 | PR | 4 | 6.1 | — | — |
| 16 | 12.9 | PNET | 2 | BX | 0 | 36.0 | PR | 3 | 5.3 | — | 88. (0.2) |
Abbreviations: CSI, craniospinal irradiation; RT, radiotherapy; HD, high dose; EFS, event-free survival; EIQ, estimated IQ; BX, biopsy; SD, stable disease; NED, no evidence of disease; CSF, cerebrospinal fluid; PNET, primitive neuroectodermal tumor; GT, gross total; NT, near total; L, local; ST, subtotal; PR, partial response; D, distant; PD, progressive disease.
Survival
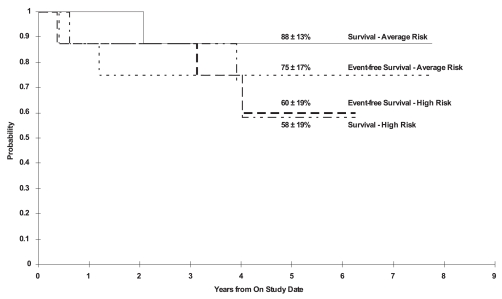
Twelve of the 16 patients were alive at a median follow-up of 5.4 years (range, 3.7–7.8 years). The 5-year EFS and OS estimates for all patients were 68% ± 14% and 73% ± 13%, respectively. The 5-year EFS and OS estimates were 75% ± 17% and 88% ± 13%, respectively, for patients with AR disease and 60% ± 19% and 58% ± 19%, respectively, for patients with HR disease (Fig. 1). The 5-year EFS for the pineal and nonpineal tumors were 54% ± 26% and 78% ± 14%, respectively, and the 5-year OS were 67% ± 22% and 78% ± 14%, respectively.
Fig. 1.
Five-year overall and event-free survival for the high-risk patients (n = 8) and average-risk patients (n = 8).
Treatment
Three of the eight HR patients (38%) received the phase II window therapy with topotecan; one had PR, one SD, and one PD. Topotecan was well tolerated with no unexpected toxicities.
The eight patients with AR disease received 23.4 Gy CSI postoperatively. Three patients with M0 disease but with >1.5 cm2 residual tumor received 36 Gy CSI. CSI doses of 39.6 Gy were given to one patient with M2 disease and four patients with M3 disease. All patients received 55.8 Gy to the tumor bed, including a 2-cm margin. Two patients with pineoblastoma received an additional 8 Gy and 10 Gy boost to the tumor bed delivered by Gamma Knife at the end of planned radiation therapy. Radiation therapy was well tolerated, with no unexpected toxicities. Evaluation of disease status prior to starting chemotherapy revealed no evidence of disease (n = 6), PR (n = 4), SD (n = 5), and PD (n = 1). One patient whose parents refused further treatment and one patient who developed PD following radiation did not receive any protocol-directed chemotherapy. This latter patient did receive another chemotherapy regimen.
Hence, 6 weeks after the completion of radiation therapy, 14 patients received four cycles of high-dose chemotherapy with stem cell rescue. Eleven patients received all four courses of chemotherapy. Three patients received either one, two, or three courses of chemotherapy because of renal toxicity, PD, or hemorrhagic cystitis, respectively. The median duration of chemotherapy was 19 weeks (range, 16–21 weeks) for the 11 patients who completed all planned courses of chemotherapy.
Of the 10 patients who had had evaluable disease after completion of radiation therapy, 5 patients had no evidence of disease at the end of chemotherapy, 1 patient had PR, 1 patient had SD, 1 patient had SD after completion of one course of chemotherapy and had to come off therapy due to toxicity, and 2 patients did not get chemotherapy.
High-Dose Chemotherapy-Related Toxicities
The toxicities that necessitated alteration of the planned chemotherapy are mentioned in the preceding section. There were no protocol-related deaths. There were 45 episodes of expected bone marrow suppression after 50 courses of high-dose chemotherapy, and six episodes of infection during chemotherapy were documented in four patients. The median times to recover absolute neutrophil counts to >500/mm3 and platelets to >20,000/mm3 were 13 and 17 days, respectively. To date, there have been no secondary malignancies in study patients.
Pattern of Failure
Disease recurrence or progression occurred in two AR patients: one local and one in the CSF. Two patients with HR disease had recurrent disease: one with local and spinal cord recurrence and one with distant recurrence in the brain. The median time to relapse (since enrollment) was 6 months for these four patients; relapse occurred after completion of radiation therapy and before initiation of chemotherapy (n = 1), after two courses of chemotherapy (n = 1), and 7 and 29 months after completion of high-dose chemotherapy (n = 2).
Intellect, Academic Achievement, and Competency
Twenty-four measures of EIQ were obtained for 10 patients using the abbreviated format of the Wechsler Scales. Four of these patients completed only single exams. Performance varied among patients, ranging from a gain of 12 EIQ points over 2.8 years from diagnosis to a loss of 22 EIQ points over 2 years from diagnosis. The mean estimated change in performance over time for the group was +1.2 EIQ points per year. Patients who were ≥ 5 years of age at time of testing (n = 12) also completed measures of reading, spelling, and mathematical reasoning (n = 24 observations). Six patients completed single exams. As a group, change in reading performance from the time of diagnosis was estimated to be a decline of 3.4 points per year. Spelling was estimated to decline 2.5 points per year, while math performance was estimated to decline 3.2 points per year. Unfortunately, the impacts of age at diagnosis, radiation dose, and tumor type were not possible to assess because of insufficient observations.
Parent ratings of child behavior (n = 15 observations) were also collected for seven patients using the Child Behavior Checklist, providing measures of social and school competency.19 While both competency measures were estimated to be in the average range upon diagnosis, over time, social competency was estimated to increase 1.4 points per year, while school competency was estimated to decline 2.4 points per year.
Discussion
This pilot study demonstrates that children with non-metastatic SPNET who undergo GTR of the tumor can be treated effectively with reduced dose CSI (23.4 Gy) and high-dose chemotherapy with stem cell rescue. In view of the relatively small number of patients in this study, we can at least state that even with a reduced dose of CSI, EFS estimates among children older than 3 years with SPNET are comparable with previous studies (Table 3). The survival estimates in this study that include 5-year EFS estimates of 75% ± 17% and 60% ± 19% for AR and HR disease, respectively, suggest that a reduced dose of CSI along with four courses of high-dose chemotherapy as given in this study may not compromise the outcome in children with localized disease who are considered to be at average risk for recurrence.4,5,25–31
Table 3.
Comparison of radiation dose, type of SPNET, and outcome in recent studies
| Study | No. Patients | Radiation Dose (CSI) | Survival Estimates | Pineal/Nonpineal |
|---|---|---|---|---|
| Reddy et al.26 | 22 | 34–40 Gy | 5-Year PFS, 37% | 13/9 |
| Cohen et al.4, 25 | 44 | 36–40 Gy | 3-Year PFS, 45% | 17/27a |
| Albright et al.25 | 27 | 36 Gy if age > 3 years (n = 18) | ||
| 23.4 Gy if age = 1.5–3 years (n = 9) | 5-Year PFS, 31% | 0/27 | ||
| Timmermann et al.28 | 63 | 35.2 Gy | 3-Year PFS, 39% | 11/52b |
| Jakacki et al.5 | 17 | 36 Gy if age > 3 years (n = 15) | ||
| 23.4 Gy if age = 1.53 years (n = 2) | 3-Year PFS, 61% | 17/0 | ||
| Mason et al.11 | 14 | Chemotherapy 10/14 consolidation with ABMR; 7/14 <3 years of age | 2-Year EFS, 43% | 3/11 |
| Pizer et al.27 | 68 | 35 Gy | 5-Year EFS, 47% | 14/54 |
| Gururangan et al.31 | 12 | 36 Gyc | 4-Year PFS, 69% | 12/0 |
| Massimino et al.29 | 15 | 31.2 Gy if age < 10 years | 3-Year EFS, 34% | 3/12d |
| 39 Gy if age ≥ 10 years (n = 6) | 3-Year PFS, 54% | |||
| Present study | 16 | 23.4 Gy if average-risk disease (n = 8) | 5-Year EFS, 75% | 2/6e |
| 36–39.6 Gy if high-risk disease (n = 8) | 5-Year EFS, 60% | 5/3e |
Abbreviations: CSI, craniospinal irradiation; PFS, progression-free survival; ABMR, autologous bone marrow rescue; EFS, event-free survival.
PFS estimate for those with pineal tumor
Disease in 46 patients with classified as M0.
Two patients younger than 2 years did not receive radiation therapy.
Ten patients received high-dose thiotepa and stem cell rescue after the completion of CSI (hyperfractionated). Second tumors developed in two patients. All patients received high-dose chemotherapy prior to radiation.
Five-year EFS for all 16 patients is 63%.
Children older than 3 years at diagnosis with pineal-region tumors are believed to have a better prognosis than do patients with SPNETs in other locations.4,5 Seven of the 16 patients in our study had tumors in the pineal region. Only two of these were classified as AR disease. Thus, the excellent EFS and OS estimates among patients with AR disease in this study cannot be attributed to an overrepresentation of pineal-region tumors.
Another factor in our study design that may have contributed to the apparent improvement in outcome of children with SPNET is the use of high-dose chemotherapy. Previous studies have shown that SPNETs are chemosensitive. For example, in the HIT 88/89 pilot trial, in which chemotherapy was administered before radiation therapy, the response rate (CR and PR) for patients with SPNET was 57%.30 In another study, high-dose chemotherapy was administered as consolidation therapy to 13 children younger than 6 years with SPNET, and this resulted in a 2-year EFS of 43%.11 In a study of 12 patients with pineoblastoma who were treated with CSI (36 Gy with local boost) followed by high-dose chemotherapy and stem cell rescue, the 4-year progression-free survival (PFS) estimate was 69%.31 Importantly, this study included two patients who were 0.3 and 1.1 years old who did not receive radiation therapy and were alive without disease at 35 and 125 months. High-dose chemotherapy has also demonstrated efficacy in the treatment of recurrent SPNET.10,32 Together with the results of our pilot study, these data indicate that SPNETs are chemosensitive and suggest dose-intensive chemotherapy as a successful treatment of newly diagnosed SPNET. The chemotherapy regimen in the present study is feasible to deliver after radiation therapy, and it permits the high dose intensity of agents active against SPNET.13,14
Radiation therapy is an important component of the treatment of SPNET. Although the introduction of effective chemotherapy regimens has allowed reductions to be made in the dose of radiation used to treat AR medulloblastoma, similar dose reductions have negatively affected cure rates among patients with SPNET.4,5,25,29,33–35 Timmermann et al.28 have recommended treating the entire neuraxis of patients with SPNET with at least 35 Gy and the primary tumor with at least 54 Gy. In another study by Massimino et al.,29 children older than 4 years with SPNET were treated with initial chemotherapy and then were treated with hyperfractionated accelerated CSI therapy (31.2–39 Gy) and boost to the tumor site to a total dose of 59.7–60 Gy. After the completion of hyperfractionated accelerated radiation therapy, these patients received either standard or high-dose chemotherapy followed by autologous stem cell rescue. The 3-year EFS and PFS for all 15 patients was 34% and 54%, respectively. Three of 10 patients who received myeloablative chemotherapy with autologous stem cell rescue relapsed, compared with three of five who received conventional chemotherapy after completion of radiation therapy. Massimino et al. concluded that in addition to higher doses of radiation therapy, high-dose chemotherapy with autologous stem cell rescue may have a positive effect on the outcome. In a recent report of the SIOP/UKCCSG (Societé Internationale Oncologie Pediatrique/U.K. Children’s Cancer Study Group) by Pizer et al.,27 68 patients were treated with radiation therapy that consisted of CSI (35 Gy) with an additional 20-Gy boost to the primary tumor site. Forty-four of these patients also received four cycles of chemotherapy before the start of radiation therapy, whereas the others received radiation therapy alone. The 5-year EFS was 40.7% for patients with nonpineal PNET and 71.4% for those with pineal PNET (14 patients). The overall 5-year EFS estimate was 47% for the entire group. The addition of preirradiation chemotherapy did not favorably affect EFS. These results once again con-firm the relatively good outcome for pineal SPNET compared with nonpineal SPNET with all patients receiving at least 35 Gy to the craniospinal axis.
Results from the evaluations of cognitive function indicate a deterioration of academic achievement skills over time from diagnosis. This deterioration was seen in all three domains—reading, spelling, and math—placing successful completion of school at risk. These declines in academic function are not surprising given the supratentorial locations of the tumors and the uniform administration of focal tumor bed boosts to 55.8 Gy, often at midline. Despite the expectation, because successful completion of school is a critical requirement for later adult productivity and vocational choice, the survivors evaluated in the present study are vulnerable to experiencing a diminished quality of life.36,37 The results in the present study are limited by the small sample size and refusal of some patients to complete serial cognitive evaluation.
We have shown that patients with newly diagnosed AR SPNET treated with reduced-dose CSI followed by high-dose chemotherapy have excellent EFS. We consider the four courses of nonmyeloablative high-dose chemotherapy with stem cell rescue to be effective in delivering active agents at high dose intensity (in our study, the planned cyclophosphamide dose intensity was 1,000 mg/m2 per week). In addition, patients with HR disease treated with higher doses of CSI and adjuvant chemotherapy also experienced durable disease control.
Acknowledgments
This work was supported by Cancer Center (CORE) support grant CA 21765 from the National Institutes of Health, Musicians against Childhood Cancer, the Noyes Brain Tumor Foundation, Ryan McGhee Foundation, the Anderson Foundation, and the American Lebanese Syrian Associated Charities.
References
- 1.Gaffney CC, Sloane JP, Bradley NJ, et al. Primitive neuroectodermal tumours of the cerebrum. Pathology and treatment. J Neurooncol. 1985;3(1):23–33. doi: 10.1007/BF00165168. [DOI] [PubMed] [Google Scholar]
- 2.Bruno LA, Rorke LB, Norris DG. Primitive neuroectodermal tumors of infancy and childhood. In: Humphrey GB, Dehner LP, Grindey GB, et al., editors. Pediatric Oncology. Vol. 1. Boston, MA: Nijhoff; 1985. pp. 265–267. [Google Scholar]
- 3.Rorke LB. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumours. J Neuropathol Exp Neurol. 1983;42(1):1–15. [PubMed] [Google Scholar]
- 4.Cohen BH, Zeltzer PM, Boyett JM, et al. Prognostic factors and treatment results for supratentorial primitive neuroectodermal tumors in children using radiation and chemotherapy. A Children’s Cancer Group randomized trial. J Clin Oncol. 1995;13:1687–1696. doi: 10.1200/JCO.1995.13.7.1687. [DOI] [PubMed] [Google Scholar]
- 5.Jakacki RI, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children. A report of the Children’s Cancer Group. J Clin Oncol. 1995;13:1377–1383. doi: 10.1200/JCO.1995.13.6.1377. [DOI] [PubMed] [Google Scholar]
- 6.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 7.Russo C, Pellarin M, Tingby O, et al. Comparative genomic hybridization in patients with supratentorial and infratentorial primitive neuroectodermal tumors. Cancer. 1999;86:331–339. doi: 10.1002/(sici)1097-0142(19990715)86:2<331::aid-cncr18>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson J, Wickramasinghe C, Ross F, Crolla J, Ellison D. Imbalances of chromosome 17 in medulloblastomas determined by comparative genomic hybridisation and fluorescence in situ hybridisation. Mol Pathol. 2000;53:313–319. doi: 10.1136/mp.53.6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett ME, White EC, Sih S, et al. Chromosome arm 17p deletion analysis reveals molecular genetic heterogeneity in supratentorial and infratentorial primitive neuroectodermal tumors of the central nervous system. Cancer Genet Cytogenet. 1997;97:25–31. doi: 10.1016/s0165-4608(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 10.Graham ML, Herndon JE, Casey JR, et al. High-dose chemotherapy with autologous stem-cell rescue in patients with recurrent and high-risk pediatric brain tumors. J Clin Oncol. 1997;15(5):1814–1823. doi: 10.1200/JCO.1997.15.5.1814. [DOI] [PubMed] [Google Scholar]
- 11.Mason WP, Grovas A, Halpern S, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 12.Broniscer A, Nicolaides TP, Dunkel IJ, et al. High-dose chemotherapy with autologous stem-cell rescue in the treatment of patients with recurrent non-cerebellar primitive neurectodermal tumors. Pediatr Blood Cancer. 2004;42(3):261–267. doi: 10.1002/pbc.10369. [DOI] [PubMed] [Google Scholar]
- 13.Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol. 2001;19(10):2696–2704. doi: 10.1200/JCO.2001.19.10.2696. [DOI] [PubMed] [Google Scholar]
- 14.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma–96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 15.Stewart CF, Iacono LC, Chintagumpala M, et al. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor (PNET) J Clin Oncol. 2004;22(16):3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [Accessed August 9, 2006];Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Available at http://ctep.cancer.gov/reporting/ctc.html.
- 17.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. New York: Psychological Corporation; 1991. [Google Scholar]
- 18.Wechsler D. Wechsler Individual Achievement Test. New York: Psychological Corporation; 1992. [Google Scholar]
- 19.Sattler JM. Assessment of Children. San Diego, CA: 1992. p. 1170. [Google Scholar]
- 20.Achenbach TM. Manual for the CBCL/4–18. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 21.Kaplan EJ, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–482. [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- 25.Albright AL, Wisoff JH, Zeltzer P, et al. Prognostic factors in children with supratentorial (nonpineal) primitive neuroectodermal tumors. A neurosurgical perspective from the Children’s Cancer Group. Pediatr Neurosurg. 1995;22(1):1–7. doi: 10.1159/000121292. [DOI] [PubMed] [Google Scholar]
- 26.Reddy AT, Janss AJ, Phillips PC, et al. Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer. 2000;88(9):2189–2193. doi: 10.1002/(sici)1097-0142(20000501)88:9<2189::aid-cncr27>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Pizer BL, Weston CL, Robinson KJ, et al. Analysis of patients with supratentorial primitive neuroectodermal tumors entered into the SIOP/UKCCSG PNET 3 study. Eur J Cancer. 2006;42:1120–1128. doi: 10.1016/j.ejca.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 28.Timmermann B, Kortmann RD, Kuhl J, et al. Role of radiotherapy in the treatment of supratentorial primitive neuroectodermal tumors in childhood: results of the prospective German brain tumor trials HIT 88/89 and 91. J Clin Oncol. 2002;20(3):842–849. doi: 10.1200/JCO.2002.20.3.842. [DOI] [PubMed] [Google Scholar]
- 29.Massimino M, Gandola L, Spreafico F, et al. Supratentorial primitive neuroectodermal tumors (S-PNET) in children: a prospective experience with adjuvant intensive chemotherapy and hyperfractionated accelerated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(4):1031–1037. doi: 10.1016/j.ijrobp.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Kuhl J, Muller HL, Berthold F, et al. Preradiation chemotherapy of children and young adults with malignant brain tumors: results of the German pilot trial HIT’88/’89. Klin Padiatr. 1998;210(4):227–233. doi: 10.1055/s-2008-1043883. [DOI] [PubMed] [Google Scholar]
- 31.Gururangan S, McLaughlin C, Quinn J, et al. High-dose chemotherapy with autologous stem-cell rescue in children and adults with newly diagnosed pineoblastomas. J Clin Oncol. 2003;21:2187–2191. doi: 10.1200/JCO.2003.10.096. [DOI] [PubMed] [Google Scholar]
- 32.Gururangan S, Dunkel I, Goldman S, et al. Myeloablative chemotherapy with autologous bone marrow rescue in young children with recurrent malignant brain tumors. J Clin Oncol. 1998;16:2486–2493. doi: 10.1200/JCO.1998.16.7.2486. [DOI] [PubMed] [Google Scholar]
- 33.Goldwein JW, Radcliffe J, Johnson J, et al. Updated results of a pilot study of low dose craniospinal irradiation plus chemotherapy for children under five with cerebellar primitive neuroectodermal tumors (medulloblastoma) Int J Radiat Oncol. 1996;34:889–904. doi: 10.1016/0360-3016(95)02080-2. [DOI] [PubMed] [Google Scholar]
- 34.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 35.Packer RJ, Gajjar A, Vezina G, et al. Phase III prospective randomized study of craniospinal radiation therapy followed by one of two adjuvant chemotherapy regimens for newly-diagnosed average risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 36.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long-terms survivors of childhood cancer. Cancer. 2003;97:1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- 37.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]